Abstract
Brown adipose tissue (BAT) is a metabolically active tissue that improves glucose metabolism and protects against the development of type 2 diabetes and obesity. However, the role of BAT to improve cardiovascular health has only recently been investigated. In this review, we discuss multiple mechanisms through which both the thermogenic and endocrine functions of BAT mediate cardiac health. β-adrenergic stimulation activates the thermogenic function of BAT, resulting in reduced circulating lipids and glucose, and enhanced clearance of hepatic cholesterol-enriched remnants leading to reduced atherosclerotic region size. Additionally, the thermogenic role of BAT has been implicated in activation of the protein kinase B-extracellular-signal-regulated kinase (ERK) 1/2 pathway after myocardial infarction (MI), contributing to reduced injury size. The endocrine function of BAT has also been implicated to improve both systemic metabolic health and cardiac health. Specifically, the batokines fibroblast growth factor 21 (FGF21) and 12,13-diHOME improve cardiovascular health via reduced hypertension, hypertrophy and MI injury size (FGF21) or by directly improving cardiac function via calcium cycling (12,13-diHOME). Finally, we discuss relevant pharmacological treatment methods currently aiming to activate BAT, typically through sympathetic activation.
SIGNIFICANCE STATEMENT
This mini-review discusses the role of BAT to improve cardiac health via thermogenic and endocrine effects in both rodents and humans and highlights the need for therapeutic methods which activate or mimic BAT activity.
1. Introduction
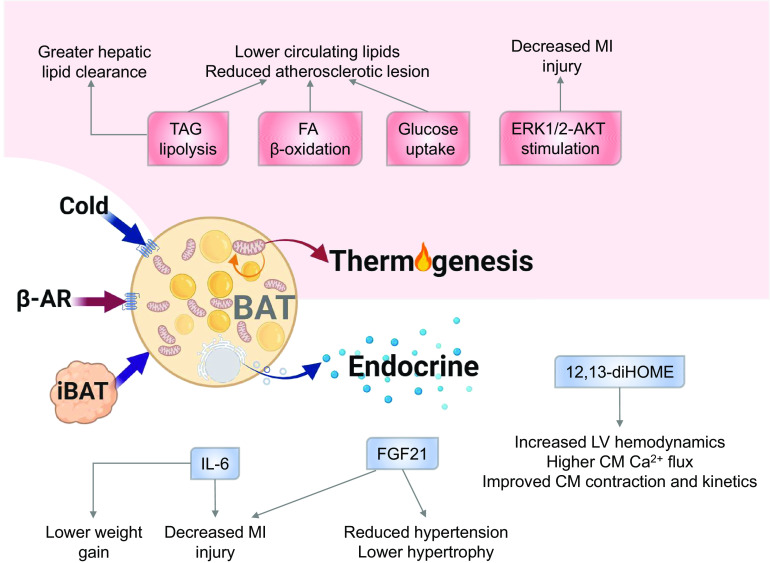
Cardiovascular disease (CVD) is the leading cause of death in the U.S. and worldwide (Prevention, 2018; Organization, 2018). The presence of obesity and type 2 diabetes (T2D) significantly increase the risk for development of CVD (Van Gaal, Mertens, and De Block 2006; Carbone et al., 2019). Obesity, T2D, and CVD are increasing at rapid rates across the United States and worldwide (Hales CM 2017; Moore, Chaudhary, and Akinyemiju 2017); thus, determining potential tools to combat these diseases is of utmost importance. An important tissue to combat both obesity and T2D is brown adipose tissue (BAT); however, a role for BAT to provide a protective role in CVD has only recently been investigated. Recent studies have now identified a direct role for BAT to mediate cardiac function in mice and reduce CVD risk in rodents and humans (Fig. 1) (Berbée et al., 2015; Ruan et al., 2018; Thoonen et al., 2015; Pinckard et al., 2021; Becher et al., 2021).
Fig. 1.
Brown adipose tissue mediates cardiac function via thermogenic and endocrine action. Abbreviations: β-AR, β-adrenergic receptors; iBAT, intrascapular brown adipose tissue; TAG, triacylglycerol; FA, fatty acid; ERK, extracellular-signal-regulated kinase; AKT, protein kinase B; IL6, interleukin 6; FGF21, fibroblast growth factor 21; CM, cardiomyocyte.
2. Brown Adipose Tissue (BAT)
Adipose tissue is a specialized connective tissue consisting of several cell types, including preadipocytes, adipose-derived stem cells and immune cells, and lipid-rich adipocytes (Esteve Rafols 2014). Adipose tissue functions in both lipid storage and lipolysis and is a vital tissue for maintaining energy intake and expenditure. There are three main types of adipose tissue found in rodents and humans, white adipose tissue (WAT), brown adipose tissue (BAT), and beige, or brite (or brown-in-white) adipose tissue. WAT and BAT differ markedly in morphology and function. White adipocytes consist of a single large lipid droplet and possess only a few mitochondria, while brown adipocytes contain multiple lipid droplets per cell and are packed with mitochondria (Betz and Enerback 2015). BAT is densely innervated by the sympathetic nervous system (SNS) and is highly vascularized. Beige adipocytes are found in WAT depots, but have morphologic and functional similarities to BAT, with high levels of mitochondria, increased expression of uncoupling protein 1 (UCP1), and multi-locular lipid droplets (Wu et al., 2012).
2.1 BAT in humans versus rodents
While the importance of BAT in rodents for thermogenesis and whole-body metabolism is well-established, it was historically believed that functional BAT only existed in human infants, rapidly decreasing through childhood and disappearing in adulthood. However, in the late 2000s, several studies identified functional BAT in adult humans through the use of retrospective analysis of nuclear medicine images of radiolabeled fluorodexyglucose positron emission tomography (PET) and computer tomography (CT) (Cypess et al., 2009; Nedergaard, Bengtsson, and Cannon 2007; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). This “rediscovery” of BAT in humans invigorated interest in BAT as a therapeutic target and has led to several important studies which identified BAT as a mediator of metabolic health in humans (Bakker et al., 2014; van der Lans et al., 2013; Lee et al., 2014).
BAT differs markedly among humans and rodents. Rodents possess five BAT depots, with the largest being a large well-delineated interscapular BAT (iBAT) depot (Lehnig et al., 2019). Conversely, human BAT depots are not well defined and are located in the supraclavicular and neck regions, as well as the paravertebral, mediastinal, para-aortic, and suprarenal areas (Nedergaard, Bengtsson, and Cannon 2007). Some human BAT depots have been described as more similar in appearance to rodent beige adipocytes rather than the traditional BAT depots; however, the gene expression profile of human BAT is similar to classic rodent BAT (Cannon et al., 2020; Cypess et al., 2009; Cypess et al., 2013).
In addition to differences in depot location, there is also a large difference in the amount of BAT found in humans versus rodents. In humans, BAT accounts for 0.1% of an individual’s body weight, which is 5-10 times lower than that of the mouse (van Marken Lichtenbelt and Schrauwen 2011; Virtanen et al., 2009). BAT is thus responsible for less of the resting metabolic rate in humans than in rodents. Despite this, there is still a significant correlation between cold-induced BAT activity, non-shivering thermogenesis, and insulin sensitivity in humans (Bakker et al., 2014; van der Lans et al., 2013; Lee et al., 2014). These data suggest that although the BAT depots may be different in quantity and location between rodents and humans, both have a profound effect on systemic metabolism.
BAT activation also differs between mice and humans. While β3-adrenergic receptors (ARs) are the predominant regulators of BAT thermogenesis in rodents (Zhao et al., 1994), β1- and β2-ARs are the primary regulators of BAT metabolism and thermogenesis in humans (Riis-Vestergaard et al., 2020; Blondin et al., 2020). However, treatment with the β3-AR agonist mirabegron is somewhat effective at eliciting a response in human BAT. One study in humans found that maximal oral treatment with mirabegron (200mg) increased BAT oxidative metabolism, blood flow, and glucose uptake in vivo, although at a much lower rate than cold exposure (Blondin et al., 2020). In contrast, treatment with a therapeutic dose of mirabegron (50 mg) did not affect BAT (Blondin et al., 2020). The authors suggest that at high enough doses, mirabegron treatment induces non-specific adrenergic responses in human tissues, as indicated by adrenergic response to 200 mg mirabegron treatment in the heart (an organ primarily driven by β1-ARs) (Blondin et al., 2020). In addition, an in vitro study found that mirabegron treatment (10 μM) stimulated lipolysis and thermogenesis in human brown/beige adipocytes in vitro, although treatment with β1- and β2-AR agonist treatments had stronger impacts on brown adipocyte respiration (Cero et al., 2021). Together, these data suggest β1- and β2 are the main ARs responsible for BAT activation in humans, but that β3-AR agonist mirabegron treatment may still stimulate BAT in humans at high doses.
There are several factors that contribute to the functionality of BAT in humans. Human studies show that BAT is negatively correlated with body mass index (BMI), fasting glucose, and age (Cypess et al., 2009; Saito et al., 2009). Young lean women have higher BAT mass (more BAT-positive PET-CT scans) and activity (higher fluorodexyglucose uptake) compared to men and older individuals in human studies (Cypess et al., 2009). BAT mass and activity are therefore affected by sex, age, and body composition, highlighting the need to find therapeutic methods to maintain BAT mass and activity in such circumstances.
2.2 BAT thermogenic activity
BAT is a highly thermogenic tissue which dissipates chemical energy as heat by uncoupling oxidative phosphorylation in the mitochondria via UCP1 (Cannon and Nedergaard 2004). In both rodents and humans, BAT is densely innervated by the sympathetic nervous system (SNS), which controls BAT activation of thermogenesis via norepinephrine. Norepinephrine binds to β-adrenergic cell surface receptors (β1–3); all three subtypes of which are expressed in brown adipocytes (Rohlfs et al., 1995) although their relative abundance varies by location and species (as discussed above) (Lafontan and Berlan 1993). The signaling pathway invoked by β-stimulation is mediated by cyclic adenosine monophosphate and protein kinase A (Thoonen, Hindle, and Scherrer-Crosbie 2016; Pagnon et al., 2012). Activation of hormone-sensitive lipase (HSL) and adipose triglyceride lipase leads to lipolysis of intracellular triglycerides (TGs), resulting in the release of fatty acids. The fatty acids are metabolized in the mitochondria by β-oxidation, and additionally activate UCP1.
UCP1 is a mitochondrial inner membrane protein and proton channel, which is stabilized by cardiolipin. This proton pump allows thermogenic capacity to be controlled by the central nervous system via norepinephrine. Fatty acids (FAs) derived from TGs are the main energy source for BAT thermogenesis, but glucose is also used by BAT for ATP generation (via glycolysis) (Carpentier et al., 2018).
Thus, increased BAT mass and activity decrease circulating FA and glucose, protecting against the development of T2D and obesity (Cypess et al., 2013; Stanford et al., 2013; Cannon and Nedergaard 2004). Because of its role in thermogenesis and energy expenditure, BAT is an attractive topic of investigation for the regulation of both systemic metabolism and cardiovascular diseases.
2.3 BAT endocrine activity
In rodents, BAT also has a crucial secretory role which contributes to the systemic consequences of BAT activity. BAT releases ‘batokines’, including signaling lipids, proteins, metabolites or microRNAs (Villarroya et al., 2019; Lee et al., 2019). These batokines can act in an autocrine, paracrine, or endocrine manner to mediate systemic physiology. Most paracrine factors released from BAT (adenosine, nitric oxide, neuregulin-4, S100b protein, bone morphogenetic protein-8b, C-X-C motif chemokine ligand-14) function to promote hypertrophy and hyperplasia of BAT. This can include increased BAT vascularization, innervation, and blood flow, all processes associated with BAT recruitment and thermogenic activity (Villarroya et al., 2019; Villarroya, Cereijo, et al., 2017).
A multitude of studies have demonstrated the efficacy of increased BAT mass or activity through transplantation, activation via cold exposure, or direct β-adrenergic stimulation to improve human and rodent metabolic health is likely related to the thermogenic or endocrine functions of BAT (Cypess et al., 2013; Stanford et al., 2013; Cannon and Nedergaard 2004). The role of BAT to improve cardiac function, however, has only recently become a topic of investigation.
3. BAT and Cardiovascular Disease
Studies in rodents show that increasing BAT mass or activity has the capacity to reduce many cardiovascular risk factors, including obesity (White et al., 2019; Wang et al., 2015c; Yoneshiro et al., 2013), insulin resistance (Stanford et al., 2013), and T2D (Cypess and Kahn 2010). However, BAT mass and activity are known to decrease with increasing BMI, fasting glucose, and age in humans (Cypess et al., 2009)
Studies in humans and rodents have also identified reduced BAT mass or activity in instances of cardiovascular disease, independent of the presence of metabolic disease (Takx et al., 2016; Thoonen et al., 2015). Here, we will discuss the impact of both the thermogenic and endocrine functions of BAT on various cardiovascular diseases including atherosclerosis, hypertension, and myocardial infarction, as well as on general cardiac function (Fig. 1).
3.1 Atherosclerosis
Atherosclerosis or coronary artery disease (CAD) is the most common form of CVD. Obesity and hyperlipidemia are two of the main causes of atherosclerosis; thus, BAT may be an attractive therapy for prevention of CAD development (Rocha and Libby 2009; Berbée et al., 2015; Becher et al., 2021). In a study of 443 human patients (44% male, 56% female, mean age 55 years, mean BMI 26), BAT activity (determined by 18F-FDG uptake in supraclavicular BAT) was negatively correlated with arterial inflammation, even after correcting for age and BMI (Takx et al., 2016). A longitudinal study in healthy human adults found that cold-induced BAT activity was negatively correlated with atherosclerosis markers and positively associated with healthy vessel function 5 years after baseline BAT measurements were recorded (Raiko et al., 2020). Together, these studies indicate that BAT may be athero-protective in healthy adults. However, whether the correlation between BAT and reduced atherosclerotic risk is direct or indirect in response to improved metabolic health in these individuals (and therefore reduced risk for CVD) has not been determined.
Mouse studies also suggest that BAT thermogenic activity is correlated with atherosclerosis; both C57BL6 wild-type (WT) and apolipoprotein E (ApoE−/−) mice housed at thermoneutrality have accelerated onset of atherosclerosis (Tian et al., 2016; Giles et al., 2016). It is not clear if lack of BAT activity at thermoneutrality is causative or correlated to the accelerated atherosclerosis development; studies measuring BAT activity in thermoneutral versus ambient temperature housed mice utilizing BAT transplant or inhibition methods are necessary to investigate this mechanistic link.
Other rodent studies have investigated the mechanistic roles of BAT to directly affect CAD. Berbée et al. used Apolipoprotein E3 human Cholesteryl Ester Transfer Protein (E3L.CETP) mice, a well-established model for human-like atherosclerosis that expresses functional ApoE and low-density lipoprotein receptor (LDLR), to study the role of BAT activity in a model of CAD (Berbée et al., 2015). The authors found that BAT activation by β3-AR stimulation enhanced the selective uptake of fatty acids from TG-rich lipoproteins into BAT, accelerating the hepatic clearance of the cholesterol-enriched remnants (Berbée et al., 2015). As a result, BAT activation reduced atherosclerotic lesion size and disease severity in Apolipoprotein E3 human Cholesteryl Ester Transfer Protein (E3L.CETP) mice; however, this effect was not seen in ApoE−/− or LDLR−/− mice. These exciting data indicate that BAT mediates local lipolysis of TG-rich lipoproteins and stimulates hepatic clearance through the ApoE–LDLR pathway (Berbée et al., 2015). Together, these studies show a correlation between BAT activity and atherosclerosis in mice and humans (Tian et al., 2016; Giles et al., 2016; Takx et al., 2016) and also indicate a mechanistic role for BAT to directly mediate atherosclerosis development via hepatic clearance of cholesterol-enriched remnants (Berbée et al., 2015), indicating the potential therapeutic role of BAT as a treatment of atherosclerosis.
3.2 Hypertension
Hypertension, or high blood pressure, is one of the most common risk factors for CVD, causing pathologic cardiac remodeling eventually leading to heart failure (Messerli, Rimoldi, and Bangalore 2017). A review of 18F-FDG PET/CT reports conducted in 53,475 human patients found lower rates of cardiometabolic diseases, including hypertension, among patients with active BAT (Becher et al., 2021). Interestingly, the correlation between increased BAT activity and reduced cardiometabolic diseases was more pronounced in obese individuals, indicating that active BAT can offset the detrimental effects of obesity in humans (Becher et al., 2021). It is surprising that increased BAT activity is correlated with reduced rates of hypertension, given that hypertension is associated with increased sympathetic activity, which should be associated with more active BAT. This suggests divergent pathways of SNS activation to mediate hypertension versus BAT activation.
To understand the relationship between BAT activity and hypertension, Ruan, et al. used three mouse models of hypertension: a WT deoxycorticosterone acetate (DOCA)-salt-sensitive mouse, an adenosine 2A receptor (A2AR) knockout mouse, and a combined DOCA-A2AR−/− mouse (Ruan et al., 2018). The DOCA-salt model is commonly used to study hypertension; in this model, DOCA is administered to the animal (50 mg/pellet) creating an imbalance of renal sodium handling with a high-salt diet [1% NaCl in drinking water (Ruan et al., 2018)] incorporated to increase the onset of hypertension (Basting and Lazartigues 2017). Adenosine is a central nervous system neuromodulator and initiates vasodilation when it binds to the A2AR (Khayat and Nayeem 2017); thus, A2AR knockout [A2AR−/−] mice exhibit high blood pressure (Ledent et al., 1997). Unsurprisingly, DOCA-A2AR−/− mice had accelerated cardiac remodeling (fibrosis and hypertrophy) compared with WT DOCA-hypertensive mice (Ruan et al., 2018). Interestingly, A2AR−/− mice had dysfunctional interscapular BAT (iBAT) demonstrated by reduced Ucp1, Pparg, PR domain containing 16 (Prdm16), and Cidea gene expression. Additionally, surgical depletion of iBAT accelerated cardiac hypertrophy in WT DOCA-hypertensive mice, but not in A2AR−/− hypertensive mice. Together, these data indicate that impaired BAT contributes to salt-induced hypertension and cardiac dysfunction and suggest a role for BAT to attenuate hypertension-induced cardiac hypertrophy via A2A receptors (Ruan et al., 2018).
To further elucidate the relationship between BAT and hypertension, a recent study used Prdm16f/fAdipoCre+ mice. The Prdm16f/f AdipoCre+ mice were investigated based on transcriptomic profiling, indicating that murine beige fat closely approximates the inducible brown fat detected by PET/CT in adult humans (Becher et al., 2021). PRDM16 is the master regulator of beiging and a coregulator of brown adipocyte development, and mice deficient in adipose tissue PRDM16 are unable to induce browning in their WAT (Cohen et al., 2014). Additionally, the white and brown adipose tissue of Prdm16f/f AdipoCre+ mice have severely reduced thermogenic function (Cohen et al., 2014; Harms et al., 2014). Blood pressure was elevated in adipocyte-specific Prdm16f/f AdipoCre+ mice compared with WT animals (Becher et al., 2021). Mesenteric arteries isolated from Prdm16f/f AdipoCre+ mice had increased contraction in response to angiotensin II treatment, suggesting the hypertension in these animals was the result of increased arterial sensitivity to angiotensin II (Becher et al., 2021).
Together, these studies indicate that a role for beige and BAT to mediate hypertension (via A2A receptors and angiotensin II type 1 receptors within the vasculature) and cardiac remodeling, thus identifying BAT as a potential tool to combat hypertension.
3.3 Myocardial infarction
Myocardial infarction (MI) commonly results from CAD. As the arterial walls thicken and plaque blocks or slows the blood flow through the coronary arteries, cell death occurs in that area of the heart (Prabhu and Frangogiannis 2016). The necrotized myocardium is eventually replaced with fibrosis, resulting in impaired cardiac systolic and diastolic function (Prabhu and Frangogiannis 2016), often culminating in heart failure. A recent study in humans found that higher BAT activity (measured by 18FDG/PET/CT) was associated with fewer cardiovascular events, including cardiac arrest and MI, in both male and female patients (Takx et al., 2016). While these human studies indicate a protective role for BAT against CVD, it is unclear whether the correlation between BAT and reduced CVD risk is direct or an indirect response due to improved metabolic health in these individuals.
Rodent studies have indicated that BAT has a direct role to minimize cardiac injury size in response to MI. WT mice have been shown to have increased Ucp1 levels in BAT after MI, indicating BAT activation (Thoonen et al., 2015). To elucidate the role of BAT in cardiac recovery and repair after MI, Ucp1−/− and WT mice underwent catecholamine-induced (isoproterenol) cardiomyopathy (Thoonen et al., 2015). Ucp1−/− mice had a more significant increase in cardiac fibrosis and hypertrophy, higher mortality rates, and a larger decrease in fractional shortening in response to fourteen days of isoproterenol treatment compared with WT mice.
Importantly, transplantation of WT BAT into Ucp1−/− mice reduced myocardial injury size and increased mouse survival, while transplantation of Ucp1−/− BAT into either Ucp1−/− or WT mice did not have protective effects. Additionally, the isoproterenol treatment was correlated with an increase in phosphorylation of protein kinase B (AKT) and extracellular-signal-regulated kinase (ERK) 1/2 in the left ventricles of WT mice, but not Ucp1−/− mice. This is important because ERK1/2 and AKT are cardioprotective in ischemia–reperfusion injury models (Murphy and Steenbergen 2008; van Berlo, Maillet, and Molkentin 2013), suggesting that the cardioprotective effect of the AKT-ERK1/2 pathway in mice after catecholamine-induced cardiomyocyte injury may require BAT activation. These data indicate that BAT thermogenic activity is vital for cardiac repair after MI. Future studies are required to fully define the pathway of BAT-activated AKT-ERK1/2 to determine targets for potential MI therapies.
4. Batokines
While several studies have focused on the thermogenic role of BAT, BAT also has an important endocrine function which can mediate systemic health benefits (Ahmad et al., 2021; Lee, Lee, and Oh 2019; Villarroya et al., 2019). BAT releases signaling molecules, such as lipids, proteins, metabolites, or microRNAs, which can be broadly categorized as ‘batokines’ that act on other tissues and organs. These batokines have significant effects on systemic metabolism, and more recently have been identified to play a role in cardiac health (Fig. 1, Table 1). Here, we will focus primarily on the batokines with a role in cardiovascular health and function.
TABLE 1.
Batokines and their known functions
| Batokine | Class | Endogenous Tissue(s) | Confirmed Stimulation | Function(s) | References |
|---|---|---|---|---|---|
| Adenosine | Nucleoside | — | β-adrenergic stimulation | Activates thermogenesis in BAT; induces beiging in WAT; reduces obesity in mice | (Lee, Lee, and Oh 2019; Gnad et al., 2014) |
| Adiponectin | Protein hormone | BAT, WAT | Cold exposure | Promotes WAT beiging | (Ahmad et al., 2021; Hui et al., 2015) |
| Angiopoietin-Like8 (ANGPTL8)Or Lipasin | Protein | BAT, WAT, liver | Cold exposure | Not fully elucidated, but assumed to negatively regulate BAT thermogenesis | (Lee, Lee, and Oh 2019; Fu et al., 2013) |
| BMP8-b | Protein | BAT, hypothalamus | — | Regulates thermogenesis, promotes sympathetic innervation of adipose tissue via NRG-4, enhances lipolysis via HSL | (Ahmad et al., 2021; Pellegrinelli et al., 2018; Whittle et al., 2012) |
| CXCL14 | Cytokine | BAT | Cold exposure | Promotes WAT beiging, alternatively activated M2 macrophage recruitment | (Ahmad et al., 2021; Cereijo et al., 2018) |
| Endothelin 1 | 21-amino acid peptide | BAT, beige adipocytes, vascular endothelial cells, brain | Gq signaling, inhibited by β-adrenergic stimulation | Suppresses UCP1 expression, beige and brown adipogenesis and whole-body energy expenditure | (Ahmad et al., 2021; Klepac et al., 2016; Lee, Lee, and Oh 2019) |
| EPDR1 | Protein | BAT, WAT | — | Thermogenic (beige/brown) adipocyte differentiation; β-adrenergic signal response, important in BAT mitochondrial respiration and whole body metabolism | (Ahmad et al., 2021; Deshmukh et al., 2019) |
| FGF21 | Protein | BAT, liver, skeletal muscle, heart | Cold exposure, β-adrenergic stimulation, BAT Tx | Protection against hypertension, cardiac hypertrophy and MI injury. Promotes WAT beiging, increases thermogenic function in BAT, and reduces dyslipidemia and insulin resistance in T2D patients | (Ahmad et al., 2021; Stanford et al., 2013; Lee et al., 2014; Ruan et al., 2018; Planavila et al., 2013; Liu et al., 2013; Hondares et al., 2011; Wang, Tao, et al., 2015; Gaich et al., 2013; Villarroya et al., 2017b) |
| Follistatin (Fst) | Glycoprotein | BAT, nearly all tissues | Cold exposure | Inhibits TGF-β/Smad3/myostatin signaling and therefore promotes BAT function and improves lipid homeostasis and whole body metabolism. | (Lee, Lee, and Oh 2019; Singh, Braga, and Pervin 2014) |
| GDF-15 | Cytokine | BAT, liver, kidney, heart, and lung | Cold exposure, β-adrenergic (requires FGF21) | Targets macrophages; anti-inflammatory | (Ahmad et al., 2021; Campderros et al., 2019) |
| IL-6 | Interleukin | BAT, heart, smooth muscle, skeletal muscle | BAT Tx, β-adrenergic | Promotes glucose uptake into BAT, WAT, and heart; promotes adipocyte browning; cardioprotection against MI injury; promotes alternative M2 macrophage activation | (Ahmad et al., 2021; Stanford et al., 2013; Kristof et al., 2019; Burysek and Houstek 1997; Villarroya et al., 2017b; Mauer et al., 2014) |
| IGF1 | Protein hormone | BAT, liver | Cold exposure, BAT Tx | Promotes β-cell function, protects against cytotoxicity and insulitis, anti-inflammatory; anti-diabetic | (Ahmad et al., 2021; Gunawardana and Piston 2012, 2015; Duchamp et al., 1997; Villarroya et al., 2017b) |
| IGFBP-2 | Protein | Beige adipocytes | — | Enhances the differentiation of osteoclasts and increases bone density | (Ahmad et al., 2021; Rahman et al., 2013; DeMambro et al., 2012; Kawai et al., 2011) |
| METRNL | Cytokine | Beige adipocytes, mucosal tissues, skin | Cold exposure | Promotes activation of eosinophils, recruits alternatively activated M2 macrophages in WAT | (Ahmad et al., 2021; Rao et al., 2014) |
| Myostatin | Cytokine | BAT, skeletal muscle, heart | Activation of Agouti-related peptide neurons (by an energy deficit) promotes the expression of GFP8 in BAT which activates myostatin | Impairs skeletal muscle function, insulin stimulated glucose uptake, and brown adipocyte differentiation | (Ahmad et al., 2021; Kong et al., 2018; Steculorum et al., 2016; Fournier et al., 2012; Kim et al., 2012; Lee, Lee, and Oh 2019) |
| Neuregulin 4 (NRG-4) | Protein | BAT, liver | Cold exposure | Enhances WAT beiging, represses hepatic lipogenesis, protects against obesity, insulin resistance, and hepatic steatosis | (Ahmad et al., 2021; Rosell et al., 2014; Wang et al., 2014; Christian 2014; Villarroya 2017b; Chen et al., 2017) |
| NGF | Protein | BAT | Cold exposure | Increases sympathetic innervation and promotes neurite outgrowth | (Ahmad et al., 2021; Néchad et al., 1994; Zeng et al., 2019) |
| Retinol binding protein 4 (RBP4) | Protein | BAT, WAT, liver | Cold exposure, β3-adrenergic stimulation | Involved in the transport of vitamin A derivatives | (Villarroya et al., 2017b) |
| SLIT2-C | Glycoprotein (extracellular matrix protein) | Beige adipocytes | — | Stimulates thermogenesis and improves glucose homeostasis; promotes WAT beiging | (Ahmad et al., 2021; Kang et al., 2017; Svensson et al., 2016; Lee, Lee, and Oh 2019) |
| Triiodothyronine (T3) | Protein hormone | BAT | Cold exposure, β3-adrenergic stimulation (stimulates thyroxin deiodinase type II, the enzyme which converts thyroxin into the active form of Triiodothyronine) | Required for adaptive thermogenesis in BAT; several systemic effects including control of metabolism, cardiac and digestive functions, brain development, and bone maintenance | (Ahmad et al., 2021; de Jesus et al., 2001; Silva and Larsen 1985; Villarroya et al., 2017b) |
| 12-HEPE | Oxylipin | BAT | Cold exposure, β3-adrenergic stimulation | Promotes glucose uptake into BAT and skeletal muscle | (Leiria et al., 2019). |
| 12,13-diHOME | Oxylipin | BAT, liver | Cold, exercise, BAT Tx | Promotes fatty acid uptake into BAT skeletal muscle, and cardiomyocytes, increases CM function and LV hemodynamics | (Lynes et al., 2017; Stanford et al., 2018; Pinckard et al., 2021) |
4.1 Interleukin 6 (IL-6)
Interleukin 6 (IL-6) is a protein which acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine. It is important to note that the tissue source of IL-6 influences the nature of its action (Han et al., 2020). Specifically, IL-6 has been identified as a batokine that mediates the improved metabolic response after BAT transplantation in mice (Stanford et al., 2013). BAT transplantation increased insulin-stimulated glucose uptake in vivo into endogenous BAT, WAT, and heart muscle. However, when BAT was transplanted from interleukin 6 (IL6)−/− mice into WT mice, these beneficial effects on metabolism were lost, demonstrating that BAT-derived IL-6 is required for the profound effects of BAT transplantation on glucose homeostasis and insulin sensitivity. In support of these data, an increase of IL-6 by gene transfer (hydrodynamic delivery of 1 μg pLIVE-IL6 plasmid) reverses weight gain and hepatic steatosis in obese mice (Ma et al., 2015).
IL-6 also has a role in thermoregulation. Body temperature is decreased in total body IL-6−/− mice and central nervous systemic specific IL-6 receptor α [IL6Rα] knockdown (IL6RαNesCre) mice, indicating impaired thermogenesis in these animals (Egecioglu et al., 2018). However, the effects of IL-6 on the heart are unclear; when IL-6 is produced acutely it is protective against MI or ischemia injury in rodents (Chandrasekar et al., 1999; Yamauchi-Takihara et al., 1995), but when chronically released, IL-6 causes pathologic cardiac hypertrophy and impaired cardiomyocyte contraction (Wollert et al., 1996; Terrell et al., 2006; Prabhu 2004). It is important to note, however, that these studies were specifically examining the effects of cardiomyocyte-derived IL-6; therefore, the effects of BAT-derived IL-6 on cardiac function remain elusive.
4.2 Fibroblast Growth Factor 21
Fibroblast growth factor 21 (FGF21) is a metabolic regulator released from several organs, including the liver and BAT. FGF21 is highly expressed in human BAT (Di Franco et al., 2016) with circulating levels increased upon cold exposure (Lee et al., 2014) via activation of the c-AMP pathways (Hondares et al., 2011).
Although the role of FGF21 in human cardiovascular disease has not been investigated, it has been shown to have both antihypertrophic and cardioprotective actions in mouse models of hypertension (Ruan et al., 2018), hypertrophy (Planavila et al., 2013; Ruan et al., 2018), and ischemia (Liu et al., 2013).
Mouse studies have shown that Fgf21−/− animals have increased heart weight, enhanced dilatation, and cardiac dysfunction compared with WT mice in response to isoproterenol infusion, indicating development of pathologic hypertrophy, a sign of heart failure (Planavila et al., 2013). Conversely, FGF21 has been shown to be upregulated in response to myocardial injury (in liver and WAT), with FGF receptor cofactor β-Klotho upregulated in cardiomyocytes for 5 days post-MI (Liu et al., 2013). FGF21 has been determined to interact with FGF receptor 1 in cardiomyocytes, and to induce PI3K phosphorylation, activating a cardioprotective signaling pathway. While these effects on cardiac function are clearly mediated by FGF21, the role of BAT-released FGF21 was not specifically addressed.
A study using hypertensive mouse models (WT DOCA-salt sensitive mice, adenosine 2A receptor (A2AR) knockout mice, and DOCA-A2AR−/− mice) has confirmed the role of batokine FGF21 in cardiovascular health (Ruan et al., 2018). Surgical removal of BAT in WT, DOCA-hypertensive mice resulted in reduced serum FGF21 levels and increased cardiac remodeling; however, these effects were absent in A2AR−/− mice (Ruan et al., 2018). Importantly, recombinant FGF21 treatment prevented these adverse effects in abate-depleted DOCA-hypertensive mice. Further investigation revealed that CGS21680 (A2AR agonist) promotes FGF21 expression and release in brown adipocytes, whereas KW6002 (A2AR antagonist) inhibits the release of FGF21 from BAT (Ruan et al., 2018). Additionally, FGF21 expression was decreased in A2AR−/− brown adipocytes compared with WT brown adipocytes. These data show that A2AR activation may induce the release of BAT-derived FGF21, resulting in reduced cardiac hypertrophy in response to hypertension.
4.3 12-HEPE
Oxylipins are lipid metabolites of polyunsaturated fatty acids and act as signaling molecules. There are several oxylipins known to be released from BAT, some of which have beneficial effects on metabolic and cardiac health. 12-HEPE is derived from arachidonate 12-lipoxygenase, which catalyzes the oxidation of polyunsaturated fatty acids, and has recently been identified to be released from BAT in response to cold and β3-adrenergic stimulation. Both cold and β3-adrenergic stimulation promote biosynthesis and release of 12-lipoxygenase metabolites from BAT (Leiria et al., 2019). 12-HEPE improves glucose metabolism by promoting the PI3K/Akt/Glut pathway and stimulating glucose uptake into adipocytes and skeletal muscle in a mouse model (Leiria et al., 2019). Although not yet studied in the context of cardiovascular function, 12-HEPE represents an exciting potential for BAT activity to regulate metabolic health, and, in turn, reduce risk factors for CVD.
4.4 12,13-diHOME
The linoleic acid derivative 12,13-diHOME is a batokine that is released in mice and humans in response to cold (Lynes et al., 2017) and exercise (Stanford et al., 2018). Mouse studies show that 12,13-diHOME increases FA uptake into brown adipocytes, skeletal muscle, and cardiomyocytes (Lynes et al., 2017; Stanford et al., 2018; Pinckard et al., 2021). Studies in humans have shown that 12,13-diHOME is correlated with reduced fat mass, fasting insulin, and TGs, improved mitochondrial function, and is increased after an acute bout of exercise in both male and female subjects (Stanford et al., 2018; Lynes et al., 2017; Nayor et al., 2020; Vasan et al., 2019).
Recent work from our laboratory investigated the effects of increasing BAT by transplantation on cardiac function and determined that increasing BAT improved in vivo cardiac hemodynamics and remodeling in mice (Pinckard et al., 2021). To determine the mechanism behind these cardiac improvements, non-targeted lipidomics were performed and revealed 12,13-diHOME to be significantly upregulated in mice after BAT transplantation. Acute injection of 12,13-diHOME improved systolic and diastolic function, similar to the effects of increasing BAT. Further investigation revealed a direct role for 12,13-diHOME to increase cardiomyocyte function (shortening, kinetics, and calcium cycling) and mitochondrial respiration. Sustained overexpression of 12,13-diHOME via tissue nano-transfection (TNT) of mice fed a high-fat diet (HFD) prevented HFD-induced impairments on cardiac function, suggesting potential translatability of 12,13-diHOME as a clinical treatment of CVD. In humans, both male and female patients with cardiovascular disease had reduced circulating levels of 12,13-diHOME compared with healthy individuals. Taken together, our data suggest that the beneficial cardiac adaptions of BAT are dependent on 12,13-diHOME, providing a possible translatable therapeutic for CVD.
Together, these studies indicate that in addition to its thermogenic role, the endocrine function of BAT (specifically IL6, FGF21, and 12-13-diHOME) can have vast systemic effects on metabolism and cardiac health. Importantly, the role of these batokines in cardiac disease (atherosclerosis, hypertension, and MI) have not been extensively investigated but will likely be an avenue of future investigation.
5. Pharmacological strategies
BAT has significant potential as a preventive and therapeutic tool against CVD. However, BAT mass and activity decrease with increasing CVD risk factors, including BMI, insulin resistance, and age (Zoico et al., 2019; Cypess et al., 2009; Vijgen et al., 2011). To use BAT for disease prevention and treatment, translational approaches aimed toward increasing BAT mass/activity or mimicking BAT endocrine function must be addressed. Because of this, recent studies have focused on pharmacological agents that activate BAT; here, we will discuss their effects on CVD.
5.1 β3-adrenergic activators
As a potent activator of BAT in both rodents and humans, sympathetic activation as a possible therapeutic for metabolic diseases has been an area of investigation for several years. However, there have been varying results as to the effectiveness of these treatments, possibly due to the specific focus on β3-AR agonists, rather than β1- and β2-ARs, which are the primary regulators of BAT activity in humans (Riis-Vestergaard et al., 2020; Blondin et al., 2020). In mice, long-term treatment with β3-AR agonist or thyroid hormone, both recognized BAT activators, lowers plasma lipid and glucose levels (Peirce and Vidal-Puig 2013; Wang, Li, and Guo 2013). Mirabegron, a β3-adrenergic receptor agonist, which has been shown to stimulate BAT glucose uptake in humans (Baskin et al., 2018), is of particular interest. Several other β3-adrenergic agonists have been investigated in both rodent studies and humans but have shown minimal benefits and therefore are not current topics of interest (Mukherjee, Baranwal, and Schade 2016).
A recent paper by O’Mara, et al. showed that chronic mirabegron treatment increases BAT thermogenesis and improves glucose effectiveness and insulin sensitivity in healthy women. Women who had less BAT mass/activity before treatment showed greater increases in both glucose effectiveness and insulin sensitivity compared with women who started with more BAT mass/activity (O'Mara et al., 2020). Additionally, patients who underwent mirabegron treatment had increased fasting high-density lipoprotein (HDL) levels and a lower ApoB/ApoA1 ratio (a biomarker of cardiovascular risk), suggesting the mirabegron treatment may provide some cardiovascular benefits. In a separate study, mirabegron also improved glucose tolerance, insulin sensitivity, and beta cell function in obese, insulin-resistant males and females (Finlin et al., 2020). These data suggest mirabegron treatment has several beneficial effects, including increased BAT mass and activity, improved glucose tolerance and insulin sensitivity, and improved cholesterol profile.
It is important to note that long-term SNS activation by drugs such as mirabegron increase blood pressure, potentially accelerating the risk for development of cardiac overload or heart failure (Zhang and Anderson 2014; Mancia and Grassi 2014). The O’Mara study showed mirabegron treatment caused both an acute and sustained increase in resting heart rate, systolic blood pressure, and rate-pressure product (an indirect measurement of myocardial oxygen consumption), indicating chronic adrenergic stimulation. These data indicate a promising role for BAT activators to improve cardiovascular health, but more work is required to determine the potential side effects of mirabegron and other β-AR agonists.
5.2 Norepinephrine reuptake inhibition
Inhibition of norepinephrine (NE) reuptake can also increase BAT activity. Atomoxetine is a drug used clinically for attention deficit hyperactivity disorder treatment in humans but has also been shown to increase glucose uptake of BAT in fasted rats (Mirbolooki et al., 2013). In mice, atomoxetine activation of BAT was associated with higher BAT temperature and lower blood glucose, indicating therapeutic potential. In humans, atomoxetine treatment has been shown to contribute to modest weight loss in adolescents (Wernicke and Kratochvil 2002) and adult women with obesity (Gadde et al., 2006). However, this drug has similar side effects as SNS activators on cardiac health, including increased heart rate and blood pressure in humans (Habel et al., 2011).
5.3 Indirubin
A recent study used connectivity mapping to identify a drug to study BAT activation focusing on Ucp1 upregulation rather than SNS activation (Wei et al., 2020). The drug indirubin is currently used for treatment of chronic myelogenous leukemia. Indirubin incubation increased mitochondrial respiration in C3H10T1/2 cells differentiated into adipocytes, and injection protected against HFD-induced weight gain, glucose-intolerance, and fatty liver in WT mice. Immunohistochemistry and gene expression analysis indicated that these improvements were in response to increased BAT thermogenic activity (Ucp1, Pgc1α, Prdm16, Dio2, Elovl3, Cideα, HSL). However, no cardiac parameters were investigated in this study; determining if indirubin protects against HFD-induced cardiovascular dysfunctions in mice is an important area of future investigation.
Discussion
There is significant evidence for increased BAT mass or activity as a potential therapeutic target for the prevention and treatment of obesity and other metabolic diseases (Stanford et al., 2013; Liu et al., 2015; Thoonen et al., 2015), and in this review, we discuss the growing evidence supporting a role for BAT as a therapeutic target for cardiovascular diseases (Berbee et al., 2015; Thoonen, Hindle, and Scherrer-Crosbie 2016; Thoonen et al., 2015). Increased BAT mass or activation in mice consistently results in dramatic improvements in cardiovascular health; this includes reduction of the atherosclerotic region, reduced myocardial injury size, and direct improvements on cardiac systolic and diastolic function (Berbee et al., 2015; Ruan et al., 2018; Thoonen et al., 2015; Pinckard et al., 2021). These benefits are partially mediated by increased thermogenic function of BAT, specifically in the case of atherosclerosis and MI: BAT activation accelerates hepatic clearance of cholesterol-enriched remnants (Berbée et al., 2015), improves TG clearance from the blood stream, and increases activation of the AKT-ERK1/2 pathways during myocardial injury (Thoonen et al., 2015). Additionally, several studies have now highlighted the endocrine action of BAT to protect against cardiovascular dysfunction and to act directly upon cardiomyocytes to improve cardiac systolic and diastolic function, including through the release of FGF21 and 12,13-diHOME (Ruan et al., 2018; Pinckard et al., 2021).
As cardiovascular diseases continue to be the number one cause of death across the globe, novel methods for preventing and treating these diseases are vital (Benjamin EJ 2018; Prevention 2017; Blacks 2018). Several studies highlight the ability of BAT to mediate cardiac function. However, BAT mass and activity decreases with increasing age and BMI, two top risk factors for CVD, highlighting the need for further investigation on the role of BAT and cardiac function in an aged population (Zoico et al., 2019; Cypess et al., 2009; Vijgen et al., 2011). It is additionally important to note that several of the animal studies discussed in this review only investigated male mice. This is an important omission since, while functional BAT can be detected upon stimulation in > 50% of adults (Cronin et al., 2012; Cypess et al., 2009; Ouellet et al., 2011), young lean women have been shown to possess BAT at a higher percentage compared with young, lean men (Ouellet et al., 2011). Thus, future studies should use mouse models of both sexes to fully understand the potential sexual dimorphisms seen with BAT mass and activation.
The data summarized in this review indicate that both the thermogenic and endocrine function of BAT play an important role in cardiovascular health. Importantly, these studies expand on previous data by showing that BAT improves cardiovascular health not only by improving systemic metabolism (Wang et al., 2015a), clearing lipids from the bloodstream (Wang et al., 2015c; Stanford et al., 2013), and reducing obesity (Thoonen et al., 2016; Peres Valgas da Silva et al., 2019), but also by activating cardioprotective pathways (Berbée et al., 2015; Thoonen et al., 2015), and directly affecting cardiomyocyte function (Pinckard et al., 2021). As a relatively new field, there is a lot to learn about the effects of BAT on cardiovascular function, specifically with regard to the endocrine role of BAT and batokines.
Increased sympathetic activation of BAT poses a possible therapeutic method; however long-term SNS activation is known to increase blood pressure and risk of heart failure (Zhang and Anderson 2014; Mancia and Grassi 2014). Mirabegron (β3-adrenergic receptor agonist) has confounding results in terms of cardiovascular risks/benefits; treatment increased resting heart rate, systolic blood pressure, and rate-pressure product, but these patients also had increased HDL levels and reduced ApoB/A1 ratio (O'Mara et al., 2020). A possible method to bypass the off-target effects of SNS activation is the use of direct batokines as therapeutics, several of which have been shown to have cardioprotective effects when treated exogenously in mice. To our knowledge, no studies to date have investigated the efficacy of batokine treatment in humans.
In summary, BAT has exciting potential to prevent CVD through both its thermogenic and endocrine functions (via batokines). Thermogenic BAT activity increases TG clearance from the bloodstream, accelerates hepatic clearance of cholesterol-enriched remnants, improves TG clearance from the blood stream, and activates the protective AKT-ERK1/2 pathway during myocardial injury (Berbée et al., 2015; Thoonen et al., 2015). A2AR-induced release of the batokine FGF21 results in reduced cardiac hypertrophy in response to hypertension, while the cold- and exercise-induced batokine 12,13-diHOME directly improves cardiac systolic and diastolic function via increased calcium cycling in cardiomyocytes (Ruan et al., 2018; Pinckard et al., 2021). Sympathetic activation of BAT via β3-AR agonists have been investigated as therapeutics for metabolic and cardiovascular diseases with varying results; however, targeting β1 or β2 -ARs (rather than β3) may be more effective at activating BAT in humans. Regardless, more research on the side effects of SNS-activators is warranted, as sustained SNS activation can cause undesirable cardiovascular effects. Future research may consider batokines or other BAT mimetics as therapeutic methods to elucidate the beneficial effects of BAT on CVD, rather than SNS-agonists.
Abbreviations
- A2AR
adenosine 2A receptor
- AKT
protein kinase B
- ApoE
apolipoprotein E
- AR
adrenergic receptor
- BAT
brown adipose tissue
- BMI
body mass index
- CAD
coronary artery disease
- CT
computer tomography
- CVD
cardiovascular disease
- DOCA
deoxycorticosterone acetate
- ERK
extracellular-signal-regulated kinase
- FA
fatty acid
- FGF21
fibroblast growth factor 21
- HDL
high-density lipoprotein
- HSL
hormone sensitive lipase
- iBAT
intrascapular brown adipose tissue
- IL6
interleukin 6
- LDLR
low-density lipoprotein receptor
- MI
myocardial infarction
- PET
positron emission tomography
- PRDM16
PR domain containing 16
- SNS
sympathetic nervous system
- T2D
type II diabetes
- TG
triglyceride
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
- WT
wild-type
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Pinckard, Stanford.
Footnotes
Financial Disclosure: This work was supported by National Institutes of Health [Grant R01-HL138738] and [Grant R01-AG060542] (to K.I.S.), and [Grant 1F31HL152648-01A1] (to K.M.P.).
No author has an actual or perceived conflict of interest with the contents of this article.
References
- Ahmad B, Vohra MS, Saleemi MA, Serpell CJ, Fong IL, Wong EH (2021) Brown/Beige adipose tissues and the emerging role of their secretory factors in improving metabolic health: The batokines. Biochimie 184:26–39. [DOI] [PubMed] [Google Scholar]
- Bakker LEBoon MRvan der Linden RAArias-Bouda LPvan Klinken JBSmit FVerberne HJJukema JWTamsma JTHavekes LM, et al. (2014) Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol 2:210–217. [DOI] [PubMed] [Google Scholar]
- Baskin ASLinderman JDBrychta RJMcGehee SAnflick-Chames ECero CJohnson JWO’Mara AEFletcher LALeitner BP, et al. (2018) Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by a β3-adrenergic receptor agonist. Diabetes 67:2113–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basting T, Lazartigues E (2017) DOCA-salt hypertension: an update. Curr Hypertens Rep 19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher TPalanisamy SKramer DJEljalby MMarx SJWibmer AGButler SDJiang CSVaughan RSchöder H, et al. (2021) Brown adipose tissue is associated with cardiometabolic health. Nat Med 27:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJVirani SSCallaway CWChang ARCheng SChiuve SECushman MDelling FNDeo Rde Ferranti SD, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee (2018) Heart Disease and Stroke Statistics 2018 At-a-Glance, American Heart Association. [Google Scholar]
- Berbée JFBoon MRKhedoe PPBartelt ASchlein CWorthmann AKooijman SHoeke GMol IMJohn C, et al. (2015) Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 6:6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz MJ, Enerbäck S (2015) Human brown adipose tissue: what we have learned so far. Diabetes 64:2352–2360. [DOI] [PubMed] [Google Scholar]
- Blacks, NH 2018. 'Heart Disease Statistics and Maps', Accessed January 6.
- Blondin DPNielsen SKuipers ENSeverinsen MCJensen VHMiard SJespersen NZKooijman SBoon MRFortin M, et al. (2020) Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metab 32:287–300.e7. [DOI] [PubMed] [Google Scholar]
- Burýsek L, Houstek J (1997) beta-Adrenergic stimulation of interleukin-1alpha and interleukin-6 expression in mouse brown adipocytes. FEBS Lett 411:83–86. [DOI] [PubMed] [Google Scholar]
- Campderrós L, Moure R, Cairó M, Gavaldà-Navarro A, Quesada-López T, Cereijo R, Giralt M, Villarroya J, Villarroya F (2019) Brown adipocytes secrete GDF15 in response to thermogenic activation. Obesity (Silver Spring) 27:1606–1616. [DOI] [PubMed] [Google Scholar]
- Cannon B, de Jong JMA, Fischer AW, Nedergaard J, Petrovic N (2020) Human brown adipose tissue: classical brown rather than brite/beige? Exp Physiol 105:1191–1200. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359. [DOI] [PubMed] [Google Scholar]
- Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, Lavie CJ (2019) Obesity paradox in cardiovascular disease: where do we stand? Vasc Health Risk Manag 15:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte EE (2018) Brown adipose tissue energy metabolism in gumans. Front Endocrinol (Lausanne) 9:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijo RGavaldà-Navarro ACairó MQuesada-López TVillarroya JMorón-Ros SSánchez-Infantes DPeyrou MIglesias RMampel T, et al. (2018) CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab 28:750–763.e6. [DOI] [PubMed] [Google Scholar]
- Cero C, Lea HJ, Zhu KY, Shamsi F, Tseng YH, Cypess AM (2021) β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B, Mitchell DH, Colston JT, Freeman GL (1999) Regulation of CCAAT/Enhancer binding protein, interleukin-6, interleukin-6 receptor, and gp130 expression during myocardial ischemia/reperfusion. Circulation 99:427–433. [DOI] [PubMed] [Google Scholar]
- Chen ZWang GXMa SLJung DYHa HAltamimi TZhao XYGuo LZhang PHu CR, et al. (2017) Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Mol Metab 6:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M (2014) Transcriptional fingerprinting of “browning” white fat identifies NRG4 as a novel adipokine. Adipocyte 4:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PLevy JDZhang YFrontini AKolodin DPSvensson KJLo JCZeng XYe LKhandekar MJ, et al. (2014) Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156:304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin CG, Prakash P, Daniels GH, Boland GW, Kalra MK, Halpern EF, Palmer EL, Blake MA (2012) Brown fat at PET/CT: correlation with patient characteristics. Radiology 263:836–842. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Kahn CR (2010) Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 17:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AMLehman SWilliams GTal IRodman DGoldfine ABKuo FCPalmer ELTseng YHDoria A, et al. (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AMWhite APVernochet CSchulz TJXue RSass CAHuang TLRoberts-Toler CWeiner LSSze C, et al. (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19:635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC (2001) The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMambro VE, Maile L, Wai C, Kawai M, Cascella T, Rosen CJ, Clemmons D (2012) Insulin-like growth factor-binding protein-2 is required for osteoclast differentiation. J Bone Miner Res 27:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh ASPeijs LBeaudry JLJespersen NZNielsen CHMa TBrunner ADLarsen TJBayarri-Olmos RPrabhakar BS, et al. (2019) Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab 30:963–975.e7. [DOI] [PubMed] [Google Scholar]
- Di Franco AGuasti DSquecco RMazzanti BRossi FIdrizaj EGallego-Escuredo JMVillarroya FBani DForti G, et al. (2016) Searching for classical brown fat in humans: development of a novel human fetal brown stem cell model. Stem Cells 34:1679–1691. [DOI] [PubMed] [Google Scholar]
- Duchamp C, Burton KA, Géloën A, Dauncey MJ (1997) Transient upregulation of IGF-I gene expression in brown adipose tissue of cold-exposed rats. Am J Physiol 272:E453–E460. [DOI] [PubMed] [Google Scholar]
- Egecioglu E, Anesten F, Schéle E, Palsdottir V (2018) Interleukin-6 is important for regulation of core body temperature during long-term cold exposure in mice. Biomed Rep 9:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve Ràfols M (2014) Adipose tissue: cell heterogeneity and functional diversity. Endocrinol Nutr 61:100–112. [DOI] [PubMed] [Google Scholar]
- Finlin BSMemetimin HZhu BConfides ALVekaria HJEl Khouli RHJohnson ZRWestgate PMChen JMorris AJ, et al. (2020) The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Invest 130:2319–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier BMurray BGutzwiller SMarcaletti SMarcellin DBergling SBrachat SPersohn EPierrel EBombard F, et al. (2012) Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol Cell Biol 32:2871–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Yao F, Abou-Samra AB, Zhang R (2013) Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem Biophys Res Commun 430:1126–1131. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Yonish GM, Wagner HR 2nd, Foust MS, Allison DB (2006) Atomoxetine for weight reduction in obese women: a preliminary randomised controlled trial. Int J Obes 30:1138–1142. [DOI] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE (2013) The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 18:333–340. [DOI] [PubMed] [Google Scholar]
- Giles DARamkhelawon BDonelan EMStankiewicz TEHutchison SBMukherjee RCappelletti MKarns RKarp CLMoore KJ, et al. (2016) Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wild type C57BL/6 mice. Mol Metab 5:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnad TScheibler Svon Kügelgen IScheele CKilić AGlöde AHoffmann LSReverte-Salisa LHorn PMutlu S, et al. (2014) Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516:395–399. [DOI] [PubMed] [Google Scholar]
- Gunawardana SC, Piston DW (2012) Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 61:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardana SC, Piston DW (2015) Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am J Physiol Endocrinol Metab 308:E1043–E1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel LACooper WOSox CMChan KAFireman BHArbogast PGCheetham TCQuinn VPDublin SBoudreau DM, et al. (2011) ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA 306:2673–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, Ogden CL2017. “Prevalence of Obesity Among Adults and Youth: United States, 2015 − 2016.” In U.S. Department of Health and Human Services, edited by Centers for Disease Control and Prevention. [Google Scholar]
- Han MS, White A, Perry RJ, Camporez JP, Hidalgo J, Shulman GI, Davis RJ (2020) Regulation of adipose tissue inflammation by interleukin 6. Proc Natl Acad Sci USA 117:2751–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, Kurokawa M, Won KJ, Seale P (2014) Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab 19:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F (2011) Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 286:12983–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui XGu PZhang JNie TPan YWu DFeng TZhong CWang YLam KS, et al. (2015) Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab 22:279–290. [DOI] [PubMed] [Google Scholar]
- Kang YE, Choung S, Lee JH, Kim HJ, Ku BJ (2017) The role of circulating slit2, the one of the newly batokines, in human diabetes mellitus. Endocrinol Metab (Seoul) 32:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Breggia AC, DeMambro VE, Shen X, Canalis E, Bouxsein ML, Beamer WG, Clemmons DR, Rosen CJ (2011) The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. J Biol Chem 286:14670–14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat MT, Nayeem MA (2017) The role of adenosine A2A receptor, CYP450s, and PPARs in the regulation of vascular tone. BioMed Res Int 2017:1720920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer FW (2017) The significance of beige and brown fat in humans. Endocr Connect 6:R70–R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Choi HR, Park SG, Ko Y, Bae KH, Lee SC (2012) Myostatin inhibits brown adipocyte differentiation via regulation of Smad3-mediated β-catenin stabilization. Int J Biochem Cell Biol 44:327–334. [DOI] [PubMed] [Google Scholar]
- Klepac KKilić AGnad TBrown LMHerrmann BWilderman ABalkow AGlöde ASimon KLidell ME, et al. (2016) The Gq signalling pathway inhibits brown and beige adipose tissue. Nat Commun 7:10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XYao TZhou PKazak LTenen DLyubetskaya ADawes BATsai LKahn BBSpiegelman BM, et al. (2018) Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab 28:631–643.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristóf EKlusóczki ÁVeress RShaw ACombi ZSVarga KGyőry FBalajthy ZBai PBacso Z, et al. (2019) Interleukin-6 released from differentiating human beige adipocytes improves browning. Exp Cell Res 377:47–55. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Berlan M (1993) Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res 34:1057–1091. [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388:674–678. [DOI] [PubMed] [Google Scholar]
- Lee MW, Lee M, Oh KJ (2019) Adipose tissue-derived signatures for obesity and type 2 diabetes: adipokines, batokines and microRNAs. J Clin Med 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, Werner CD, Chen KY, Celi FS (2014) Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63:3686–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnig AC, Dewal RS, Baer LA, Kitching KM, Munoz VR, Arts PJ, Sindeldecker DA, May FJ, Lauritzen HPMM, Goodyear LJ, Stanford KI(2019) Exercise training induces depot-specific adaptations to white and brown adipose tissue. iScience 11:425–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiria LOWang CHLynes MDYang KShamsi FSato MSugimoto SChen EYBussberg VNarain NR, et al. (2019) 12-Lipoxygenase regulates cold adaptation and glucose metabolism by producing the omega-3 lipid 12-HEPE from brown fat. Cell Metab 30:768–783.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, Zhang LQ, Wu YH (2013) Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep 3:2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XWang SYou YMeng MZheng ZDong MLin JZhao QZhang CYuan X, et al. (2015) Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology 156:2461–2469. [DOI] [PubMed] [Google Scholar]
- Lynes MDLeiria LOLundh MBartelt AShamsi FHuang TLTakahashi HHirshman MFSchlein CLee A, et al. (2017) The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med 23:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Gao M, Sun H, Liu D (2015) Interleukin-6 gene transfer reverses body weight gain and fatty liver in obese mice. Biochim Biophys Acta 1852:1001–1011. [DOI] [PubMed] [Google Scholar]
- Mancia G, Grassi G (2014) The autonomic nervous system and hypertension. Circ Res 114:1804–1814. [DOI] [PubMed] [Google Scholar]
- Mauer JChaurasia BGoldau JVogt MCRuud JNguyen KDTheurich SHausen ACSchmitz JBrönneke HS, et al. (2014) Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 15:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli FH, Rimoldi SF, Bangalore S (2017) The transition from hypertension to heart failure: contemporary update. JACC Heart Fail 5:543–551. [DOI] [PubMed] [Google Scholar]
- Mirbolooki MR, Constantinescu CC, Pan ML, Mukherjee J (2013) Targeting presynaptic norepinephrine transporter in brown adipose tissue: a novel imaging approach and potential treatment for diabetes and obesity. Synapse 67:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JX, Chaudhary N, Akinyemiju T (2017) Metabolic syndrome prevalence by race/ethnicity and sex in the United States, national health and nutrition examination survey, 1988-2012. Prev Chronic Dis 14:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Baranwal A, Schade KN (2016) Classification of therapeutic and experimental drugs for brown adipose tissue activation: potential treatment strategies for diabetes and obesity. Curr Diabetes Rev 12:414–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C (2008) Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88:581–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayor MShah RVMiller PEBlodgett JBTanguay MPico ARMurthy VLMalhotra RHoustis NEDeik A, et al. (2020) Metabolic architecture of acute exercise response in middle-aged adults in the community. Circulation 142:1905–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Néchad M, Ruka E, Thibault J (1994) Production of nerve growth factor by brown fat in culture: relation with the in vivo developmental stage of the tissue. Comp Biochem Physiol Part A Physiol 107:381–388. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293:E444–E452. [DOI] [PubMed] [Google Scholar]
- O’Mara AEJohnson JWLinderman JDBrychta RJMcGehee SFletcher LAFink YAKapuria DCassimatis TMKelsey N, et al. (2020) Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Invest 130:2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, World Health. 2018. 'Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000- 2016.'. http://www.who.int/healthinfo/global_burden_disease/estimates/en/.
- Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D (2011) Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 96:192–199. [DOI] [PubMed] [Google Scholar]
- Pagnon J, Matzaris M, Stark R, Meex RC, Macaulay SL, Brown W, O’Brien PE, Tiganis T, Watt MJ (2012) Identification and functional characterization of protein kinase A phosphorylation sites in the major lipolytic protein, adipose triglyceride lipase. Endocrinology 153:4278–4289. [DOI] [PubMed] [Google Scholar]
- Peirce V, Vidal-Puig A (2013) Regulation of glucose homoeostasis by brown adipose tissue. Lancet Diabetes Endocrinol 1:353–360. [DOI] [PubMed] [Google Scholar]
- Pellegrinelli VPeirce VJHoward LVirtue STürei DSenzacqua MFrontini ADalley JWHorton ARBidault G, et al. (2018) Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat Commun 9:4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres Valgas Da Silva C, Hernández-Saavedra D, White J, Stanford K(2019) Cold and exercise: therapeutic tools to activate brown adipose tissue and combat obesity. Biology 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinckard KMShettigar VKWright KRAbay EBaer LAVidal PDewal RSDas DDuarte-Sanmiguel SHernández-Saavedra D, et al. (2021) A novel endocrine role for the BAT-released lipokine 12,13-diHOME to mediate cardiac function. Circulation 143:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planavila ARedondo IHondares EVinciguerra MMunts CIglesias RGabrielli LASitges MGiralt Mvan Bilsen M, et al. (2013) Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun 4:2019. [DOI] [PubMed] [Google Scholar]
- Prabhu SD (2004) Cytokine-induced modulation of cardiac function. Circ Res 95:1140–1153. [DOI] [PubMed] [Google Scholar]
- Prabhu SD, Frangogiannis NG (2016) The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention, Division for Heart Disease and Stroke (2017) Heart Disease Fact Sheet, Accessed 1/19.
- Centers for Disease Control and Prevention (2018) “Underlying Cause of Death, 1999. –2018.” In CDC WONDER Online Database., edited by Centers for Disease Control and Prevention. Atlanta, GA.
- Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B (2013) Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 154:2687–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiko J, Orava J, Savisto N, Virtanen KA (2020) High brown fat activity correlates with cardiovascular risk factor levels cross-sectionally and subclinical atherosclerosis at 5-year follow-up. Arterioscler Thromb Vasc Biol 40:1289–1295. [DOI] [PubMed] [Google Scholar]
- Rao RRLong JZWhite JPSvensson KJLou JLokurkar IJedrychowski MPRuas JLWrann CDLo JC, et al. (2014) Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis-Vestergaard MJ, Richelsen B, Bruun JM, Li W, Hansen JB, Pedersen SB (2020) Beta-1 and not Beta-3 adrenergic receptors may be the primary regulator of human brown adipocyte metabolism. J Clin Endocrinol Metab 105:dgz298. [DOI] [PubMed] [Google Scholar]
- Rocha VZ and Libby P (2009) Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 6:399–409. [DOI] [PubMed] [Google Scholar]
- Rohlfs EM, Daniel KW, Premont RT, Kozak LP, Collins S (1995) Regulation of the uncoupling protein gene (Ucp) by beta 1, beta 2, and beta 3-adrenergic receptor subtypes in immortalized brown adipose cell lines. J Biol Chem 270:10723–10732. [DOI] [PubMed] [Google Scholar]
- Rosell MKaforou MFrontini AOkolo AChan YWNikolopoulou EMillership SFenech MEMacIntyre DTurner JO, et al. (2014) Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab 306:E945–E964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB, Gao PJ (2018) A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab 28:476–489.e5. [DOI] [PubMed] [Google Scholar]
- Saito MOkamatsu-Ogura YMatsushita MWatanabe KYoneshiro TNio-Kobayashi JIwanaga TMiyagawa MKameya TNakada K, et al. (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58:1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JE, Larsen PR (1985) Potential of brown adipose tissue type II thyroxine 5′-deiodinase as a local and systemic source of triiodothyronine in rats. J Clin Invest 76:2296–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Braga M, Pervin S (2014) Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol 2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KILynes MDTakahashi HBaer LAArts PJMay FJLehnig ACMiddelbeek RJWRichard JJSo K, et al. (2018) 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab 27:1111–1120.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KIMiddelbeek RJTownsend KLAn DNygaard EBHitchcox KMMarkan KRNakano KHirshman MFTseng YH, et al. (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steculorum SMRuud JKarakasilioti IBackes HEngström Ruud LTimper KHess METsaousidou EMauer JVogt MC, et al. (2016) AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson KJLong JZJedrychowski MPCohen PLo JCSerag SKir SShinoda KTartaglia JARao RR, et al. (2016) A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell Metab 23:454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takx RA, Ishai A, Truong QA, MacNabb MH, Scherrer-Crosbie M, Tawakol A (2016) Supraclavicular brown adipose tissue 18F-FDG uptake and cardiovascular disease. J Nucl Med 57:1221–1225. [DOI] [PubMed] [Google Scholar]
- Terrell AM, Crisostomo PR, Wairiuko GM, Wang M, Morrell ED, Meldrum DR (2006) Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock 26:226–234. [DOI] [PubMed] [Google Scholar]
- Thoonen RErnande LCheng JNagasaka YYao VMiranda-Bezerra AChen CChao WPanagia MSosnovik DE, et al. (2015) Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol 84:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoonen R, Hindle AG, Scherrer-Crosbie M (2016) Brown adipose tissue: The heat is on the heart. Am J Physiol Heart Circ Physiol 310:H1592–H1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, Tangirala RK, Tontonoz P, Chawla A (2016) Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab 23:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Maillet M, Molkentin JD (2013) Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest 123:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lans AAHoeks JBrans BVijgen GHVisser MGVosselman MJHansen JJörgensen JAWu JMottaghy FM, et al. (2013) Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 123:3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE (2006) Mechanisms linking obesity with cardiovascular disease. Nature 444:875–880. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Schrauwen P (2011) Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol 301:R285–R296. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508. [DOI] [PubMed] [Google Scholar]
- Vasan SK, Noordam R, Gowri MS, Neville MJ, Karpe F, Christodoulides C (2019) The proposed systemic thermogenic metabolites succinate and 12,13-diHOME are inversely associated with adiposity and related metabolic traits: evidence from a large human cross-sectional study. Diabetologia 62:2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD (2011) Brown adipose tissue in morbidly obese subjects. PLoS One 6:e17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya F, Cereijo R, Villarroya J, Giralt M (2017a) Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13:26–35. [DOI] [PubMed] [Google Scholar]
- Villarroya F, Gavaldà-Navarro A, Peyrou M, Villarroya J, Giralt M (2017) The lives and times of brown adipokines. Trends Endocrinol Metab 28:855–867. [DOI] [PubMed] [Google Scholar]
- Villarroya J, Cereijo R, Gavaldà-Navarro A, Peyrou M, Giralt M, Villarroya F (2019) New insights into the secretory functions of brown adipose tissue. J Endocrinol 243:R19–R27. [DOI] [PubMed] [Google Scholar]
- Virtanen KALidell MEOrava JHeglind MWestergren RNiemi TTaittonen MLaine JSavisto NJEnerbäck S, et al. (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525. [DOI] [PubMed] [Google Scholar]
- Wang GX, Zhao XY, Lin JD (2015a) The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab 26:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GXZhao XYMeng ZXKern MDietrich AChen ZCozacov ZZhou DOkunade ALSu X, et al. (2014) The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med 20:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QATao CJiang LShao MYe RZhu YGordillo RAli ALian YHolland WL, et al. (2015b) Distinct regulatory mechanisms governing embryonic versus adult adipocyte maturation. Nat Cell Biol 17:1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang M, Xu M, Gu W, Xi Y, Qi L, Li B, Wang W (2015c) Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One 10:e0123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZH, Li YF, Guo YQ (2013) β3-Adrenoceptor activation attenuates atherosclerotic plaque formation in ApoE(-/-) mice through lowering blood lipids and glucose. Acta Pharmacol Sin 34:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Sun H, Liu JL, Dong K, Liu J, Zhang M (2020) Indirubin, a small molecular deriving from connectivity map (CMAP) screening, ameliorates obesity-induced metabolic dysfunction by enhancing brown adipose thermogenesis and white adipose browning. Nutr Metab (Lond) 17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke JF, Kratochvil CJ (2002) Safety profile of atomoxetine in the treatment of children and adolescents with ADHD. J Clin Psychiatry 63 (Suppl 12):50–55. [PubMed] [Google Scholar]
- White JD, Dewal RS, Stanford KI (2019) The beneficial effects of brown adipose tissue transplantation. Mol Aspects Med 68:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle AJCarobbio SMartins LSlawik MHondares EVázquez MJMorgan DCsikasz RIGallego RRodriguez-Cuenca S, et al. (2012) BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149:871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert KCTaga TSaito MNarazaki MKishimoto TGlembotski CCVernallis ABHeath JKPennica DWood WI, et al. (1996) Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem 271:9535–9545. [DOI] [PubMed] [Google Scholar]
- Wu JBoström PSparks LMYe LChoi JHGiang AHKhandekar MVirtanen KANuutila PSchaart G, et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi-Takihara K, Ihara Y, Ogata A, Yoshizaki K, Azuma J, Kishimoto T (1995) Hypoxic stress induces cardiac myocyte-derived interleukin-6. Circulation 91:1520–1524. [DOI] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M (2013) Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 123:3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Ye M, Resch JM, Jedrychowski MP, Hu B, Lowell BB, Ginty DD, Spiegelman BM (2019) Innervation of thermogenic adipose tissue via a calsyntenin 3β-S100b axis. Nature 569:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Anderson AS (2014) The sympathetic nervous system and heart failure. Cardiol Clin 32:33–45, vii vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Unelius L, Bengtsson T, Cannon B, Nedergaard J (1994) Coexisting beta-adrenoceptor subtypes: significance for thermogenic process in brown fat cells. Am J Physiol 267:C969–C979. [DOI] [PubMed] [Google Scholar]
- Zoico E, Rubele S, De Caro A, Nori N, Mazzali G, Fantin F, Rossi A, Zamboni M (2019) Brown and beige adipose tissue and aging. Front Endocrinol (Lausanne) 10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]