Abstract
Resveratrol is a polyphenolic phytochemical found in fruits, nuts, and vegetables that contributes to the remarkable dietary effects of polyphenolic as inhibitors of aging and multiple aging related diseases. In addition, resveratrol has been extensively investigated as an inhibitor of inflammatory diseases including cancer; however, the underlying mechanisms of these chemotherapeutic effects of resveratrol are not completely understood. In cancer cells, resveratrol inhibits cell growth, survival, migration, and invasion, and many of the effects of resveratrol resemble those observed for bis-indole derived (CDIM) compounds that bind the pro-oncogenic nuclear receptor 4A1 (NR4A1, Nur77) and act as receptor antagonists. Using an isothermal titration calorimetry binding assay, we observed that resveratrol bound to the ligand binding domain of NR4A1 with a KD value of 2.4 µM and a ΔG of -32.2 kJ/mol. Resveratrol also inhibited NR4A1-dependent transactivation in H460 and H1299 lung cancer cells, suggesting that resveratrol is an NR4A1 antagonist. This observation was confirmed in a series of functional (cell proliferation, survival, migration, and invasion) and gene expression assays in H460 and H1299 cells, showing that treatment with resveratrol mimicked the effects of NR4A1 knockdown and were similar to results of previous studies using CDIM/NR4A1 antagonists. These data indicate that applications of resveratrol may be more effective in patients that overexpress NR4A1, which is a negative prognostic factor for patients with some solid tumor-derived cancers.
SIGNIFICANCE STATEMENT
This study has examined the mechanism of action of resveratrol and shows binding to NR4A1 (KD = 2.4 µM) and inhibition of NR4A1-dependent transactivation in lung cancer cells. Treatment of H460 and H1299 lung cancer cells with resveratrol inhibits cell growth, survival, migration/invasion, and related genes and acts as an NR4A1 antagonist. Resveratrol can now be used more effectively in cancer chemotherapy by targeting patients that overexpress NR4A1 in lung cancer.
Introduction
Dietary polyphenolics produced in vegetables, fruits, and nuts have been associated with multiple beneficial health effects, including longer lifespans and other age-related diseases (Liu et al., 2019; Hano and Tungmunnithum, 2020; Wu et al., 2021). Polyphenols associated with these health benefits include phenolics acids, coumarins, flavonoids, lignans, and stilbenes including 3,4’,5-trihydroxy-trans-stilbene (resveratrol), which is enriched in foods such as blueberries, grapes, peanuts, and red wine and exhibits prototypical polyphenolic health benifits (Saiko et al., 2008; Koh et al., 2021; Raj et al., 2021; Zhou et al., 2021; Santana et al., 2022). Resveratrol has been extensively investigated as a therapeutic agent for treatment of multiple diseases in both in vitro cell culture and animal models. Resveratrol inhibits proliferation and inflammation in cell culture models of endometriosis (Rudzitis-Auth et al., 2013; Kolahdouz and Arablou, 2017), and this includes inhibition of nuclear factor kB (NFkB) and other kinases (Bruner-Tran et al., 2011; Ergenoğlu et al., 2013), downregulation of estrogen receptor (ESR1) (Amaya et al., 2014), activation of NAD-dependent deacetylase sirtuin-1 (SIRT1) (Taguchi et al., 2014), and decreased matrix metallopeptidase 9 (MMP9) and MMP2 activities (Bayoglu Tekin et al., 2015). Resveratrol also has multiple effects on neuronal cells in culture and in vivo, and this includes inhibition of brain inflammation, damage, and enhanced memory (Le et al., 2019; Teertam et al., 2020; Tang et al., 2021). For example, in rat brain resveratrol upregulates Sirt1/microRNA 149-Sp to protect against ischemia (Teertam et al., 2020), whereas resveratrol protection in neonatal hypoxic-ischemic brain injury involves SIRT1-regulated inhibition of high mobility group box 1 protein (HMGB1) and downstream NFkB signaling (Le et al., 2019). Moreover, induction of inflammation in BV2 cells enhances toll-like receptor 4 (TLR4), myeloid differentiation primary response 88 (MyD88), NFkB, and multiple cytokines, and these responses are inhibited by resveratrol (Le et al., 2019). Resveratrol also activates a SIRT1/NFkB to protect against sevoflurane-induced cognitive impairment in mice (Tang et al., 2021).
Resveratrol is a highly effective inhibitor of cancer cell and tumor growth, migration, and invasion in multiple cell lines (rev. in Saiko et al., 2008), and in most of these cell lines this was accompanied by altered regulation of pathways/gene products associated with these anticancer activities. The results of many studies on the anticancer activities of resveratrol have been variable and cell context-specific; however, some pathways and genes such as resveratrol-dependent inhibition of the mammalian target of rapamycin (mTOR) pathway have been reported in many different cancer cell lines (He et al., 2011; Rashid et al., 2011; Wu and Liu, 2013; Alayev et al., 2015; Selvaraj et al., 2016; Chang et al., 2017; Liu et al., 2018; Wang et al., 2018; Bian et al., 2020; Khan et al., 2020).
Research in our laboratory has been focused on the orphan nuclear receptor 4A1 (NR4A1, Nur77) and its functions in cancer and noncancer cell lines and animal models (Selvaraj et al., 2016). Nr4a1 and other members of this family (Nr4a2 and Nr4a3) are immediate early genes that are induced by diverse stressors to maintain cellular homeostasis, and NR4A1 is overexpressed in solid tumor-derived cancers and other inflammatory diseases (Pearen and Muscat, 2010; Safe and Karki, 2021). Results primarily of NR4A1 knockdown studies show that this receptor regulates cancer cell growth, survival, migration, and invasion, and this includes mTOR signaling pathways on multiple cancer cell lines. NR4A1 regulates prosurvival and growth promoting genes such as epidermal growth factor receptor, survivin, and B-cell lymphoma 2 (Bcl-2), as well as β1-integrin and other integrins in many different cancer cell lines. Isocitrate dehydrogenase-1 (Idh-1) and thioredoxin domain containing 5 (Txndc5) are also NR4A1-regulated genes in solid tumor-derived cell lines, and these genes serve to maintain high reductant levels in cancer cells (rev. in Safe and Karki, 2021). Bis-indole derived compounds (CDIMs) have been characterized as ligands that bind NR4A1 and act as NR4A1 antagonists that inhibit cancer cell growth, survival, migration, and invasion (Lee et al., 2014; Safe and Karki, 2021). CDIM-NR4A1 antagonists also inhibit mTOR in lung and other cancer cell lines (Lee et al., 2012; Lacey et al., 2016; Safe and Karki, 2021), and many of the effects of these NR4A1 antagonists have also been observed for resveratrol (Saiko et al., 2008; Lacey et al., 2016; Wright et al., 2017; Wang et al., 2018; Koh et al., 2021). We hypothesize that one of the underlying mechanisms of action of resveratrol in cancer cells is that of an NR4A1 ligand that acts as an antagonist, and this study demonstrates for the first time that resveratrol is an NR4A1 ligand.
Materials and Methods
Ligand – Receptor Binding Assays
Isothermal titration calorimetry (ITC) was used to determine the ligand binding constant (Kd) to NR4A1 utilizing an Affinity ITC (TA Instruments, New Castle, DE). Briefly, the experimental setup was as follows. The ITC sample cell contained 250 μl of NR4A1 protein [ligand binding domain (LBD)] at a concentration of 20 μmol/l in buffer containing 20 mmol sodium phosphate/l (pH 7.4), 5% glycerol, and 1.0% ethanol. The ligand titrant was prepared in the same buffer as above at a ligand concentration of 66.6 μmol/l. The ligand titration into protein was performed at 25°C with a stir rate of 125 rpm. Each ligand injection volume was 5 μl followed by 200 seconds to measure the total heat flow required to maintain constant temperature. A total of 20 injections were done for each ligand/NR4A1 combination. Each ligand titration into protein experiment was repeated for a total of three separate and independent experiments to generate the curves shown in the figure. In a separate set of injections, the same ligand was injected into buffer only (no protein) to determine heat flow as a result of ligand dilution into buffer. The ligand/buffer values were subtracted from the ligand/protein values prior to data analysis using the Affinity ITC manufacturer-supplied data analysis software package. Sigmoidal curve fitting was performed using the Affinity ITC manufacturer-supplied data analysis software package to determine the following binding parameters: Kd, the equilibrium binding dissociation constant (μmol/l); n, the equilibrium ligand-to-protein binding stoichiometry (mol ligand per mol NR4A1); and ΔG, the equilibrium free energy of ligand binding (kJ/mol). The resulting data are plotted as heat flow/area data (μJ) versus the cumulative resveratrol concentration (μmol/l) present in the sample cell. Statistical analysis of the triplicate data was performed utilizing SigmaPlot 14.5 (Systat Software, Inc.) to determine the parameter mean (Kd, n, ΔG) and standard deviation. In addition, we also used a direct binding assay by determining the loss of fluorescence of a tryptophan residue in the LBD as previously described (Lee et al., 2014).
Computation-Based Molecular Modeling
Molecular modeling studies were conducted using Maestro (Schrödinger Release 2020-1, Schrödinger, LLC, New York, NY, 2020). The version of Maestro used for these studies is licensed to the Laboratory for Molecular Simulation, a Texas A&M University core user facility for molecular modeling and is associated with the Texas A&M University High Performance Research Computing facility. All Maestro-associated applications were accessed via the graphical user interface (GUI) VNC interactive application through the HPRC Ada OnDemand portal. The crystal structure coordinates for human orphan nuclear receptor NR4A1 ligand binding domain (LBD) (Zhan et al., 2012) were downloaded from the Protein Data Bank (https://www.rcsb.org; PDB ID 3V3Q). The human NR4A1 LBD crystal structure was prepared for ligand docking utilizing the Maestro Protein Preparation Wizard; restrained minimization of the protein structure was performed utilizing the OPLS3e force field. Each ligand (resveratrol or DIM-3,5-Cl2) three-dimensional structure was prepared for docking utilizing the Maestro LigPrep, again using the OPLS3e force field. Maestro Glide (Halgren et al., 2004; Friesner et al., 2006) was used with the default settings to dock each prepared ligand to the prepared protein, predict the lowest energy ligand binding orientation, and calculate the predicted binding energy in units of kcal/mol.
Cell Culture, Reagents, and Antibodies
H460 and H1299 lung cancer cells are purchased from American Type Culture Collection (Manassas, VA). Both cell lines were derived from male patients with nonsmall cell lung cancer (H1299) or large cell lung cancer (H460). Cells are cultured in RPMI1640 medium with 10% FBS at 37°C in the presence of 5% CO2. The details of antibodies used for Western blots and for chromatin immunoprecipitation (ChIP) assays are summarized in Supplemental Table 1.
Cell Proliferation Assay
Cell proliferation was investigated using XTT Cell Viability Kit (Cell Signaling Biotechnology) according to the manufacturer's instructions. Cells (1.5 × 104/well) were plated in 100 μl of plating medium (as above) on 96-well plates and allowed to attach for 24 hours. The medium was then changed to RPMI 1640 containing 2.5% charcoal-stripped FBS, and either vehicle DMSO or different concentrations of compounds in DMSO were added. After 24, 48, and 72 hours of culture, 25 μl of XTT reaction solution (sodium 3′-[1-(phenyl-aminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzenesulfonic acid hydrate and N-methyl dibenzopyrazine methyl sulfate (mixed in proportion 50:1) were added to the each well. The optical density was read at 490 nm wavelength in a plate reader after incubation for 4 hours. All determinations were replicated in at least three separate experiments.
Transfection and Luciferase Assay
Cells were plated on 12-well plates at 5 × 104/well in RPMI 1640 medium supplemented with 2.5% charcoal-stripped FBS. After 24-hour growth, various amounts of DNA [i.e., UASx5-Luc (400 ng), GAL4-NR4A1 (50 ng) and β-gal (50 ng)] were cotransfected into each well by Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. After 6 hours of transfection, cells were treated with plating media (as above) containing either solvent (DMSO) or the indicated concentration of compound for 18 hours. Cells were then lysed using a freeze-thaw protocol, and 30 μl of cell extract was used for luciferase and β-gal assays. LumiCount (Packard, Meriden, CT) was used to quantify luciferase and β-gal activities. Luciferase activity values were normalized against corresponding β-gal activity values as well as protein concentrations determined by Bradford assay.
Annexin V Staining Assay
Annexin V staining assay was performed using Dead Cell Apoptosis Kits with Annexin V for Flow Cytometry (Invitrogen, Carlsbad, CA). Briefly, cells were seeded in 6-well plates followed by various drug treatments. The cells were then washed with ice cold PBS, and 5 μl Alexa Fluor 488 Annexin V with 100 μg/ml PI (as per the manufacturer instructions) were added to the cells and incubated for 15 min. The cells were determined by Accuri flow cytometer.
Boyden Chamber Invasion Assay and Scratch Migration Assay
Attached cells (2.0 × 105) were treated with DMSO or with different concentrations of resveratrol in medium supplemented with 2.5% charcoal stripped FBS for 24 hours or transfected with different small interfering RNAs (siRNAs) with RNA imax transfection for 72 hours as manufacturer’s protocol. Then, for Boyden chamber invasion assay, 1.0 × 105 cells from each treatment condition were allowed to invade through the Boyden Chamber for 48 hours. Cells that invaded into the Boyden Chamber were fixed using formaldehyde, stained, and then counted. For scratch migration assay, cells were grown to 90% confluency in 6-well plates, then scratched with a 200 μl sterile pipette tip and washed with PBS to remove detached cells from the plates. Cells were kept in incubator with DMSO or indicated treatments for 48 hours. After 48 hours, cells were fixed with 4% formaldehyde and stained with crystal violate solution. The wound gap was observed under AMG EVOS fl microscope. At least 3 replicates were performed for each treatment group.
Western Blot Analysis
Cells (3.0 × 105) were seeded on 6-well plates, and after various treatments, whole cell lysates were obtained by treating them with high salt lysis buffer RIPA (Thermo Scientific, Waltham, MA) that contained protease and phosphatase inhibitors (GenDEPOT, Baker, TX). The total protein in the lysates was quantified by Bradford assay. Equal amounts of protein from each lysate were then loaded on SDS polyacrylamide gel. The proteins on the gel were transferred to a polyvinylidene fluoride (PVDF) membrane, then blocked for an hour using 5% skimmed milk. The membranes were then incubated with primary antibody for overnight at 4°C. It was then washed with Tris-buffered saline and Polysorbate 20 and incubated with horseradish peroxidase-linked secondary antibody for 1 hour at room temperature. The membranes were further washed with Tris-buffered saline and treated with Immobilon western chemiluminescence horseradish peroxidase-substrates to detect the protein bands using Kodak 4000 MM Pro image station (Molecular Bioimaging, Bend, OR). Protein levels in various treatment groups were normalized to β-actin.
Transfection and Small Interfering RNAs
For RNA interference experiment, cells were seeded on 6-well plates at 3 × 105/well then allowed 24 h to attach and grow. Then, they were transfected with siRNA of 100 nmol each/well for 6-well plates using 6.5 μl/well RNA iMax transfection reagent for 72 hours. siRNAs targeting NR4A1 (siNR4A1), Sp1 (siSp1), and Sp4 (siSp4) were purchased from Sigma-Aldrich. Negative Control Ig L2 siRNA were purchased from Qiagen. The oligonucleotides used were as follows:
siNR4A1_1, SASI_Hs02_00333289; siNR4A1_2, SASI_Hs02_00333290; siSp1_1: SASI_HS01-00070994; siSp1_2: SASI_Hs02_00333289; siSp4_1: SASI_HS01-00114420; siSp4_2: SASI_HS01-00114421.
ChIP Assay
The chromatin immunoprecipitation (ChIP) assay was performed using the ChIP-IT Express magnetic chromatin immunoprecipitation kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. All cells (3 × 107) were treated with DMSO or indicated concentration of resveratrol for 3 hours. Cells were then fixed with 1% formaldehyde, and the cross-linking reaction was stopped by addition of 0.125 M glycine. After washing twice with phosphate-buffered saline, cells were scraped and pelleted. Collected cells were hypotonically lysed, and nuclei were collected. Nuclei were then sonicated to the desired chromatin length (200–1,500 bp). The sonicated chromatin was immunoprecipitated with normal IgG (Cell signaling), NR4A1 (Abcam), Sp1 (Abcam), Sp4 (Santa Cruz), or RNA polymerase II (pol II; GeneTex) antibodies and protein A-conjugated magnetic beads at 4°C for overnight. After the magnetic beads were extensively washed, protein-DNA cross-links were reversed and eluted. DNA was prepared by proteinase K digestion followed by polymerase chain reaction (PCR) amplification. The primers for detection of the β1 integrin promoter region were 5 = -TCA CCA CCC TTC GTG ACA C-3 = (sense) and 5 = -GAG ATC CTG CAT CTC GGA AG-3 = (antisense). PCR products were resolved on a 2% agarose gel in the presence of ethidium bromide (EtBr).
Real Time-PCR
RNA was isolated using Qiagen RNeasy Mini kit (Irvine, CA). Quantification of mRNA (β1-integrin) was performed using Bio-Rad iTaq Universal SYBR Green 1-Step Kit (Richmond, CA) using the manufacturer's protocol with real-time PCR. Human GAPDH mRNA was used as a control to determine relative mRNA expression. The primers for detection of the β1 integrin mRNA were 5′-GAA GGG CGT GTT GGT AGA CA-3′ (Forward) and 5′-GTT GCA CTC ACA CAC ACG AC-3′ (Reverse).
Statistical Analysis
Each assay was performed in triplicate and the results were presented as means with S.D. The statistical significance of differences between the treatment groups was determined by Dunnett’s multiple comparison test in ordinary one-way ANOVA. Analysis of Western blotting was done using ImageJ (1.53K) software. GraphPad Prism 8 (Version 8.4.3) software was used for analysis of variance and determined statistical significance. Data with a P value of less than 0.05 were considered statistically significant and indicated with “*” in figures.
Results
1. Binding and Transactivation
Based on the similarities between the effects of resveratrol and NR4A1 antagonists on solid tumor derived cancer cells, we initially investigated the binding of resveratrol (Fig. 1A) to the ligand binding domain of human NR4A1 using an isothermal titration calorimetry (ITC) assay procedure. The results showed that resveratrol bound to NR4A1 with a calculated KD value of 2.4 ± 0.7 µM (S.D., 3 determinations) and a ΔG value of -32.2 ± 0.8 kJ/mol (Fig. 1B). The stoichiometry of binding (n) is 0.82 mol ligand bound/mol protein. The direct interaction of resveratrol with NR4A1 was also confirmed in a fluorescence quenching assay of a Trp in the NR4A1 binding pocket (Lee et al., 2014) and the KD value was 1.4 µM. We also used a computer modeling approach and compared the predictive interactions of resveratrol in the ligand binding pocket of NR4A1 (Fig. 1C) to that observed for the bis-indole NR4A1 ligand bis(3′-indolyl)-1-(3,5-dichlorophenyl)methane (DIM-3,5-Cl2) (Karki et al., 2021) (Fig. 1D). The simulations predicted that both compounds interact with common amino acids side chains Ser110, Glu114, Arg184, and Thr236; in addition, they also interacted uniquely with amino acid side chains Leu113, Leu239, and Ile260 (resveratrol), and Arg232 (DIM-3,5-Cl2), demonstrating some ligand structure-dependent differences in binding of resveratrol and DIM-3,5-Cl2 to NR4A1.
Fig. 1.
Resveratrol as an NR4A1 ligand. (A) Structure of resveratrol B. Binding of resveratrol to NR4A1 (LBD) by isothermal titration calorimetry (ITC) (B) as outlined in the Methods. Molecular modeling of the interaction of resveratrol (C) and 1,1-bis(3′-indolyl)-1-(3,5-dichlorophenyl)methane (DIM-3,5-Cl2) (D) with NR4A1 (LBD) was carried out using Maestro and crystal structure coordinates for the human orphan nuclear receptor NR4A1 ligand binding domain as outlined in the Methods. Effects of resveratrol on luciferase activity in H460 (E) and H1299 (F) cells transfected with GAL4-NR4A1 and UAS-luc as outlined in the Methods. Results are expressed as means ± S.D. for at least 3 replicate determinations for each treatment group and significant (P < 0.05) effects compared with control are indicated (*). The ITC binding assay was repeated (3X) and the means KD and ΔG values ± S.D. are indicated in panel 1B.
The activity of resveratrol as an NR4A1 ligand was confirmed in transactivation assays in H460 and H1299 lung cancer cells transfected with a yeast GAL4-NR4A1 chimera construct and a UAS-luc reported gene containing 5 tandem yeast GAL4 binding elements. Resveratrol decreased transactivation in both cell lines (Fig. 1, E and F), indicating NR4A1 antagonist activity, which has previously been observed for the CDIM/NR4A1 ligands in lung and other cancer cell lines (Lee et al., 2010, 2014; Lacey et al., 2016; Safe and Karki, 2021).
2. Resveratrol and NR4A1 Knockdown Inhibit Lung Cancer Cell Growth, Survival, Migration, and Invasion
Treatment of lung cancer cells with resveratrol decreased H460 and H1299 cell growth by approximately 40%–50% (Fig. 2, A and B) and also decreased NR4A1 protein expression (Fig. 2C). Knockdown of NR4A1 also decreased lung cancer cell growth by 40%–65% (Fig. 2, D and E), and NR4A1 protein (Fig. 2F) and levels of NR4A1 protein are quantitated (Supplemental Fig. 1A). Cell growth inhibition was observed over a range of concentrations (50–150 µM) and previous studies in lung cancer cells used concentrations of 100 or 200 µM to investigate effects of resveratrol on multiple endpoints (Wright et al., 2017; Wang et al., 2018). Based on preliminary studies we used two concentrations (125 and 150 µM) of resveratrol, which changed most pathways and levels of gene products investigated in this study. We also observed that resveratrol (125 and 150 µM) induced markers of apoptosis (PARP and caspase-3 cleavage; Bcl-2 downregulation) (Fig. 2G). Similar results were observed after knockdown of NR4A1 (Fig. 2H), and quantitation of the western blots (G and H) are summarized in Supplemental Fig. S1B and S1C. Resveratrol (125 and 150 µM) also induced Annexin V staining in H460 and H1299 cells (by >12-fold) (Fig. 2I). In addition, we also observed that resveratrol inhibited cell migration in a scratch assay by >45% at the high dose, and similar results were observed after NR4A1 knockdown (approximately 25% inhibition) (Fig. 3, A and B). Resveratrol (125 and 150 µM) inhibited cell invasion by 75% in a Boyden chamber assay by >25% after receptor knockdown (Fig. 3, C and D). These results demonstrate that the functional inhibitory effects of resveratrol on H460 and H1299 cell growth, survival, migration, and invasion mimic those obtained after knockdown of NR4A1, suggesting that the anticancer activity of resveratrol is due, in part, to its activity as an NR4A1 ligand.
Fig. 2.

Resveratrol and NR4A1 knockdown (siNR4A1) inhibit growth and induce apoptosis in H460 and H1299 cells. H460 (A) and H1299 (B) cells were treated with resveratrol for up to 72 hours, and effects on cell proliferation and NR4A1 protein expression (C) were determined using an XTT assay. H460 and H1299 cells were transfected with siNR4A1 (2 oligonucleotides), and effects on proliferation of H460 (D) and H1299 (E) cells and NR4A1 protein expression (F) were determined. (G) H460 and H1299 cells were treated with resveratrol, or whole cell lysates were analyzed by western blots, and bands were quantitated. H460 and H1299 cells were transfected with siNR4A1 (H), whole cell lysates were analyzed by western blots, and bands were quantitated. (I) H460 and H1299 cells were treated with resveratrol, and effects on Annexin V staining were determined as outlined in the Methods. Results are expressed as means ± S.D. for at least 3 separate determinations for each treatment group, and significant (P < 0.05) changes compared with control (DMSO) are indicated. Calculations of changes in intensity of protein bands are also normalized to the β-actin loading control for each treatment group. Quantitation of blots in 2C/2F, 2G, and 2H are summarized in Supplemental Fig. 1A, B, and C, respectively.
Fig. 3.
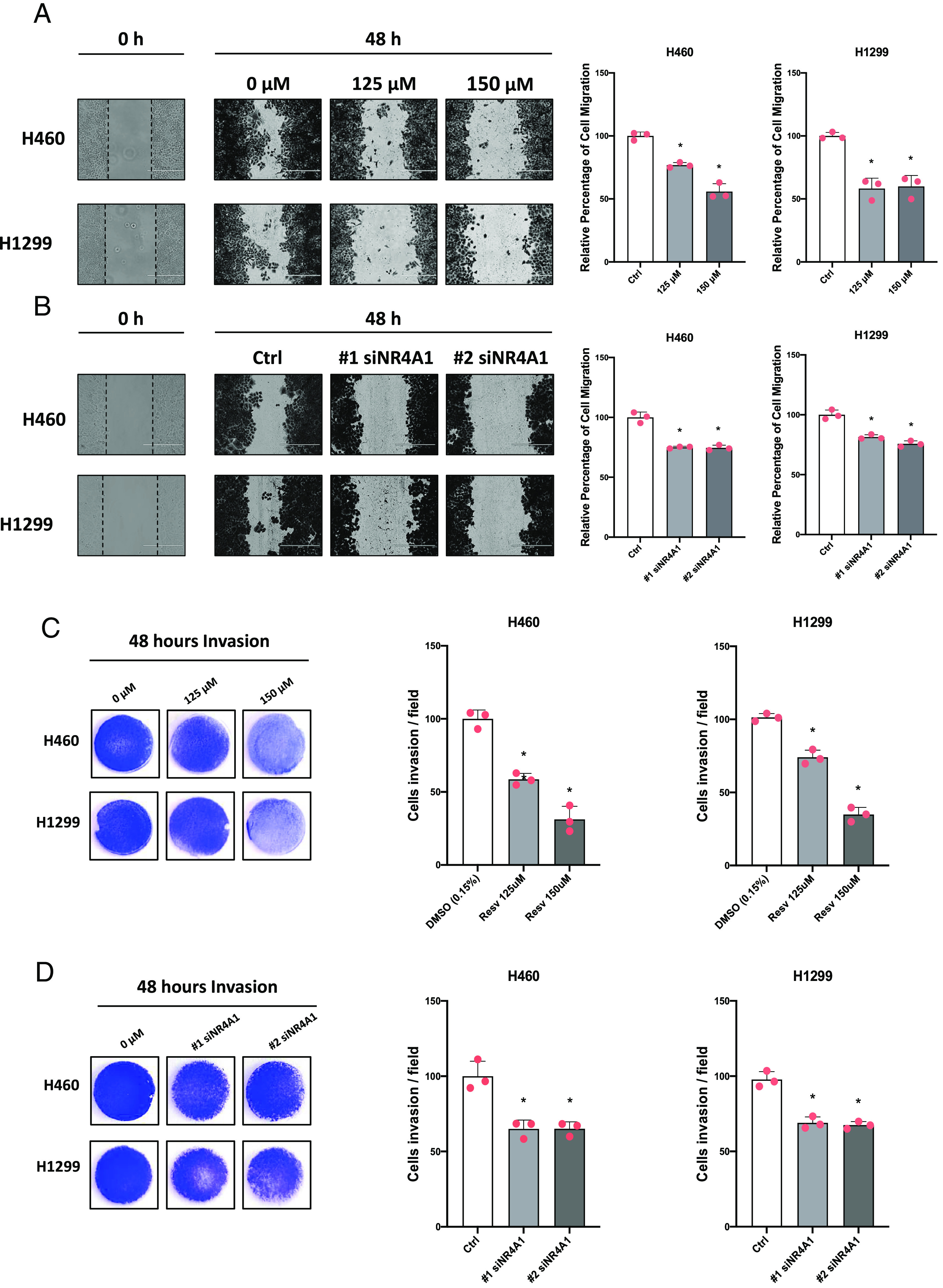
Resveratrol inhibits cell migration and invasion. (A) H460 and H1299 cells were treated with resveratrol (A) or transfected with siNR4A1 oligonucleotides (B), and effects on cell migration were determined in scratch assays. Cells were treated with resveratrol (C) or transfected with siNR4A1 oligonucleotides (D), and effects on cell invasion were determined in a Boyden chamber assay. Results of the effects of resveratrol (A and C) and knockdown (B and D) have been quantitated and appear in the same panels (right side). Quantitative results are expressed as means ± S.D. for at least 3 separate determinations per treatment group, and significant (P < 0.05) inhibition is indicated (*). Resveratrol (125 and 150 µM) decreased migration ability in H460 cells by 23.22% with a 95% CI of 13.37%–33.06% and 44.19% with a 95% CI of 34.34%–54.04%, respectively. Resveratrol (125 and 150 µM) decreased cell migration ability in H1299 cells by 41.76% with a 95% CI of 25.16%–58.35% and 40.10% with a 95% CI of 23.50%–56.70%, respectively. Resveratrol (125 and 150 µM) decreased invasion ability in H460 cells by 41.43% with a 95% CI of 25.94%–56.92% and 68.78% with a 95% CI of 53.29%–84.27%, respectively. Resveratrol (125 and 150 µM) decreased invasion ability in H1299 cells by 27.30% with a 95% CI of 17.34%–37.26% and 66.46% with a 95% CI of 56.50%–76.41%, respectively.
3. Resveratrol and NR4A1 Knockdown Modulate Expression of Several Gene Products (Proteins) and mRNAs in Common
Previous studies in multiple solid tumor derived cell lines have identified several NR4A1-regulated genes and proteins, and they include thioredoxin containing domain 5 (TXNDC5), isocitrate dehydrogenase-1 (IDH-1), and inhibition of phospho-AMP-activated protein kinase-α (p-AMPKα) (Lee et al., 2012, 2014; Lacey et al., 2016; Safe and Karki, 2021). Treatment of H460 and H1299 cells with resveratrol (125 and 150 µM) decreased expression of TXNDC5 and IDH-1 and induced p-AMPKα proteins (Fig. 4, A and B), and similar results were obtained in H460 and H1299 cells transfected with oligonucleotides targeted to NR4A1 (#1 siNR4A1, #2 siNR4A1) (Fig. 4, C and D), confirming comparable effects of resveratrol and NR4A1 knockdown. Previous studies show that resveratrol and CDIM/NR4A1 antagonists inhibit mTOR and downstream pathways, and results in Fig. 5, A and B (quantitation) show that resveratrol (125 and 150 µM) inhibited phosphorylation of mTOR and the downstream kinases S6RP and 4E-BP1 in H460 and H1299 cells. These results are consistent with activation of AMPK by resveratrol (Fig. 4); however, in this study resveratrol also downregulated the kinase proteins (mTOR, S6RP, and 4E-BP1), and this would also contribute to their decreased phosphorylation. The effects of NR4A1 knockdown in H460 and H1299 cells (Fig. 5, C and D) also resulted in decreased expression of mTOR, S6RP, and 4E-BP1 and their phosphorylated forms, and thus resembled effects observed in cells treated with resveratrol.
Fig. 4.

Effects of resveratrol and NR4A1 knockdown on selected NR4A1-regulated gene products. H460 and H1299 cells were treated with resveratrol (A; B-quantitation of bands in A) or transfected with siNR4A1 oligonucleotides (C; D-quantitation of bands in C), and whole cell lysates were analyzed by western blots as outlined in the Methods. Quantitative results (B and D) are expressed as means ± S.D. for at least 3 separate gels per treatment group, and significantly (P < 0.05) induced changes in band densities compared with CTL (DMSO or empty vector) are indicated (*). Levels for all proteins were normalized to β-actin.
Fig. 5.

Effects of resveratrol and siNR4A1 on mTOR signaling. H460 and H1299 cells were treated with resveratrol (A; B-quantitation of bands in A) or transfected with siNR4A1 oligonucleotides (C; D, quantitation of bands in C), and whole cell lysates were analyzed by western blots as outlined in the Methods. Results (B and D) are expressed as means ± S.D. for at least 3 separate gels per treatment group, and significantly (P < 0.05) induced changes in band intensities compared with CTL (DMSO or empty vector) are indicated (*). Levels for all proteins were normalized to β-actin.
NR4A1 not only directly binds promoter DNA, but also acts as a nuclear cofactor that activates expression of several genes and proteins in cancer cells through protein-protein interactions with Sp1 or Sp4 bound to GC-rich promoter sites (Lee et al., 2010; Lacey et al., 2016; Hedrick et al., 2016, 2017 a, 2017b; Safe and Karki, 2021; Shrestha et al., 2021). This is commonly observed for other nuclear receptors that act as ligand-dependent nuclear cofactors (Safe and Kim, 2008). β1-integrin is regulated by NR4A1/Sp1 and NR4A1/Sp4 in rhabdomyosarcoma, breast, colon, and pancreatic cancer cells, and interactions with Sp1 or Sp4 are cell context dependent (Lacey et al., 2016; Hedrick et al., 2016, 2017a, 2017b; Shrestha et al., 2021). Fig. 6A illustrates that treatment of H460 or H1299 cells with resveratrol or knockdown of NR4A1 by RNA interference (RNAi) decreases levels of β1-integrin protein (quantitation in Supplemental Fig. 1D), and resveratrol also decreased β1-integrin mRNA levels (Fig. 6B), confirming that β1-integrin is an NR4A1-regulated gene. Knockdown of Sp1 in H460 and H1299 cells decreased Sp1 but only minimally affected Sp4 expression, and this was accompanied by decreased levels of β1-integrin protein, confirming a role for NR4A1/Sp1 (Fig. 6C). In contrast, knockdown of Sp4 by RNAi decreases expression of both Sp1 and Sp4 proteins and also β1-integrin protein (Fig. 6D). Thus, it is not possible to demonstrate unambiguously whether NR4A1/Sp4 regulates expression of β1-integrin, and quantitation of these data are illustrated in Supplemental Fig. 1, E and F. ChIP analysis shows that in H460 cells treated with DMSO (control) or resveratrol that NR4A1, Sp1, and Sp4 were associated with the GC-rich promoter region of the β1-integrin gene, and treatment with resveratrol resulted in some loss of NR4A1, Sp1, and Sp4 binding (Fig. 6E) to the promoter. Similar results were observed in previous studies with CDIM/NR4A1 antagonist (Lacey et al., 2016; Hedrick et al., 2016, 2017a, 2017b; Shrestha et al., 2021), demonstrating that the anticancer activity of resveratrol in H460 and H1299 lung cancer cells is due, in part, to the activity of resveratrol as an NR4A1 antagonist.
Fig. 6.
Mechanism of β1-integrin regulation by resveratrol. H460 and H1299 cells were treated with resveratrol for 24 hours, and whole cell lysates were analyzed by western blots or transfected with siNR4A1 oligonucleotides (A). (B) H460 and H1299 cells were treated with resveratrol for 24 hours, and β1-integrin mRNA levels were determined by real-time PCR as outlined in the Methods. H460 and H1299 cells were transfected with oligonucleotides that target Sp1 (siSp1) (C) or Sp4 (siSp4) (D) expression, and whole cell lysates were analyzed by western blots. (E) Effects of resveratrol on interactions of NR4A1 Sp1, Sp4, and polII with the GC-rich region of the β1-integrin promoter were determined in a ChIP assay as outlined in the Methods. Quantitative results (B) are means ± S.D. for at least 3 replicate determinations, and significant (P < 0.05) changes relative to untreated control values are given (*). Quantitation of western blots in A, C, and D are summarized in Supplemental Fig. 1D, E, and F, respectively.
Discussion
Polyphenolic compounds, including resveratrol, are enriched in diets containing fruits, nuts, and vegetables, and their consumption is associated with numerous health benefits, including longer lifespans and protection from aging-related and inflammatory diseases including cancer (Saiko et al., 2008; Liu et al., 2019; Hano and Tungmunnithum, 2020; Koh et al., 2021; Raj et al., 2021; Wu et al., 2021; Zhou et al., 2021; Santana et al., 2022). These compounds act as antioxidant, anti-inflammatory, and antiviral agents and also regulate multiple pathways and genes that contribute to diverse disease states and serve as an important class of dietary chemo-preventive agents. Resveratrol has been extensively investigated in preclinical cell culture and in vivo models and exhibits impressive cancer chemotherapeutic properties that have been attributed to the effects of this compound on expression of multiple genes. This also includes activation of AMPK and subsequent inhibition of mTOR signaling, as well as the effects of resveratrol on activation of SIRT1 and other histone and nonhistone deacetylase, which are two pathways linked to the chemotherapeutic effects of resveratrol (He et al., 2011; Rashid et al., 2011; Wu and Liu, 2013; Alayev et al., 2015; Selvaraj et al., 2016; Chang et al., 2017; Liu et al., 2018; Wang et al., 2018; Bian et al., 2020; Khan et al., 2020; Koh et al., 2021). Results of preliminary studies showed that effects of resveratrol on SIRT1 expression in H460 and H1299 cells were cell context dependent and highly variable (data not shown).
Despite the remarkable activities of resveratrol, the effects of this compound in human clinical trials have not matched the promise of results from preclinical cell culture and animal models of disease (Jazirehi and Bonavida, 2004; Berman et al., 2017; Ramirez-Garza et al., 2018; Singh et al., 2019). Although resveratrol is generally well tolerated and provides some indications of benefits, poor bioavailability has been a problem, and detrimental effects have been observed for some cancers. For example, resveratrol inhibited several kinases in models of multiple myeloma (Popat et al., 2013); however, treatment of drug-resistant multiple patients with myeloma with resveratrol resulted in several toxic side effects including renal failure.
It is also possible that the modest results obtained for resveratrol in human clinical trials may be due, in part, to unknown mechanisms of action that prevent a more targeted or precision medicine approach. Studies in this laboratory have identified NR4A1 as a pro-oncogenic factor in solid tumor-derived cells and animal models (Safe and Karki, 2021). NR4A1 also regulates cancer cell growth, survival, and migration/invasion, and this includes inactivation of AMPK. Activation of mTOR and these responses can be reversed by bis-indole derived (CDIM) NR4A1 antagonists. Many of the effects of CDIMs are similar to those caused by resveratrol in cancer.
In this study, we used H460 and H1299 lung cancer cells as models, and treatment with resveratrol decreased lung cancer cell growth, enhanced apoptosis, and decreased migration and invasion (Figs. 2 and 3). These results have previously been observed in lung cancer cells treated with resveratrol (Wright et al., 2017; Wang et al., 2018), and our studies also show that comparable effects have been observed in H460 and H1299 cells after NR4A1 knockdown (Figs. 2 and 3) and after treatment with CDIM/NR4A1 antagonists (Lee et al., 2012). These data suggested that resveratrol may be an NR4A1 ligand, and this was confirmed in direct binding and ITC assays, where the KD value for binding was in the low µM range (Fig. 1). Docking resveratrol to the NR4A1 LBD (Fig. 1C) utilizing the Schrodinger Maestro modeling approach resulted in several favorable interactions (yellow dotted line) between resveratrol and specific amino acid residues of NR4A1 LBD [Ser110, Gul114, Arg184, Thr236 (aromatic, Leu239, and Ile260)]. Two unfavorable interactions (orange dotted line) between resveratrol and the NR4A1 LBD were also predicted (Leu239 and Ile260). Docking studies with the newly developed high affinity CDIM ligand bis(3′-indolyl)-1-(3,5-dichlorophenyl)methane (DIM-3,5-Cl2) (Karki et al., 2021) to the NR4A1 LBD (Fig. 1D) resulted in similar favorable interactions (yellow dotted line), as predicted for resveratrol, including specific interactions with Ser110, Glu114 (halogen bond), Arg184, Arg232 (aromatic), and Thr236 side chains, but also some differences. These differences in the interactions of resveratrol and DIM-3,5-Cl2 with amino acids in the ligand binding domain of NR4A1 are consistent with designation of these compounds as selective receptor modulators. The binding results coupled with the inhibitory effects of resveratrol on NR4A1-dependent transactivation (Fig. 1) demonstrate for the first time that resveratrol is an NR4A1 ligand that acts as a receptor antagonist and inhibits NR4A1-dependent transactivation in lung cancer cells.
We also examined a number of gene products previously shown to be regulated by CDIM/NR4A1 antagonists in cancer cells (Safe and Karki, 2021), and these include decreased expression of TXNDC5, IDH1, mTOR, and β1-integrin and induction of apoptosis gene products and activation of pAMPK (Figs. 4, 5, and 6). Responses observed for resveratrol and NR4A1 knockdown were comparable, and β1-integrin was regulated by NR4A1 through interactions of NR4A1 as a cofactor of Sp1 bound to the GC-rich sites of the β1-integrin gene. Since knockdown of Sp4 in H1299 and H460 cells also decreases Sp1 expression, it was not possible to determine unequivocally a role for Sp4 in regulating β1-integrin gene expression via NR4A1/Sp4. The ChIP assay shows that both Sp1 and Sp4 bind the GC-rich integrin promoter, and it is possible that NR4A1 may coactivate both Sp1 and Sp4, and this process is blocked by resveratrol, as previously observed for CDIM/NR4A1 ligands (Lacey et al., 2016; Hedrick et al., 2016, 2017a, 2017 b). Thus, like CDIM/NR4A1 antagonists’ resveratrol also inactivates NR4A1/Sp-regulated genes such as β1-integrin, and this further confirms that the mechanisms and functions of resveratrol are due, in part, to its activity as a NR4A1 antagonist.
This study demonstrates for the first time that resveratrol binds with high affinity to NR4A1 and acts as an NR4A1 antagonist in lung cancer cell lines. Although the KD value for resveratrol is in the low μM range, indicating strong ligand-receptor interactions, the dose-response functional effects of resveratrol are in the 100–200 µM range in lung cancer cells (Wright et al., 2017; Wang et al., 2018), and this is several orders of magnitude higher than the KD value. This difference may be due to several factors, including the effectiveness of the bound receptor complex to interact with nuclear cofactors, cellular uptake of resveratrol, and rapid metabolism (conjugation), which is commonly observed for other polyphenolics. Like many solid tumors, NR4A1 is overexpressed in many solid tumors and is a negative prognostic factor for patient survival (Lee et al., 2012). This suggests that clinical applications of resveratrol in lung cancer chemotherapy may be more effective in treating patients with tumors that overexpress NR4A1. It should also be noted that there is a long list of potential targets of resveratrol that include kinases, cytokines, cell signaling molecules, key genes involved in cancer cell proliferation, survival, and migration/invasion (Saiko et al., 2008; Bruner-Tran et al., 2011; Ergenoğlu et al., 2013; Rudzitis-Auth et al., 2013; Amaya et al., 2014; Taguchi et al., 2014; Bayoglu Tekin et al., 2015; Kolahdouz and Arablou, 2017; Le et al., 2019; Teertam et al., 2020; Koh et al., 2021; Raj et al., 2021; Tang et al., 2021; Zhou et al., 2021; Santana et al., 2022). This list also includes interactions with other receptors including nuclear receptor superfamily members. This study highlights the contribution of resveratrol as an NR4A1 ligand (antagonist) in lung cancer cells, and the effectiveness and contributions of this response to the overall anticancer activity of resveratrol may also be tumor-type specific and needs to be further investigated.
Acknowledgments
The authors would like to acknowledge the support provided by the Kleberg Foundation (S. Safe) and the Sid Kyle Chair Endowment (S. Safe).
Abbreviations
- AMPK
AMP-activated protein kinase
- CDIM
bis-indole derived compound
- ChIP
chromatin immunoprecipitation
- ΔG
the equilibrium free energy of ligand binding
- IDH-1
isocitrate dehydrogenase-1
- ITC
isothermal titration calorimetry
- Kd
ligand binding constant
- LBD
ligand binding domain
- NR4A
nuclear receptor 4A
- PCR
polymerase chain reaction
- siRNA
small interfering RNA
- SIRT1
NAD-dependent deacetylase sirtuin-1
- TXNDC5
thioredoxin domain containing 5
Authorship Contributions
Participated in research design: Zhang, Safe.
Conducted experiments: Zhang, Martin, Mohankumar, Hampton.
Contributed new reagents or analytical tools: Hampton, Liu.
Performed data analysis: Zhang, Martin.
Wrote or contributed to the writing of the manuscript: Zhang, Safe.
Footnotes
This work was funded by National Institutes of Health National Institute of Environmental Health Sciences [Grant P30-ES029067] (to S.S.) and the Welch Foundation [A-1715] (to W.R.L.).
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Alayev A, Berger SM, Holz MK (2015) Resveratrol as a novel treatment for diseases with mTOR pathway hyperactivation. Ann N Y Acad Sci 1348:116–123. [DOI] [PubMed] [Google Scholar]
- Amaya SC, Savaris RF, Filipovic CJ, Wise JD, Hestermann E, Young SL, Lessey BA (2014) Resveratrol and endometrium: a closer look at an active ingredient of red wine using in vivo and in vitro models. Reprod Sci 21:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoglu Tekin Y, Guven S, Kirbas A, Kalkan Y, Tumkaya L, Guvendag Guven ES (2015) Is resveratrol a potential substitute for leuprolide acetate in experimental endometriosis? Eur J Obstet Gynecol Reprod Biol 184:1–6. [DOI] [PubMed] [Google Scholar]
- Berman AY, Motechin RA, Wiesenfeld MY, Holz MK (2017) The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol 1:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian P, Hu W, Liu C, Li L (2020) Resveratrol potentiates the anti-tumor effects of rapamycin in papillary thyroid cancer: PI3K/AKT/mTOR pathway involved. Arch Biochem Biophys 689:108461. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, Osteen KG, Taylor HS, Sokalska A, Haines K, Duleba AJ (2011) Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol Reprod 84:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM, Tsao JW, Juan YN, Chiu HY, Yang JS, Wang CC (2017) Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: a key role of AMPK and Akt/mTOR signaling. Int J Oncol 50:873–882. [DOI] [PubMed] [Google Scholar]
- Ergenoğlu AM, Yeniel AO, Erbaş O, Aktuğ H, Yildirim N, Ulukuş M, Taskiran D (2013) Regression of endometrial implants by resveratrol in an experimentally induced endometriosis model in rats. Reprod Sci 20:1230–1236. [DOI] [PubMed] [Google Scholar]
- Friesner RABanks JLMurphy RBHalgren TAKlicic JJMainz DTRepasky MPKnoll EHShelley MPerry JK, et al. (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749. [DOI] [PubMed] [Google Scholar]
- Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 49:6177–6196. [DOI] [PubMed] [Google Scholar]
- Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759. [DOI] [PubMed] [Google Scholar]
- Hano C, Tungmunnithum D (2020) Plant polyphenols, more than just simple natural antioxidants: oxidative stress, aging and age-related diseases. Medicines (Basel) 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Wang Y, Zhu J, Orloff M, Eng C (2011) Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett 301:168–176. [DOI] [PubMed] [Google Scholar]
- Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S (2016) NR4A1 antagonists inhibit β1-integrin-dependent breast cancer cell migration. Mol Cell Biol 36:1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick E, Lee SO, Safe S (2017a) The nuclear orphan receptor NR4A1 regulates β1-integrin expression in pancreatic and colon cancer cells and can be targeted by NR4A1 antagonists. Mol Carcinog 56:2066–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick E, Li X, Safe S (2017b) Penfluridol represses integrin expression in breast cancer through induction of reactive oxygen species and downregulation of Sp transcription factors. Mol Cancer Ther 16:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazirehi AR, Bonavida B (2004) Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin’s lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther 3:71–84. [PubMed] [Google Scholar]
- Karki K, Mohankumar K, Schoeller A, Martin G, Shrestha R, Safe S (2021) NR4A1 ligands as potent inhibitors of breast cancer cell and tumor growth. Cancers (Basel) 13:2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Quispe C, Javed Z, Iqbal MJ, Sadia H, Raza S, Irshad A, Salehi B, Reiner Ž, Sharifi-Rad J (2020) Resveratrol, curcumin, paclitaxel and miRNAs mediated regulation of PI3K/Akt/mTOR pathway: go four better to treat bladder cancer. Cancer Cell Int 20:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YC, Ho CT, Pan MH (2021) Recent advances in health benefits of stilbenoids. J Agric Food Chem 69:10036–10057. [DOI] [PubMed] [Google Scholar]
- Kolahdouz Mohammadi R, Arablou T (2017) Resveratrol and endometriosis: in vitro and animal studies and underlying mechanisms (Review). Biomed Pharmacother 91:220–228. [DOI] [PubMed] [Google Scholar]
- Lacey A, Hedrick E, Li X, Patel K, Doddapaneni R, Singh M, Safe S (2016) Nuclear receptor 4A1 (NR4A1) as a drug target for treating rhabdomyosarcoma (RMS). Oncotarget 7:31257–31269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le K, Chibaatar Daliv E, Wu S, Qian F, Ali AI, Yu D, Guo Y (2019) SIRT1-regulated HMGB1 release is partially involved in TLR4 signal transduction: a possible anti-neuroinflammatory mechanism of resveratrol in neonatal hypoxic-ischemic brain injury. Int Immunopharmacol 75:105779. [DOI] [PubMed] [Google Scholar]
- Lee SO, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, Wang H, Safe S (2010) Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res 70:6824–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SO, Andey T, Jin UH, Kim K, Singh M, Safe S (2012) The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene 31:3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SO, Li X, Hedrick E, Jin UH, Tjalkens RB, Backos DS, Li L, Zhang Y, Wu Q, Safe S (2014) Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Mol Endocrinol 28:1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tong L, Luo Y, Li X, Chen G, Wang Y (2018) Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J Cell Biochem 119:6162–6172. [DOI] [PubMed] [Google Scholar]
- Liu Y, Weng W, Gao R, Liu Y (2019) New insights for cellular and molecular mechanisms of aging and aging-related diseases: herbal medicine as potential therapeutic approach. Oxid Med Cell Longev 2019:4598167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearen MA, Muscat GE (2010) Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol 24:1891–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat R, Plesner T, Davies F, Cook G, Cook M, Elliott P, Jacobson E, Gumbleton T, Oakervee H, Cavenagh J (2013) A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol 160:714–717. [DOI] [PubMed] [Google Scholar]
- Raj P, Thandapilly SJ, Wigle J, Zieroth S, Netticadan T (2021) A comprehensive analysis of the efficacy of resveratrol in atherosclerotic cardiovascular disease, myocardial infarction and heart failure. Molecules 26:6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Garza SL, Laveriano-Santos EP, Marhuenda-Muñoz M, Storniolo CE, Tresserra-Rimbau A, Vallverdú-Queralt A, Lamuela-Raventós RM (2018) Health effects of resveratrol: results from human intervention trials. Nutrients 10:1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid A, Liu C, Sanli T, Tsiani E, Singh G, Bristow RG, Dayes I, Lukka H, Wright J, Tsakiridis T (2011) Resveratrol enhances prostate cancer cell response to ionizing radiation. Modulation of the AMPK, Akt and mTOR pathways. Radiat Oncol 6:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzitis-Auth J, Menger MD, Laschke MW (2013) Resveratrol is a potent inhibitor of vascularization and cell proliferation in experimental endometriosis. Hum Reprod 28:1339–1347. [DOI] [PubMed] [Google Scholar]
- Safe S, Karki K (2021) The paradoxical roles of orphan nuclear receptor 4A (NR4A) in cancer. Mol Cancer Res 19:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Kim K (2008) Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol 41:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiko P, Szakmary A, Jaeger W, Szekeres T (2008) Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res 658:68–94. [DOI] [PubMed] [Google Scholar]
- Santana TM, Ogawa LY, Rogero MM, Barroso LP, Alves de Castro I (2022) Effect of resveratrol supplementation on biomarkers associated with atherosclerosis in humans. Complement Ther Clin Pract 46:101491. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Sun Y, Sukumaran P, Singh BB (2016) Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol Carcinog 55:818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R, Mohankumar K, Jin UH, Martin G, Safe S (2021) The histone methyltransferase gene G9A is regulated by nuclear receptor 4A1 in alveolar rhabdomyosarcoma cells. Mol Cancer Ther 20:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Singh R, Verma SS, Rai V, Kaschula CH, Maiti P, Gupta SC (2019) Health benefits of resveratrol: evidence from clinical studies. Med Res Rev 39:1851–1891. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wada-Hiraike O, Kawana K, Koga K, Yamashita A, Shirane A, Urata Y, Kozuma S, Osuga Y, Fujii T (2014) Resveratrol suppresses inflammatory responses in endometrial stromal cells derived from endometriosis: a possible role of the sirtuin 1 pathway. J Obstet Gynaecol Res 40:770–778. [DOI] [PubMed] [Google Scholar]
- Tang XL, Wang X, Fang G, Zhao YL, Yan J, Zhou Z, Sun R, Luo AL, Li SY (2021) Resveratrol ameliorates sevoflurane-induced cognitive impairment by activating the SIRT1/NF-κB pathway in neonatal mice. J Nutr Biochem 90:108579. [DOI] [PubMed] [Google Scholar]
- Teertam SK, Jha S, Prakash Babu P (2020) Up-regulation of Sirt1/miR-149-5p signaling may play a role in resveratrol induced protection against ischemia via p53 in rat brain. J Clin Neurosci 72:402–411. [DOI] [PubMed] [Google Scholar]
- Wang J, Li J, Cao N, Li Z, Han J, Li L (2018) Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. OncoTargets Ther 11:7777–7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Iyer AKV, Yakisich JS, Azad N (2017) Anti-tumorigenic effects of resveratrol in lung cancer cells through modulation of c-FLIP. Curr Cancer Drug Targets 17:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Luo Q, Nie R, Yang X, Tang Z, Chen H (2021) Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit Rev Food Sci Nutr 61:2175–2193. [DOI] [PubMed] [Google Scholar]
- Wu Y, Liu F (2013) Targeting mTOR: evaluating the therapeutic potential of resveratrol for cancer treatment. Anticancer Agents Med Chem 13:1032–1038. [DOI] [PubMed] [Google Scholar]
- Zhan YYChen YZhang QZhuang JJTian MChen HZZhang LRZhang HKHe JPWang WJ, et al. (2012) The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nat Chem Biol 8:897–904. [DOI] [PubMed] [Google Scholar]
- Zhou DD, Luo M, Huang SY, Saimaiti A, Shang A, Gan RY, Li HB (2021) Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid Med Cell Longev 2021:9932218. [DOI] [PMC free article] [PubMed] [Google Scholar]




