Abstract
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening disease that may be triggered by inflammation, including infection or vaccination. Since the start of the COVID-19 pandemic, several case reports were published on de novo or relapsed immune TTP (iTTP) in COVID-19-infected patients. Case reports of iTTP episodes following vaccination against COVID-19 are also emerging. We report a case of relapsed iTTP in a patient who received Moderna mRNA-1273 SARS-CoV-2 vaccine and developed concurrent severe COVID-19 infection. The patient’s iTTP was successfully managed with caplacizumab, therapeutic plasma exchange and high-dose steroids. We summarise published cases of iTTP associated with COVID-19 infection or vaccination.
Keywords: Thrombotic Thrombocytopenic purpura, COVID-19, Vaccination/immunisation
Background
Immune thrombotic thrombocytopenic purpura (iTTP) is a rare, life-threatening disorder caused by severe ADAMTS13 deficiency due to autoantibodies and resulting in the accumulation of ultra-large von Willebrand factor (vWF) multimers and microvascular thrombi.1 Management includes therapeutic plasma exchange (TPE), immunosuppression and anti-vWF therapy.1
The role of ADAMTS13 and vWF in COVID-19 pathophysiology is not completely understood. Recent studies demonstrated an increased vWFAg/ADAMTS13 ratio, possibly driven by endothelial injury and excess ADAMTS13 consumption due to elevated vWF.2 This disruption of the ADAMTS13–vWF axis was associated with increased mortality in patients with COVID-19 in one study.3 However, another study found no pathological role for ADAMTS13 in COVID-19.4 Several cases of COVID-19-associated iTTP were reported,5–20 yet causation has not been demonstrated. iTTP following vaccination has been previously reported21 and cases of COVID-19 vaccine-associated iTTP are also emerging.22–25 We present a complicated case of relapsed iTTP following concurrent COVID-19 vaccination and infection.
Case presentation
A man in his 70s with a history of stroke, left ventricular aneurysm thrombosis (on chronic anticoagulation) and chronic kidney disease (baseline creatinine 130 µmol/L) presented with iTTP relapse. iTTP was first diagnosed 7 years ago, had no identifiable trigger and was complicated by refractoriness and severe acute kidney injury (AKI). He recovered following TPE, steroids and rituximab. He subsequently had three relapses, which were successfully treated with TPE and immunosuppression. He was well for the past 4 years and his remission ADAMTS13 activity was normal (71%).
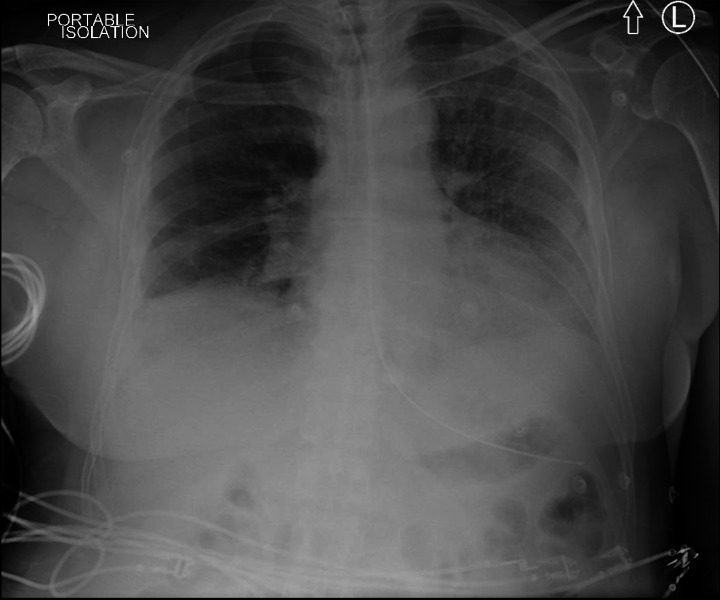
The patient became unwell 7 days after his first dose of Moderna COVID-19 vaccine and 3–4 days after exposure to an individual infected with COVID-19. On admission to the hospital, the patient was febrile and dyspnoeic, with a chest X-ray (CXR) suggestive of left lower lobe pneumonia. Blood work (see table 1) revealed microangiopathic haemolytic anaemia, thrombocytopenia, AKI and elevated troponin. There were no neurological symptoms and CT of the head showed no acute abnormalities. On admission day 3, his oxygen requirements increased, necessitating transfer to the critical care for non-invasive respiratory support. On day 6, CXR revealed bilateral pneumonia (see figure 1) and the patient was intubated for severe respiratory failure.
Table 1.
Laboratory presentation on admission
| Laboratory parameter | Value |
| Haemoglobin (g/L) | 107 |
| Platelet (109/L) | 11 |
| Lactate dehydrogenase (U/L) | 2776 (upper limit of normal 195 U/L) |
| Indirect bilirubin (µmol/L) | 32 |
| Haptoglobin (g/L) | Undetectable (lower limit of normal 0.3 g/L) |
| Reticulocyte count (×109/L) | 20 |
| Blood film | Moderate red blood cell fragments |
| INR (on warfarin) | 2.25 |
| aPTT (s) | 41.9 |
| Fibrinogen (g/L) | 2.32 |
| ADAMTS13 activity (U/mL) | <0.01 (less than 1%) |
| ADAMTS13 inhibitor (U/mL) | >15 |
| Creatinine (µmol/L) | 732 (upper limit of normal 112 µmol/L) |
| High-sensitivity troponin (ng/L) | 323 (upper limit of normal <18 ng/L) |
aPTT, activated partial thromboplastin time; INR, international normalised ratio.
Figure 1.
Chest X-ray on day 6 of admission.
Differential diagnosis
On admission, COVID-19 infection was suspected and the patient was placed in isolation. The initial COVID-19 nasopharyngeal swab was negative (Altona RealStar SARS-CoV-2 RT-PCR Kit 1.0). COVID-19 swab was repeated on day 6 and was positive. iTTP relapse was confirmed by undetectable ADAMTS13 activity and positive ADAMTS13 inhibitor (see table 1).
Treatment
Corticosteroids (1 mg/kg/day (day 1–20) followed by 10 mg/week taper) and TPE (1.0–1.5 plasma volume exchange with solvent detergent-treated plasma daily for 8 days) were started on presentation empirically. After confirmation of iTTP diagnosis, caplacizumab (anti-vWF nanobody) 11 mg once daily was added, while rituximab was deferred. The patient was also started on haemodialysis. Following confirmation of COVID-19 infection on day 6, he was switched to dexamethasone for a few days and then resumed on high-dose prednisone. Warfarin was held on admission; prophylactic dose low molecular weight heparin was started once platelets were above 30 000 and switched to therapeutic dose following platelet normalisation. The patient was subsequently resumed on warfarin. Fifteen doses of caplacizumab were given; caplacizumab was discontinued once clinical remission and ADAMTS13 activity over 10% were reached.
Outcome and follow-up
Treatment with TPE, corticosteroids, and caplacizumab induced a rapid and sustained clinical remission. No bleeding was noted with caplacizumab use. The patient developed deep vein thrombosis and several infections, including pulmonary aspergillosis. He underwent tracheostomy on day 24, was decannulated on day 47 and finally weaned off oxygen by day 58. Treatment of iTTP with rituximab (anti-CD20 antibody) was deferred due to concerns about exacerbating infections. The patient eventually recovered and was discharged from the hospital on day 74. Haemodialysis was discontinued and renal function returned to baseline by discharge. A few months later, he received rituximab (375 mg/m2 weekly for 4 weeks) for persistent severe ADAMTS13 deficiency (less than 10%) and his activity improved (34%). He eventually received the second dose of COVID-19 vaccine (Pfizer-BioNTech) without complications.
Discussion
iTTP is a life-threatening blood disease that may be triggered by immune response to infections, vaccinations and tissue injury.26 It is treated with TPE, immunosuppression and anti-vWF therapy.1 We described an iTTP relapse in a patient with recent COVID-19 vaccination and concurrent COVID-19 infection. The patient responded well to iTTP treatment, however, suffered significant complications of COVID-19. There were several unique features in this case. The patient presented with severe AKI during two of his iTTP episodes. Severe renal dysfunction necessitating renal replacement therapy is uncommon in iTTP.1 Following treatment, he returned to baseline kidney function both times. When added to TPE and immunosuppression, caplacizumab leads to faster platelet count response (HR, 1.65; p<0.001) and decreased refractoriness and mortality in iTTP.27 However, there are no studies on the safety of caplacizumab in patients on haemodialysis. Our patient received caplacizumab and tolerated it without any complications. Further studies on the safety of caplacizumab in patients with severe renal dysfunction are needed. Due to severe concurrent COVID-19 infection, rituximab was not administered. Rituximab is highly effective in treatment of antibody-mediated diseases such as iTTP.1 It causes rapid and sustained depletion of B cells, impairing humoral response to COVID-19 infection, and can potentially place patients at risk of reactivation or reinfection.28 Among reported cases of iTTP in patients with COVID-19,5–20 8 of 19 patients were treated with rituximab (see online supplemental table 2). Of these eight patients, three died; two of the deaths were due to COVID-19-related severe lung disease and multiorgan failure. It remains unclear whether immunosuppressive effect of rituximab could result in more severe COVID-19 and hence should be avoided during acute infection. Our team opted to delay rituximab and monitor for iTTP exacerbation. Considering the ongoing pandemic and the patient’s immunosuppressed state, we offered a second dose of COVID-19 vaccine. We debated the timing of the second dose, as well as using a different vaccine. However, the patient was worried about the vaccine triggering iTTP relapse and initially opted to defer the second dose.
bcr-2021-247524supp001.pdf (242.7KB, pdf)
In our case, iTTP relapse occurred within 7 days of COVID-19 vaccination and 3–4 days post-COVID-19 exposure, after 4 years of remission. Timing of vaccination and exposure in this case, as well as pathophysiology of iTTP, suggest that both events may have contributed to this relapse. Previous publications reported imbalance of the vWF/ADAMTS13 axis in patients with COVID-192 and remarked that ADAMTS13 activity ranged from normal to significantly reduced.2 4 However, ADAMTS13 deficiency alone may not be sufficient to cause TTP.1 On the other hand, association between iTTP and infections is well described; it could be a result of inflammation-mediated increase in ADAMTS13 autoantibodies, complement activation and reduced cleavage of vWF by ADAMTS13.26 Hence, it is conceivable that COVID-19 infection could trigger iTTP. Similarly, there may be a causative link between vaccination and iTTP. Case reports of iTTP associated with influenza, pneumococcal and rabies vaccination were published previously.21 iTTP following different types of COVID-19 vaccines has also been reported.22–25 The time between vaccination and iTTP relapse was consistent at 5–7 days in most cases, whereas the time between vaccination and de novo iTTP was variable at 5–37 days (see online supplemental table 3). Ongoing surveillance for vaccine-associated complications, including iTTP, is warranted. Almost 500 million COVID-19 infections were reported globally to WHO and over 11 billion doses of COVID-19 vaccines were administered as of 13 April 2022.29 Our literature review identified 20 cases of de novo or relapsed iTTP in COVID-19-infected adult patients and 14 reports of iTTP after COVID-19 vaccination (see online supplemental tables). Considering the total number of infections and vaccinations, the reported number of COVID-19 infection or vaccine-associated iTTP cases remains very low. The medical and patient community should feel reassured about the safety of COVID-19 vaccine vis-à-vis risk of iTTP. COVID-19 infection appears to be a rare trigger for iTTP.
In conclusion, we presented a case of relapsed iTTP within 7 days of the first dose of Moderna mRNA COVID-19 vaccine with concurrent COVID-19 infection. The literature review identified a very low number of iTTP cases associated with either COVID-19 infection or vaccination, suggesting that neither COVID-19 infection nor vaccination represents a strong trigger for iTTP. Ongoing surveillance is warranted. Careful analysis of risks and benefits is required to decide on whether to proceed with anti-CD20 therapy in patients with iTTP and COVID-19 infection.
Patient’s perspective.
Being in the ICU with COVID were the darkest days of my life, I feel lucky to have recovered.
Learning points.
COVID-19 infection or vaccination-associated immune thrombotic thrombocytopenic purpura (iTTP) is very rare.
Post-vaccination monitoring for relapse may be warranted in patients with iTTP.
Risk of anti-CD20 therapy in patients with iTTP and COVID-19 infection is unclear.
Footnotes
Twitter: @BrandonTse3
Contributors: FF collected data, conducted literature review and prepared the first draft of the manuscript. BT participated in literature review and writing of the manuscript. KP supervised the project and contributed to writing of the manuscript. All authors have read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: KP participated in industry-sponsored randomised controlled trials and received honoraria for speaking and consulting for Ablynx/Sanofi, Shire/Takeda.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Parental/guardian consent obtained.
References
- 1.Sukumar S, Lämmle B, Cataland SR. Thrombotic thrombocytopenic purpura: pathophysiology, diagnosis, and management. J Clin Med 2021;10:536. 10.3390/jcm10030536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mancini I, Baronciani L, Artoni A, et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost 2021;19:513–21. 10.1111/jth.15191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweeney JM, Barouqa M, Krause GJ, et al. Low ADAMTS13 activity correlates with increased mortality in COVID-19 patients. TH Open 2021;5:e89–103. 10.1055/s-0041-1723784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escher R, Breakey N, Lämmle B. Adamts13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb Res 2020;192:174–5. 10.1016/j.thromres.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AL-Ansari R, Bakkar M, Abdalla L. Critical care COVID-19 patient with a picture of thrombotic thrombocytopenia purpura. EJCRIM 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albiol N, Awol R, Martino R. Autoimmune thrombotic thrombocytopenic purpura (TTP) associated with COVID-19. Ann Hematol 2020;99:1–2. 10.1007/s00277-020-04097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altowyan E, Alnujeidi O, Alhujilan A, et al. COVID-19 presenting as thrombotic thrombocytopenic purpura (TTP). BMJ Case Rep 2020;13:e238026. 10.1136/bcr-2020-238026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aminimoghaddam S, Afrooz N, Nasiri S, et al. A COVID-19 pregnant patient with thrombotic thrombocytopenic purpura: a case report. J Med Case Rep 2021;15:104. 10.1186/s13256-020-02577-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaulieu M-C, Mettelus DS, Rioux-Massé B, et al. Thrombotic thrombocytopenic purpura as the initial presentation of COVID-19. J Thromb Haemost 2021;19:1132–4. 10.1111/jth.15231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capecchi M, Mocellin C, Abbruzzese C, et al. Dramatic presentation of acquired thombotic thrombocytopenic purpura associated with COVID-19. Haematologica 2020;105:e540. 10.3324/haematol.2020.262345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MK, Sheena L, Shafir Y. An early unexpected immune thrombotic thrombocytopenic purpura relapse associated with SARS-CoV-2 infections: a case report and literature review. Acta Haematol 2021;144. 10.1159/000514283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darnahal M, Azhdari Tehrani H, Vaezi M, et al. Covid-19 and thrombotic thrombocytopenic purpura: a case report. Int J Hematol Oncol Stem Cell Res 2021;15:72–4. 10.18502/ijhoscr.v15i1.5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhingra G, Maji M, Mandal S. COVID 19 infection associated with thrombotic thrombocytopenic purpura. J Thromb Thrombolysis 2021:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorooshi G, Lalehzar SS, Nasri M, et al. Thrombotic thrombocytopenic purpura with conjunctivitis in a patient with coronavirus disease 2019 infection. Adv Biomed Res 2020;9:71. 10.4103/abr.abr_190_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hindilerden F, Yonal-Hindilerden I, Akar E, et al. Covid-19 associated autoimmune thrombotic thrombocytopenic purpura: report of a case. Thromb Res 2020;195:136–8. 10.1016/j.thromres.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law L, Ho G, Cen D, et al. Atypical manifestations of coronavirus disease 2019 (COVID-19)-associated autoimmune thrombotic thrombocytopenic purpura. Clin Case Rep 2021;9:1402–4. 10.1002/ccr3.3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maharaj S, Xue R, Rojan A. Thrombotic thrombocytopenic purpura (TTP) response following COVID-19 infection: implications for the ADAMTS-13-von Willebrand factor axis. J Thrombo Haemost 2020;19. 10.1111/jth.15230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolotti D, Bignami EG, Rossi S, et al. A case of thrombotic thrombocytopenic purpura associated with COVID-19. J Thromb Thrombolysis 2021;52:468–70. 10.1007/s11239-020-02362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tehrani HA, Darnahal M, Vaezi M, et al. COVID-19 associated thrombotic thrombocytopenic purpura (TTP); A case series and mini-review. Int Immunopharmacol 2021;93:107397. 10.1016/j.intimp.2021.107397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma DP, Dandu H, Yadav G, et al. Complicated case of COVID-19 disease with overlapping features of thrombotic thrombocytopenic purpura and haemophagocytic lymphohistiocytosis. BMJ Case Rep 2021;14:e242202. 10.1136/bcr-2021-242202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yavaşoğlu İrfan,. Vaccination and thrombotic thrombocytopenic purpura. Turk J Haematol 2020;37:218–9. 10.4274/tjh.galenos.2020.2020.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bruijn S, Maes M-B, De Waele L, et al. First report of a de novo iTTP episode associated with an mRNA-based anti-COVID-19 vaccination. J Thromb Haemost 2021;19:2014–8. 10.1111/jth.15418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sissa C, Al-Khaffaf A, Frattini F, et al. Relapse of thrombotic thrombocytopenic purpura after COVID-19 vaccine. Transfus Apher Sci 2021;60:103145. 10.1016/j.transci.2021.103145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yocum A, Simon EL. Thrombotic thrombocytopenic purpura after Ad26.COV2-S vaccination. Am J Emerg Med 2021;49:441.e3–441.e4. 10.1016/j.ajem.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavenski K. Relapse of immune thrombotic thrombocytopenic purpura following vaccination with COVID19 mRNA vaccine. TH Open 2021;5:e335–7. 10.1055/s-0041-1732342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavenski K, Huang S-HS, Patriquin CJ. Predictors of relapse and preventative strategies in immune thrombotic thrombocytopenic purpura. Expert Rev Hematol 2021;14:1027–40. 10.1080/17474086.2021.2003703 [DOI] [PubMed] [Google Scholar]
- 27.Peyvandi F, Cataland S, Scully M, et al. Caplacizumab prevents refractoriness and mortality in acquired thrombotic thrombocytopenic purpura: integrated analysis. Blood Adv 2021;5:2137–41. 10.1182/bloodadvances.2020001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levavi H, Lancman G, Gabrilove J. Impact of rituximab on COVID-19 outcomes. Ann Hematol 2021;100:2805–12. 10.1007/s00277-021-04662-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . Who coronavirus (COVID-19) Dashboard, 2022. Available: https://covid19.who.int/ [Accessed 13 Apr 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2021-247524supp001.pdf (242.7KB, pdf)