The Agenzia Italiana del Farmaco (Aifa) and the Italian Ministry of Health authorized the fourth dose of the BNT162b2 vaccine (BioNTech/Pfizer) to subjects with a marked impairment of the immune response from 1 March 2022.1 Immunocompromised patients have heterogeneous levels of immunosuppression, with subsequent heterogeneous vaccine responses.2 Knowledge of these differences between various patients’ subgroups may improve the preventive measures. We characterized the humoral and cellular immune responses before and after the fourth BNT162b2 vaccine dose in patients with solid tumors.
The current analysis included 23 patients with solid tumors (6 females, 17 males; median age 66 years, range 48-86 years) who were on active treatment at the time of the fourth dose. Lung cancer (82%) was the most common tumor subtype. Twenty-one patients (91%) were on immunotherapy alone and two patients (9%) were on chemo-immunotherapy (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2022.07.012). We have considered only patients without a documented previous COVID-19 infection to exclude the confounder of ‘hybrid immunity’. Patients received the fourth BNT162b2 vaccine dose 6 months after their third dose. The most common side-effect was pain at the injection site (65.2%). Nobody had grade 3-4 side-effects (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2022.07.012).
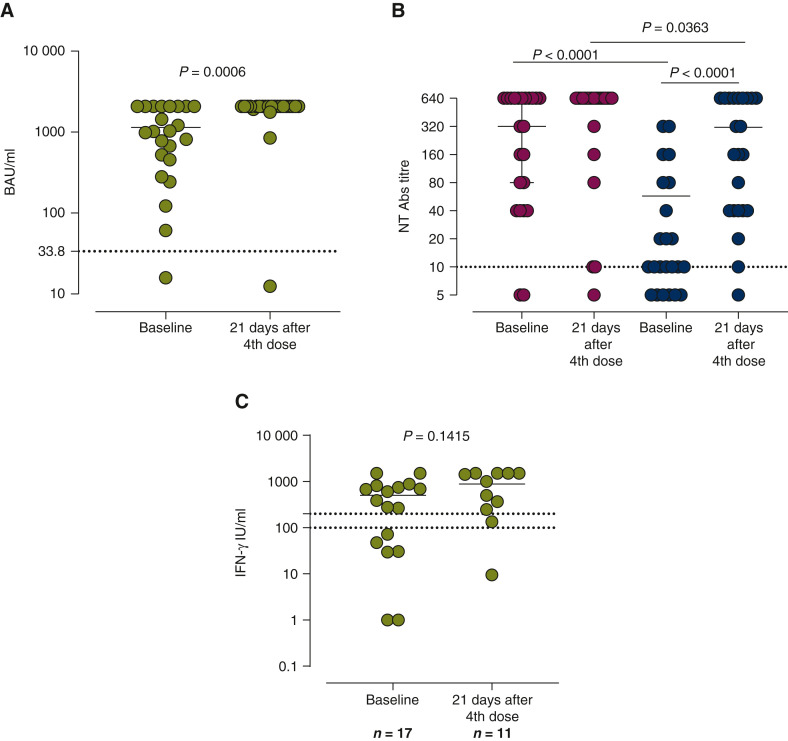
Three weeks after the fourth dose, all subjects except one (on anti-programmed cell death protein 1) developed positive levels of anti-spike antibodies. A significant difference was detected between the baseline, median 1020 [interquartile range (IQR) 456-2020] BAU/ml and 3 weeks after the fourth dose, when the majority of patients (82.6%) reached levels >2080 BAU/ml (P = 0.006) (Figure 1 A). As regards neutralizing antibodies titer 3 weeks after the fourth dose, the percentage of responders against wild type (WT) strain was 91% (21/23) and the median titer was 1 : 320 (IQR 1 : 40-1 : 640). Before the fourth dose, positive values were detected in 22/23 patients and the median titer was 1 : 640 (IQR 1 : 320-1 : 640). No difference between the baseline and 3 weeks after the fourth dose was detected (Figure 1B). At the baseline, the percentage of responders against the Omicron strain was 73% (17/23) and 95% (22/23) after the fourth dose, respectively. A significant difference between the baseline and 3 weeks after the fourth dose was observed [median 1 : 10 (IQR <1 : 10-1 : 160) versus 1 : 160 (IQR 1 : 40-1 : 640); P < 0.0001] (Figure 1B). A significant difference between WT and Omicron was detected, not only 3 weeks after the fourth dose (P < 0.0001) but also before (P = 0.0363). The percentage of subjects with positive T-cell response was 65% before and 90% after the fourth dose (P = 0.1914). The median level of response was higher at 3 weeks after the fourth dose, but this difference was not statistically significant (P = 0.1415; Figure 1C). The third dose is able to reinforce anti-COVID-19 immunity in patients with cancer,3 but the duration of protection is difficult to predict, because the exact mechanisms of protective immunity against SARS-CoV-2 are unknown yet.
Figure 1.
(A) Immunoglobulin G (IgG) antibody levels. (B) Neutralizing antibody titers. (C) Spike-specific T-cell response.
We measured IgG antibody levels, the serum neutralizing antibody (NT Ab) titers and the increase of spike-specific T-cell response between the baseline and 3 weeks after the fourth dose of BNT162b2 SARS-CoV-2 vaccine. According to our protocol,5 anti-S IgG levels were determined with a Trimeric assay (Liaison, DiaSorin, Saluggia, Italy) and the results were given as BAU/ml (positive results >33.8 BAU/ml). The NT Ab titers against the wild type (WT) strain (dark gray) and B.1.1.529 (omicron) variant strain (light gray) were measured as previously reported6; we defined the results as positive when NT Ab was ≥1 : 10. T-cell response against the spike protein was detected by the interferon-γ (IFN-γ) release assay (IGRA from Euroimmun, Lübeck, Germany).6 A positive T-cell response was defined as >200 mIU/ml whereas the results ranging from 100 to 200 mIU/ml were defined as borderline. GraphPad Prism 8.3.0 (GraphPad Software, La Jolla, CA) was used for statistical analyses. A two-sided P value <0.05 is considered statistically significant. Data were described with the median and interquartile range if continuous and as counts and percentage if categorical. The comparison between two groups was carried out using the Mann–Whitney (unpaired samples) or Wilcoxon (paired samples) test while Spearman’s test was used for the correlation analysis. Fisher’s exact test was used for the comparison of categorical variables.
Our data suggest that the fourth dose may increase the vaccine effectiveness also against the Omicron variant. Future larger studies are warranted to confirm our results. Finally, repeated booster vaccination in patients with solid tumors on active treatment appears without any safety concerns, as previously reported by Waissengrin et al.4
Acknowledgments
Funding
This work was supported by Ricerca Corrente [grant number 08067620], Fondazione IRCCS Policlinico San Matteo, Ricerca Finalizzata [grant BIAS number 2020-12371760], and from European Commission—Horizon 2020 (EU project 101003650 – ATAC).
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Available at. https://www.salute.gov.it/imgs/C_17_pagineAree_5452_43_file.pdf
- 2.See K.C. Vaccination for the prevention of infection among immunocompromised patients: a concise review of recent systematic reviews. Vaccines (Basel) 2022;10(5):800. doi: 10.3390/vaccines10050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro L.C., Thakkar A., Campbell S.T., et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2021;40(1):3–5. doi: 10.1016/j.ccell.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22(5):581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasagna A., Agustoni F., Percivalle E., et al. A snapshot of the immunogenicity, efficacy and safety of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors: a longitudinal cohort study. ESMO Open. 2021;6(5) doi: 10.1016/j.esmoop.2021.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasagna A., Bergami F., Lilleri D., et al. Immunogenicity and safety after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with cancer on active treatment: a prospective cohort study. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.