Abstract
The association between vaccines and peripheral facial palsy (PFP), an issue that has been the subject of debate for many years, has been raised again following results of clinical trials assessing mRNA based COVID-19 vaccines. To review the available literature on this topic, PubMed was searched from inception until February 25, 2022. Inclusion criteria were case reports with documented rechallenge and comparative epidemiological studies. Cases of COVID-19 vaccine-induced PFP with available data on vaccine rechallenge were also identified from Vigibase until December 31, 2021. Of the 347 articles retrieved, 32 comparative epidemiological studies, 1 meta-analysis and 4 case reports met our criteria, of which 13 involved COVID-19 vaccines. Eight studies found an association between at least one vaccine and the occurrence of PFP, whereas 24 did not. Positive studies involved seasonal or pandemic H1N1 influenza vaccines administered parenterally (4 studies) or intranasally (1 study with a toxin-adjuvanted vaccine), BNT162b2, a mRNA COVID-19 vaccine (1 disproportionality analysis and 1 observed-to-expected analysis) and an inactivated virus COVID-19 vaccine (CoronaVac®) (1 study combining a case-control and an observed-to-expected approach). Strong evidence was found only for the intranasal influenza vaccine while other positive studies detected only a marginal association between PFP and vaccination. Of the four case reports with documented rechallenge, only two were positive and involved an influenza vaccine and tozinameran in one case each. In Vigibase, rechallenge was documented in 49 reports with 29 (59.2%) cases being negative and 20 (40.8%) positive. The available data did not confirm an excess risk of PFP after vaccination in most studies. Moreover, of the eight epidemiological studies suggesting a possible excess risk of PFP after any vaccine, three were disproportionality analyses and two observed-to excepted analyses, suggesting great caution should be taken when interpreting these results.
Keywords: Facial paralysis, Bell's palsy, Vaccination, COVID-19 vaccines, Immunization
Abbreviations
- ADR
adverse drug reaction
- AESI
adverse events of special interest
- CDC
Centers for Disease Control
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- FDA
Food and Drug Administration
- GPRD
General Practice Research Database
- HR
hazard ratio
- IC
information component
- IRR
incidence rate ratio
- LT
heat-labile E. coli enterotoxin
- OE
observed-to-expected
- OR
odds ratio
- PFP
peripheral facial paralysis
- PRR
proportional reporting ratio
- RI
relative incidence
- ROR
reporting odds ratio
- RR
relative risk
- SCCS
self-controlled case series
- SIR
standardized incidence ratio
- SPEAC
Safety Platform for Emergency vACcines
- TTO
time to onset
- VAERS
Vaccine Adverse Event Reporting Qystem
- WHO
World Health Organization
Introduction
The global estimated incidence of Bell's palsy or peripheral facial palsy (PFP) range from 11 to 40 cases per 100,000 person-years in adults and 11.5 to 30 per 100,000 person-years in children [1]. Although the etiology of PFP remains frequently unknown, various causes should be investigated, in particular head traumas, tumors, viral infections, inflammatory diseases affecting the cranial nerves, strokes or diabetes.
Vaccination has long been suggested as a possible cause and was first mentioned in 1946 after the rabies vaccination [2]. Since then, this association has been studied for most of the currently used vaccines and included in the priority list of adverse events of special interest (AESI) generated by the Safety Platform for Emergency vACcines (SPEAC) [3].
More recently, this issue was raised in clinical trials involving mRNA-based vaccines (Pfizer/BioNTech BNT162b2 [tozinameran] and Moderna mRNA-1273 [elosameran]) and later analyzed thoroughly in a number of epidemiological studies. Spontaneous reporting was also a major source of information by gathering detailed individual data. Owing to the need for close monitoring of coronavirus disease 2019 (COVID-19) vaccine safety, a number of countries have therefore considerably reinforced their pharmacovigilance monitoring program to allow better spontaneous reporting. This was illustrated by the intensive pharmacovigilance program that was implemented in France with weekly expertise of COVID-19 vaccine adverse reactions [4]. Besides, PFP resulting from a COVID-19 infection has also been described as the initial manifestation or the sole major neurological manifestation of the disease in several case reports [5].
Our aim was to review and summarize the published data that evaluated the possible relationship between the occurrence of PFP and vaccination with a particular focus on COVID-19 vaccines. The identification of reports providing data on COVID-19 vaccine rechallenge was also performed in Vigibase.
Methods
Relevant publications regarding PFP and vaccines were retrieved by searching the PubMed/Medline database until February 25, 2022. Two independent search strategies were used. A broad search included all vaccines except COVID-19 vaccines “(facial paralysis OR facial palsy OR Bell's OR Bell Palsy) AND (vaccine OR vaccines) NOT COVID” and another focused on COVID-19 vaccines only “(facial paralysis OR facial palsy OR Bell's OR Bell Palsy) AND (vaccine OR vaccines) AND COVID”.
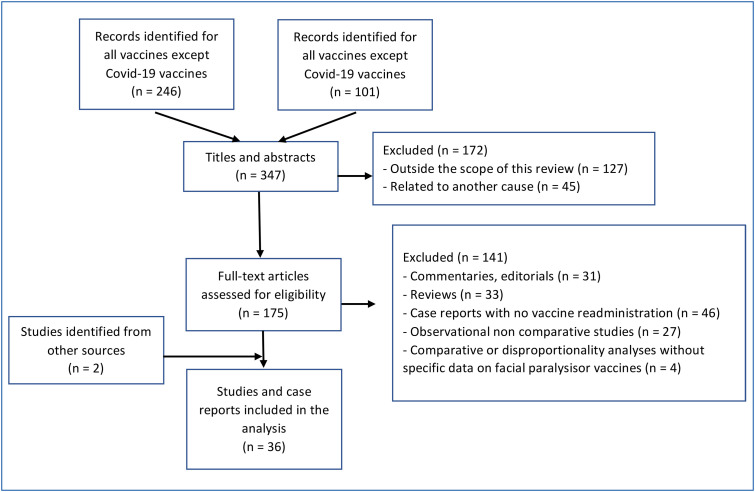
Experimental studies, studies related to COVID-19 without mention of vaccine, editorial or author opinions were excluded based on their titles or abstracts when available. All other articles were screened for their abstracts and full texts. The inclusion criteria were all clinically relevant articles including case reports with available data on vaccine readministration, observational or case-control studies, observed-to-expected analyses, and disproportionality analyses performed in pharmacovigilance databases. General reviews and the reference lists of all the originally retrieved articles were also screened to further identify additional relevant articles. Fig. 1 summarizes the flow diagram of study selection for both searches.
Figure 1.
Flow diagram of study selection.
Vigibase, the World Health Organization (WHO) global pharmacovigilance database that contains spontaneous reports on adverse drug reactions from 149 countries, was used to assess the outcome of documented COVID-19 vaccine rechallenge after a previous episode of COVID-19 vaccine-induced PFP. For this purpose, all cases of PFP (preferred terms: “Bell's palsy”, “facial paralysis”, “facial paresis”, “oculofacial paralysis”, “diplegia”, and “facial nerve disorders”) involving mRNA-based vaccines or adenovirus-vectored vaccines were extracted as of December 31, 2021. Cases with available information on rechallenge were subsequently identified from the Individual Case Safety Report form. The rate of PFP recurrence was calculated for each vaccine.
Results
All vaccines except COVID-19 vaccines
Using the broad search including all vaccines except COVID-19 vaccines, 246 articles were identified, of which 23, published between 1988 and 2021, met our inclusion criteria [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]. After including 4 studies, which combined 2 different designs, there were 1 meta-analysis of controlled trials, 12 self-controlled case series or case-centered analyses, 7 observed-to-expected analyses, 2 disproportionality analyses performed in pharmacovigilance databases, 3 controlled cohort studies, 1 case-control study, and 1 case report with documented rechallenge. The vaccines, study designs and main results of the pharmacoepidemiological studies are summarized in Table 1 [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27].
Table 1.
Published studies on vaccine-associated PFP (except COVID-19 vaccines).
| Ref | Country/Source of data | Vaccine, study design and population | Main results |
|---|---|---|---|
| Miscellaneous vaccines | |||
| [6] | US/Spontaneous reports to CDC, FDA or the manufacturer (1982–1985) |
Observed-to-expected analysis based on a comparison of the incidence rate of PFP spontaneously reported within a 3-week risk interval after the most proximate 1st to 3rd hepatitis B vaccine dose in 838,215 persons to the US background rate in a representative population | OE incidence: 31.4 observed vs. 42.5 expected per 100,000 person-years (NS) |
| [7] | Spontaneous reports to the manufacturer (2007–2011) |
Observed-to-expected analysis based on a comparison of the incidence rate of FP spontaneously reported within 30 days of human papillomavirus vaccination in European countries to the background rate from literature sources and stratified for age | OE (95%CI) number of cases of PFP − 10 (4.8–18.4) vs. 34 within 7 d – 13 (6.9–22.2) vs. 147 within 30 d |
| [8] | Scottish National Health Service (2004–2012) |
Observed-to expected population-based study comparing the incidence rate of prespecified diagnoses including PFP in 246,954 girls aged 12–18 years in the 5 years following initiation of the human papillomavirus vaccination campaign to the expected values calculated in the 5 years preceding initiation of the campaign | OE incidence ratio during the 2008–2012 period of vaccination ranged from 1.25 to 1.7 with a lower boundary of the CI never crossing 1 |
| [9] | Canada/Ontario's administrative health and vaccination databases (2007–2013) | Population-based SCCS comparing the incidence rate of autoimmune disorders including PFP (65 cases overall) diagnosed 7–60 days after any of the 3 doses of the quadrivalent human papillomavirus (99,841 person-years) to that diagnosed at any other time (825,160 person-years) in girls aged 12–17 years vaccinated with a quadrivalent human papillomavirus | Adjusted rate ratio (95%CI): 1.73 (0.77–3.89) a |
| [10] | US/Kaiser Permanente Northern California database (2005–2006) |
Population-based SCCS comparing the incidence rate of prespecified ADR including PFP in a risk interval of 0–30 days after quadrivalent meningococcal conjugate ACWY (Menactra®) vaccination to that in a comparison interval of 31–60 days post-vaccination in 31,561 patients aged 11–55 years | IRR not significantly increased (detailed data not reported) |
| [11] | US/Kaiser Permanente Southern California database (2011–2013) |
Population-based SCCS comparing the incidence rate of prespecified ADR including PFP in a risk interval of 1–84 days after quadrivalent meningococcal conjugate ACWY (Menveo®) vaccination to that in a comparison interval of 85–365 days post-vaccination in 48,899 patients aged 11–21 years | RI (95%CI) b: 2.9 (1.1–7.5) RI (95%CI) stratified on: − concomitantly used vaccines: 5.0 (1.4–17.8) − meningococcal vaccine alone: 1.1 (0.2–5.5) |
| [12] | US/Vaccine Safety Datalink Project (2007–2008) |
Population-based study with 2 self-comparisons to address a potential increased risk of prespecified ADR in 193,083 patients aged ≥ 50 years receiving a live, attenuated strainzoster vaccine: 1/Case-centered approach with comparison of the number of vaccinated patients during the 14 days prior to the onset of PFP to that vaccinated in a remote follow-back period 2/SCCS comparing the incidence rate of PFP in a risk interval of 1–14 days post-vaccination to that in a comparison interval of 45–59 days post-vaccination |
1/Relative risk (95%CI): 0.63 (0.29–1.38) 2/Relative risk (95%CI): 0.78 (0.29–2.09) |
| [13] | Spontaneous reports to the manufacturer GlaxoSmithKline (2017–2019) |
Observed-to-expected analysis with comparison of the incidence rate of spontaneously reported ADR including PFP within 7 or 30 days following vaccination with a recombinant zoster vaccine (9,323,118 doses in patients aged ≥50 years) to age and country-stratified background rate from the literature in populations with similar characteristics to the zoster vaccine target population | OE (95%CI) number of cases of PFP − 9 (4.1–17.1) vs. 73 within 7 d − 17 (9.9–27.2) vs. 312 within 30 d OE analyses also found that the observed number of cases were below the expected number by using scenarios of underreporting and background rates |
| [14] | UK/GPRD (1992–2005) |
Population-based SCCS comparing the incidence of PFP within 4 risk intervals (1–91; 1–30; 31–60; 61–91 days) after a pneumococcal or seasonal influenza vaccine to that outside this period and the 14 days prior to vaccination (2128 patients for 2263 episodes of PFP) | RI (95%CI) for the 1–91 days risk interval: − 0.92 (0.78–1.08) c for influenza vaccine – 0.67 (0.38–1.17) for pneumococcal vaccine |
| [15] | US/Vaccine Safety Datalink Project (2015) |
Retrospective cohort study involving 6 managed care organizations comparing the incidence rate of prespecified ADR including PFP in a 1–42 day risk interval after vaccination of patients ≥ 65 years with either the 13-valent pneumococcal conjugate (PCV13; 313,136 doses) or the 23-valent pneumococcal polysaccharide (PPSV23: 232,591 doses) vaccines | Adjusted RI (95%CI): 0.69 (0.41–1.15) d |
| [16] | US/Kaiser Permanente Northern California (2001–2006) |
Population-based case centered analysis comparing the rate of immunization with an influenza vaccine, an hepatitis B vaccine or any vaccine combined within 3 risk intervals (1–14; 1–28 and 29–56 days) prior to the onset of PFP in children ≤ 18 years to the expected rate of exposure in age- and sex-matched children who received the same vaccine during an observation period of 1 year prior to the diagnosis of PFP (233 episodes in total) | OR for the 1–28 day risk interval: − influenza vaccine: 0.7 (0.2–2.8)e − hepatitis B vaccine: 0.8 (0.2–2.4) e − any vaccine: 0.9 (0.6–1.4) e |
| Influenza vaccines | |||
| [17] | Meta–analysis of controlled trials | Meta-analysis of 14 controlled trials including 13,325 patients vaccinated with an AS03-adjuvanted influenza vaccine (H5N1 or pandemic A/H1N1) compared to 6,361 patients vaccinated with a non adjuvanted influenza vaccine (H5N1, A/H1N1, seasonal) or with placebo | RR (95% CI) for PFP: 2.3 (0.2–118.1) |
| [18] | Korea Disease Control Prevention Agency and the National Health Insurance Service database (2015–2018) |
Population-based self-controlled risk interval study comparing the incidence rate of PFP in a risk interval of 1–42 days after seasonal influenza vaccination to that in a comparison interval of 57–98 days post vaccination in 4,653,440 patients ≥ 65 years (11,656,966 doses) | IRR (95%CI): 0.99 (0.92–1.07) f |
| [19] | US/Vaccine Safety Datalink Project (2009–2010) |
Population-based study using two approaches to evaluate the risk of prespecified ADR in 2,898,988 recipients of seasonal trivalent inactivated (TIV) or live, attenuated (LAIV) influenza vaccines and 1,613,348 recipients of monovalent inactivated (MIV) or live, attenuated (LAMV) monovalent H1N1 vaccines 1/Self-controlled risk interval comparing the incidence rate of PFP during a risk interval of 1–42 days post vaccination to that in a comparison interval of 15–56 days before vaccination 2/Current versus historical comparison of the rate of PFP in the same risk interval after vaccination with a historical background rate calculated in the risk interval in the database |
Statistical signals for MIV in adults aged ≥25 years with − RR of 1.67 in the self-controlled approach − RR of 1.60 in the OE analyses No statistical signals were evidenced for LAMV, TIV and LAIV in adults aged ≥25 years in the self-controlled approach for the 42-day risk interval |
| [20] | UK/THIN (The Health Improvement Network database) (2009–2013) |
Population-based SCCS comparing the incidence rate of PFP during a risk interval of 1-42 days following vaccination with the pandemic (2009 season) or seasonal (2010–2013 seasons) influenza vaccines containing A/H1N1-like viral strains to that outside this risk interval using all person time (5,732,656 patients of whom 6288 experienced 6381 episodes of PFP) | Adjusted RI (95% CI) g − any H1N1: 0.85 (0.72–1.01) − seasonal H1N1: 0.96 (0.82–1.13) − pandemic H1N1: 0.73 (0.47–1.12) |
| [21] | UK/THIN (The Health Improvement Network database) (2012–2015) | Population-based study comparing the incidence of several ADR observed within a 3-month to 6 month period after trivalent seasonal influenza vaccination in 4578 patients aged ≥ 18 years to that expected for the considered ADR. For PFP, the observed number of cases in a risk period of 1–90 days after vaccination was compared to that expected outside this period after exclusion of a pre-exposure period of –60 to 0 days | OE rate ratio was not estimable as no cases of PFP were observed in vaccine recipients within the risk period |
| [22] | US/Kaiser Permanente Northern California (2013–2014) |
Population-based study using two approaches to evaluate the risk of prespecified ADR in 62,040 recipients of the quadrivalent live attenuated influenza vaccine (Q/LAIV) aged 2–49 years: 1/SCCS comparing the incidence rate of PFP during a risk interval of 1–14 or 1–42 days post-vaccination to that in a comparison interval of 15–29 or 43–84 days post vaccination, respectively (within a cohort analysis) 2/Comparison of the rate of PFP in this cohort to rates observed in 2 frequency-matched (age, medical center) groups (61,803 unvaccinated controls and 57,185 inactivated influenza vaccine recipients) |
HR (95% CI) was not estimable for any of the comparisons as no cases of PFP were observed in Q/LVAI recipients within the 1–42 day risk interval |
| [23] | Sweden/Stockholm county (2009–2010) |
Population-based study comparing the incidence rate of selected ADR including PFP after pandemic A/H1N1 influenza vaccination in 1,024,019 patients to that in 921,005 unvaccinated patients | Adjusted HR (95% CI) h − 1.25 (1.06–1.48) for the whole period − 1.60 (1.25–2.05) for the 6-week period after vaccination Excess risk (95% CI) per 100,000 vaccinated person-years − 8.4 (2.3–13.4) cases for the whole period − 65 (1.5 to 89) cases for the 6 weeks after vaccination |
| [24] | US/VAERS (1991–2001) |
Disproportionality analysis performed in the VAERS database (128,717 ADR reports) comparing the reporting ratio of PFP after seasonal influenza vaccination (154 cases of PFP) to that of all other vaccines | PRR: 3.78 (χ2: 203.81) i |
| [25] | US/VAERS (2015–2019) |
Disproportionality analysis performed in the VAERS database (224,241 ADR reports) comparing the reporting ratio of PFP after seasonal influenza vaccination (250 cases) to that of all other vaccines (346 cases) | PRR: 2.44 (χ2: 122.32) j ROR (95% CI): 2.44 (2.08–2.88) |
| [26] | Taiwan/National Adverse Drug Reaction Reporting System (NADRRS) and a nationwide large–linked database (LLDB) for 2009 H1N1 vaccination | Population-based study comparing the estimated number of several ADR including PFP within 0–42 days after pandemic A(H1N1) influenza vaccination (5,688,517 doses) to the expected background incidence in the general Taiwanese population. The estimated true number of PFP was calculated by using a capture-recapture method based on the number of spontaneous reports of PFP to the NADRRS and the number of this diagnosis in the National Health Insurance database linked to the prescription database |
OE ratio (95% CI): 1.48 (1.11–1.98) for the 0–42 day interval post vaccination k 525 (CI 95%: 398–692) estimated cases compared with 354 expected cases |
| [27] | Switzerland (2000–2001) |
Study dedicated to inactivatedintranasal influenza vaccines containing Escherichia coli heat-labile toxin as a mucosal adjuvant and conducted with physicians from 19 German-speaking regions of Switzerland by combining two approaches: 1/a case-series analysis comparing the incidence rate of PFP during a risk interval of 1–30, 31–60 and 61–91 days post vaccination to that in a comparison interval of 92 or more days in 412 patients aged ≥ 18 years and diagnosed with PFP 2/a case control study comparing the rate of exposure to the inactivated intranasal or parenteral influenza vaccine in the 91 days preceding vaccination in 250 patients diagnosed with PFP to that of 722 patients matched to age, date of visit and physician |
1/RI (95% CI) for the risk interval: − 1–30 day: 14 (5.2–37.9) − 31–60 day: 35.6 (14.1–89.8) − 61–91 day: 11.8 (4.3–32.3) 2/Adjusted OR (95% CI) − Intranasal: 84.0 (20.1–351.9) – Parenteral: 1.1 (0.6–2.0) |
ADR: adverse drug reaction; CDC: Centers for Disease Control; CI: confidence interval; COVID-19: coronavirus disease 2019; FDA: Food and Drug Administration; PFP: peripheral facial paralysis; GPRD: general practice research database; HR: hazard ratio; IRR: incidence rate ratio; NR: not reported; NS: not significant; OE: observed-to-expected; OR: odds ratio; PRR: proportional reporting ratio (PRR ≥2, χ2 ≥4 and ≥3 cases were considered as a signal); RI: relative incidence; ROR: reporting odds ratio (ROR > 2 with a lower boundary 95%CI > 1 is considered significant); RR: relative risk; SCCS: self-controlled case series; VAERS: vaccine adverse event reporting system
Adjusted for age at diagnosis, seasonality, receipt of non-HPV vaccines and recent infections (7–60 d before diagnosis).
Adjusted for seasonality.
No difference was evidenced whatever the risk window and the age group (0–44; 45–64 and ≥ 65 years).
Adjustment based on a propensity score analysis with the inverse probability of treatment weighting (IPTW) approach.
No associations were also found between these vaccines and the occurrence of FP during the risk interval 1–14 and 29–56 days preceding the onset of FP.
Similar non significant results were found in a sensitivity analysis for each season (2015/2016; 2016/2017; 2017/2018) or with risk intervals of 1–14 or 1–28 days post vaccination and in a subgroup analysis (age groups, sex and comorbidities).
Adjusted for seasonality, acute respiratory infections, influenza diagnoses and pregnancies.
Adjustment for age, sex, socioeconomic status, and healthcare utilization.
The PRR exceeded the criteria for a signal in all age groups (< 18, 18–64, ≥ 65 years) and was the highest in patients ≥ 65 years (3.91).
After excluding cases of FP associated with Guillain Barre syndrome, PRR (χ2) and ROR (95%CI) were 2.3 (89.37) and 2.3 (1.93–2.75), respectively.
The capture–recapture method estimated that spontaneous reporting of FP after vaccination represented 9% of the true estimated number of cases for the 0–42 day risk interval.
No increased risk of PFP was found with vaccines against hepatitis B virus (one observed-to-expected analysis) [6], human papillomavirus (two observed-to-expected analyses and one self-controlled case series) [7], [8], [9], meningococcus (two self-controlled case-series) [10], [11], herpes zoster (one case-centered approach combined to one self-controlled case series and one observed-to-expected analysis) [12], [13] and pneumococcus (one self-controlled case series) [14]. In a retrospective cohort study, the incidence rate of PFP was also not different after administrating the 13-valent pneumococcal conjugate or the 23-valent pneumococcal polysaccharide vaccines [15]. A self-case-centered analysis study focusing on children and performed in a healthcare database did not find a significantly increased risk of PFP associated with commonly used pediatric vaccines [16].
Most of the data originated from studies assessing parenteral or intranasal influenza vaccines. Overall, 1 meta-analysis and 12 pharmacoepidemiological studies (including references [14] and [16] cited previously) evaluated seasonal or pandemic H1N1 influenza vaccines and provided conflicting results.
The meta-analysis included 14 controlled trials assessing H1N1 influenza virus vaccines and found an incidence rate of PFP close to that observed in the general population (45.2 versus 20.1 cases per 100,000 person-year, respectively) with a RR of 2.3 [95%CI] 0.2–118.1). However, the very low number of events in each group (4 and 1, respectively) precluded any definite conclusion [17].
Seven of the pharmacoepidemiological studies did not evidence a significantly increased risk:
-
•
Four self-controlled case series performed in the US, Korea, and the UK did not show any significantly increased risk of PFP with seasonal influenza vaccination [14], [18] or influenza A (H1N1) vaccination [19], [20]. By using an additional case-centered logistic regression adjusted for seasonality, one of these studies also concluded that seasonality likely contributed to the Bell's palsy signal [19].
-
•
One case centered analysis involving children ≤ 18 years also failed to evidence an increased risk of PFP following immunization with an influenza vaccine [16].
-
•
One observed-to-expected analysis, based on UK electronic healthcare records, reported an incidence rate of 44.1 per 100,000 person-years for PFP associated with trivalent seasonal influenza vaccination in the 6-month post-exposure period [21]. However, no cases were recorded in the predefined risk period of three months and the observed-to-expected ratio could not be estimated.
-
•
In the last study, no cases of PFP were observed in more than 60,000 recipients of quadrivalent live attenuated or inactivated influenza vaccines while 2 cases occurred in unvaccinated controls [22].
Four studies suggested a slight increase in the risk of PFP after influenza vaccination:
-
•
A Swedish retrospective cohort found a marginal increased risk in recipients of the H1N1 influenza vaccine compared to unvaccinated patients (HR 1.25 [1.06–1.8]) [23].
-
•
Two US case-non-case pharmacovigilance studies performed 15 years apart in the Vaccine Adverse Event Reporting System (VAERS) database found a significant signal of disproportionality associated with seasonal influenza vaccination with a proportional reporting ratio of 2.4 to 3.8 (>2 were reported as significant) as compared to any other vaccines [24], [25].
-
•
A study performed in 2 Taiwanese databases indicated a modest increased risk of PFP within 42 days of the H1N1 influenza vaccination with an observed-to-expected ratio of 1.48 (95% CI 1.11–1.98) [26].
The only vaccine for which strong evidence of an excess risk of PFP has been reported was the intranasal influenza vaccine used in Switzerland in 2000–2001 (NasalFlu®). According to a case-control study based on 63 medically confirmed cases of PFP, the intranasal vaccine significantly increased the risk of PFP (adjusted OR, 84.0; 95% CI 20.1–351.9) [27]. By using the most conservative assumptions, this resulted in 13 excess cases per 10,000 vaccinated persons within 1 to 91 days of vaccination. The inclusion of an E. coli heat labile toxin (LT) as an adjuvant for the intranasal route of administration was the suggested cause and similar cases of PFP were in fact reported with other experimental intranasal vaccines using another LT [28]. No such association has been later found with another intranasal trivalent live, attenuated influenza vaccine, supporting the hypothesis that the heat LT is the cause of PFP rather than the intranasal route [29].
Among all the available published data, only one case with positive rechallenge, the gold standard of causality in pharmacovigilance, has been published [30]. A 57-year-old female patient experienced a first episode of PFP 2 days after H1N1 influenza vaccination with no other causes found after extensive investigations. She later tolerated a seasonal influenza vaccine performed 5 and 6 years later but relapsed the next year within 3 days of receiving the same influenza vaccine. Although not ruled out, a true positive rechallenge remains doubtful in this case.
COVID-19 vaccines
Literature review
In phase 3 trials assessing mRNA-based vaccines, eight cases of PFP were observed among the 73,868 volunteers, of whom 36,901 received the vaccine [31]. Seven cases occurred in vaccinated patients and one in placebo groups. Although these data raised some concerns, there were no formal statistical differences between groups. The time to onset (TTO) ranged from 3 to 48 days after injection and 6 of the 8 cases were observed after the second dose. The only patient who developed PFP after the first dose did not receive the second dose. From these data and based on an observed-to-expected comparison, Ozonoff et al. [32] estimated that the incidence rate of PFP ranged from 44 to 106 cases per 100,000 person-years in the vaccinated groups, an incidence 3.5 to 7 times higher than that expected in the general population. By using a longer observation period after the last dose and by considering either the number of volunteers or the number of doses, the estimates found by others were rather 1.5 to 3 times higher than the baseline incidence [33]. These estimates based on a very small number of cases obtained from clinical trials and calculated by using an observed-to-expected approach are questionable and an imbalance due to chance in these two trials remains possible. By contrast, there was no numerical imbalance in the number of PFP in the clinical trials involving adenovirus-vectored vaccines ChAdOx1nCoV-19 (Oxford/AstraZeneca) and Ad26.COV2-S (Janssen/Johnson & Johnson) with three and two cases in the treated and placebo groups, respectively.
Besides the results of clinical trials, our search strategy for COVID-19 vaccines associated with PFP identified 101 articles, of which 13 met our inclusion criteria (10 pharmacoepidemiological studies and 3 case reports).
The 10 comparative studies are summarized in Table 2 [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]. One study, which comprised of both a nested case-control study and an observed-to-expected analysis [42], was counted as two separate studies. Overall, there were two disproportionality analyses [34], [35], three retrospective cohort studies [36], [38], [39], two self-controlled case series [37], [40], two case-control studies [41], [42] and two observed-to-expected analyses [42], [43]. All involved at least 1 mRNA-based vaccine (tozinameran in 10 and elasomeran in 4), while 1 also involved ChadOx1nCov-19, an adenovirus-vectored vaccine, and 2 CoronaVac®, an inactivated virus COVID-19 vaccine. These cohort studies and observed-to-expected analyses derived from observational studies and encompassed more than 40 million patients who received at least 1 dose of any mRNA or adenovirus-based vaccines, of whom nearly 1700 developed PFP.
Table 2.
Published studies on COVID-19 vaccine-associated PFP.
| Ref | Country/data source | Study design | Vaccine and sample size | FP in vaccine groups | Controls and sample size | FP in control groups | Main results |
|---|---|---|---|---|---|---|---|
| Disproportionality analyses | |||||||
| [34] | US/Vaccine Adverse Event Reporting System | Disproportionality analysis | BNT162b2 | 405 |
Any other vaccinesa |
NR |
ROR (95%CI): BNT162b2: 1.84 (1.65-2.06)b |
| mRNA-1273 | 512 | mRNA-1273: 1.54 (1.39-1.70) | |||||
| Influenza vaccines | 462 | Influenza: 2.04 (1.76-2.36) | |||||
| [35] | WHO Vigibase | Disproportionality analysis | mRNA vaccines: 133,883c | 844d | Influenza vaccine: 314,980e | 5734 | IC f: -1.26 (-1.36 to -1.17) b |
| Other viral vaccines e: 1,265,182 | 2087 | ICf: 0.09 (-0.01 to 0.19) b | |||||
| Cohort studies | |||||||
| [36] | Israel/Clalit Health Service | Observational cohort study comparing the incidence of AE within 42 days of vaccination to unvaccinated patients matched 1:1 on sociodemographic and clinical variable g | BNT162b2: 884,828 | 81 | Unvaccinated patients: 884,828 | 59 | Risk ratio (95%CI): 1.32 (0.92-1.86) |
| [37] | US/Vaccine Safety Datalink | Self-controlled case series comparing the incidence of AE during a risk interval of 1 to 21 days after either mRNA vaccine dose 1 or 2 versus 22-42 days after the most recent mRNA vaccine dose h | BNT162b2/mRNA-1273: 660,766 person-years |
535 | BNT162b2/mRNA-1273: 364,988 person-years |
301 | Adjusted rate ratio (95%CI): 1.00 (0.86-1.17) |
| [38] | US/Mayo Clinic Health Systems (curation methods of Electronic health records) | Retrospective cohort study comparing the incidence rate of AE within 7 days after the 1st or 2nd mRNA vaccine dose to that of unvaccinated patients propensity matched 1:1 according to demographic, geographic and clinical features | Number of person-days: BNT162b2: |
Number of person-days (unvaccinated) | Incidence rate ratio (95%CI) | ||
| Dose 1: 361,554 | 4 | 359,560 | 10 | Dose 1: 0.4 (0.091-1.4) | |||
| Dose 2: 273,167 | 4 | 263,612 | 7 | Dose 2: 0.55 (0.12-2.2) | |||
| mRNA-1273: | |||||||
| Dose 1: 114,992 | 1 | 114,637 | 4 | Dose 1: 0.25 (0.0051-2.5) | |||
| Dose 2: 82,941 | 1 | 79,822 | 3 | Dose 2: 0.32 (0.0061-1.4) | |||
| [39] | Israel/Meuhedet Health Maintenance Organization | Historical cohort study comparing the number of cases of FP in vaccinated and unvaccinated patients matched 1:1 according to sex, age, comorbidities and population sectori | BNT162b2: | Unvaccinated | Relative risk (95%CI) | ||
| Dose 1: 233,159 | 23 | 233,159 | 24 | Dose 1: 0.96 (0.54 -1.70) | |||
| Dose 2: 131,033 | 8 | 131,033 | 12 | Dose 2: not significant (calculation not provided) | |||
| [40] | UK/Linkage of the national database of Covid-19 vaccination to national databases for mortality, hospital admissions and SARS-CoV-2 infection | Self-controlled case series comparing the incidence rate ratio of neurological AE in a risk interval of 1 to 28 days after either the first dose of mRNA vaccine or ChadOx1nCov-19 and in SARS-CoV-2-positive patients relative to baseline period (≥ 29 days before or after exposure) |
BNT162b2: 12,134,782 |
247 |
Incidence rate ratio (95%CI) within 1-28 days after exposure 1.06 (0.9-1.26) |
||
| Ch adOx1nCov-19: 20,417,752 | 430 | 1.07 (0.94-1.21)j | |||||
| SARS-Cov-2 infection: 2,005,280 | 77 | 1.34 (0.91-1.97)k | |||||
| Case control studies | |||||||
| [41] | Israel Emergency department of the Shamir Medical Center | Case control study comparing the rate of exposure to BNT162b2 within the previous 30 days in 37 patients admitted for a new onset of FP to that of 74 patients admitted for any other reasons and matched (1:2) on age, sex and admission date |
BNT162b2: 65 |
21 |
Unvaccinated: 46 |
16 |
Adjusted OR (95%CI): 0.84 (0.37-1.90) |
| [42] | Hong Kong/Hospital Authority electronic health record system | Nested case control study comparing the rate of exposure to BNT162b2 within the previous 42 days in 298 patients admitted for a new onset of FP to that of 1,181 controls admitted for any other reason during the same period and matched (1:4) by age, sex, admission setting, and date of hospital attendance |
BNT162b2: 45 |
14 |
Unvaccinated: 1181 |
256 |
Adjusted OR (95%CI) - BNT162b2: 1.76 (0.89-3.48) |
| CoronaVac®: 81 | 28 | - CoronaVac®: 2.39 (1·42-4.02) | |||||
| Observed-to-expected analyses | |||||||
| Hong Kong/Adverse events reported in the online Vaccine Adverse Event Reporting System | Comparison of the reported incidence rate of FP within 42 days of the first dose of CoronaVac® or BNT162b2 to the background incidence rate of FP in the same area for the same study period in 2020 |
BNT162b2 : 537,235 |
16 |
NA l |
NA l |
Age-standardized rate ratio (95% CI): BNT162b: 1.66 (0.95-2.91) |
|
| CoronaVac® : 451,939 | 18 |
CoronaVac®: 2.64 (1.67-4.17) |
|||||
| [43] | Israel Database of Clalit Health Services (CHS) | Comparison of the observed number of cases of FP within 21 or 30 days after the first or second dose in patients with a previous history of FP to the background rate of FP estimated from the CHS data in 2019 during the same period | BNT162b2 | Expected number | SIR (95% CI) | ||
| - Dose 1: 2,594,990 | 132 | 97.1 | - 1.36 (1.14-1.61)m | ||||
| - Dose 2: 2,434,674 | 152 | 130.5 | - 1.16 (0.99-1.36) | ||||
| Previous history of FP | |||||||
| - Dose 1: 7,567 | 4 | 3.5 | - 1.15 (0.36-2.76) | ||||
| - Dose 2: 7,045 | 10 | 4.7 | - 2.15 (1.09-3.83) | ||||
AE: adverse effects; CI: confidence interval; COVID-19: coronavirus disease 2019, FP: facial paralysis, IC: information component; NR: not reported; OR: odds ratio; ROR: reporting odds ratio; SIR: standardized incidence ratio.
The study involved a total of 303,589 reports of AE following the use of any vaccine but the total number of AE for each vaccine was not provided
Results were adjusted based on sex and age
Total number of AE reported with BNT162b2 and 1273mRNA vaccines in Vigibase.
Of the 844 cases of FP, 749 were reported with BNT162b2 and 95 with mRNA-1273 vaccines.
Total number of AE with other viral vaccines or influenza vaccines alone.
IC: Information Component. A disproportionality signal is significant if the lower boundary of the 95% credibility interval of the IC025 is >0.
Patients with a previous PCR-positive test for SARS-Cov-2 or with a previous history of FP were excluded.
A total of 6.2 million individuals were administered BNT162b2 (3,539,611 first and 3,214,737 second doses) or mRNA-1273 (2,636,202 first and 2,454,578second doses) vaccines.
Patients with a COVID-19 infection at any time before or after the vaccine were excluded.
For the ChadOx1nCov-19 vaccine, there was a significant increased risk only for the 15–21 day period after the first dose (IRR, 1.29; 95% CI: 1.08–1.56).
For patients with a positive SARS-CoV-2 test, there was an increased risk at day 0 (IRR, 33.23; 95% CI: 22.57–48.94), days 1–7 (IRR, 5.84; 95% CI: 4.09–8.33) and days 8–14 (IRR, 2.17; 95% CI: 1.30–3.63).
The crude expected number of cases of FP was not provided. The age-standardized incidence rate per 100,000 person-years (95%CI) was 42.8 (19.4–66.1) for BNT162b2 and 66.9 (37.2–96.6) for CoronaVac® with a background incidence calculated for the same study period for each vaccination program of 25.7 (22.7–28.8) to 25.3 (22.6–28.1), respectively.
The highest SIR was 2.51 (95%CI: 1.65–3.68) and observed in females older than 64 years, only after the first dose. The SIR did not significantly increased in males whatever the dose.
As shown in Table 2, 8 of the 11 study designs did not show any significant increased risk of PFP with both tozinameran or elosameran vaccines. In one study involving both tozinameran and CoronaVac®, only CoronaVac® was significantly associated with a risk of PFP, either in the nested case-control study or the observed-to-expected analysis [42].
An increased risk of PFP after mRNA vaccines was found in two studies. The first was a disproportionality analysis performed in the US VAERS, which evidenced a moderately increased risk compared to any other vaccines [34]. However, the reporting odds ratio was low and below or close to that calculated for influenza vaccines. The second study used an observed-to-expected analysis approach and found a higher than expected risk of PFP after the first dose (standardized incidence ratio [SIR]: 1.36 [CI 95% 1.14–1.61]) but not after the second dose [43]. Conversely, the SIR significantly increased only after the second dose in patients with a previous history of PFP.
Several clinical characteristics of mRNA vaccines associated with PFP could be derived from these studies. The mean TTO after vaccination calculated from 16 and 21 patients included in 2 case-control studies was 11.3 ± 7.1 days in the first study [42], and 9.3 ± 4.2 days and 14.0 ± 4.2 days after the first and second dose, respectively, in the second study [41]. In both studies, the maximum TTO was 23 days. Except for the study by Shibli et al. [43], two other studies did not find risk differences in the incidence between the first and the second dose [38], [39]. Shibli et al. [43] also specifically focused on vaccine recipients who had a previous history of PFP and found very similar SIR to those calculated for the whole vaccinated population. This study also found a higher than expected incidence in females older than 45 years with the highest SIR (2.51, 95% CI 1.65–3.68) in those aged ≥65 years, but this was observed only after the first dose. By contrast, there was no significant increase in the SIR in males whatever the dose. Finally, the House-Brackman score used to assess the severity of the nerve damage was available in 13 of 21 patients included in a case-control study [41]. Of these, 7 patients scored slight to moderate, 5 moderate and 1 had total facial palsy.
Only one case report described PFP recurrence after a second vaccine dose [44]. A 61-year-old patient with a history of diabetes, hypercholesterolemia, and who was overweight experienced a first episode of right-sided PFP 5 hours after receiving the first dose of tozinameran. He presented a recurrence of more severe facial paralysis on the opposite side after the second dose performed 6 weeks later even though the previous episode had been completely resolved. Both episodes were promptly resolved following corticosteroid administration. By contrast, two other reports documented no recurrence of PFP after a second dose of tozinameran and an unspecified COVID-19 viral vector vaccine [45], [46].
Data from Vigibase
As of December 31,2021, 22,770 reports of facial paralysis or related disorders associated with mRNA- or adenovirus-based vaccines were identified from Vigibase with tozinameran in 15,638 cases (68.2%), elosameran in 3,864 (16.9%), ChAdOx1 nCoV-19 in 2,388 (10.4%) and Ad26.CoV2.S in 880 (3.8%). Among them, rechallenge was documented in 49 reports and was negative in 29 (59.2%) and positive in 20 (40.8%) (Table 3 ). Unfortunately, the number of cases reporting rechallenge is very low, either due to low reporting, lack of follow-up or because the patient did not receive the subsequent dose.
Table 3.
Rechallenge data available in Vigibase, the WHO global pharmacovigilance database.
| Rechallenge | Tozinameran | Elasomeran | ChAdOx1 nCoV-19 | Ad26.CoV2.S | Total |
|---|---|---|---|---|---|
| Positive | 14 | 4 | 1 | 1 | 20 |
| Negative | 20 | 1 | 8 | – | 29 |
| Rate (95% CI) of positive rechallenge | 41 (25–59) | 80 (28–99) | 11 (0–48) | – | 41 (27–56) |
CI: confidence interval; WHO: World Health Organization.
Discussion
Across several studies using various methodological approaches, there is no sufficient evidence allowing us to confirm or refute a link between vaccine-induced immune stimulation and the occurrence of PFP in general, with the noticeable exception of the LT-adjuvanted intranasal influenza vaccine. Interestingly, of the eight studies indicating a possible excess in PFP after any vaccine, three were disproportionality analyses performed in pharmacovigilance databases and three observed-to-expected analyses. As such designs mostly allow a signal to be to detected rather than confirmed these results should be interpreted with great caution.
More specifically, regarding mRNA-based vaccines, the mention of this adverse effect in the summary of product characteristics of both tozinameran and elasomeran resulted from the imbalance observed in the distribution of PFP cases between vaccinated and control patients in clinical trials. Based on a suggestive temporal association, several isolated case reports later supported a possible relationship [47] while other authors suggested a type 1 interferon response to mRNA vaccines as a possible pathophysiological mechanism of PFP [31]. However, according to the available epidemiological data that did not confirmed an excess risk in most studies using reliable designs and the very limited number of published or spontaneously reported cases indicating positive rechallenge despite billions of vaccinated patients, reasonable doubts on this association still remain.
The occurrence of PFP after COVID-19 vaccination does not currently constitute a contraindication to the continuation of the vaccination schedule. In such a case, an individual assessment of the risk-benefit ratio should be offered and an alternative vaccine can eventually be discussed. Similar conclusions can be made for all other vaccines, especially influenza vaccines.
Funding
The study did not receive any specific funding.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
The authors acknowledge the assistance of Brigitte Tourliere, documentalist of the Hospital University Pharmacotoxicology Department, Hospices civils de Lyon, France and Gaelle Simeon for proofreading this manuscript.
References
- 1.Karlsson S., Arnason S., Hadziosmanovic N., Laestadius Å., Hultcrantz M., Marsk E., et al. The facial nerve palsy and cortisone evaluation (FACE) study in children: protocol for a randomized, placebo-controlled, multicenter trial, in a Borrelia burgdorferi endemic area. BMC Pediatr. 2021;21:220. doi: 10.1186/s12887-021-02571-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zabludovich S. Rabies vaccine accidents; pseudo peritoneal syndrome and successive bilateral facial paralysis. Dia Med. 1946;18:1454–1458. [PubMed] [Google Scholar]
- 3.2020. Priority list of Adverse Events of Special Interest: COVID 19. https://brightoncollaboration.us/priority-list-aesi-covid/. [Accessed 28 July 2022] [Google Scholar]
- 4.Lacroix C., Salvo F., Gras-Champel V., Gautier S., Massy N., Valnet-Rabier M.B., et al. French organization for the pharmacovigilance of COVID-19 vaccines: A major challenge. Therapie. 2021;76:297–303. doi: 10.1016/j.therap.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S., Jawanda M.K. Surge of Bell's Palsy in the era of COVID-19: systematic review. Eur J Neurol. 2022;29:2526–2543. doi: 10.1111/ene.15371. [DOI] [PubMed] [Google Scholar]
- 6.Shaw F.E., Graham D.J., Guess H.A., Milstien J.B., Johnson J.M., Schatz G.C., et al. Postmarketing surveillance for neurologic adverse events reported after hepatitis B vaccination. Experience of the first three years. Am J Epidemiology. 1988;127:337–352. doi: 10.1093/oxfordjournals.aje.a114808. [DOI] [PubMed] [Google Scholar]
- 7.Angelo M.G., Zima J., Tavares Da Silva F., Baril L., Arellano F. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiology Drug Saf. 2014;23:456–465. doi: 10.1002/pds.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron R.L., Ahmed S., Pollock K.G. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452–457. doi: 10.1111/imj.13005. [DOI] [PubMed] [Google Scholar]
- 9.Liu E.Y., Smith L.M., Ellis A.K., Whitaker H., Law B., Kwong J.C., et al. Quadrivalent human papillomavirus vaccination in girls and the risk of autoimmune disorders: the Ontario Grade 8 HPV Vaccine Cohort Study. CMAJ. 2018;190:E648–E655. doi: 10.1503/cmaj.170871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen J., Zhang L., Klein N.P., Robertson C.A., Decker M.D., Greenberg D.P., et al. Post-licensure safety surveillance study of routine use of quadrivalent meningococcal diphtheria toxoid conjugate vaccine. Vaccine. 2017;35(49 PtB):6879–6884. doi: 10.1016/j.vaccine.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Tseng H.F., Sy L.S., Ackerson B.K., Hechter R.C., Tartof S.Y., Haag M., et al. Safety of quadrivalent meningococcal conjugate vaccine in 11- to 21-year-olds. Pediatrics. 2017;139:e20162084. doi: 10.1542/peds.2016-2084. [DOI] [PubMed] [Google Scholar]
- 12.Tseng H.F., Liu A., Sy L., Marcy S.M., Fireman B., Weintraub E., et al. Safety of zoster vaccine in adults from a large managed-care cohort: a Vaccine Safety Datalink study. J Intern Med. 2012;271:510–520. doi: 10.1111/j.1365-2796.2011.02474.x. [DOI] [PubMed] [Google Scholar]
- 13.Tavares-Da-Silva F., Co M.M., Dessart C., Herve C., Lopez-Fauqued M., Mahaux O., et al. Review of the initial post-marketing safety surveillance for the recombinant zoster vaccine. Vaccine. 2020;38:3489–3500. doi: 10.1016/j.vaccine.2019.11.058. [DOI] [PubMed] [Google Scholar]
- 14.Stowe J., Andrews N., Wise L., Miller E. Bell's palsy and parenteral inactivated influenza vaccine. Human Vaccines. 2006;2:110–112. doi: 10.4161/hv.2790. [DOI] [PubMed] [Google Scholar]
- 15.Tseng H.F., Sy L.S., Qian L., Liu I.A., Mercado C., Lewin B., et al. Pneumococcal conjugate vaccine safety in elderly adults. Open Forum Infect Dis. 2018;5 doi: 10.1093/ofid/ofy100. ofy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowhani-Rahbar A., Klein N.P., Lewis N., Fireman B., Ray P., Rasgon B., et al. Immunization and Bell's palsy in children: a case-centered analysis. Am J Epidemiol. 2012;175:878–885. doi: 10.1093/aje/kws011. [DOI] [PubMed] [Google Scholar]
- 17.Vaughn D.W., Seifert H., Hepburn A., Dewe W., Li P., Drame M., et al. Safety of AS03-adjuvanted inactivated split virion A(H1N1)pdm09 and H5N1 influenza virus vaccines administered to adults: Pooled analysis of 28 clinical trials. Hum Vaccin Immunother. 2014;10:2942–2957. doi: 10.4161/21645515.2014.972149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong N., Kim Y., Kim C., Park S., Lee J., Choi N. Association between influenza vaccination and the risk of Bell's palsy in the Korean elderly. Vaccines (Basel) 2021;9:746. doi: 10.3390/vaccines9070746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee G.M., Greene S.K., Weintraub E.S., Baggs J., Kulldorff M., Fireman B.H., et al. H1N1 and seasonal influenza vaccine safety in the Vaccine Safety Datalink Project. Am J Prev Med. 2011;41:121–128. doi: 10.1016/j.amepre.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Wijnans L., Dodd C.N., Weibel D., Sturkenboom M. Bell's palsy and influenza(H1N1)pdm09 containing vaccines: a self-controlled case series. PLoS One. 2017;12:e0175539. doi: 10.1371/journal.pone.0175539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall G.C., Davies P.T.G., Karim M.Y., Haag M.D.M., O’Leary C. Observational safety study of specific outcomes after trivalent cell culture seasonal influenza vaccination (Optaflu®) among adults in THIN database of electronic UK primary healthcare records. Pharmacoepidemiol Drug Saf. 2018;27:52–58. doi: 10.1002/pds.4347. [DOI] [PubMed] [Google Scholar]
- 22.Baxter R., Eaton A., Hansen J., Aukes L., Caspard H., Ambrose C.S. Safety of quadrivalent live attenuated influenza vaccine in subjects aged 2-49 years. Vaccine. 2017;35:1254–1258. doi: 10.1016/j.vaccine.2017.01.062. [DOI] [PubMed] [Google Scholar]
- 23.Bardage C., Persson I., Ortqvist A., Bergman U., Ludvigsson J.F., Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ. 2011;343:d5956. doi: 10.1136/bmj.d5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou W., Pool V., DeStefano F., Iskander J.K., Haber P., Chen R.T. VAERS Working Group. Pharmacoepidemiol. A potential signal of Bell's palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS)-United States, 1991-2001. Drug Saf. 2004;13:505–510. doi: 10.1002/pds.998. [DOI] [PubMed] [Google Scholar]
- 25.Kamath A., Maity N., Nayak M.A. Facial paralysis following influenza vaccination: a disproportionality analysis using the Vaccine Adverse Event Reporting System Database. Clin Drug Investig. 2020;40:883–889. doi: 10.1007/s40261-020-00952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W.T., Huang W.I., Huang Y.W., Hsu C.W., Chuang J.H. The reporting completeness of a passive safety surveillance system for pandemic (H1N1) 2009 vaccines: a capture-recapture analysis. Vaccine. 2012;30:2168–2172. doi: 10.1016/j.vaccine.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R.T., Linder T., et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 28.Lewis D.J., Huo Z., Barnett S., Kromann I., Giemza R., Galiza E., et al. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izurieta H.S., Haber P., Wise R.P., Iskander J., Pratt D., Mink C., et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA. 2005;294:2720–2725. doi: 10.1001/jama.294.21.2720. [DOI] [PubMed] [Google Scholar]
- 30.Gocko X., Poulteau S., Beyens M.N., Bertholon P., Pozzetto B. Case report: recurrent peripheral facial paralysis following two influenza vaccinations in 2009 and 2016. Vaccine. 2019;37:4864–4866. doi: 10.1016/j.vaccine.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Soeiro T., Salvo F., Pariente A., Grandvuillemin A., Jonville-Béra A.P., Micallef J. Type I interferons as the potential mechanism linking mRNA COVID-19 vaccines to Bell's palsy. Therapie. 2021;76:365–367. doi: 10.1016/j.therap.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozonoff A., Nanishi E., Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021;21:450–452. doi: 10.1016/S1473-3099(21)00076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cirillo N., Doan R. Bell's palsy and SARS-CoV-2 vaccines - an unfolding story. Lancet Infect Dis. 2021;21:1210–1211. doi: 10.1016/S1473-3099(21)00273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K., Mano T., Niimi Y., Toda T., Iwata A., Iwatsubo T. Facial nerve palsy following the administration of COVID-19 mRNA vaccines: analysis of a self-reporting database. Int J Infect Dis. 2021;111:310–312. doi: 10.1016/j.ijid.2021.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renoud L., Khouri C., Revol B., Lepelley M., Perez J., Roustit M., et al. Association of facial paralysis with mRNA COVID-19 vaccines: a disproportionality analysis using the World Health Organization pharmacovigilance database. JAMA Intern Med. 2021;181:1243–1245. doi: 10.1001/jamainternmed.2021.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMurry R., Lenehan P., Awasthi S., Silvert E., Puranik A., Pawlowski C., et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA COVID-19 vaccines. Med (N Y) 2021;2 doi: 10.1016/j.medj.2021.06.006. 965-968.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shasha D., Bareket R., Sikron F.H., Gertel O., Tsamir J., Dvir D., et al. Real-world safety data for the Pfizer BNT162b2 SARS-CoV-2 vaccine: historical cohort study. Clin Microbiol Infect. 2022;28:130–134. doi: 10.1016/j.cmi.2021.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patone M., Handunnetthi L., Saatci D., Pan J., Katikireddi S.V., Razvi S., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. J Nat Med. 2021;27:2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shemer A., Pras E., Einan-Lifshitz A., Dubinsky-Pertzov B., Hecht I. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg. 2021;147:739–743. doi: 10.1001/jamaoto.2021.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan E.Y.F., Chui C.S.L., Lai F.T.T., Chan E.W.Y., Li X., Yan V.K.C., et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22:64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibli R., Barnett O., Abu-Full Z., Gronich N., Najjar-Debbiny R., Doweck I., et al. Association between vaccination with the BNT162b2 mRNA COVID-19 vaccine and Bell's palsy: a population-based study. Lancet Reg Health Eur. 2021;11:100236. doi: 10.1016/j.lanepe.2021.100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burrows A., Bartholomew T., Rudd J., Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14:e243829. doi: 10.1136/bcr-2021-243829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kharoubi S. Paralysie faciale périphérique post-vaccination COVID-19 : à propos d’un cas. Pan Afr Med J. 2021;40:244. doi: 10.11604/pamj.2021.40.244.31498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mussatto C.C., Sokol J., Alapati N. Bell's palsy following COVID-19 vaccine administration in HIV+ patient. Am J Ophthalmol Case Rep. 2022;25:101259. doi: 10.1016/j.ajoc.2022.101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sriwastava S., Sharma K., Khalid S.H., Bhansali S., Shrestha A.K., Elkhooly M., et al. COVID-19 vaccination and neurological manifestations: a review of case reports and case series. Brain Sci. 2022;12:407. doi: 10.3390/brainsci12030407. [DOI] [PMC free article] [PubMed] [Google Scholar]