We compared the height of wild female orphan and non-orphan African elephants, finding that orphans are shorter for their age than non-orphans. Stunted growth suggests elephant orphans may suffer reduced fitness. Our results further indicated the presence of peers may increase juvenile elephant growth, while competition with adults may reduce growth.
Keywords: von Bertalanffy, stunting, orphan, growth, conservation, African elephant
Orphans of several species suffer social and physiological consequences such as receiving more aggression from conspecifics and lower survival. One physiological consequence of orphaning, stunted growth, has been identified in both humans and chimpanzees, but has not been assessed in a non-primate species. Here, we tested whether wild African elephant orphans show evidence of stunted growth. We measured individually known female elephants in the Samburu and Buffalo Springs National Reserves of Kenya, with a rangefinder capable of calculating height, to estimate a von Bertalanffy growth curve for female elephants of the study population. We then compared measurements of known orphans and non-orphans of various ages, using a Bayesian analysis to assess variation around the derived growth curve. We found that orphans are shorter for their age than non-orphans. However, results suggest orphans may partially compensate for stunting through later growth, as orphans who had spent a longer time without their mother had heights more similar to non-orphans. More age mates in an individual’s family were associated with taller height, suggesting social support from peers may contribute to increased growth. Conversely, more adult females in an individual’s family were associated with shorter height, suggesting within-group competition for resources with older individuals may reduce juvenile growth. Finally, we found a counterintuitive result that less rainfall in the first 6 years of life was correlated with taller height, potentially reflecting the unavoidable bias of measuring individuals who were fit enough to survive conditions of low rainfall as young calves. Reduced growth of individuals has been shown to reduce survival and reproduction in other species. As such, stunting in wildlife orphans may negatively affect fitness and represents an indirect effect of ivory poaching on African elephants.
Introduction
Post-weaning maternal care is vital to the well-being of many social mammal species, providing benefits to offspring such as defence against predators and conspecifics, provisioning and knowledge of social and ecological landscapes (Clutton-Brock, 1991). The loss of such care results in lower survival for weaned orphans of species such as African elephants (Loxodonta africana), spotted hyenas (Crocuta crocuta), red deer (Cervus elaphus), chimpanzees (Pan troglodytes) and orcas (Orcinus orca) (Watts et al., 2009; Foster et al., 2012; Andres et al., 2013; Stanton et al., 2020; Parker et al., 2021). In the case of at least one species (the African elephant), this lowered orphan survivorship depresses population growth (Parker et al., 2021), making orphaning and the consequences of being orphaned relevant to conservation.
Weaned orphans who manage to survive may also suffer consequences that could lead to reduced fitness. For example, wild chimpanzee orphans secreted more stress hormones than non-orphans for a period of time following their mothers’ deaths (Girard-Buttoz et al., 2021); African elephant orphans suffered more aggression from conspecifics and had less access to mature adult females who are repositories of social and ecological knowledge (McComb et al., 2001; Wittemyer et al., 2007b; Foley et al., 2008; Goldenberg and Wittemyer, 2017, 2018); and orphans of primate species including muriquis (Brachyteles hypoxanthus), blue monkeys (Cercopithecus mitis) and savannah baboons (Papio cynocephalus) have had lower reproductive success because their offspring were more likely to die than the offspring of non-orphans (Zipple et al., 2021). Evidence also suggests humans (Homo sapiens) whose mother has died have stunted growth when compared to humans with both parents, but the degree of stunting was influenced by their social situation, for example if the surviving father lived with a maternal orphan their growth was less stunted (Finlay et al., 2016).
Similar studies of orphan stunting in wildlife are sparse, likely because it is challenging to noninvasively measure growth of known wild individuals. Yet such studies are valuable because stunted growth reliably indicates reduced fitness (Johnson, 2003; Altmann and Alberts, 2005; Dmitriew, 2011; de Onis and Branca, 2016). Animals who can allocate energy to growth tend to be in better condition and have access to more resources (Altmann and Alberts, 2005; Dmitriew, 2011). Larger individuals are also more likely to survive attacks and have more surviving offspring (Johnson, 2003; Dmitriew, 2011). Despite logistical challenges, at least two studies of wild primates have succeeded in relating growth to maternal characteristics. Researchers showed that growth in wild juvenile baboons positively correlates with maternal rank by placing weighing scales in areas frequented by study subjects (Johnson, 2003; Altmann and Alberts, 2005). Another study used urinary creatine, a by-product of metabolism in muscle tissue, to show that non-orphan chimpanzees (P. troglodytes) excreted more creatine than orphans, and therefore that prolonged maternal care is important for immature chimpanzee growth (Samuni et al., 2020).
In this study, we investigated whether the loss of prolonged maternal care stunts growth in a non-primate species, the African elephant. The African elephant population that uses the Samburu and Buffalo Springs National Reserves of Kenya has been closely monitored since 1998, such that over 1000 elephants are individually identified (Wittemyer, 2001; Wittemyer et al., 2013, 2021). Increased poaching in the population from the years 2009–2013 left many individuals orphaned, and these orphans have been the focal point of several studies assessing the consequences of orphaning in a highly social species of conservation concern (Goldenberg et al., 2016; Goldenberg and Wittemyer, 2017, 2018; Parker et al., 2020, 2021). Building on these studies, we compared orphan and non-orphan elephant heights, as shoulder height is a known correlate of body mass in elephants (Larramendi, 2015). Male and female African elephants grow at different rates (Shrader et al., 2006); therefore, we included only females in this study. We defined ‘orphan’ as an individual whose mother died while the individual was still immature, with maturity marked by giving birth. Our hypothesis was that the loss of maternal care stunts growth in African elephants as found for humans and chimpanzees (Finlay et al., 2016; Samuni et al., 2020), and therefore predicted orphan elephants would be shorter for their age than non-orphans. We incorporated rainfall in early life and estimated weaning age because available resources and early weaning affect nutrition and growth (Altmann and Alberts, 2005; Dmitriew, 2011; Meale et al., 2015). We also assessed potential effects of an individual’s social environment, including the number of consistently available adult females and age mates, as these are important to juvenile elephant social interactions and physiology (Goldenberg and Wittemyer, 2017, 2018; Parker et al., 2022) and may influence degree of stunting for orphans. Our findings add to the literature on the effects of orphaning for weaned but immature individuals of social wildlife species.
Methods
Study system
The Samburu and Buffalo Springs National Reserves are located at 0.3–0.8°N and 37–38°E in Kenya, separated by the Ewaso Ngiro River and together encompassing 220 km2. The ecosystem is semi-arid and prone to drought, with rain averaging ~350 mm per year between the two wet seasons: April–May and November–December (Wittemyer, 2001; Wittemyer et al., 2021). A long-term monitoring project of elephants began in the reserves in late 1997. To date, roughly 1000 elephants, some regular residents and others wet season visitors, have been individually identified in a continually updated photo ID file that distinguishes them according to ear tear patterns, tusk configurations and other unique characteristics such as the presence of a crooked tail (Douglas-Hamilton, 1973; Wittemyer et al., 2007b, 2021). Detailed demographic history is available for each identified elephant because a long-term monitoring team drives throughout the reserves daily to record which elephants are present together in aggregations, as well as births, deaths when individuals have been consistently missing from families (about a third of deaths are confirmed through carcass surveys), estrous females, matings, injuries and other noteworthy events (Wittemyer et al., 2005a, 2013, 2021). We have precise age estimates for individuals born during the study because newborn calves are generally sighted within 3 weeks of birth (Wittemyer et al., 2013). Age estimates for individuals who were present at the start of the study are also reliable, confirmed with dental molds to be within 3 years for 75% of individuals and within 5 years for 95% of individuals (Rasmussen et al., 2005).
Height measurements
We used an Impulse 200LR laser rangefinder with zoom scope 7003824 (Laser Technology, Inc.), equipped with an attached tripod and bubble level, to measure elephant height in centimetres. We (JP and GW) measured elephants opportunistically because they had to be standing on level ground with a straight front leg such that a vehicle could be parked parallel to their body with an unobstructed view. When an elephant was resting in proper position, we parked with the observer on the side nearest the elephant, situated the tripod on the vehicle window ledge, aligned the rangefinder using the bubble level and measured perpendicular distance to the elephant. Next, ensuring that the rangefinder bubble level remained in place, we pivoted the rangefinder to measure the angle down from the perpendicular to where the distal portion of the elephant’s front foot touched the ground, and then the angle up from the perpendicular to the top of the elephant’s shoulder blade. The Impulse rangefinder is equipped with a function that then calculated height based on trigonometry. In this way, we repeatedly took and recorded as many measurements as possible before the elephant shifted position.
For this study, elephants were measured from 1999 to 2005 and 2011 to 2019 (GW) and 2015 to 2019 (JP). We discarded measurements of an individual if there was only one taken within 6 months, and where measurements taken on a single date were highly variable (i.e. <3 measurements with a range >5 cm, <6 measurements with a range >10 cm, <9 measurements with a range >15 cm or measurements with a range >20 cm). Following these quality control steps, the total number of female elephants measured was 222 (GW 158, JP 75 with some overlap). To remove concerns about interobserver variability, we used only GW’s measurements of 158 elephants to calculate the female population’s growth curve (described below), as he measured more elephants across a larger age range (rounded age range, 1–58 years; mean ± SD = 20.24 ± 14.14 years). Of these elephants, 113 were measured on only one date, 39 on two dates separated by at least 6 months (and usually separated by 3 or more years), and 5 on three dates with at least 6 months between consecutive measurement dates. An average of 5.83 (± SD 2.13) measurements were taken from an individual on a single date. We calculated a median height for each individual on each date they were measured, for a total of 206 median heights used to calculate the growth curve.
When addressing our main question we controlled for interobserver variability by using only JP’s measurements, as she focused on measuring orphans and age-matched non-orphans and included only subjects who were born during the study period to ensure precise age estimates. Therefore, we analysed 611 total measurements from 59 elephants of 28 families, consisting of 32 orphans and 27 non-orphans aged 6.87–19.61 years (mean ± SD = 13.85 ± 2.78 years), and compared these data according to the derived growth curve (see below). Some of the 59 elephants were measured on more than one date at different ages, with mean of 2.00 (±1.40) dates of measure per elephant, and an average of 5.14 (± SD 3.31) measurements taken from an individual on a single date. We were able to include all 611 measurements in our primary analysis (described below) regardless of the number of days separating measurement dates for an individual and despite variation in the number of measurements taken per individual on a single date, because the analysis model accounted for variation in age and the estimated precision parameter incorporated variation in number of measurements per individual. (See supplementary material to view height measurement data.)
Determination of growth curve
We paired the 206 median height measurements of 158 individuals with their estimated or known age on date of measure, rounded ages to the nearest year, then used FSA version 0.9.1 (Ogle et al., 2021), FSAdata version 0.3.8 (Ogle, 2019) and nlstools (Baty et al., 2015) packages in Rstudio version 1.4.1106-5 (R Core Team, 2020; Rstudio Team, 2020) to calculate a standard von Bertalanffy growth curve (von Bertalanffy, 1938; Lee and Moss, 1995; Ogle, 2013) for female elephants of the Samburu population (code for this calculation is available in supplementary materials). The formula for the curve was 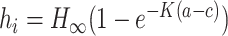 , where
, where 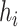 is the height of an individual,
is the height of an individual, 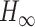 is the estimated average asymptotic height reached by individuals in the population,
is the estimated average asymptotic height reached by individuals in the population, 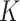 is the growth rate coefficient,
is the growth rate coefficient, 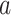 is age and
is age and 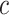 is a constant calculated to achieve the best fit to data under curve constraints (von Bertalanffy, 1938; Ogle, 2013).
is a constant calculated to achieve the best fit to data under curve constraints (von Bertalanffy, 1938; Ogle, 2013).
Calculation of covariates
We included six covariates for the 59 individuals in our primary analysis. The first covariate was (i) rainfall. We summed rainfall (mm) recorded the month prior to an individual’s birth and over her first 6 years of life as measured in Archer’s Post, a town on the Samburu National Reserve’s eastern boundary. Rainfall during the first 2–3 years captured conditions during the most dependent period of a calf’s life (Lee and Moss, 1986), and we included as many additional years as possible given the age of the youngest calf measured was 6.87 years old. We included rainfall the month prior to birth to approximate conditions at birth because vegetative growth lags behind rainfall.
The next four covariates captured aspects of juvenile social status: (ii) orphan status (binary variable: 0 non-orphan, 1 orphan); (iii) years spent without mother, calculated by subtracting the mother’s estimated death date from date of measure and dividing by 365 such that partial years were represented by a decimal amount (non-orphans had a value of ‘0’ for this covariate); (iv) the number of adult multiparous females within an individual’s core group (Wittemyer et al., 2005b; Parker et al., 2022); and (v) the number of age mates within an individual’s core group (Parker et al., 2022). To define associate adult females and age mates, we used long-term monitoring data for the 10 years between 2009 and 2019 to calculate association indices between each subject and each member of her extended family. Association index (AI) between two individuals A and B was calculated as follows:
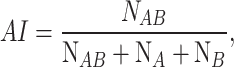 |
where NAB is the number of times individual A and B were seen together, NA is the number of times individual A was seen without individual B when B was available in the study population and NB is the number of times individual B was seen without individual A when A was available in the study population (Goldenberg et al., 2016). We only used observations for which observers were confident they recorded all individuals present in a group (Wittemyer et al., 2005b) and only observations taken during the wet seasons of April–May and November–December to ensure seasonal differences in reserve use did not influence interpretation of social units (some measured individuals typically only came to the reserves in wet seasons when elephants group together in larger aggregations). Our multiparous female covariate was defined as the number of females who had given birth at least twice with which a subject had an AI of ≥ 0.5. Our age mate covariate was the number of associates within ±4 years of age of the measured individual with which she had an AI of ≥ 0.5, as 4 years is the average interbirth interval for a Samburu elephant female (Wittemyer et al., 2013).
Finally, we included a covariate of (vi) weaning age. We estimated this covariate by using an individual’s age when their first younger sibling was born. This is an imperfect estimate as some mothers have been observed attempting to wean their calves before a younger sibling was born, and there have been infrequent observations of older calves nursing even after a younger sibling was born. Additionally, for one individual of a family that is not resident to the study area, observers may have missed counting a younger sibling that died shortly after birth. This individual was 14.12 years when her next youngest recorded sibling was born, therefore we capped weaning age at 6.5 years, slightly above the maximum age of 6.35 years for which we are certain we observed the next youngest sibling. We also capped weaning age at 6.5 years for an orphan of another family not resident to the study area whose mother died when she was 9.92 years old without a record of a younger sibling. Finally, in one case a 3.78-year-old individual’s next youngest sibling died less than a month after birth and she may have resumed suckling, but we kept her weaning age at 3.78 years. The weaning estimates ranged from 2.73 to 6.5 years, mean 4.23 (± SD 0.80) years. Only three of the included orphans lost their mother before a younger sibling was born, at ages 3.68, 4.42 and 5.75 years.
Statistical analysis
We analysed the influence of (i) rainfall in the first 6 years, (ii) orphan status, (iii) time since mother’s death, (iv) number of adult females, (v) number of age mates and (vi) estimated weaning age with a Bayesian hierarchical regression model (Hobbs and Hooten, 2015) fitted on the von Bertalanffy growth curve estimated for the population, using uninformative priors for all parameters (see supplementary materials). The first level of the model organized individuals by family, estimating a family-level asymptote (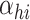 ) to account for genetic constraints on the height that an individual can reach:
) to account for genetic constraints on the height that an individual can reach:
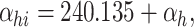 |
where 240.135 is the H∞ parameter determined from the fitted growth curve (see results) and h is an index from 1–28 according to the family of individual i. The resulting asymptotes (αhi’s) were incorporated into the second level of the model that estimated height for individual i on date l according to measurement j (μilj) by incorporating their age (a) and adding the other covariates of b = first 6 years of rainfall, o = orphan status, t = years spent without mother, f = number of multiparous females in core group, m = number of age mates in core group and w = estimated weaning age:
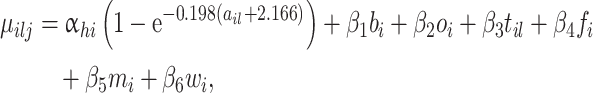 |
where 0.198 and −2.166 are the K and c parameters determined from the fitted growth curve (see results). Following examination of a histogram of the height measurement data (yilj) we assigned it a normal distribution:
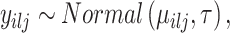 |
where τ is a precision parameter associated with uncertainty surrounding measurement and the process by which height is determined.
We standardized all covariates prior to analysis by subtracting mean and dividing by SD (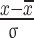 ) and ran our analysis in RStudio version 1.1.463 (R Core Team, 2020; Rstudio Team, 2020) with the package rjags version 4-10 (Plummer, 2019) using Markov-Chain Monte Carlo and three parallel chains of 100 000 iterations. We used 1000 iterations for adaptation and discarded 10 000 iterations as burn-in. We examined post-burn-in chains of the model using the MCMCvis package (Youngflesh, 2018), calculated Gelman-Rubin diagnostic Rc values (Gelman and Rubin, 1992; Brooks and Gelman, 1998) and ran the Heidelberger–Welch test (Heidelberger and Welch, 1983) to test for convergence. We also assessed model fit by simulating data according to our top model and overlaying a plot of the simulated data and real data with the package bayesplot (Gabry and Mahr, 2020) (see supplementary materials for all analysis and model check code). Figures were made with the ggplot2 (Wickham, 2016) and MCMCvis (Youngflesh, 2018) packages.
) and ran our analysis in RStudio version 1.1.463 (R Core Team, 2020; Rstudio Team, 2020) with the package rjags version 4-10 (Plummer, 2019) using Markov-Chain Monte Carlo and three parallel chains of 100 000 iterations. We used 1000 iterations for adaptation and discarded 10 000 iterations as burn-in. We examined post-burn-in chains of the model using the MCMCvis package (Youngflesh, 2018), calculated Gelman-Rubin diagnostic Rc values (Gelman and Rubin, 1992; Brooks and Gelman, 1998) and ran the Heidelberger–Welch test (Heidelberger and Welch, 1983) to test for convergence. We also assessed model fit by simulating data according to our top model and overlaying a plot of the simulated data and real data with the package bayesplot (Gabry and Mahr, 2020) (see supplementary materials for all analysis and model check code). Figures were made with the ggplot2 (Wickham, 2016) and MCMCvis (Youngflesh, 2018) packages.
Results
Growth curve
The von Bertalanffy growth curve for female elephants of the Samburu population was
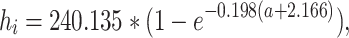 |
where 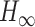 = 240.135,
= 240.135, 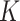 = 0.198 and c = −2.166 (Fig. 1). The curve begins to asymptote between 20 and 30 years, but does not come to a true asymptote until after 50 years (Supplementary Fig. 1) (Lee et al., 2016).
= 0.198 and c = −2.166 (Fig. 1). The curve begins to asymptote between 20 and 30 years, but does not come to a true asymptote until after 50 years (Supplementary Fig. 1) (Lee et al., 2016).
Figure 1.
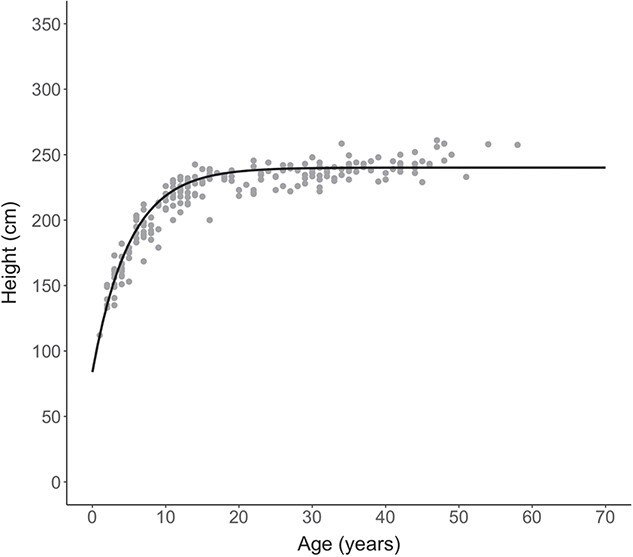
Estimated von Bertalanffy growth curve for the Samburu African elephant female population, with axes scaled to match Lee and Moss (1995) and Shrader et al. (2006) for comparison. Grey dots show data points (n = 206 median heights from n = 158 female elephants aged 1–58 years).
Primary analysis
Rc values for the analysis model were all <1.1 (Gelman and Rubin, 1992; Brooks and Gelman, 1998), and all parameters passed the Heidelberger–Welch test (Heidelberger and Welch, 1983). There was no concerning lack of fit in the posterior check plot overlaying real and simulated data (Supplementary Fig. S2).
As predicted, orphans were shorter for their age relative to non-orphans, displayed by the negative correlation of the non-orphan/orphan (0/1) covariate with height (Table 1; Fig. 2). Orphans were estimated to be 13.08 cm (with a 95% confidence interval of 11.51–14.35 cm) shorter than non-orphans of the same age, and the orphan status covariate had the largest coefficient value among the covariates assessed. The variable showing the next highest correlation with height was also related to orphaning. Orphans who had spent a longer time without their mother were estimated to be taller for their age than orphans who had spent shorter periods of time without their mother, indicated by the positive correlation of years without mother and height (Table 1; Fig. 2).
Table 1.
Primary analysis results, ordered from greatest to least by magnitude of estimated effect
| Coefficient | Covariate | Estimate | 95% CI lower | 95% CI upper |
|---|---|---|---|---|
| β2* | Orphan status* | −13.08 | −14.35 | −11.81 |
| β3* | Years without mom* | 5.56 | 4.66 | 6.45 |
| β1* | Rainfall first 6 years* | −2.01 | −2.75 | −1.28 |
| β5* | Age mates in core group* | 1.41 | 0.52 | 2.29 |
| β4* | Multiparous females in core group* | −1.23 | −2.16 | −0.30 |
| β6 | Estimated weaning age | 0.42 | −0.31 | 1.16 |
Asterisks denote variables whose 95% confidence interval did not overlap 0.
Figure 2.
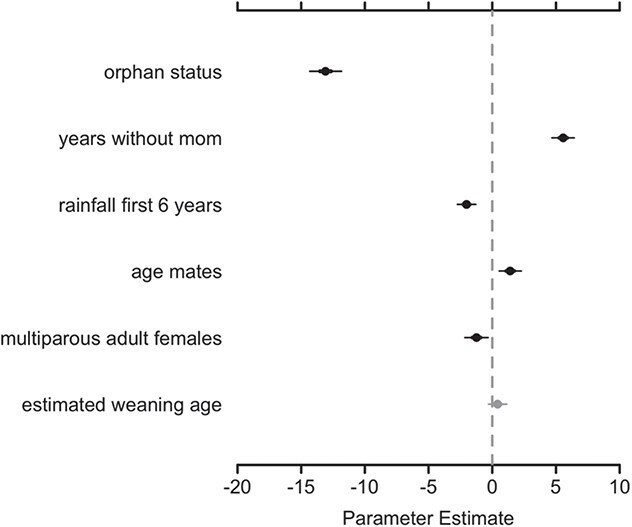
Results from the primary analysis, with 95% confidence intervals, ordered from greatest to least by magnitude of estimated effect. Grey-filled circles signify variables whose 50% confidence interval did not overlap zero, and black-filled circles signify variables whose 95% confidence interval did not overlap 0.
We unexpectedly found that the amount of rainfall in the first 6 years of life showed a negative correlation with height (Table 1; Fig. 2). The estimated correlation was small, but the 95% confidence interval did not overlap 0. Results also suggest that individuals with more age mates in their group were slightly taller for their age than individuals with fewer age mates, while individuals with more multiparous females in their group were marginally shorter for their age than individuals with fewer multiparous females (Table 1; Fig. 2). The 95% confidence interval for estimated weaning age overlapped 0 (Table 1; Fig. 2).
Discussion
The benefits of prolonged maternal care are well known (Clutton-Brock, 1991). Studies in wild orphans have underscored its importance by showing that weaned orphans suffer social and physiological consequences, including lower survival (Foster et al., 2012; Andres et al., 2013; Goldenberg and Wittemyer, 2018; Girard-Buttoz et al., 2021; Parker et al., 2021). However, few studies have quantified the costs of orphaning in terms of growth, even though growth is a reliable proxy for fitness and stress (Dmitriew, 2011; Dickens and Romero, 2013). Our results showed orphan elephants were shorter for their age than non-orphans, similar to studies of humans and chimpanzees that showed decreased growth in orphans (Finlay et al., 2016; Samuni et al., 2020).
Our study was correlational and did not determine proximate causes of decreased growth, but at least two factors related to the loss of maternal care were likely involved. First, mother elephants may increase access to food for weaned calves because they reduce displacement by other elephants (Goldenberg and Wittemyer, 2018), thereby increasing access to nutrition and energy available for growth (Dmitriew, 2011). Secondly, increased stress resulting from both the experience of losing a mother and the absence of her care could stunt growth. Reduced body mass and stress are known correlates (Dickens and Romero, 2013; Wu, 2021); the release of glucocorticoids during stress diverts energy away from nonurgent uses like growth (Sapolsky, 2004), and elevated glucocorticoid levels have been associated with lower levels of growth hormones and their binding proteins (Gabbitas et al., 1996; Bernstein, 2010). Previous study suggests orphans do not sustain higher levels of stress hormones ≥2 years after their mother’s death (Parker et al., 2022), but if there are short-term alterations that may affect growth. In chimpanzee orphans, short-term but not long-term alterations in glucocorticoid concentrations were observed (Girard-Buttoz et al., 2021). We note that the majority of orphans in this study were weaned when their mother died (all but three) and we did not find a correlation between estimated weaning age and height, suggesting loss of milk from mothers was not the cause of their stunted growth.
Our results that orphans who had spent more time without their mother were closer in height to non-orphans may indicate that surviving orphans can habituate to the absence of maternal care, likely by strengthening other social bonds (Goldenberg et al., 2016; Goldenberg and Wittemyer, 2017), and have an opportunity for compensatory growth (Dmitriew, 2011). However, compensatory growth can itself be costly (Metcalfe and Monaghan, 2001; Douhard et al., 2017); therefore even if orphans make up for initial stunting following their mother’s death, this does not necessarily equate to an absence of physiological costs associated with reduced growth. Alternatively, older orphans measured in this study generally had survived a longer time without their mother than younger orphans; given the lower overall survival of orphans (Parker et al., 2021), older orphans were likely fitter (and potentially taller) relative to their counterparts that did not survive and therefore were not part of this study.
Rainfall in the first 6 years of life was significantly, but weakly, negatively correlated with elephant growth, a counterintuitive result as early life environmental conditions are recognized as influencing growth in several mammal populations (Plard et al., 2015; Hamel et al., 2016; Veylit et al., 2021). Further, the positive influence of rainfall on growth and fitness has been documented in another African elephant population in Amboseli, Kenya (Moss, 2001; Moss et al., 2011), and rainfall’s effect on elephant demographics such as calf survival has been well established in our semi-arid study system as well (Wittemyer et al., 2007a, 2013, 2021). The drivers of this study’s counterintuitive results are unclear. Possibly, the elephants we measured who were able to survive conditions of drought as young calves represented more fit individuals than others who would have died under similar conditions but were born into more productive times. In addition, natural orphaning disproportionately occurs in low rainfall periods and poaching pressure in the study area increased during a particularly dry period (Wittemyer et al., 2021), causing unbalanced sampling relative to rainfall.
Our findings showed small but interesting correlations between height and the number of multiparous females and age mates within a core group. A previous study found similar relationships between faecal glucocorticoids (stress) and social conditions, notably that social buffering from age mates may reduce stress in wild African elephants (Parker et al., 2022), which could foster growth (Wu, 2021). However, the effect of adult females on the stress response was uncertain (Parker et al., 2022), and our results here suggest the presence of more adult females marginally reduces growth. Possibly adult females increase competition and limit access to resources for growth. Social rank among elephants is strongly correlated with age (Wittemyer and Getz, 2007), meaning adults would outcompete the younger elephants included in our orphan/non-orphan comparison. A greater number of multiparous females also indicates larger group size and thus more individuals to compete with overall. Elephants split into smaller groups during the dry season when there are fewer resources (Wittemyer et al., 2005b), presumably to reduce competition. Our results suggest these fission events may be important for growing juveniles, and thus the need for young elephants to access resources for growth may be a driver of social fissioning in African elephants.
Our study used rich data on elephant individuals from a long-term study, but a notable limitation was our inability to adequately assess genetic effects beyond grouping families together according to a common estimated asymptote. The analysis model could not optimally control for certain families within the study population being generally taller or shorter than others as has been anecdotally observed. We further could not account for paternal effects because paternity is unknown for many individuals in the Samburu population (Rasmussen et al., 2008).
In addition to our findings concerning orphaning, the derived growth curve provides valuable insight into the study population. The Samburu growth curve for females was comparable with that calculated by Lee and Moss (1995) for known-age elephants in Amboseli, Kenya, but indicated a slightly taller population. Lee and Moss (1995) used photogrammetry and a calibrated lens, deriving an H∞ of 232 cm when including measurements from animals with estimated older ages and 236 cm when using only animals born during the study period. Our estimate of 240 cm using all data points was similar, and between Amboseli’s asymptotes and the 246 cm asymptote recorded in Kruger National Park of South Africa (Shrader et al., 2006). The work by Shrader et al. (2006) demonstrated that elephant growth is similar across Africa; however, other asymptote estimates given in their study were derived from dead elephants on their sides, meaning there was no compression due to body mass and limiting comparability of asymptotic growth across populations other than with the two populations mentioned above.
We recommend similar studies of potential orphan stunting within other long-term projects of terrestrial mammals. Research into possible stunting would be especially interesting in a species in which adoption is common such as the mountain gorilla (Morrison et al., 2021), or with a larger sample size of adopted individuals in chimpanzees as suggested by Samuni et al. (2020). Adopted orphans may grow at the same rate as non-orphans, or at least show less stunting than unadopted orphans. Even more interesting would be if growth correlates with the quality of care provided. In humans, adopted children with a kind caretaker grew at a faster rate than adopted children with a strict caretaker, then growth rates of the two groups flipped when the caretakers switched positions with one another (Sapolsky, 2004).
Stunted growth is relevant to conserving the endangered African elephant (Gobush et al., 2021) because growth positively correlates with fitness (Altmann and Alberts, 2005; Dmitriew, 2011). Poaching, rising human–elephant conflict and increased drought severity due to climate change that remove adult females from elephant populations indirectly decrease population growth by generating more orphans, who have lower survival than non-orphans (Kinyanjui et al., 2020; Gobush et al., 2021; Li et al., 2021; Parker et al., 2021). Our results suggest these factors may have even further-reaching indirect effects by decreasing the fitness of orphans who manage to survive (Dmitriew, 2011).
Supplementary Material
Acknowledgments
We thank S.Z. Goldenberg for assistance with long-term monitoring and guidance to J.P. in measuring elephants. Thanks you to Save the Elephants: the long-term monitoring team, especially D. Daballen, for assistance with ageing; manager G. Sabinga, CFO W. Kimani, CEO F. Pope and founder I. Douglas-Hamilton for providing logistical support; and the camp staff for providing a base. Thank you to A. Feuka for double checking the analysis model. The comments of two anonymous reviewers greatly improved the manuscript. We thank the National Commission of Science and Technology of Kenya, the Kenya Wildlife Service and the county governments of Samburu and Isiolo for permission to conduct this research in the Samburu and Buffalo Springs National Reserves of Kenya.
Contributor Information
Jenna M Parker, San Diego Zoo Wildlife Alliance, 15600 San Pasqual Valley Road, Escondido, CA 92027, USA; Save the Elephants, Marula Manor, Marula Lane, Karen, Nairobi 00200, Kenya.
George Wittemyer, Save the Elephants, Marula Manor, Marula Lane, Karen, Nairobi 00200, Kenya; Graduate Degree Program in Ecology, Colorado State University, 102 Johnson Hall, Fort Collins, CO 80523, USA; Department of Fish, Wildlife and Conservation Biology, Colorado State University, 1474 Campus Delivery, Fort Collins, CO 80523, USA.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
This work was supported by the National Science Foundation Graduate Research Fellowship Program [grant number 006784] and Postdoctoral Research Fellowship in Biology Program [grant number 2109816], Save the Elephants and S. Rankin of WorldWomenWork.
Data Availability
The height measurement data and analysis code used in this manuscript can be found in the supplementary material. Some of the long-term monitoring data are sensitive due to the endangered status of the African elephant, but may be viewable upon request.
References
- Altmann J, Alberts SC (2005) Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol 57: 490–501. 10.1007/s00265-004-0870-x. [DOI] [Google Scholar]
- Andres D, Clutton-Brock TH, Kruuk LEB, Pemberton JM, Stopher KV, Ruckstuhl KE (2013) Sex differences in the consequences of maternal loss in a long-lived mammal, the red deer (Cervus elaphus). Behav Ecol Sociobiol 67: 1249–1258. 10.1007/s00265-013-1552-3. [DOI] [Google Scholar]
- Baty F, Ritz C, Charles S, Brutsche M, Flandrois J-P, Delignette-Muller M-L (2015) A toolbox for nonlinear regression in R: the Package nlstools. J Stat Softw 66: 1–21. 10.18637/jss.v066.i05. [DOI] [Google Scholar]
- Bernstein RM (2010) The big and small of it: how body size evolves. Am J Phys Anthropol 143: 46–62. 10.1002/ajpa.21440. [DOI] [PubMed] [Google Scholar]
- Bertalanffy L (1938) A quantitative theory of organic growth. Hum Biol 10: 181–213. [Google Scholar]
- Brooks SP, Gelman A (1998) General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 7: 434–455. [Google Scholar]
- Clutton-Brock T (1991) The Evolution of Parental Care. Princeton University Press, Princeton, NJ. 10.1515/9780691206981. [DOI] [Google Scholar]
- Onis M, Branca F (2016) Childhood stunting: a global perspective. Matern Child Nutr 12: 12–26. 10.1111/mcn.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens MJ, Romero LM (2013) A consensus endocrine profile for chronically stressed wild animals does not exist. Gen Comp Endocrinol 191: 177–189. 10.1016/j.ygcen.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Dmitriew CM (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev 86: 97–116. 10.1111/j.1469-185X.2010.00136.x. [DOI] [PubMed] [Google Scholar]
- Douglas-Hamilton I (1973) On the ecology and behaviour of the Lake Manyara elephants. Afr J Ecol 11: 401–403. 10.1111/j.1365-2028.1973.tb00101.x. [DOI] [Google Scholar]
- Douhard F, Gaillard JM, Pellerin M, Jacob L, Lemaître JF (2017) The cost of growing large: costs of post-weaning growth on body mass senescence in a wild mammal. Oikos 126: 1329–1338. 10.1111/oik.04421. [DOI] [Google Scholar]
- Finlay JE, Fink G, Mccoy DC, Tavárez LC, Chai J, Danaei G, Ezzati M, Fawzi W, Fawzi MCS, Journal Set al. (2016) Stunting risk of orphans by caregiver and living arrangement in low-income and middle-income countries. J Epidemiol Community Health 70: 784–790. 10.1136/jech-2015-206346. [DOI] [PubMed] [Google Scholar]
- Foley C, Pettorelli N, Foley L (2008) Severe drought and calf survival in elephants. Biol Lett 4: 541–544. 10.1098/rsbl.2008.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster EA, Franks DW, Mazzi S, Darden SK, Balcomb KC, Ford JKB, Croft DP (2012) Adaptive prolonged postreproductive life span in killer whales. Science 337: 1313. 10.1126/science.1224198. [DOI] [PubMed] [Google Scholar]
- Gabbitas B, Pash JM, Delany AM, Canalis E (1996) Cortisol inhibits the synthesis of insulin-like growth factor-binding protein-5 in bone cell cultures by transcriptional mechanisms. J Biol Chem 271: 9033–9038. 10.1074/jbc.271.15.9033. [DOI] [PubMed] [Google Scholar]
- Gabry J, Mahr T (2020) Bayesplot: plotting for Bayesian models. https://mc-stan.org/bayesplot.
- Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7: 457–511. 10.1214/ss/1177011136. [DOI] [Google Scholar]
- Girard-Buttoz C, Tkaczynski PJ, Samuni L, Fedurek P, Gomes C, Löhrich T, Manin V, Preis A, Valé PF, Deschner Tet al. (2021) Early maternal loss leads to short- but not long-term effects on diurnal cortisol slopes in wild chimpanzees. Elife 10: e64134. 10.7554/eLife.64134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobush KS, Edwards CT, Balfour D, Wittemyer G, Maisels F, Taylor RD (2021) Loxodonta africana (African Savanna elephant). Loxodonta africana IUCN Red List Threat Species 2021. https://www.iucnredlist.org/species/181008073/181022663.
- Goldenberg SZ, Douglas-Hamilton I, Wittemyer G (2016) Vertical transmission of social roles drives resilience to poaching in elephant networks. Curr Biol 26: 75–79. 10.1016/j.cub.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Goldenberg SZ, Wittemyer G (2017) Orphaned female elephant social bonds reflect lack of access to mature adults. Sci Rep 7: 14408. 10.1038/s41598-017-14712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg SZ, Wittemyer G (2018) Orphaning and natal group dispersal are associated with social costs in female elephants. Anim Behav 143: 1–8. 10.1016/j.anbehav.2018.07.002. [DOI] [Google Scholar]
- Hamel S, Gaillard JM, Yoccoz NG, Albon S, Côté SD, Craine JM, Festa-Bianchet M, Garel M, Lee P, Moss Cet al. (2016) Cohort variation in individual body mass dissipates with age in large herbivores. Ecol Monogr 86: 517–543. 10.1002/ecm.1232. [DOI] [Google Scholar]
- Heidelberger P, Welch PD (1983) Simulation run length control in the presence of an initial transient. Oper Res 31: 1109–1144. 10.1287/opre.31.6.1109. [DOI] [Google Scholar]
- Hobbs NT, Hooten MB (2015) Bayesian Models: A Statistical Primer for Ecologists. Princeton University Press, Princeton, NJ. 10.23943/princeton/9780691159287.001.0001. [DOI] [Google Scholar]
- Johnson SE (2003) Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboons. Am J Phys Anthropol 120: 83–98. 10.1002/ajpa.10139. [DOI] [PubMed] [Google Scholar]
- Kinyanjui MW, Raja NR, Brennan EJ, King LE, Tiller LN (2020) Local attitudes and perceived threats of human-elephant conflict: a case study at Lake Jipe, Kenya. Pachyderm 2020: 120–130. [Google Scholar]
- Larramendi A (2015) Proboscideans: shoulder height, body mass and shape. Acta Palaeontol Pol 61: 537–574. 10.4202/app.00136.2014. [DOI] [Google Scholar]
- Lee PC, Fishlock V, Webber CE, Moss CJ (2016) The reproductive advantages of a long life: longevity and senescence in wild female African elephants. Behav Ecol Sociobiol 70: 337–345. 10.1007/s00265-015-2051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Moss CJ (1986) Early maternal investment in male and female African elephant calves. Behav Ecol Sociobiol 18: 353–361. 10.1007/BF00299666. [DOI] [Google Scholar]
- Lee PC, Moss CJ (1995) Statural growth in known-age African elephants (Loxodonta africana). J Zool 236: 29–41. 10.1111/j.1469-7998.1995.tb01782.x. [DOI] [Google Scholar]
- Li H, Li Z, Chen Y, Xiang Y, Liu Y, Kayumba PM, Li X (2021) Drylands face potential threat of robust drought in the CMIP6 SSPs scenarios. Environ Res Lett 16: 114004. 10.1088/1748-9326/ac2bce. [DOI] [Google Scholar]
- McComb K, Moss C, Durant SM, Baker L, Sayialel S (2001) Matriarchs as repositories of social knowledge in African elephants. Science 292: 491–494. 10.1126/science.1057895. [DOI] [PubMed] [Google Scholar]
- Meale SJ, Leal LN, Martín-Tereso J, Steele MA (2015) Delayed weaning of Holstein bull calves fed an elevated plane of nutrition impacts feed intake, growth and potential markers of gastrointestinal development. Anim Feed Sci Technol 209: 268–273. 10.1016/j.anifeedsci.2015.08.008. [DOI] [Google Scholar]
- Metcalfe NB, Monaghan P (2001) Compensation for a bad start: grow now, pay later? Trends Ecol Evol 16: 254–260. 10.1016/S0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Morrison RE, Eckardt W, Colchero F, Vecellio V, Stoinski TS (2021) Social groups buffer maternal loss in mountain gorillas. Elife 10: 1–22. 10.7554/eLife.62939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss CJ (2001) The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya. J Zool 255: 145–156. 10.1017/S0952836901001212. [DOI] [Google Scholar]
- Moss CJ, Croze H, Lee PC (2011) The Amboseli Elephants: A Lont-Term Perspective on a Long-Lived Mammal. University of Chicago Press, Chicago. 10.7208/chicago/9780226542263.001.0001. [DOI] [Google Scholar]
- Ogle DH (2013) fish R Vignette–Von Bertalanffy Growth Models. http://derekogle.com/fishR/examples/oldFishRVignettes/VonBertalanffy.pdf.
- Ogle DH (2019) FSAdata: fisheries stock analysis. https://cran.r-project.org/web/packages/FSAdata/FSAdata.pdf.
- Ogle DH, Doll JC, Wheeler P, Dinno A (2021) FSA: fisheries stock analysis. https://cran.r-project.org/web/packages/FSA/FSA.pdf.
- Parker JM, Brown JL, Hobbs NT, Boisseau NP, Letitiya D, Douglas-Hamilton I, Wittemyer G (2022) Social support correlates with glucocorticoid concentrations in wild African elephant orphans. Commun Biol. 5: 630. 10.1038/s42003-022-03574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JM, Goldenberg SZ, Letitiya D, Wittemyer G (2020) Strongylid infection varies with age, sex, movement and social factors in wild African elephants. Parasitology 147: 348–359. 10.1017/S0031182019001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JM, Webb CT, Daballen D, Goldenberg SZ, Lepirei J, Letitiya D, Lolchuragi D, Leadismo C, Douglas-Hamilton I, Wittemyer G (2021) Poaching of African elephants indirectly decreases population growth through lowered orphan survival. Curr Biol 31: 1–7. [DOI] [PubMed] [Google Scholar]
- Plard F, Gaillard JM, Coulson T, Hewison AJM, Douhard M, Klein F, Delorme D, Warnant C, Bonenfant C (2015) The influence of birth date via body mass on individual fitness in a long-lived mammal. Ecology 96: 1516–1528. 10.1890/14-0106.1. [DOI] [Google Scholar]
- Plummer M (2019) rjags: Bayesian graphical models using MCMC. https://cran.r-project.org/package=rjags.
- R Core Team (2020) A language and environment for statistical computing. https://www.r-project.org/
- Rasmussen HB, Okello JBA, Wittemyer G, Siegismund HR, Arctander P, Vollrath F, Douglas-Hamilton I (2008) Age- and tactic-related paternity success in male African elephants. Behav Ecol 19: 9–15. 10.1093/beheco/arm093. [DOI] [Google Scholar]
- Rasmussen HB, Wittemyer G, Douglas-Hamilton I (2005) Estimating age of immobilized elephants from teeth impressions using dental silicon. Afr J Ecol 43: 215–219. 10.1111/j.1365-2028.2005.00571.x. [DOI] [Google Scholar]
- Rstudio Team (2020) RStudio: Integrated development for R. http://www.rstudio.com/
- Samuni L, Tkaczynski P, Deschner T, Löhrrich T, Wittig RM, Crockford C (2020) Maternal effects on offspring growth indicate post-weaning juvenile dependence in chimpanzees (Pan troglodytes verus). Front Zool 17: 1–12. 10.1186/s12983-019-0343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM (2004) Why Zebras Don’t Get Ulcers, Ed3rd. Henry Holt and Company, LLC, New York. [Google Scholar]
- Shrader AMÃ, Ferreira SM, Mcelveen ME, Lee PC, Moss CJ, Van ARJ (2006) Growth and age determination of African savanna elephants. J Zool 270: 40–48. 10.1111/j.1469-7998.2006.00108.x. [DOI] [Google Scholar]
- Stanton MA, Lonsdorf EV, Murray CM, Pusey AE (2020) Consequences of maternal loss before and after weaning in male and female wild chimpanzees. Behav Ecol Sociobiol 74: 22. 10.1007/s00265-020-2804-7. [DOI] [Google Scholar]
- Veylit L, Sæther BE, Gaillard JM, Baubet E, Gamelon M (2021) Many lifetime growth trajectories for a single mammal. Ecol Evol 11: 14789–14804. 10.1002/ece3.8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts HE, Tanner JB, Lundrigan BL, Holekamp KE (2009) Post-weaning maternal effects and the evolution of female dominance in the spotted hyena. Proc R Soc B Biol Sci 276: 2291–2298. 10.1098/rspb.2009.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016) ggplot2: Elegant graphics for data analysis. https://ggplot2.tidyverse.org, 10.1007/978-3-319-24277-4. [DOI]
- Wittemyer G (2001) The elephant population of Samburu and Buffalo Springs National Reserves, Kenya. Afr J Ecol 39: 357–365. 10.1046/j.1365-2028.2001.00324.x. [DOI] [Google Scholar]
- Wittemyer G, Daballen D, Douglas-Hamilton I (2013) Comparative demography of an at-risk African elephant population. PLoS One 8: e53726. 10.1371/journal.pone.0053726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittemyer G, Daballen D, Douglas-Hamilton I (2021) Differential influence of human impacts on age-specific demography underpins trends in an African elephant population. Ecosphere 12: e03720. 10.1002/ecs2.3720. [DOI] [Google Scholar]
- Wittemyer G, Daballen D, Rasmussen H, Kahindi O, Douglas-Hamilton I (2005a) Demographic status of elephants in the Samburu and Buffalo Springs National Reserves, Kenya. Afr J Ecol 43: 44–47. 10.1111/j.1365-2028.2004.00543.x. [DOI] [Google Scholar]
- Wittemyer G, Douglas-Hamilton I, Getz WM (2005b) The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim Behav 69: 1357–1371. 10.1016/j.anbehav.2004.08.018. [DOI] [Google Scholar]
- Wittemyer G, Ganswindt A, Hodges K (2007a) The impact of ecological variability on the reproductive endocrinology of wild female African elephants. Horm Behav 51: 346–354. 10.1016/j.yhbeh.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Wittemyer G, Getz WM (2007) Hierarchical dominance structure and social organization in African elephants, Loxodonta africana. Anim Behav 73: 671–681. 10.1016/j.anbehav.2006.10.008. [DOI] [Google Scholar]
- Wittemyer G, Getz WM, Vollrath F, Douglas-Hamilton I (2007b) Social dominance, seasonal movements, and spatial segregation in African elephants: a contribution to conservation behavior. Behav Ecol Sociobiol 61: 1919–1931. 10.1007/s00265-007-0432-0. [DOI] [Google Scholar]
- Wu A (2021) Social buffering of stress—physiological and ethological perspectives. Appl Anim Behav Sci 239: 105325. 10.1016/j.applanim.2021.105325. [DOI] [Google Scholar]
- Youngflesh C (2018) MCMCvis: tools to visualize, manipulate, and summarize MCMC output. JOSS 3: 640. 10.21105/joss.00640. [DOI] [Google Scholar]
- Zipple MN, Altmann J, Campos FA, Cords M, Fedigan LM, Lawler RR, Lonsdorf EV, Perry S, Pusey AE, Stoinski TSet al. (2021) Maternal death and offspring fitness in multiple wild primates. Proc Natl Acad Sci 118: e2015317118. 10.1073/pnas.2015317118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The height measurement data and analysis code used in this manuscript can be found in the supplementary material. Some of the long-term monitoring data are sensitive due to the endangered status of the African elephant, but may be viewable upon request.


