Abstract
Mu opioid receptor (MOR) agonists produce locomotor hyperactivity in mice as one sign of opioid-induced motor disruption. The goal of this study was to evaluate the degree of MOR efficacy required to produce this hyperactivity. Full dose-effect curves were determined for locomotor activation produced in male and female Institute of Cancer Research (ICR) mice by (1) eight different single-molecule opioids with high to low MOR efficacy and (2) a series of fixed-proportion fentanyl/naltrexone mixtures with high to low fentanyl proportions. Data from the mixtures were used to quantify the efficacy requirement for MOR agonist-induced hyperactivity relative to efficacy requirements determined previously for other MOR agonist effects. Specifically, efficacy requirement was quantified as the EP50 value, which is the “Effective Proportion” of fentanyl in a fentanyl/naltrexone mixture that produces a maximal effect equal to 50% of the maximal effect of fentanyl alone. Maximal hyperactivity produced by each drug and mixture in the present study correlated with previously published data for maximal stimulation of GTPɣS binding in MOR-expressing Chinese hamster ovary cells as an in vitro measure of relative efficacy. Additionally, the EP50 value for hyperactivity induced by fentanyl/naltrexone mixtures indicated that opioid-induced hyperactivity in mice has a relatively high efficacy requirement in comparison with some other MOR agonist effects, and in particular is higher than the efficacy requirement for thermal antinociception in mice or fentanyl discrimination in rats. Taken together, these data show that MOR agonist-induced hyperactivity in mice is efficacy dependent and requires relatively high levels of MOR agonist efficacy for its full expression.
SIGNIFICANCE STATEMENT
Mu opioid receptor (MOR) agonist-induced hyperlocomotion in mice is dependent on the MOR efficacy of the agonist and requires a relatively high degree of efficacy for its full expression.
Introduction
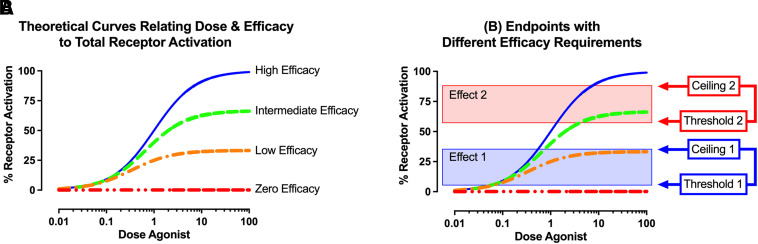
Morphine and other mu opioid receptor (MOR) agonists produce a wide range of physiologic and behavioral effects that include both therapeutically useful effects like analgesia and undesirable effects that include disrupted motor function (Yaksh and Wallace, 2018). One key determinant of the effects produced by any given MOR ligand is the relationship between two factors: (a) drug efficacy to activate MOR-coupled signaling mechanisms, and (b) efficacy requirements for different MOR-mediated effects (Selley et al., 2021). This relationship is illustrated in Fig. 1. For any in vitro or in vivo test system, increasing drug doses will produce increasing MOR occupation and increasing levels of MOR activation. Maximal receptor activation plateaus at high doses that saturate receptors, and high-efficacy MOR ligands will produce higher plateaus than lower efficacy ligands. Within this framework of dose- and efficacy-dependent MOR activation, different effects require different thresholds of receptor activation for their expression and are constrained by different ceilings imposed by either experimental or biologic limits. Together, the threshold and ceiling for a given effect define its efficacy requirement, which specifies the range of receptor-activation levels across which increasing doses will produce increasing effect. Moreover, the degree to which different effects have different efficacy requirements can be exploited in drug development, because low-efficacy drugs may have sufficient efficacy to produce some therapeutic effects but lack sufficient efficacy to produce some undesirable effects.
Fig. 1.
Dose and efficacy of the drug and efficacy requirement of the effect as determinants of drug effects. Drugs produce their effects by acting on a population of target receptors in the biologic system to which they are administered. For drugs with both affinity and efficacy at the target receptor, receptor theory predicts that increasing drug doses will produce increasing levels of receptor occupancy and activation. (A) Peak levels of total receptor activation are determined by drug efficacy, such that drugs with higher efficacy will produce higher plateau levels of receptor activation than drugs with lower efficacy. Abscissa: Drug dose in arbitrary units. Ordinate: % Total Possible Activation of all available receptors. (B) Different effects mediated by the receptor require different levels of receptor activation for their expression. Effects with a low efficacy requirement (Effect 1) require low levels of receptor activation to surpass the threshold and reach the ceiling for their expression. Even low-efficacy drugs have sufficient efficacy to produce these effects. Effects with a high efficacy requirement (Effect 2) require higher levels of receptor activation to surpass the threshold and reach the ceiling for their expression. High-efficacy drugs can produce sufficient receptor activation to produce these effects, although this will require higher doses than for effects with low efficacy requirements. Lower efficacy drugs may have sufficient efficacy to surpass the threshold but not reach the ceiling and may therefore function as partial agonists for these effects. Even lower efficacy drugs may lack sufficient efficacy even to reach the threshold, in which case they will function as antagonists.
Mice are commonly used in preclinical drug development studies, and one MOR agonist effect in mice is a stimulation of horizontal locomotor activity (Frischknecht et al., 1983; Michael-Titus et al., 1989; Narita et al., 1993; Raehal et al., 2005; Osborn et al., 2010; Varshneya et al., 2019). Opioid-induced locomotor activation in mice is one manifestation of MOR-mediated motor disruption, and as such, it can be considered an undesirable opioid effect. Additionally, opioid-induced locomotor stimulation involves activation of the mesolimbic dopamine system and serves as one behavioral consequence of enhanced mesolimbic dopamine signaling (Funada et al., 1993; Chefer et al., 2003; Walters et al., 2005; Urs and Caron, 2014; Severino et al., 2020; Botz-Zapp et al., 2021). Lastly, locomotor activation is an unconditioned behavioral effect that requires no prior training for its expression, and it can be continuously and quantitatively measured in commercially available locomotor-activity chambers (Raehal et al., 2005; Varshneya et al., 2019; Chakraborty et al., 2021). These features make opioid-induced locomotor activation useful as an endpoint for early evaluation of the in vivo potency, effectiveness, and time course of novel opioids. The utility of opioid-induced locomotor activation as a preclinical endpoint in drug evaluation would be further enhanced by clarification of its efficacy requirement relative to other opioid effects in mice and in other in vitro and in vivo test systems.
Accordingly, the goal of the present study was to evaluate the efficacy requirements for opioid-induced locomotor activation in male and female mice. Two parallel sets of studies were conducted. First, dose-effect curves were determined for a panel of eight MOR ligands with a range of maximal effects to stimulate GTPɣS binding as an in vitro measure of relative MOR efficacy (Selley et al., 1998; Thompson et al., 2004; Yuan et al., 2013; Thomsen et al., 2014). Second, dose-effect curves were also determined for a panel of drug mixtures composed of the high-efficacy MOR agonist fentanyl and antagonist naltrexone. We have previously shown that the fixed proportion of fentanyl to naltrexone in fentanyl/naltrexone mixtures can be precisely manipulated to yield mixtures with graded maximal effects in both in vitro assays of ligand-stimulated GTPɣS binding and in vivo assays across multiple endpoints in multiple species of test subject (Cornelissen et al., 2018; Schwienteck et al., 2019; Selley et al., 2021). Additionally, fentanyl/naltrexone mixtures can be used to identify the effective proportion of fentanyl sufficient to produce 50% of the maximum effect of fentanyl alone (defined as the EP50 value) as a metric of the efficacy requirement for any in vitro and in vivo MOR-mediated effect (Cornelissen et al., 2018; Schwienteck et al., 2019; Selley et al., 2021). Prevailing evidence suggests that locomotor activation may have a higher efficacy requirement than some other opioid effects, such as thermal antinociception in mice (Varshneya et al., 2019; Chakraborty et al., 2021; Varshneya et al., 2021). As a result, we predicted that the EP50 value for locomotor activation in mice would be high relative to EP50 values we have determined previously for other opioid effects in mice, rats, and rhesus monkeys.
Methods
Animals
Subjects were male and female ICR mice (Envigo, Frederick, MD) that were 6–8 weeks old upon arrival to the laboratory. Males weighed 27–50 g and females weighed 22–50 g throughout the study. Mice were housed in same-sex, littermate groups in cages with corncob bedding (Envigo), a “nestlet” composed of pressed cotton (Ancare, Bellmore, NY), a cardboard tube for enrichment, and ad libitum access to food (Teklad LM-485 Mouse/Rat Diet; Envigo). Cages were mounted in a RAIR HD Ventilated Rack (Laboratory Products, Seaford, DE) in a temperature-controlled room with a 12-hour light/dark cycle (lights on from 6:00 AM to 6:00 PM) in a facility approved by the American Association for Accreditation of Laboratory Animal Care. All experiments were performed during the light phase of the daily light/dark cycle beginning 1 week after arrival at the laboratory. Ethical animal-use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee (Protocol #AD10001093) and complied with the National Research Council Guide for the Care and Use of Laboratory Animals.
Apparatus
Horizontal locomotor activity was assessed during 60-minute sessions in test boxes (16.8 × 12.7 cm2 floor area × 12.7 cm high) housed in sound-attenuating chambers (Med Associates, St. Albans, VT) and located in a procedure room separate from the housing room. Each box had black plexiglass walls, a clear plexiglass ceiling equipped with a house light, bar floors, and six photobeams arranged at 3-cm intervals across the long wall and 1 cm above the floor. Beam breaks were monitored by a microprocessor operating Med Associates software. The primary dependent variable was the total number of beam breaks, excluding consecutive interruptions of the same beam, during the 60-minute session.
Pharmacological Procedure
The primary goal of the study was to test a range of MOR ligands that varied in their relative efficacy at the MOR as quantified by maximum agonist-stimulated GTPɣS binding in Chinese hamster ovary (CHO) cells expressing the mouse or human MOR in previously published studies (Selley et al., 1998; Thompson et al., 2004; Obeng et al., 2018; Selley et al., 2021). This was accomplished by testing two different categories of treatments. First, a range of eight different single-molecule MOR ligands was tested. These drugs and their associated dose ranges were as follows (listed from highest to lowest maximum effect (Emax) in studies of agonist-stimulated GTPɣS binding): methadone, 0.32–32 mg/kg (Middaugh and Zemp, 1976), fentanyl, 0.032–3.2 mg/kg (Varshneya et al., 2019), morphine, 1.0–100 mg/kg (Loggi et al., 1991), hydrocodone, 1.0–100 mg/kg (Jacob et al., 2017), buprenorphine, 0.01 – 3.2 mg/kg (Cowan et al., 1977), nalbuphine, 0.32–32 mg/kg (Patrick et al., 1999), NAQ (17-Cyclopropyl-methyl-3,14β- dihydroxy-4,5α-epoxy-6α-[(3′-isoquinolyl)acetamido]-morphinan), 1.0–100 mg/kg (Zhang et al., 2014), and naltrexone, 0.1–3.2 mg/kg (Castellano and Puglisi-Allegra, 1982). Nalbuphine, NAQ, and naltrexone produced little or no locomotor stimulation across the dose-range tested, so each of these drugs was further evaluated for effectiveness to block locomotor activation by 10 mg/kg of morphine.
The second category of treatments consisted of a series of fixed-proportion fentanyl/naltrexone mixtures. In these mixtures, the proportion of fentanyl to naltrexone was fixed at a constant value for a given mixture (e.g., 1:1 fentanyl/naltrexone), and changes in the dose of one drug of the mixture were matched by equivalent changes in the other drug. We have reported previously that the net MOR efficacy of fentanyl/naltrexone mixtures can be precisely calibrated both in vitro and in vivo by adjusting the fentanyl proportion in the mixture, such that increasing fentanyl proportions result in increasing levels of MOR efficacy for the mixture. The present study compared effects of five different fentanyl/naltrexone mixtures ranging from 100:1 to 3.2:1 fentanyl/naltrexone.
With two exceptions noted below, a different group of 12 mice (six females, six males) were used to test each drug or mixture, and we have previously presented a detailed rationale for this group size and sex allocation (Diester et al., 2019). For this study, cohorts of up to 36 mice were generally used at any one time to test three different drugs or mixtures, and mice in each cohort were randomly assigned to the different treatments. Within each group, test sessions were conducted twice a week with at least 48 hours between sessions. All mice received a vehicle control and all doses of the designated test drug or mixture, and dose order was randomized across mice using a Latin-square design. The experimenter was not blinded to treatment because data collection was automated by the Med Associates software. There were no exclusion criteria, and all data were included in final analysis. On test days, mice were brought to the procedure room at least 2 hours before session onset. After subcutaneous test-drug administration, mice were returned to their home cages for the 5-minute pretreatment interval and then placed into the locomotor activity boxes at session onset. Doses for each drug or mixture were varied in 0.5 or 1.0 log-unit increments across a ≥10-fold dose range with the intent of progressing from low doses that produced little or no effect to high doses that produced maximal increases in locomotor activation for that drug. For nalbuphine, NAQ, and naltrexone, antagonism studies were conducted after completion of drug-alone studies in the same mice. Doses of the test drug were administered 10 minutes before 10 mg/kg of morphine, and locomotor sessions began 5 minutes after morphine administration. There were two exceptions to this general design. First, in the case of hydrocodone, only six of the original mice (three of each sex) were tested at the high dose of 100 mg/kg due to limited drug supply. Because a clear effect plateau had not been reached at this dose, more drug was acquired and a higher dose of 320 mg/kg of hydrocodone was tested in four other mice (two of each sex); however, all mice died, and further studies with hydrocodone were not pursued. Second, in the case of buprenorphine, the initial group was tested only up to a dose of 1.0 mg/kg due again to limited drug supply. Because a clear effect plateau had not been reached at this dose, more drug was acquired, and a higher dose of 3.2 mg/kg was tested in six other mice (three of each sex).
Data and Statistical Analysis
The primary dependent variable was the total number of beam breaks, excluding consecutive interruptions of the same beam, during each 60-minute session. These data were first analyzed within each drug or mixture to assess dose-dependent effects. Initial within-drug analysis proceeded in four phases as described previously for studies that include both females and males but are not intended a priori to detect sex differences (Diester et al., 2019). First, because sex was not the primary variable of interest, pooled data from both females and males were analyzed by repeated-measures one-way ANOVA with dose as the single variable, and a significant ANOVA was followed by a Holm-Sidak post-hoc test to both (a) identify doses producing effects different from vehicle and (b) evaluate presence or absence of a significant difference between the highest doses to identify an effect plateau for Emax determination. For this and all other analyses described below, the criterion for significance was P < 0.05. Data for the highest doses of hydrocodone and buprenorphine were not included in the one-way ANOVA for these drugs because of the lower number of mice tested; rather, effects of these doses were compared with the next lower dose by t test (paired for hydrocodone, unpaired for buprenorphine). Second, data were segregated by sex and again submitted to repeated-measures one-way ANOVA followed by Holm-Sidak post-hoc test to assess dose-dependent effects within each sex. Third, male and female data were directly compared by two-way ANOVA with sex as a between-subjects factor and drug dose as a within-subjects factor. A significant sex × dose interaction was followed by a Holm-Sidak post-hoc test. These first three steps of data analysis were performed using GraphPad Prism 9.0 (La Jolla, CA). Lastly, the two-way ANOVA results were submitted to power analyses to calculate the Cohen’s f effect size, achieved power (1 - β), and the total number of animals predicted as necessary to detect a significant effect of sex, dose, and the sex × dose interaction given the effect size, α = 0.05, and power (1 - β) = 0.8 using the free statistical analysis program G*Power (Faul et al., 2007). Regarding the antagonism studies, data analysis was performed as described above in steps 1–3 with the exception that test drugs were evaluated for their effectiveness to decrease locomotor stimulant effects of morphine. Taken together, this strategy for experimental design and data analysis is intended to treat sex as an important but secondary variable of interest and to provide exploratory power analysis that can guide future studies explicitly designed to explore sex as a biologic variable (Diester et al., 2019).
Following this within-drug analysis, three additional types of analyses were conducted. First, the maximal effects of each drug or fentanyl/naltrexone mixture at any dose were compared, and these Emax values were considered to be different if 95% confidence limits did not overlap. Second, the Emax of each drug or mixture for locomotor stimulation was transformed to a percentage of the fentanyl-alone Emax (% Fent Max) using the equation (Test Drug Emax – Vehicle Baseline)/(Fentanyl Emax – Vehicle Baseline))*100, where “Emax” was the maximum number of locomotor counts for a test drug or fentanyl at any dose, and “Baseline” was the number of counts after vehicle treatment in that group. Values for % Fent Max of each drug and mixture were then graphed as a function of previously published Emax values of each drug or mixture to stimulate GTPɣS binding in CHO cells expressing cloned MOR (Selley et al., 1998; Thompson et al., 2004; Obeng et al., 2018; Selley et al., 2021). Data for single-molecule ligands and fentanyl/naltrexone mixtures were submitted separately to linear regression analyses for the linear sections of their respective curves to identify the magnitudes of GTPɣS binding (95% CL) associated with 50% Fent Max. Values were considered to be statistically similar if 95% confidence limits overlapped, and we predicted that these values would be similar for both single-molecule MOR ligands and fentanyl/naltrexone mixtures. Lastly, data for the fentanyl/naltrexone mixtures were used to determine an EP50 value, defined as the proportion of fentanyl in the fentanyl/naltrexone mixture that produces an Emax equal to 50% of the fentanyl-alone Emax. As we have described previously, the EP50 value determined from a series of fentanyl/naltrexone mixtures can be used to quantify the efficacy requirement for a given endpoint of MOR agonist-induced effects, such that higher EP50 values indicate higher efficacy requirements. To calculate the EP50 value, the Emax of each mixture was again expressed as % Fent Max and graphed as a function of the fentanyl proportion for each mixture. These fentanyl proportion-Emax data were submitted to nonlinear regression to determine the EP50 (95% CL). This EP50 value for locomotor activity in mice determined in the present study was then compared with previously determined EP50 values for fentanyl/naltrexone mixtures to produce a range of other effects in previously published studies (Cornelissen et al., 2018; Schwienteck et al., 2019; Selley et al., 2021). EP50 values across endpoints were considered to be different if 95% confidence limits did not overlap.
Materials
(±)Methadone HCl, fentanyl HCl, morphine sulfate, hydrocodone bitartrate, buprenorphine HCl, nalbuphine HCl, and naltrexone HCl were all provided by the National Institute on Drug Abuse Drug Supply Program. 17-Cyclopropyl-methyl-3,14β- dihydroxy-4,5α-epoxy-6α-[(3′-isoquinolyl)acetamido]-morphinan (NAQ) was synthesized by Dr. Yan Zhang (Virginia Commonwealth University). In addition to these single-molecule test drugs, five fentanyl/naltrexone (FENT/NTX) mixtures were tested with fentanyl-to-naltrexone proportions of 100:1, 56:1, 32:1. 10:1, and 3.2:1. All compounds were administered subcutaneously (SC) per body weight in volumes of 0.1–0.9 ml and dissolved in sterile saline, except for NAQ, which was dissolved in 10% DMSO and 90% water.
Results
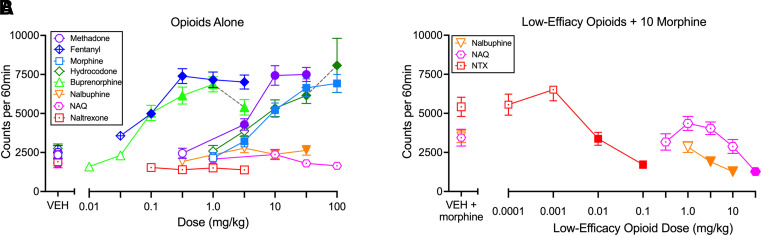
Figure 2 shows pooled data from both sexes for locomotor effects of all single-molecule opioids. One-way ANOVA results and Emax values for each drug are shown in Table 1, and Fig. 3 shows the time course of effects produced by selected doses of each drug over the 60-minute session. Methadone, fentanyl, morphine, hydrocodone, buprenorphine, and nalbuphine produced dose-dependent and significant locomotor stimulation, whereas NAQ and naltrexone did not. Each drug was tested up to an effect plateau at which increasing doses failed to produce further significant increases in locomotion. Note that, for hydrocodone, a higher dose of 320 mg/kg was tested in a subset of four male and female mice, and all died in ≤30 minutes. No dose of any other drug produced lethality in any other mice. Emax values for methadone, fentanyl, morphine, hydrocodone, and buprenorphine were similar to each other and higher than for nalbuphine. Nalbuphine, NAQ, and naltrexone all produced a dose-dependent blockade of morphine-induced locomotor stimulation.
Fig. 2.
Locomotor activating effects of opioids with differing MOR efficacy. (A) Effects of opioids administered alone. (B) Effects of nalbuphine, NAQ and naltrexone administered as a pretreatment to 10 mg/kg morphine. Abscissae: Dose in mg/kg. Ordinates: Locomotor counts per 60 minutes. In general, all points show mean±S.E.M. for N = 12 mice, and filled symbols indicate a significant difference compared with vehicle within each drug as determined by repeated-measures one-way ANOVA followed by the Holm-Sidak post hoc test, P < 0.05. There were two exceptions. The dashed line to 3.2 mg/kg of buprenorphine indicates low sample size (N = 6) and a different cohort of mice for this dose, and the filled point indicates different from vehicle by unpaired t test. The dashed line at 100 mg/kg hydrocodone indicates low sample size (N = 6) but in the same cohort of mice for this dose, and the filled symbol indicates different from vehicle by paired t test. A different group of four male and female mice tested with a higher hydrocodone dose (320 mg/kg) all died, so further studies at this dose were not conducted, and these data are not included in the graph. Statistical results for Panel A are shown in Table 1. For Panel B, one-way ANOVA results were as follows. Nalbuphine: F(1.64, 18.02) = 14.42; P = 0.0003; NAQ: F(4.15, 45.68) = 8.67; P < 0.0001; naltrexone: F(2.59, 28.45) = 27.35; P < 0.0001.
TABLE 1.
One-way ANOVA results and Emax values for data shown in Fig. 2A.
Hydrocodone and buprenorphine data in this table do not include high doses due to different N.
| Opioid | One-Way ANOVA | Emax (95% Confidence Interval) |
|---|---|---|
| Methadone | F(2.89, 31.87) = 38.81; P < 0.0001 | 7500 (6505, 8495) |
| Fentanyl | F(2.97, 32.63) = 26.94; P < 0.0001 | 7393 (6346, 8440) |
| Morphine | F(2.76, 30.40) = 31.75; P < 0.0001 | 6925 (5662, 8188) |
| Hydrocodone | F(2.08, 22.86) = 13.81; P = 0.0001 | 6153 (5025, 7280) |
| Buprenorphine | F(2.58, 28.34) = 39.39; P < 0.0001 | 6867 (5802, 7933) |
| Nalbuphine | F(3.45, 37.90) = 3.30; P = 0.0254 | 2639 (1954, 3324) |
| NAQ | F(2.52, 27.67) = 2.06; P = 0.1376 | — |
| Naltrexone | F(2.41, 26.46) = 1.69; P = 0.2001 | — |
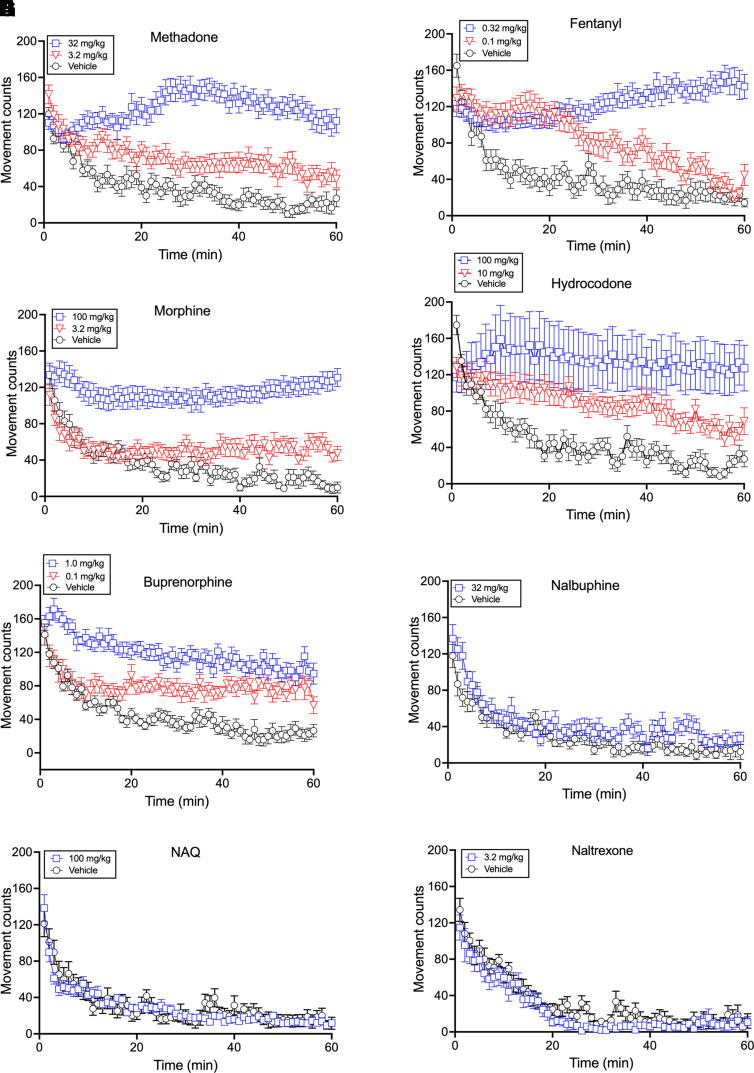
Fig. 3.
Time course of locomotor activating effects produced by opioids with differing MOR efficacy. Each panel shows time course data over a 60-minute session for a different drug. For most drugs, data are shown for vehicle, the lowest dose to significantly increase locomotion, and the Emax dose producing the highest level of locomotor activation. Nalbuphine, NAQ, and naltrexone show only data for vehicle and the highest dose tested. Abscissae: Time in min for the 60-minute session. Ordinates: movement counts over the 60-minute session. Each point shows mean±S.E.M. from 12 mice except the high dose for hydrocodone, which shows N = 6.
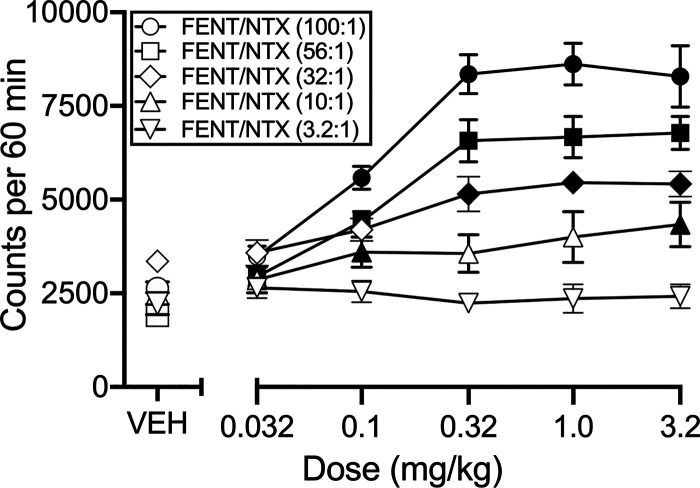
Figure 4 shows pooled data from both sexes for locomotor effects of the fentanyl/naltrexone mixtures. One-way ANOVA results and Emax values for each mixture are shown in Table 2. The 100:1, 56:1, 32:1, and 10:1 fentanyl/naltrexone mixtures produced dose-dependent and significant increases in locomotor activity, whereas the 3.2:1 mixture did not. The mixture with the highest fentanyl proportion (100:1) produced the highest Emax value, which was not significantly different from the Emax for fentanyl alone (see Table 1), and mixtures with progressively lower fentanyl proportions produced progressively lower Emax values.
Fig. 4.
Locomotor activating effects of fentanyl/naltrexone mixtures. Abscissa: Dose fentanyl in mg/kg. The naltrexone dose was proportional to the fentanyl dose as indicated by fixed fentanyl/naltrexone (FENT/NTX) proportions for each mixture. Ordinate: Locomotor counts per 60 minutes. All points show mean±S.E.M. for N = 12 mice, and filled symbols indicate a significant difference compared with vehicle within each mixture as determined by repeated-measures one-way ANOVA followed by the Holm-Sidak post hoc test, P < 0.05. Statistical results are shown in Table 2.
TABLE 2.
One-way ANOVA results and Emax values for fentanyl/naltrexone mixtures in Fig. 4.
| Fentanyl/ Naltrexone Mixture | One-Way ANOVA | Emax (95% Confidence Interval) |
|---|---|---|
| 100:1 | F(2.40, 26.37) = 40.84; P < 0.0001 | 8615 (7386, 9843) |
| 56:1 | F(2.16, 23.79) = 36.28; P < 0.0001 | 6777 (5805, 7748) |
| 32:1 | F(3.27, 35.94) = 14.06; P < 0.0001 | 5458 (4854, 6061) |
| 10:1 | F(2.68, 29.43) = 6.60; P = 0.0021 | 4338 (3033, 5643) |
| 3.2:1 | F(3.82, 41.99) = 0.76; P = 0.5506 | — |
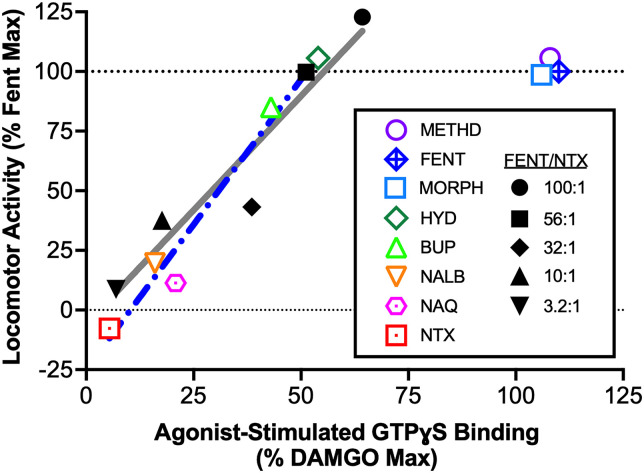
Results in Figs. 2–4 indicate that, within boundaries described below, increasing MOR efficacy is associated with increasing locomotor activation in mice. The efficacy requirements for locomotor activation were quantified in two ways. First, Fig. 5 shows the relationship between (a) the Emax value of each single-molecule opioid and fentanyl/naltrexone mixture in the present study of locomotor activation and (b) the Emax value in prior studies of ligand-stimulated GTPɣS binding in CHO cells expressing cloned MOR. Drugs or mixtures with in vitro Emax values from 0% to approximately 50% of the DAMGO Emax produced graded increases in locomotor activity; however, further increases in the in vitro Emax values (with morphine, fentanyl, and methadone) did not produce further increases in locomotor activity. The mean (95% CL) magnitude of ligand-stimulated GTPɣS binding associated with a locomotor Emax equal to 50% of the fentanyl-alone Emax was similar for both single-molecule opioids [30.8 (25.1-37.2)] and fentanyl/naltrexone mixtures [29.2 (10.2-41.9)]. The mean (95% CL) slopes of the regressions were also similar [2.43 (1.66-3.21) for single-molecule opioids; 1.91 (0.81-3.01) for fentanyl/naltrexone mixtures].
Fig. 5.
Relationship between MOR agonist and fentanyl/naltrexone mixture effects on in vitro activation of GTPɣS binding and in vivo locomotor stimulation. Abscissa: Emax for each drug or mixture to stimulate GTPɣS binding in CHO cells expressing cloned MOR from previously published studies (see text for citations). Data are expressed as a percentage of the maximum effect of the high-efficacy MOR agonist DAMGO, which was included as a standard in each study. Ordinate: Emax for each drug or mixture to stimulate locomotor activity in the present study. Data are expressed as a percentage of the maximum effect produced by fentanyl. The blue dotted line shows linear regression for single-molecule opioids on the linear portion of the curve (morphine, fentanyl, and methadone excluded). The gray solid line shows linear regression for the fentanyl/naltrexone mixtures. Abbreviations: BUP, buprenorphine; HYD, hydrocodone; METHD, methadone; MORPH, morphine; .
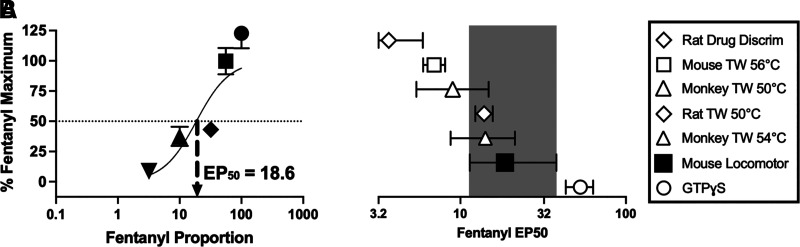
Second, Fig. 6 shows determination of the locomotor EP50 value, with EP50 value defined as the fentanyl proportion of the fentanyl/naltrexone mixture sufficient to produce an Emax equal to 50% of the fentanyl-alone Emax. Insofar as the EP50 value serves as a metric of the efficacy requirement for a given MOR-mediated effect, these results indicate that the efficacy requirement determined in the present study for locomotor activation in mice [EP50 (95% CL) = 18.6 (11.4-38.0)] is higher than in assays of opioid discrimination in rats or thermal antinociception in mice, similar to thermal antinociception in rats and rhesus monkeys, and lower than for stimulation of GTPɣS binding in CHO cells expressing cloned MOR.
Fig. 6.
EP50 values as a metric of efficacy requirement for different effects produced by fentanyl/naltrexone mixtures. (A) Abscissa: Fentanyl proportion in different fentanyl/naltrexone mixtures. Ordinate: Maximum locomotor activating effects of each mixture expressed as a percentage of the fentanyl-alone maximum. Each point shows mean±S.E.M. for 12 mice, and nonlinear regression was used to calculate the EP50, which is defined as the fentanyl proportion in a fentanyl/naltrexone mixture that would produce a maximum effect equal to 50% of the fentanyl-alone maximum effect. (B) EP50 value (95% CL) for locomotor activation in the present study relative to EP50 values for fentanyl/naltrexone mixtures determined in previous studies using various behavioral endpoints in mice, rats, and rhesus monkeys or in the in vitro assay of ligand-stimulated GTPɣS binding in CHO cells expressing cloned MOR. Assays with EP50 values to the left of the shaded box have lower efficacy requirements than locomotor activation, whereas points to the right of the shaded box have higher efficacy requirements than locomotor activation. Abbreviations: Drug Discrim, drug discrimination; TW, warm-water tail-withdrawal with water temperature specified in °C.
Although the present study was not intended to rigorously evaluate sex differences in opioid effects, it did include both male and female subjects and did permit two-way dose x sex ANOVAs and subsequent post hoc power analysis for preliminary evaluation of sex as determinant of opioid effects. Results of these analyses are shown in Supplemental Table 1 (main effects of dose), Table 2 (main effects of sex) and Table 3 (dose x sex interaction), which show two-way ANOVA results, Cohen’s effect size, current power, and projected sample size to achieve power ≥0.8 for all treatments. These analyses confirmed a main effect of dose for most single-molecule opioids and fentanyl/naltrexone mixtures, but not for NAQ, naltrexone, or the 3.2:1 fentanyl/naltrexone mixture. Main effects of sex or dose x sex interactions were rare, and in general, post-hoc power analysis indicated that power and associated sample sizes were too low to detect sex differences. Nonetheless, there were main effects of sex for the lowest two fentanyl/naltrexone mixtures (10:1 and 3.2:1) as shown in Supplemental Fig. 1, with males showing higher locomotion regardless of dose, including after vehicle treatment. There was also a significant dose × sex interaction for both the 32:1 fentanyl/naltrexone mixture and for hydrocodone, but for both treatments, post-hoc analysis did not identify a significant effect of sex at any dose of the mixture as shown in Supplemental Fig. 2. Thus, even these significant sex effects provided weak evidence for a role of sex as a determinant of opioid-induced hyperactivity.
Discussion
This study evaluated locomotor activation produced in mice by a panel of single-molecule opioids and fentanyl/naltrexone mixtures. There were three main findings. First, these results provide evidence for efficacy-dependent MOR agonist effects on maximal locomotor activation in mice. This finding suggests that in vivo assessment of mouse locomotor activity can serve as an efficient tool for in vivo stratification of the MOR efficacies of opioid ligands. Second, the apparent efficacy requirement for locomotor activation was relatively high in comparison with previously determined efficacy requirements for other in vivo opioid effects in mice, such as antinociception. To the degree that locomotor activation in mice is an undesirable sign of opioid-induced motor disruption, these findings suggest the potential for low-efficacy MOR ligands to produce effects of potential therapeutic benefit (e.g., thermal antinociception) with minimal motor disruption. Lastly, the present results provided weak evidence for sex differences in opioid-induced locomotor stimulation, but when differences were observed, locomotor activity was higher in males. These results could provide a foundation for future efforts to explore sex differences in opioid-induced locomotor activation.
Efficacy Dependence of MOR Agonist-Induced Hyperactivity in Mice
MOR agonists produce locomotor activation in several strains of mice, including ICR mice (Rethy et al., 1971; Brase et al., 1977; Bailey et al., 2010; Szumlinski et al., 2020). This hyperactivity is expressed as continuous, unidirectional, and thigmotactic rotation around the perimeter of available space with reduced vertical activity (i.e., rearing, climbing) (Marcais-Collado et al., 1983; Michael-Titus et al., 1989; Mickley et al., 1989), and it can be viewed as a sign of adverse MOR-agonist-induced motor disruption relative to other effects, such as antinociception, associated with therapeutic benefit.
The present study expands on these previous findings in its explicit examination of MOR efficacy as a determinant of MOR agonist-induced hyperactivity. Most single-molecule opioids and fentanyl/naltrexone mixtures produced dose-dependent increases in locomotion, and peak levels of activity across different drugs and mixtures were associated with peak levels of MOR-coupled G-protein signaling as measured by in vitro assays of ligand-stimulated GTPɣS binding in CHO cells expressing cloned MORs. This relationship was well described by a linear function up to a point, suggesting that MOR agonist-induced locomotor activation is mediated by MOR-coupled G-protein signaling; however, drugs or mixtures that exceeded an in vitro Emax value of ∼50% of the reference agonist DAMGO all produced similar Emax values for locomotor activation. These findings suggest that biologic or procedural constraints impose a ceiling on maximal locomotor activation by high-efficacy MOR agonists. Conversely, no significant locomotor activation was produced by the low-efficacy MOR agonist NAQ or by the low-proportion 3.2:1 fentanyl/naltrexone mixture, both of which produce low but detectable levels of ligand-stimulated GTPɣS binding (Yuan et al., 2013; Selley et al., 2021). Taken together, these results show that MOR agonist-induced hyperactivity in mice is efficacy dependent, with graded Emax values within a range of low- to intermediate-efficacy agonists and a plateau of peak hyperactivity for high-efficacy agonists. Additionally, these results indicate that in vivo hyperactivity had a slightly higher efficacy threshold to detect agonist activity, a substantially lower ceiling, and a lower overall efficacy requirement than in vitro stimulation of GTPɣS binding for detection of MOR agonist effects.
This study examined efficacy dependence of MOR agonist-induced hyperactivity in mice using both single-molecule opioids and fentanyl/naltrexone mixtures. Results with the single-molecule opioids were suggestive of efficacy dependence, but the low-efficacy agonists nalbuphine and NAQ in this series have relatively low MOR selectivity (Pick et al., 1992; Yuan et al., 2011), and nalbuphine in particular produces agonist effects mediated by kappa opioid receptors (KOR) in mice (Patrick et al., 1999; Narver, 2015). Because KOR agonists decrease locomotor activity in mice (Gwynn and Domino, 1984; Kuzmin et al., 2000), it is possible that low locomotor activity with these drugs in general and nalbuphine in particular resulted from low selectivity for MOR versus KOR rather than from low MOR efficacy. However, fentanyl/naltrexone mixtures with low fentanyl proportions also produced low peak levels of hyperactivity. With the mixtures, all agonist effects are produced by the highly MOR-selective opioid fentanyl, and net efficacy is controlled by the inclusion of naltrexone to block MORs and limit the maximal number of receptors that can be occupied by fentanyl. Moreover, linear regression indicated that the magnitude of GTPɣS binding associated with an intermediate level of hyperactivity (50% of the Emax for fentanyl alone) was the same for single-molecule opioids and fentanyl/naltrexone mixtures. This suggests that low MOR efficacy is sufficient to explain the low levels of hyperactivity produced by nalbuphine and NAQ in this study, although any additional KOR agonist effects may also have contributed. Overall, the inclusion of data with fentanyl/naltrexone mixtures strengthens the conclusion of efficacy dependence for MOR agonist-induced hyperactivity in mice.
Efficacy Requirements for MOR Agonist-Induced Hyperactivity in Mice Relative to Other in Vivo Effects
The determination of dose-effect curves and Emax values for a range of fixed-proportion fentanyl/naltrexone mixtures provides a strategy to quantify efficacy requirements across opioid endpoints as the EP50 value, or the “effective proportion” of fentanyl in a fentanyl/naltrexone mixture required to produce an Emax equal to 50% of the fentanyl-alone Emax (Cornelissen et al., 2018; Schwienteck et al., 2019; Selley et al., 2021). For example, evidence cited above indicates that MOR agonist-induced hyperactivity in mice has a lower efficacy requirement than ligand-stimulated GTPɣS binding in MOR CHO cells, and this conclusion is further supported and quantified by reference to the EP50 values, with the EP50 (95%CL) being significantly lower for hyperactivity in mice than for GTPɣS binding in MOR CHO cells.
Two other general conclusions are suggested by a comparison of the present results with our previously published results (Cornelissen et al., 2018; Schwienteck et al., 2019; Selley et al., 2021). First, the EP50 for hyperactivity in mice was high relative to other in vivo behavioral endpoints in mice, rats, and monkeys, and in particular, was significantly higher than the EP50 from an assay of thermal nociception in mice. Insofar as opioid antinociception is related to a therapeutic opioid effect (analgesia) whereas hyperactivity is related to an adverse effect (motor disruption), these results provide evidence for the potential of low-efficacy MOR agonists to produce analgesic effects without producing at least some degree of motor impairment. It should be noted that the EP50 for hyperactivity in mice was not significantly lower than that for thermal antinociception in rats or monkeys, suggesting that the window of opportunity here is narrow; nonetheless, these findings agree with other evidence to suggest that low-efficacy opioids can produce thermal antinociception without hyperactivity in mice (Varshneya et al., 2019; 2021). Second, the EP50 for hyperactivity in mice was also significantly higher than for a fentanyl discrimination assay in rats. Drug discrimination procedures model drug-induced subjective effects that may contribute to abuse potential, and as such, this finding suggests that abuse-related of MOR agonist effects have very low efficacy requirements. The fentanyl/naltrexone-mixture approach has not yet been applied to other endpoints of abuse-related opioid effects; however, evidence using other approaches to assess efficacy requirements (e.g., comparing low- and high-efficacy agonists or evaluating abuse-related effects after MOR downregulation with irreversible antagonists or genetic receptor knockdown) has also suggested that abuse-related MOR effects have relatively low efficacy requirements (Zernig et al., 1997; Sora et al., 2001; Negus and Moerke, 2019). Thus, although both hyperactivity in mice and rewarding/reinforcing effects of opioids in multiple species all appear to be mediated at least in part by mesolimbic dopamine signaling as a common neural substrate, it appears that lower MOR efficacy is required for behavioral reward/reinforcement processes than for unconditioned hyperactivity. One implication of these findings is that low-efficacy MOR agonists may produce little or no evidence of hyperactivity in mice but nonetheless produce rewarding/reinforcing effects sufficient to underlie abuse potential.
Sex Differences in MOR Agonist-Induced Hyperactivity
This study focused on MOR efficacy as the principal independent variable and was neither intended nor powered to detect sex differences in opioid effects; however, the study did include both male and female mice and permitted preliminary assessment of sex as a determinant of opioid effects (Diester et al., 2019). In general, evidence for sex differences was weak and did not vary systematically as a function of MOR efficacy. Studies with most drugs and mixtures found only small effect sizes for the main effect of sex or the sex × dose interaction, and post hoc power analysis indicated that most group sizes were underpowered to detect significance of any sex differences that might actually exist. In the cases where the main effect of sex or sex × dose interaction was significant, locomotion tended to be higher in males, but this could not be attributed to higher sensitivity to opioid-induced hyperactivity because the sex × dose interaction either was not significant or was not followed by a significant post hoc effect of sex at any dose. Previous studies have also found little evidence for sex differences in opioid-induced hyperactivity in mice. Main effects of sex have been observed suggestive of different baseline levels of activity, but the sex showing higher activity has varied (Kavaliers and Innes, 1987; Collins et al., 2016; Szumlinski et al., 2020). We could find only one study to show a significant sex × dose interaction with a significant post hoc sex difference, with male deer mice showing higher activity than females during the light phase after treatment with 1 mg/kg of morphine (Kavaliers and Innes, 1987). The power analysis of the present results could provide an empirical foundation for future studies explicitly designed to investigate sex as a determinant of opioid-induced hyperactivity in mice.
Abbreviations
- Emax
maximum effect
- EP50
agonist proportion in an agonist/antagonist mixture that produces 50% agonist alone
- FENT/NTX
fentanyl/naltrexone
- MOR
mu opioid receptor
- NAQ
17-Cyclopropyl-methyl-3,14β- dihydroxy-4,5α-epoxy-6α- [(3′-isoquinolyl)acetamido]-morphinan
Authorship Contributions
Participated in research design: Santos, Banks, Negus.
Conducted experiments: Santos.
Performed data analysis: Santos, Banks, Negus.
Wrote or contributed to the writing of the manuscript: Santos, Banks, Negus.
Footnotes
This work was supported by the National Institutes of Health [Grant P30-DA033934, National Institute on Drug Abuse, PI: Dewey WL], [Grant R25-GM090084, National Institute of General Medical Sciences, PI: Akbarali HI], and [Grant T32-DA007027, National Institute on Drug Abuse, PI: Dewey WL].
No author has an actual or perceived conflict of interest with the contents of this article.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Bailey A, Metaxas A, Al-Hasani R, Keyworth HL, Forster DM, Kitchen I (2010) Mouse strain differences in locomotor, sensitisation and rewarding effect of heroin; association with alterations in MOP-r activation and dopamine transporter binding. Eur J Neurosci 31:742–753 10.1111/j.1460-9568.2010.07104.x. [DOI] [PubMed] [Google Scholar]
- Botz-Zapp CA, Foster SL, Pulley DM, Hempel B, Bi GH, Xi ZX, Newman AH, Weinshenker D, Manvich DF (2021) Effects of the selective dopamine D3 receptor antagonist PG01037 on morphine-induced hyperactivity and antinociception in mice. Behav Brain Res 415:113506 10.1101/2020.04.07.029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brase DA, Loh HH, Way EL (1977) Comparison of the effects of morphine on locomotor activity, analgesia and primary and protracted physical dependence in six mouse strains. J Pharmacol Exp Ther 201:368–374. [PubMed] [Google Scholar]
- Castellano C, Puglisi-Allegra S (1982) Effects of naloxone and naltrexone on locomotor activity in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav 16:561–563 10.1016/0091-3057(82)90415-4. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, DiBerto JF, Faouzi A, Bernhard SM, Gutridge AM, Ramsey S, Zhou Y, Provasi D, Nuthikattu N, Jilakara R, et al. (2021) A Novel Mitragynine Analog with Low-Efficacy Mu Opioid Receptor Agonism Displays Antinociception with Attenuated Adverse Effects. J Med Chem 64:13873–13892 10.1021/acs.jmedchem.1c01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Kieffer BL, Shippenberg TS (2003) Basal and morphine-evoked dopaminergic neurotransmission in the nucleus accumbens of MOR- and DOR-knockout mice. Eur J Neurosci 18:1915–1922 10.1046/j.1460-9568.2003.02912.x. [DOI] [PubMed] [Google Scholar]
- Collins D, Reed B, Zhang Y, Kreek MJ (2016) Sex differences in responsiveness to the prescription opioid oxycodone in mice. Pharmacol Biochem Behav 148:99–105 10.1016/j.pbb.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Cornelissen JC, Obeng S, Rice KC, Zhang Y, Negus SS, Banks ML (2018) Application of Receptor Theory to the Design and Use of Fixed-Proportion Mu-Opioid Agonist and Antagonist Mixtures in Rhesus Monkeys. J Pharmacol Exp Ther 365:37–47 10.1124/jpet.117.246439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR (1977) Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 60:537–545 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diester CM, Banks ML, Neigh GN, Negus SS (2019) Experimental design and analysis for consideration of sex as a biological variable. Neuropsychopharmacology 44:2159–2162 10.1038/s41386-019-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Frischknecht HR, Siegfried B, Riggio G, Waser PG (1983) Inhibition of morphine-induced analgesia and locomotor activity in strains of mice: a comparison of long-acting opiate antagonists. Pharmacol Biochem Behav 19:939–944 10.1016/0091-3057(83)90395-7. [DOI] [PubMed] [Google Scholar]
- Funada M, Suzuki T, Narita M, Misawa M, Nagase H (1993) Modification of morphine-induced locomotor activity by pertussis toxin: biochemical and behavioral studies in mice. Brain Res 619:163–172 10.1016/0006-8993(93)91608-u. [DOI] [PubMed] [Google Scholar]
- Gwynn J, Domino F (1984) Genotype-Dependent Behavioral Sensitivity to Mu vs. Kappa Opiate Agonists. I. Acute and Chronic Effects on Mouse Locomotor Activity 231:6. [PubMed] [Google Scholar]
- Jacob JC, Poklis JL, Akbarali HI, Henderson G, Dewey WL (2017) Ethanol Reversal of Tolerance to the Antinociceptive Effects of Oxycodone and Hydrocodone. J Pharmacol Exp Ther 362:45–52 10.1124/jpet.117.241083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Innes DGL (1987) Sex and day-night differences in opiate-induced responses of insular wild deer mice, Peromyscus maniculatus triangularis. Pharmacol Biochem Behav 27:477–482 10.1016/0091-3057(87)90351-0. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Gren SOO, (2000) Dose- and time-dependent bimodal effects of - opioid agonists on locomotor activity in mice. J Pharmacol Exp Ther 295:1031–1042. [PubMed] [Google Scholar]
- Loggi G, Laviola G, Alleva E, Chiarotti F (1991) Morphine effects on mouse locomotor/exploratory activity: test dependency, test reliability, uni- and multi-variate analyses. Pharmacol Biochem Behav 38:817–822 10.1016/0091-3057(91)90248-Z. [DOI] [PubMed] [Google Scholar]
- Marcais-Collado H, Chaillet P, Costentin J (1983) Inhibition of the spontaneous climbing behavior elicited in mice by opiates. J Pharmacol Exp Ther 227:466–471. [PubMed] [Google Scholar]
- Michael-Titus A, Dourmap N, Costentin J (1989) MU and delta opioid receptors control differently the horizontal and vertical components of locomotor activity in mice. Neuropeptides 13:235–242 10.1016/0143-4179(89)90076-0. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Mulvihill MA, Postler MA (1990) Brain mu and delta opioid receptors mediate different locomotor hyperactivity responses of the C57BL/6J mouse. Psychopharmacology (Berl) 101:332–337. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Zemp JW (1976) Effects of methadone on activity and on brain monoamines in two strains of mice. Pharmacol Biochem Behav 5:367–370. [DOI] [PubMed] [Google Scholar]
- Narita M, Takahashi Y, Takamori K, Funada M, Suzuki T, Misawa M, Nagase H (1993) Effects of κ-agonist on the antinociception and locomotor enhancing action induced by morphine in mice. Jpn J Pharmacol 62:15–24 10.1254/jjp.62.15. [DOI] [PubMed] [Google Scholar]
- Narver HL (2015) Nalbuphine, a non-controlled opioid analgesic, and its potential use in research mice. Lab Anim (NY) 44:106–110 10.1038/laban.701. [DOI] [PubMed] [Google Scholar]
- Negus SS, Moerke MJ (2019) Determinants of opioid abuse potential: Insights using intracranial self-stimulation. Peptides 112:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng S, Yuan Y, Jali A, Selley DE, Zhang Y (2018) In vitro and in vivo functional profile characterization of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(isoquinoline-3-carboxamido)morphinan (NAQ) as a low efficacy mu opioid receptor modulator. Eur J Pharmacol 827:32–40 10.1016/j.ejphar.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn MD, Lowery JJ, Skorput AGJ, Giuvelis D, Bilsky EJ (2010) In vivo characterization of the opioid antagonist nalmefene in mice. Life Sci 86:624–630 10.1016/j.lfs.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CA, Ko MCH, Woods JH (1999) Comparison of Antinociceptive Effects Induced by Kappa Opioid Agonists in Male and Female Mice. Analgesia 4: 397–404 10.3727/107156999819565766. [PMC free article] [PubMed] [Google Scholar]
- Pick CG, Paul D, Pasternak GW (1992) Nalbuphine, a mixed kappa 1 and kappa 3 analgesic in mice. J Pharmacol Exp Ther 262:1044–1050. [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadée W, Bilsky EJ (2005) In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther 313:1150–1162 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- Rethy CR, Smith CB, Villarreal JE (1971) Effects of narcotic analgesics upon the locomotor activity and brain catecholamine content of the mouse. J Pharmacol Exp Ther 176:472–479. [PubMed] [Google Scholar]
- Schwienteck KL, Faunce KE, Rice KC, Obeng S, Zhang Y, Blough BE, Grim TW, Negus SS, Banks ML (2019) Effectiveness comparisons of G-protein biased and unbiased mu opioid receptor ligands in warm water tail-withdrawal and drug discrimination in male and female rats. Neuropharmacology 150:200–209 10.1016/j.neuropharm.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Banks ML, Diester CM, Jali AM, Legakis LP, Santos EJ, Negus SS (2021) Manipulating Pharmacodynamic Efficacy with Agonist + Antagonist Mixtures: In Vitro and In Vivo Studies with Opioids and Cannabinoids. J Pharmacol Exp Ther 376:374–384 10.1124/jpet.120.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Liu Q, Childers SR (1998) Signal transduction correlates of mu opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTP gamma S binding in mMOR-CHO cells and rat thalamus. J Pharmacol Exp Ther 285: 496–505. [PubMed] [Google Scholar]
- Severino AL, Mittal N, Hakimian JK, Velarde N, Minasyan A, Albert R, Torres C, Romaneschi N, Johnston C, Tiwari S, Lee AS, Taylor AM, Gavériaux-Ruff C, Kieffer BL, Evans CJ, Cahill CM, Walwyn WM (2020) μ-Opioid Receptors on Distinct Neuronal Populations Mediate Different Aspects of Opioid Reward-Related Behaviors. eNeuro 7: ENEURO.0146-20.2020 10.1523/ENEURO.0146-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS, Uhl GR (2001) Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology 25:41–54. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Coelho MA, Tran T, Stailey N, Lieberman D, Gabriella I, Swauncy I, Brewin LW, Ferdousian S (2020) Who is HOT and who is LOT? Detailed characterization of prescription opioid-induced changes in behavior between 129P3/J and 129S1/SvlmJ mouse substrains. Genes Brain Behav 19:e12609 10.1111/gbb.12609. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Wojno H, Greiner E, May EL, Rice KC, Selley DE (2004) Activation of G-proteins by morphine and codeine congeners: insights to the relevance of O- and N-demethylated metabolites at μ- and δ-opioid receptors. J Pharmacol Exp Ther 308:547–554 10.1124/jpet.103.058602. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Fulton BS, Caine SB (2014) Acute and chronic effects of the M1/M4-preferring muscarinic agonist xanomeline on cocaine vs. food choice in rats. Psychopharmacology (Berl) 231:469–479 10.1007/s00213-013-3256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Caron MG (2014) The physiological relevance of functional selectivity in dopamine signalling. Int J Obes Suppl 4 (Suppl 1):S5–S8 10.1038/ijosup.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM (2021) Fentanyl-related substances elicit antinociception and hyperlocomotion in mice via opioid receptors. Pharmacol Biochem Behav 208:173242 10.1016/j.pbb.2021.173242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshneya NB, Walentiny DM, Moisa LT, Walker TD, Akinfiresoye LR, Beardsley PM (2019) Opioid-like antinociceptive and locomotor effects of emerging fentanyl-related substances. Neuropharmacology 151:171–179 10.1016/j.neuropharm.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Godfrey M, Li X, Blendy JA (2005) Alterations in morphine-induced reward, locomotor activity, and thermoregulation in CREB-deficient mice. Brain Res 1032:193–199 10.1016/j.brainres.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Yaksh and Wallace (2018) Opioids, analgesia, and pain management, in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13e (Brunton LL, Hilal-Dandan R, Knollmann BC, eds) pp 355–386, McGraw-Hill, New York. [Google Scholar]
- Yuan Y, Elbegdorj O, Beletskaya IO, Selley DE, Zhang Y (2013) Structure activity relationship studies of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(isoquinoline-3′-carboxamido)morphinan (NAQ) analogues as potent opioid receptor ligands: preliminary results on the role of electronic characteristics for affinity and function. Bioorg Med Chem Lett 23:5045–5048 10.1016/j.bmcl.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Li G, He H, Stevens DL, Kozak P, Scoggins KL, Mitra P, Gerk PM, Selley DE, Dewey WL, et al. (2011) Characterization of 6α- and 6β-N-heterocyclic substituted naltrexamine derivatives as novel leads to development of mu opioid receptor selective antagonists. ACS Chem Neurosci 2:346–351 10.1021/cn2000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Lewis JW, Woods JH (1997) Clocinnamox inhibits the intravenous self-administration of opioid agonists in rhesus monkeys: comparison with effects on opioid agonist-mediated antinociception. Psychopharmacology (Berl) 129:233–242 10.1007/s002130050185. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Braithwaite A, Yuan Y, Streicher JM, Bilsky EJ (2014) Behavioral and cellular pharmacology characterization of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(isoquinoline-3′-carboxamido)morphinan (NAQ) as a mu opioid receptor selective ligand. Eur J Pharmacol 736:124–130 10.1016/j.ejphar.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]