Abstract
Strains of Escherichia coli carrying mutations at the relA locus are deficient in cyclopropane fatty acid (CFA) synthesis, a phospholipid modification that occurs as cultures enter stationary phase. RelA protein catalyzes the synthesis of guanosine-3′,5′-bisdiphosphate (ppGpp); therefore, ppGpp was a putative direct regulator of CFA synthesis. The nucleotide could act by increasing either the activity or the amount of CFA synthase, the enzyme catalyzing the lipid modification. We report that the effect of RelA on CFA synthesis is indirect. In vitro and in vivo experiments show no direct interaction between ppGpp and CFA synthase activity. The relA effect is due to ppGpp-engendered stimulation of the synthesis of the alternative sigma factor, RpoS, which is required for function of one of the two promoters responsible for expression of CFA synthase.
When enterobacteria such as Escherichia coli are starved for amino acids, they elicit the stringent response, characterized by the accumulation of the nucleotide guanosine 3′,5′-bisdiphosphate (ppGpp) (and the related compound guanosine 3′-diphosphate-5′-bistriphosphate) (4). Other metabolic processes such as nutrient limitation cause accumulation of ppGpp. Two different enzymes, the ribosome-associated RelA protein (ppGpp synthetase I) and the SpoT protein (ppGpp synthetase II), synthesize ppGpp (4). The latter protein can act either as a synthetase or as a hydrolyase, depending on the growth conditions (27). Alterations in the intracellular level of ppGpp have pleiotropic effects on metabolism; e.g., the nucleotide binds to the β subunit of RNA polymerase (RNAP) and modifies polymerase specificity (19), inhibits the accumulation of rRNA and protein synthesis (21), and stimulates the metabolism of certain amino acids (3, 4). Moreover, accumulation of ppGpp positively regulates rpoS expression (5, 15), leading to increased amounts of the alternative sigma factor RpoS, a major regulator involved in the transcription of many stress-induced genes (10).
Previous studies indicated that relA strains are deficient in cyclopropane fatty acid (CFA) synthesis (6, 7, 22). Since wild-type and relA strains differ in ppGpp content, these studies suggested that ppGpp acts as a positive effector of CFA synthesis, perhaps by stimulating the activity of the cytosolic enzyme, CFA synthase, that catalyzes this postsynthetic methylenation of unsaturated fatty acyl chains to their cyclopropane derivatives (8, 23, 26). CFA synthase uses a soluble substrate, S-adenosylmethionine (AdoMet), to modify an insoluble substrate, the phospholipid unsaturated fatty acyl moieties, which reside in the hydrophobic interior of the lipid bilayer (see reference 8 for a review). CFA synthesis largely occurs as cultures enter stationary phase, and this timing is regulated by a combination of increased transcription and enzyme instability (25). The cfa gene has two promoters (25) (Fig. 1); one has the consensus sequence of a ς70-dependent promoter, whereas the other promoter is growth phase dependent and recognized by the alternative sigma factor RpoS (also called ς38 and ςS). The onset of CFA synthesis as cultures enter stationary phase is due to increased transcription of cfa from the RpoS-dependent promoter, whereas the ς70-dependent promoter is responsible for the low level of CFA synthesis in exponentially growing cultures (25). The aim of the present study was to determine the level at which ppGpp effects CFA synthesis. We show that there is no direct interaction of ppGpp with the enzyme and that the ppGpp effect is due solely to an increased RpoS content.
FIG. 1.
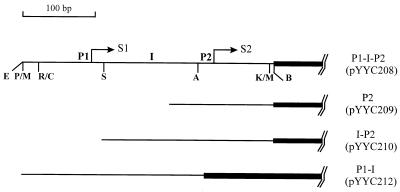
Schematic representation of the different cfa::lacZ transcriptional fusions. The thin lines denote the cfa promoter region, and the thick line denotes the lacZ gene. P1 and P2 are the two cfa promoters, S1 and S2 are the two transcriptional start sites, and I is the interpromoter region. Restriction sites used in the constructions: E, EcoRI; P, SphI; M, SmaI; R, EcoRV; C, ClaI; S, SspI; A, AccI; K, KpnI; B, BamHI.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study are E. coli K-12 derivatives (Table 1). Cultures were grown in Luria-Bertani (LB) medium (17) or in defined medium E (24) with vigorous shaking at 37°C. Growth was monitored by measuring the optical density at 600 nm. Antibiotics were used at the concentrations recommended by Miller (17). Strain YYC1098 was grown in minimal medium E containing 0.4% glucose as the carbon source and supplemented with, per liter, 50 mg of thiamine, 50 mg of methionine, 130 mg of adenosine, 20 mg of tryptophan, 50 mg of proline, and 55 mg of lysine. Strain YYC1098 was constructed by phage P1vir-mediated transduction of strain FT1 with a phage stock grown on strain CF1693 with selection for kanamycin resistance.
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Relevant genotype | Reference(s) |
|---|---|---|
| Strains | ||
| MG1655 | Wild type | 5, 27 |
| CF1693 | relA::kan spoT::cm of MG1655 | 5, 27 |
| FT1 | metE70 metB1 | 23 |
| FT17 | cfa of FT1 | 23 |
| ZK126 | W3110 lacZ | 2 |
| ZK1000 | rpoS::kan of ZK126 | 2 |
| YYC1098 | relA::kan of FT1 | This study |
| YYC1113 | λ lysogen of ZK126 carrying pYYC208 (P1-Ia-P2) fusion | This study |
| YYC1114 | λ lysogen of ZK126 carrying pYYC209 (P2) fusion | This study |
| YYC1115 | λ lysogen of ZK126 carrying pYYC210 (I-P2) fusion | This study |
| YYC1116 | λ lysogen of ZK126 carrying pYYC212 (P1-I) fusion | This study |
| YYC1117 | λ lysogen of ZK1000 carrying pYYC208 (P1-I-P2) fusion | This study |
| YYC1118 | λ lysogen of ZK1000 carrying pYYC209 (P2) fusion | This study |
| YYC1119 | λ lysogen of ZK1000 carrying pYYC210 (I-P2) fusion | This study |
| YYC1120 | λ lysogen of ZK1000 carrying pYYC212 (P1-I) fusion | This study |
| YYC1102 | relA::kan spoT::cm of ZK126 | This study |
| YYC1123 | relA::kan spoT::cm rpoS::tet of ZK126 | This study |
| Plasmids | ||
| pALS10 | Intact relA gene under tac promoter control | 21 |
| pALS13 | Truncated, active relA gene under tac promoter control | 21 |
| pALS14 | Inactive truncated relA gene under tac promoter control | 21 |
I, interpromoter region.
Chemicals and reagents.
[methyl-14C]methionine (45.5 μCi/mmol, 0.1 mCi/ml) and [γ-32P]ATP (6,000 Ci/mmol, 10 mCi/ml) were from Amersham; [methyl-3H]AdoMet (79 Ci/mmol, 0.55 mCi/ml) was from NEN; ATP, GTP, AdoMet, and S-adenosyl-l-homocysteine hydrolase were purchased from Sigma (Deisenhofen, Germany); polyethyleneimine thin-layer chromatography plates were from Macherey & Nagel (Düren, Germany).
Isolation of ribosomes and ppGpp synthesis.
For the synthesis of ppGpp, ribosomes were isolated as described by Krohn and Wagner (13). Strain ZK126 carrying plasmid pALS10 (which contains the wild-type relA gene under the isopropyl-β-d-thiogalactopyranoside [IPTG]-inducible tac promoter [21]) was grown to an A600 of 0.6, and relA expression was induced by addition of 100 μM IPTG for 2 h. Cells were harvested by centrifugation and resuspended in 50 mM potassium phosphate (pH 7.5). After disruption (French press at 11,000 lb/in2), large debris was removed by centrifugation and the supernatant was layered on top of a sucrose gradient (1 and 0.5 M sucrose in buffer A [50 mM Tris acetate {pH 8.0}, 15 mM magnesium acetate, 60 mM potassium acetate, 30 mM ammonium acetate, 1 mM dithiothreitol, 0.2 mM EDTA]) and centrifuged at 150,000 × g at 4°C for 2 h (13). The ribosomal pellet was washed with buffer A and gently resuspended in this buffer at 4°C. The concentration of the ribosomes was adjusted to 500 A260/ml of buffer and stored at −70°C.
The standard reaction mixture for the synthesis consists of 4 mM ATP, 2 mM GTP, and 50 A260 of ribosomes per ml of buffer A. After 1 to 3 h, the ribosomes were pelleted by ultracentrifugation (200,000 × g, 30 min), and the supernatant was used as the source of ppGpp in the CFA synthase assay. The reaction proceeded at 25°C for 1 to 3 h, and the yield of ppGpp was determined with a sample to which radioactive ATP was added as a tracer. RelA-mediated ppGpp(p) synthesis proceeded very rapidly such that within 30 min all of the GTP was completely converted to ppGpp. The products were separated by thin-layer chromatography on polyethyleneimine plates developed in 1.5 M sodium phosphate (pH 3.4) (13), and the radioactive areas were detected with a PhosphorImager.
Enzyme assays.
The CFA synthase assay was performed by the method of Taylor and Cronan (23). The source of the CFA synthase-containing crude extracts was either the wild-type strain MG1655 or strain CF1693 (relA spoT). The strains were grown in LB to stationary phase, harvested, washed with 50 mM Tris-HCl (pH 7.5), and resuspended in 100 mM potassium phosphate (pH 7.5) to give final concentration of 100 A600/ml buffer. Cells were broken by sonication for 3 min (Branson sonicator, microtip, output 3, cycle 50%), and debris was removed by centrifugation (12,000 × g, 10 min, 4°C) to give the crude cell extract.
A typical assay mixture consists of 10 to 20 μl of cell extract (250 to 500 μg of protein), 0.1 U of S-adenosyl-l-homocysteine hydrolase, 0.1 mg of liposomes (prepared from the cfa-deficient strain FT17 as described in reference 23), 0.1 mg of AdoMet, 2.5 μCi of [methyl-3H]AdoMet, and 10 μl of the ppGpp preparation (a final ppGpp concentration of approximately 90 μM) in a total volume of 100 μl. The reaction proceeded for 30 min at 37°C. All the other steps were done as described by Taylor and Cronan (23). Radioactivity in the lipid fraction was determined by scintillation counting.
β-Galactosidase was assayed as described by Miller (17), and activities are given in Miller units.
In vivo CFA synthase assay.
Derivatives of strain YYC1098 carrying the RelA overexpression plasmid pALS13 (which encodes a truncated, functional RelA protein) or plasmid pALS14 (which encodes a defective RelA protein) were grown in 10 ml of minimal medium E supplemented with glucose and amino acids as described above at 37°C. Synthesis of ppGpp was induced in exponentially growing cultures (A600 = 0.4) by addition of 100 μM IPTG for 20 min. Protein synthesis was inhibited with chloramphenicol (25 μg/ml of culture [final concentration]); the cells were washed in medium E plus chloramphenicol and resuspended in medium E containing l-[methyl-14C]methionine (11 μCi/ml, 24 μM). After incubation, 1-ml samples were removed and the lipids were isolated by the method of Bligh and Dyer (1) as modified by Kates et al. (12). The radioactivity in the final chloroform phase was determined by scintillation counting.
Construction of cfa promoter-lacZ transcriptional fusion plasmids.
Plasmid pYYC208 carrying both cfa promoters P1 and P2 fused to a promoterless lacZ gene was constructed from plasmids pAYW27 and pRS415. Plasmid pRS415, a lacZ-based operon fusion cloning vector, was obtained from R. W. Simons (20). Plasmid pAYW27, a deletion derivative of plasmid pAYW19 (26), contains a 340-bp fragment that includes the cfa promoter and part of the cfa coding sequence (the endpoint is the KpnI site at position 332 bp within cfa [26]). Plasmid pAYW27 was cut with KpnI and SphI (at multiple cloning sites of the vector), the ends were converted to blunt ends by T4 DNA polymerase treatment, and the 340-bp fragment was isolated from a gel and subcloned into SmaI site of plasmid pRS415 to form pYYC208. Expression of the pYYC208 lacZ from promoters P1 and P2 of cfa was detected as blue colonies on 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) medium, and the correct orientation of the insert was verified by restriction digests.
Plasmid pYYC209 carrying only the P2 cfa promoter fused to lacZ (lacking the P1 promoter and interpromoter region) was constructed from plasmids pAYW52 and pRS415. Plasmid pAYW52 was one of the nested cfa deletions derived from plasmid pAYW20 (26). Plasmid pAYW20 is identical to pAYW19 except that the cfa gene has the opposite orientation. The cfa gene in pAYW52 is the 180-bp deletion of the 5′ end of the region shown in Fig. 3 of reference 26, which removes promoter P1 and the interpromoter region sequence (see also Fig. 3 of reference 25). Plasmid pAYW52 was cut with NsiI (in the polylinker of the vector) and KpnI (in the cfa coding region), the DNA ends were converted to blunt ends by T4 DNA polymerase treatment, and the 160-bp fragment was ligated into the SmaI site of plasmid pRS415 to form plasmid pYYC209. Plasmids from blue colonies were verified by restriction digests as specified above.
Plasmid pYYC210 carrying the cfa P2 promoter and the interpromoter region sequence fused to lacZ was constructed from plasmid pYYC208. Plasmid pYYC208 was cut with SspI (at position 94 bp of the cfa sequence [see Fig. 3 of reference 25]) and BamHI (polylinker site), and the 240-bp fragment was ligated into the SmaI and BamHI sites of pRS415 to give plasmid pYYC210.
Plasmid pYYC212 carrying the P1 cfa promoter plus the interpromoter region sequence fused to lacZ was also constructed from plasmid pYYC208. Plasmid pYYC208 was cut first with AccI (at position 238 bp of the cfa sequence [see Fig. 3 of reference 25]), converted to blunt ends by DNA I (Klenow fragment) polymerase treatment, and then cut with EcoRI (polylinker site). The 270-bp EcoRI-AccI fragment was cloned between the EcoRI and SmaI sites of plasmid pRS415.
Figure 1 shows the relevant restriction sites in the cfa promoter region and the sites used to fuse the cfa promoters and lacZ in the plasmids described above. It should be noted that we constructed a cfa P1 promoter alone (without the interpromoter region) fused to lacZ (the first 85 bp of the cfa gene shown in Fig. 3 of reference 25), but the P1-lacZ fusion expression was sufficiently weak that recombination with the λRS45 (see below) could not be detected by screening for blue plaques.
Construction of lambda phage lysogens.
The cfa promoter-lacZ fusions of plasmids pYYC208, pYYC209, pYYC210, and pYYC212 were recombined into the specialized lambda phage λRS45 (20). The recombinant phages were detected by formation of blue plaques and then isolated and purified as described by Simons and coworkers (20). These phages were subsequently used to lysogenize the wild-type strain, ZK126, and its isogenic rpoS strain, ZK1000. For study of the effect of ppGpp, strains carrying each cfa promoter fusion as a lambda lysogen were transformed with either plasmid pALS10 or plasmid pALS14. In addition, strains YYC1102 and YYC1123 were lysogenized with the phages and transformed with plasmid pALS14.
SDS-PAGE and immunodetection.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (14), and the proteins were transferred with a semidry blotting device from Bio-Rad according to the manufacturer’s guidelines. Nitrocellulose blots were blocked for 2 h with 2% bovine serum albumin in phosphate-buffered saline containing 0.1% Tween. The primary antibody was a polyclonal anti-RpoS antiserum, and the secondary antibody was a goat anti-rabbit alkaline phosphatase-conjugated immunoglobulin G (Sigma). The blot was developed with 5-bromo-4-chloro-3-indolylphosphate–4-nitrotetrazolium chloride blue (Pierce) until sufficient intensity was obtained.
RESULTS AND DISCUSSION
Previous results (6, 7, 22) suggested that ppGpp could act as a positive effector of CFA synthesis perhaps at the level of CFA synthase. In other systems, metabolic regulation by ppGpp can act by directly affecting the activity of enzymes or indirectly through influencing gene expression. Three different mechanisms by which ppGpp could influence CFA synthesis have been demonstrated for other metabolic processes (4): (i) direct interaction of the nucleotide with CFA synthase; (ii) ppGpp binding to RNAP, with this complex specifically stimulating transcription from the cfa promoter; and (iii) an indirect effect due to an increased synthesis of RpoS, which in turn stimulates cfa transcription.
Effects of ppGpp on CFA synthase in vitro.
We first tested the possibility that ppGpp acted directly on CFA synthase by in vitro assay. For this purpose we synthesized ppGpp in an in vitro system (13) with a preparation in which nearly every ribosome contained RelA protein (21) (see Materials and Methods). CFA synthase assays were performed in the presence of 90 to 190 μM ppGpp. Under these conditions, enzyme activity was unaffected by the presence of ppGpp, regardless of the source of the cell extracts (i.e., strain MG1655 or strain CF1693). The activities did not differ from those of the controls containing only ATP and GTP (data not shown). In some assays we noted slight inhibitory or stimulatory effects upon addition of ppGpp (±10%), but these changes reflected only the accuracy of the CFA synthase assay. Thus, under the conditions tested, no effect of ppGpp on the CFA synthase reaction could be detected. Our results are consistent with those reported by Taguchi and coworkers (22), although no experimental details were given in that report.
In vivo effects of ppGpp on CFA synthase.
To test the possibility of a more complex effect of ppGpp on CFA synthase, we assayed CFA activities in vivo. The methionine auxotrophic strain YYC1098 was transformed with either plasmid pALS13 or the control plasmid pALS14, and the transformants were grown in minimal medium E plus glucose and amino acid supplements (see Materials and Methods). Plasmid pALS13 was used instead of pALS10, because the truncated, functional RelA protein encoded by pALS13 is not sensitive to chloramphenicol (21). After treatment of these cultures with IPTG for 20 min to induce ppGpp production, protein synthesis was blocked with chloramphenicol. The cells were then collected, washed, resuspended in the same medium, and labeled with l-[methyl-14C]methionine. Within 5 min after addition of the label, radioactivity was detected in the phospholipid fraction (as CFA moieties). The radioactivity in this fraction increased during 3 h until it reached a constant plateau, with no further changes over 18 h (data not shown). The same kinetics of CFA synthesis were seen under all conditions tested; i.e., the strain with elevated ppGpp content (carrying plasmid pALS13 induced with IPTG) or the strain carrying control plasmid pALS14 (which does not overproduce ppGpp) have nearly identical CFA synthesis curves, and these were independent of induction with IPTG. We also found that strain YYC1098 carrying pALS10 gave results identical to those obtained with the same strain carrying pALS13. Thus, consistent with the above in vitro results, ppGpp accumulation had no direct effect on CFA synthase. Therefore, although other enzymes of lipid metabolism are affected by ppGpp (9, 16, 18), this nucleotide does not directly alter the activity of CFA synthase.
The effect of ppGpp on CFA synthesis is mediated by RpoS.
Since we failed to detect a direct effect of ppGpp on the enzymatic activity of CFA synthase, we investigated the effects of ppGpp on expression of the cfa gene. Preliminary experiments showed that strain CF1693 (relA::kan spoT::cm) contained only 40 to 50% of the CFA synthase activity of the wild-type strain, MG1655. To facilitate more detailed studies, we made transcriptional fusions of various parts of the cfa promoter region to the lacZ gene and integrated these constructs into the chromosome as phage λ lysogens. The cfa gene is transcribed by two promoters, P1 and P2 (Fig. 1); P1 is recognized by ς70-RNAP whereas P2 is recognized by RpoS-RNAP (25). As depicted in Fig. 1, the λ lysogen constructs carried (i) the intact promoter region (P1 and P2), (ii) only P2, (iii) P2 plus the interpromoter region, or (iv) P1 plus the interpromoter region. Preliminary experiments showed that the β-galactosidase activities of the P2-lacZ fusions were significantly lower in a relA strain than in the wild-type strain, as expected from the reduced CFA synthase activity. In a relA::kan spoT::cm strain, the P2-lacZ fusions produced only half of the β-galactosidase activity seen when the fusion was present in a wild-type strain, whereas the β-galactosidase levels of P1-lacZ fusions were the same in the two strains (data not shown).
Since the P2 promoter requires RpoS (25) and RpoS levels decrease in ppGpp-deficient cells (5, 15), the effect of the relA mutation on CFA synthesis could be due to the decreased RpoS content resulting from decreased ppGpp levels. To test this possibility, we made lysogens of a wild-type strain and of an isogenic rpoS null mutant strain. These strains were then transformed with either the RelA overproduction plasmid pALS10 or the control plasmid pALS14 to allow production of ppGpp without amino acid starvation (in these plasmids, either functional [pALS10] or nonfunctional [pALS14] RelA is produced under control of the IPTG-inducible tac promoter). The effects of increasing concentrations of ppGpp by IPTG induction of the plasmid pALS10 relA gene were then studied. Induction with IPTG led to a dramatic increase in the ppGpp content of all strains carrying pALS10, as shown by an increase in the doubling time from 37 min in strains without plasmid (or containing the control plasmid pALS14) to 87 min in the strains containing plasmid pALS10 (21). Samples withdrawn at different time points after induction were then assayed for β-galactosidase activity (Fig. 2).
FIG. 2.
Effects of ppGpp on the expression of different cfa promoter-lacZ transcriptional fusions in rpoS (A and B) or wild-type strains (C and D). All strains (YYC1113 to YYC1120 [Table 1]) carrying plasmid pALS10 (B and D) or plasmid pALS14 (A and C) were grown to an A600 of 0.2 (time 0 min) in LB medium before induction with 100 μM IPTG, and samples were taken at 0, 20, 40, and 60 min. β-Galactosidase activities were measured as described by Miller (17). Strains containing plasmid pALS10 (encoding the active RelA protein) synthesize high amounts of ppGpp, whereas strains carrying plasmid pALS14 (encoding inactive RelA protein) do not synthesize ppGpp (21). Promoters P1 and P2 and the interpromoter (I) region of the lacZ promoter fusions (Fig. 1) are shown on the abscissa.
In strains lacking RpoS, the β-galactosidase activities of the two P2 promoter fusion constructs were barely detectable (Fig. 2A and B). The β-galactosidase activities of these strains remained at very low levels in the presence (Fig. 2B) or absence (Fig. 2A) of ppGpp, indicating that ppGpp had no direct effect on utilization of the promoter itself. The activities of the intact cfa promoter fusion and the P1 fusion were much higher, comparable to the values obtained in a wild-type background (compare Fig. 2A and C). These results are consistent with those of Wang and Cronan (25), indicating that the P2 promoter requires RpoS for activity.
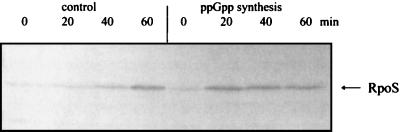
In contrast, upon IPTG induction of wild-type strains producing functional RpoS, the β-galactosidase activities of P2-lacZ and I (interpromoter region)-P2-lacZ fusions increased 25- and 19-fold, respectively (Fig. 2D), whereas the P1-lacZ and P1-I-P2-lacZ fusions showed only a 2- to 3-fold increase. The dramatic increase in the activity of the P2 fusions, which nearly matched the activity of the full-length promoter, was accompanied by elevated levels of RpoS (Fig. 3). Twenty minutes after induction, a significant increase in the RpoS level was detected, whereas the RpoS content in the control strains remained barely detectable (Fig. 3). The concomitant increase in transcription of the lacZ reporter gene and RpoS level indicated that the effect of ppGpp on the cfa P2 promoter is the result of the increased RpoS synthesis in the presence of the nucleotide. The lack of proportionality of β-galactosidase activity and RpoS levels observed in the promoter P2-lacZ fusion strains after 20 min of ppGpp synthesis may be due the maximal RpoS levels having been reached or to titration of the limited amount of RpoS-specific antiserum available.
FIG. 3.
ppGpp-dependent increase in the cellular RpoS content. Strain YYC1114 (containing the promoter P2-lacZ fusion) carrying plasmid pALS14 (control) or pALS10 (encoding the active RelA protein, ppGpp induction) were induced with 100 μM IPTG at an A600 of 0.2. Cell samples (A600 of 0.1) were harvested after 0, 20, 40, and 60 min. The proteins were dissolved in SDS-sample buffer, separated by SDS-PAGE (12% gel), and blotted; the transfer membrane was probed with a polyclonal anti-RpoS antiserum.
IPTG induction of strains carrying the P2-lacZ fusions and pALS14, which encodes an inactive RelA protein, gave β-galactosidase activities that remained low and constant for the first 40 min of induction. At 60 min we noted slightly increased activities in all strains that contained P2 promoter fusions (Fig. 2C). These small increases can be attributed to the expected (5, 11) rise in the intracellular RpoS content when cultures of wild-type strains enter stationary phase (Fig. 3). The activities of the P2-lacZ fusions increase further and reach the level of the ppGpp stimulated cells (Fig. 2D) 120 min after induction (data not shown).
It should be noted that the β-galactosidase activities of the fusion with the complete cfa promoter were two- to threefold higher than those of the P1-lacZ fusion, suggesting that either the longer promoter is more efficiently transcribed by ς70-RNAP or ς70-RNAP has some activity on the P2 promoter (or both). Finally, the twofold increase in β-galactosidase activities of the P1-lacZ fusion strain carrying plasmid pALS10 over the strain carrying plasmid pALS14 (Fig. 2A and B) is not understood. One possibility is that the presence of ppGpp stabilizes the cfa transcript, as proposed for other transcripts (15). A similar increase is evident in the case of wild-type strain (Fig. 2C and D), in which the P1-lacZ fusion in pALS10-carrying strains had β-galactosidase activities twofold higher than those of the strains carrying plasmid pALS14.
These results were confirmed in a ppGpp0 genetic background. Strains YYC1102 (relA::kan spoT::cm of ZK126) and YYC1123 (relA::kan spoT::cm rpoS::tet of ZK126) were lysogenized with the various cfa-lacZ fusion phages constructs and transformed with plasmid pALS14. However, a stable transformation with plasmid pALS10 could not be obtained despite several attempts. For reasons that are unclear, the few transformants obtained had lost their IPTG sensitivity and did not produce ppGpp. The results with the control plasmid pALS14 confirmed our results with two minor differences: (i) a slightly reduced β-galactosidase activity in all strains and (ii) a shift in the increase in RpoS to later in the growth curve.
In conclusion, our experimental results indicate that the effect of ppGpp on CFA synthesis is mediated indirectly. In wild-type cells, the increase in ppGpp levels as cells enter stationary phase results in increased levels of RpoS. RpoS then combines with core RNAP, and this holo form of RNAP utilizes the P2 promoter, resulting in increased cfa transcription and hence increased CFA synthase and the stimulation of CFA synthesis.
ACKNOWLEDGMENTS
This work was supported by grants from the DFG to D.R. and J.E. (SFB 197, project A7) and NIH grant GM26156 to J.E.C.
We thank R. Hengge-Aronis for the kind gift of the polyclonal anti-RpoS antiserum and M. Cashel for strains MG1655 and CF1693 as well as plasmids pALS10, pALS13, and pALS14.
REFERENCES
- 1.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 2.Bohannon D E, Connel N, Keener J, Tormo A, Espinosa-Urgel M, Zambrano M M, Kolter R. Stationary-phase inducible ‘gearbox’ promoters: differential effects of katF mutations and role of ςS. J Bacteriol. 1991;173:4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkovski A, Weil B, Krämer R. Glutamate excretion in Escherichia coli: dependency on the relA and spoT genotype. Arch Microbiol. 1995;164:24–28. doi: 10.1007/BF02568730. [DOI] [PubMed] [Google Scholar]
- 4.Cashel M, Gentry D R, Hernandez V J, Vinella D. The stringent response. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1458–1496. [Google Scholar]
- 5.Gentry D R, Hernandez V J, Ngyen L H, Jensen D B, Cashel M. Synthesis of the stationary-phase sigma factor ςS is positively regulated by ppGpp. J Bacteriol. 1993;175:7982–7989. doi: 10.1128/jb.175.24.7982-7989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gitter B, Riesenberg D. Influence of phospholipid composition on excretion of β-lactamase from a stringent/relaxed Escherichia coli K12 strain pair. Microbiol Res. 1996;151:337–342. doi: 10.1016/s0944-5013(96)80001-2. [DOI] [PubMed] [Google Scholar]
- 7.Gitter B, Diefenbach R, Keweloh H, Riesenberg D. Influence of stringent and relaxed response on excretion of recombinant proteins and fatty acid composition in Escherichia coli. Appl Microbiol Biotechnol. 1995;43:89–92. doi: 10.1007/BF00170628. [DOI] [PubMed] [Google Scholar]
- 8.Grogan D W, Cronan J E., Jr Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev. 1997;61:429–441. doi: 10.1128/mmbr.61.4.429-441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath R J, Jackowski S, Rock C O. Guanosine tetraphosphate inhibition of fatty acid and phospholipid synthesis in Escherichia coli is relieved by overexpression of glycerol-3-phosphate acyltransferase (plsB) J Biol Chem. 1994;269:26584–26590. [PubMed] [Google Scholar]
- 10.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1497–1512. [Google Scholar]
- 11.Jishage M, Iwata A, Ueda S, Ishishama A. Regulation of RNA polymerase sigma subunits synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J Bacteriol. 1995;178:5447–5451. doi: 10.1128/jb.178.18.5447-5451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kates M, Adam G, Martin S. Lipids of Serratia marcescens. Can J Biochem. 1964;42:461–479. doi: 10.1139/o64-054. [DOI] [PubMed] [Google Scholar]
- 13.Krohn M, Wagner R. A procedure for the rapid preparation of guanosine tetraphosphate (ppGpp) from Escherichia coli ribosomes. Anal Biochem. 1995;225:188–190. doi: 10.1006/abio.1995.1138. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lange R, Fischer D, Hengge-Aronis R. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the ςS subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1995;177:4676–4680. doi: 10.1128/jb.177.16.4676-4680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merlie J P, Pizer L I. Regulation of phospholipid synthesis in Escherichia coli by guanosine tetraphosphate. J Bacteriol. 1973;116:355–366. doi: 10.1128/jb.116.1.355-366.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 18.Polakis S E, Guchhait R B, Lane M D. Stringent control of fatty acid synthesis in Escherichia coli. Possible regulation of acetyl coenzyme A carboxylase by ppGpp. J Biol Chem. 1973;248:7957–7966. [PubMed] [Google Scholar]
- 19.Reddy P S, Raghavan A, Chatterji D. Evidence for a ppGpp-binding site on Escherichia coli RNA polymerase: proximity relationship with the rifampicin-binding domain. Mol Microbiol. 1995;15:255–265. doi: 10.1111/j.1365-2958.1995.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 20.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusion. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 21.Svitil A L, Cashel M, Zyskind J W. Guanosine tetraphosphate inhibits protein synthesis in vivo. J Biol Chem. 1993;268:2307–2311. [PubMed] [Google Scholar]
- 22.Taguchi M, Izui K, Katsuki H. Augmentation of cyclopropane fatty acid synthesis under stringent control in Escherichia coli. J Biochem. 1980;88:1879–1882. [PubMed] [Google Scholar]
- 23.Taylor F R, Cronan J E., Jr Cyclopropane fatty acid synthase of Escherichia coli: stabilization, purification and interaction with phospholipid vesicles. Biochemistry. 1979;18:3292–3300. doi: 10.1021/bi00582a015. [DOI] [PubMed] [Google Scholar]
- 24.Vogel H J, Bonner D M. Acetyl-ornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 25.Wang A-Y, Cronan J E., Jr The growth phase-dependent synthesis of cyclopropane fatty acids in Escherichia coli is the result of an RpoS (KatF)-dependent promoter plus enzyme instability. Mol Microbiol. 1994;11:1009–1017. doi: 10.1111/j.1365-2958.1994.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang A-Y, Grogan D, Cronan J E., Jr Cyclopropane fatty acid synthase of Escherichia coli: deduced amino acid sequence, purification, and studies of the enzyme active site. Biochemistry. 1992;31:11020–11028. doi: 10.1021/bi00160a011. [DOI] [PubMed] [Google Scholar]
- 27.Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem. 1991;266:5980–5990. [PubMed] [Google Scholar]