Abstract
Canonical nonshivering thermogenesis (NST) in brown and beige fat relies on uncoupling protein 1–mediated heat generation, although alternative mechanisms of NST have been identified, including sarcoplasmic reticulum (SR)–calcium cycling. Intracellular calcium is a crucial cell signaling molecule for which compartmentalization is tightly regulated, and the sarco/endoplasmic reticulum calcium ATPase (SERCA) actively pumps calcium from the cytosol into the SR. In this review, we discuss the capacity of SERCA-mediated calcium cycling as a significant mediator of thermogenesis in both brown and beige adipocytes. Here, we suggest two primary mechanisms of SR calcium–mediated thermogenesis. The first mechanism is through direct uncoupling of the ATPase and calcium pump activity of SERCA, resulting in the energy of ATP catalysis being expended as heat in the absence of calcium transport. Regulins, a class of SR membrane proteins, act to decrease the calcium affinity of SERCA and uncouple the calcium transport function from ATPase activity, but remain largely unexplored in adipose tissue thermogenesis. A second mechanism is through futile cycling of SR calcium, whereby SERCA-mediated SR calcium influx is equally offset by SR calcium efflux, resulting in ATP consumption without a net change in calcium compartmentalization. A fuller understanding of the functional and mechanistic role of calcium cycling as a mediator of adipose tissue thermogenesis and how manipulation of these pathways can be harnessed for therapeutic gain remains unexplored.
SIGNIFICANCE STATEMENT
Enhancing thermogenic metabolism in brown or beige adipose tissue may be of broad therapeutic utility to reduce obesity and metabolic syndrome. Canonical brown adipose tissue–mediated thermogenesis occurs via uncoupling protein 1 (UCP1). However, UCP1-independent pathways of thermogenesis, such as sarcoplasmic (SR) calcium cycling, have also been identified, but the regulatory mechanisms and functional significance of these pathways remain largely unexplored. Thus, this minireview discusses the state of the field regarding calcium cycling as a thermogenic mediator in adipose tissue.
Introduction
Obesity and metabolic syndrome are driven by imbalances in energy consumption, storage, and expenditure, and hallmarked by an increase in white adipose tissue (WAT), which serves as the primary lipid energy store and is relatively mitochondrial poor. White adipocytes are a dynamic cell type that play key metabolic roles in lipolysis, fatty acid synthesis and esterification, and glycogen synthesis in addition to their roles as endocrine cells (Morigny et al., 2021). However, brown adipocytes are mitochondrially dense, highly metabolically active, and the primary drivers of thermogenesis, making them of high interest for therapeutic manipulation to reduce obesity and metabolic syndrome (Townsend and Tseng, 2012). The presence of metabolically active brown adipose tissue (BAT) was conclusively demonstrated in adult humans in 2009 (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009), and has been associated with multiple physiological benefits, including improved glucose tolerance and lipid/cholesterol profiles and decreased risk of type II diabetes and cardiovascular disease (Villarroya et al., 2013; Thoonen et al., 2016; Anthony et al., 2019; Becher et al., 2021). Thus, in addition to the goal of enhancing BAT metabolism to reduce obesity, clinical manipulation of BAT activity and/or induction of the phenotypic transition of WAT to BAT (beiging) may be of broad therapeutic utility.
Maintenance of core body temperature is crucial to maintain homeostatic balance, and therefore redundant mechanisms of heat generation would intuitively be selected for. Brown and beige fat are major organs of interest in thermogenic maintenance; however, the capacity of skeletal muscle-based thermogenesis must also be considered. Skeletal muscle is the largest organ in the body, composing up to 55% body mass, and significantly contributes to heat production and metabolic energy expenditure (Zurlo et al., 1990, 1994; Janssen et al., 2000). In fact, malignant hyperthermia, a life-threatening condition, results from excessive heat production by skeletal muscle through the development of a hypermetabolic state (Rosenberg et al., 2015). The role of skeletal muscle in thermogenic homeostasis becomes more important under conditions that reduce BAT function, such as aging or obesity, leading to an increased reliance on skeletal muscle-based thermogenesis (Bal et al., 2016). Shivering is a well-known mechanism of skeletal muscle thermogenesis in which involuntary contractions of muscle fibers drive heat production. During these muscle contractions, heat is generated through two different mechanisms: myosin-mediated ATP hydrolysis and sarco/endoplasmic reticulum ATPase–driven calcium transport (Block, 1994; Rowland et al., 2015).
Alternative mechanisms of heat generation, collectively referred to as nonshivering thermogenesis (NST), primarily occur in thermogenic adipose depots, though skeletal muscle has also been suggested to mediate NST (Li et al., 2021). Canonical NST in BAT occurs through the uncoupling of the electrochemical proton gradient generated by mitochondrial oxidative phosphorylation, allowing the return of protons back across the gradient without driving ATP synthesis, thereby reducing the efficiency of the reaction and generating heat (Ikeda and Yamada, 2020). Investigation into the molecular underpinnings of NST have focused primarily on the uncoupling of mitochondrial ATP synthesis by uncoupling proteins, of which uncoupling protein 1 (UCP1) is expressed specifically in brown and beige adipocytes and is the most well-studied mediator of thermogenesis (Harper et al., 2008). As expected, UCP1-deficient (UCP1-/-) mice have an impaired ability to maintain core body temperature during acute cold challenge (Enerbäck et al., 1997). However, UCP1−/− mice unexpectedly showed resistance to chronic cold and diet-induced adiposity, which was the first indication of the existence of UCP1-independent thermogenic mechanisms, and follow-up investigations identified multiple alternative thermogenic mechanisms as adaptive cold responses in UCP1−/− mice. Specifically, it was reported that UCP1−/− mice can adapt to tolerate cold through gradual exposure to decreasing temperatures (Hofmann et al., 2001; Golozoubova et al., 2006; Ukropec et al., 2006). Several such mechanisms have since been identified to promote thermogenic energy expenditure in adipocytes through futile, non-ATP–producing substrate cycling independent of UCP1, including fatty acid oxidation (Solinas et al., 2004; Mottillo et al., 2014), creatine phosphorylation (Kazak et al., 2015), and calcium cycling (de Meis et al., 1997; Zhao et al., 1997; de Meis, 2003; Ikeda et al., 2017). Here, we review the functional role of calcium cycling as a mediator of thermogenic metabolism in adipose tissue.
SERCA-Mediated SR Calcium Transport
Calcium localization, transport, and concentration are vital components of cellular signaling pathways in numerous cell types across the body, though the functional role of calcium in each is highly dependent on cell type (Clapham, 2007). Cellular compartmentalization of calcium is tightly regulated, with the primary cellular calcium store located within the sarcoplasmic reticulum (SR) (Bootman and Bultynck, 2020). The ubiquitously expressed sarco/endoplasmic reticulum calcium ATPase (SERCA) functions to maintain resting cytosolic calcium content through the ATP-dependent transport of cytosolic calcium into the SR lumen (Vandecaetsbeek et al., 2011; Primeau et al., 2018). The SERCA pump is powered by ATP hydrolysis to move calcium ions across the concentration gradient of the SR, with two calcium ions being transported with each ATP molecule consumed (Kabashima et al., 2020). The calcium transport mechanism of SERCA is driven by stoichiometric changes occurring during the transition between its calcium-bound and free states, which is directly influenced by the calcium binding affinity of SERCA (Møller et al., 2010). SERCA is encoded by three highly conserved genes—ATP2A1, ATP2A2, and ATP2A3—which results in expression of SERCA1, SERCA2, and SERCA3, respectively (Vandecaetsbeek et al., 2011). From these three genes, multiple tissue-specific splice variants with strict developmental regulation under complex neural control have been identified and outlined in previous reviews (Vangheluwe et al., 2005). While SERCA2b is considered a ubiquitously expressed isoform, SERCA1 expression has also been identified as the primary isoform in rat BAT (de Meis, 2003). Analysis of kinetic properties revealed that calcium affinity and transport efficacy differ between the brown fat and skeletal muscle SERCA1 isoform, with the calcium released in the cytosol of brown adipocytes during adrenergic stimulation directly interacting with mitochondria and stimulating the rate of heat production (de Meis, 2003).
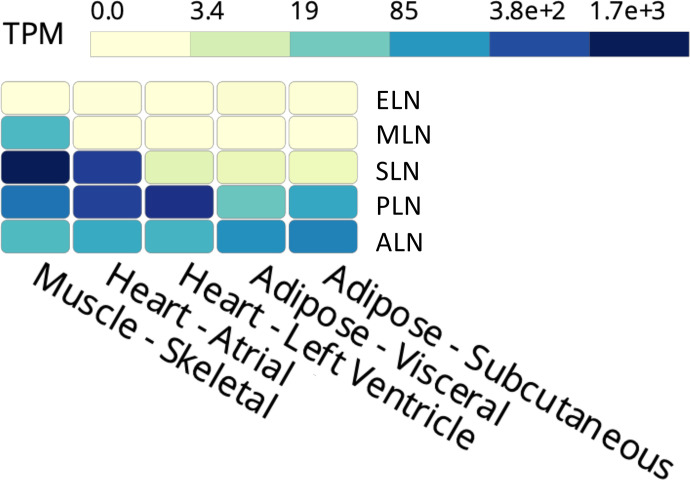
SERCA activity is modulated by a family of small transmembrane proteins collectively known as regulins. Phospholamban (PLN) was the first regulin to be identified. It’s expressed primarily in slow-twitch and cardiac muscle and, along with sarcolipin (SLN; expressed primarily in fast-twitch skeletal muscle), is the most well-studied of the regulins (Davis et al., 1983; Rathod et al., 2021). Both SLN and PLN have been shown to reduce the calcium affinity of SERCA through the distortion of the two calcium transport sites, but SLN has been shown to uncouple calcium transport from ATP hydrolysis through a unique structural mechanism in which the calcium bound in the transport site is not transferred to the SR in every catalytic cycle. This SLN-mediated uncoupling of ATPase activity from calcium transport promotes futile use of the SERCA pump without the transport of calcium and has been shown to promote thermogenesis in skeletal muscle through heat generation via unproductive ATP hydrolysis (Bal et al., 2012; Sahoo et al., 2015; Autry et al., 2016). Therefore, while SLN binding has no effect on the ATP hydrolysis activity of SERCA, it reduces the efficiency of SERCA calcium transport activity and has unsurprisingly been shown to be necessary for NST in skeletal muscle (Bal et al., 2012; Rowland et al., 2015). While SLN and PLN expression are best characterized in skeletal and cardiac muscle, respectively, additional SERCA-mediating regulins have been identified, including myoregulin (MLN), endoregulin (ELN), and another-regulin (ALN) in nonmuscle tissues (Anderson et al., 2016; Bal and Periasamy, 2020; Rathod et al., 2021). Of the regulins, ALN and PLN are the most prominently expressed in WAT (Fig. 1). However, PLN is expressed in BAT as well, and its expression in WAT was found to increase in response to chronic cold stimulation (Ukropec et al., 2006). ALN has been demonstrated to interact with SERCA in the same regulatory location as other regulins, resulting in decreased calcium binding (Anderson et al., 2016; Makarewich, 2020). To add further complexity, the Olson laboratory recently identified dwarf open reading frame as a micropeptide arising from a previously annotated long noncoding RNA that can act as a SERCA regulator by displacing regulin binding (Nelson et al., 2016). Despite the potential of these regulins to enhance thermogenesis by uncoupling SERCA activity, very little is known regarding the functional role of regulins in mediating calcium cycling or thermogenesis within adipose tissue.
Fig. 1.
Expression profile of SERCA regulins in muscle and adipose tissue. Expression of the regulins in skeletal muscle, cardiac muscle (atrium and ventricle), and adipose tissue (visceral and subcutaneous WAT) is represented in transcripts per million (TPM) as obtained and compiled from the Genotype-Tissue Expression Project (BAT expression data were not available at the time these data were compiled). ALN expression is enriched in subcutaneous and visceral adipose tissue depots. PLN is readily expressed in adipose tissue as well, although to a lesser extent than what is observed in cardiac and skeletal muscle, while SLN expression is present but limited.
Calcium in the SR can be released through interactions between SR lumen protein calsequestrin (CASQ) and the SR membrane–associated ryanodine receptor (RyR), as well as additional proteins, junctin and triadin, which compose the RyR complex and mediate interactions between CASQ and RyR (Gyorke et al., 2004; Lanner et al., 2010; Fuller-Jackson and Henry, 2018). Multiple peptide regulators of the RyR complex have also been identified, including FK506-binding proteins and calmodulin, but all of these have yet to be identified as playing a functional role in thermogenesis (Van Petegem, 2012). CASQ acts as a calcium sensor and an important mediator of calcium release by communicating changes in luminal calcium content to the ryanodine receptor complex (Wang and Michalak, 2020). Calcium release from the SR has been shown to increase in amplitude following adrenergic receptor activation in multiple cell types, including brown adipocytes (Marx et al., 2000; Leaver and Pappone, 2002; Curran et al., 2007). This traditionally occurs in muscle cells through β-adrenergic receptor–induced phosphorylation of the SERCA regulins, PLN and SLN, as well as RyR (Bhupathy et al., 2007; Zhou et al., 2009). However, α-adrenergic receptors have also been suggested to be necessary for full potentiation of SR-calcium release in brown adipocytes through phospholipase C–dependent opening of inositol triphosphate (IP3) receptors on the sarcoplasmic reticulum membrane (Zhao et al., 1997; Leaver and Pappone, 2002).
Activation of calcium release receptors, RyR or IP3, on the SR initiates rapid calcium efflux that can result in localized increases in calcium concentration near mitochondria (Clapham, 1995). Calcium is then transported into the mitochondria through voltage-dependent anion-selective channel proteins and mitochondrial calcium uniporters, resulting in the activation of several mitochondrial dehydrogenase enzymes to regulate tricarboxylic acid metabolism (Denton, 2009; Dejos et al., 2020). This calcium-driven stimulation of tricarboxylic acid dehydrogenases enhances mitochondrial ATP production, and the inhibition of mitochondrial calcium influx by mitochondrial calcium uniporters or SR calcium release by IP3 receptors has been demonstrated to disrupt oxidative phosphorylation and decrease cellular ATP levels (Mallilankaraman et al., 2012; Cardenas et al., 2020). Additionally, pharmacological inhibition of mitochondrial calcium transport has been shown to reduce ATP synthase activity (Das and Harris, 1990). Thus, modulation of cytosolic calcium content via SR calcium cycling has an established regulatory role in mitochondrial energy production, but the complex interplay of SR calcium cycling and ATP consumption with mitochondrial ATP synthesis and how these two phenomena may coordinate to mediate thermogenesis have yet to be delineated.
SR Calcium Cycling as a Mediator of Thermogenesis in Brown and Beige Adipose Tissue
The canonical pathway of UCP1-dependent thermogenesis in brown adipocytes can be induced via adrenergic signaling through a cAMP-Protein kinas A (PKA)–mediated activation of hormone sensitive lipase, leading to free fatty acids from intracellular triglyceride stores, which can then directly increase UCP1 activity (Cannon and Nedergaard, 2004). Additionally, adrenergic stimulation’s ability to amplify SR calcium cycling and increase cytosolic calcium concentration in multiple cell types through post-translational control of SERCA, RyR, and other calcium binding proteins has been well-studied (Kranias and Hajjar, 2012; Kho et al., 2012). Unsurprisingly, adrenergic stimulation also mediates increased intracellular calcium in brown adipocytes, from ∼0.05 μM at baseline to up to ∼0.7 μM post-stimulation, via calcium release from the SR (de Meis et al., 2006; Hayato et al., 2011). Calcium cycling was observed in brown adipocytes as early as the 1970s (Al-Shaikhaly et al., 1979), but the involvement of increased cytosolic calcium in thermogenesis wasn’t implicated until much later when it was shown that elevation of cytosolic calcium, originating from an intracellular source, was sufficient to potentiate adrenergic-mediated thermogenesis (Zhao et al., 1997). That same year, it was also demonstrated that heat generation via SERCA uncoupling was a variable biological process that could thus potentially be controlled by cell signaling to increase thermogenesis during NST (de Meis et al., 1997). Subsequent work confirmed the key source of intracellular calcium in BAT to be the SR (Leaver and Pappone, 2002). The expression of SERCA in BAT was demonstrated in 2003 along with direct evidence that uncoupling of SERCA ATPase activity and calcium transport in brown adipocytes can serve as a relevant means of thermogenic metabolism (de Meis, 2003; de Meis et al., 2005). The same group went on to demonstrate that increased cytosolic calcium concentration, as stimulated by adrenergic signaling, could also potentiate mitochondrial thermogenesis in brown adipocytes (de Meis et al., 2006). However, follow-up studies later suggested that the fusion of SR and mitochondrial membranes in brown adipocytes allowed for the transfer of SERCA1 from SR to BAT mitochondria (de Meis et al., 2010), but the role of SERCA1 in BAT mitochondria remains largely unknown, and the story of SR calcium as a thermogenic mediator in BAT has gone cold since.
Interest in calcium cycling as a thermogenic mediator has been renewed following a 2017 report by Ikeda et al. demonstrating SR calcium cycling as a potent mediator of thermogenic metabolism in beige adipocytes (Ikeda et al., 2017). This is the most complete work to date on thermogenic calcium cycling in adipocytes, showing that process to be driven primarily by SERCA2b, which it also demonstrated to be more highly expressed than SERCA1 in beige adipocytes. Furthermore, it showed SERCA2b localization to the SR with increased protein expression in beige, but not brown, adipocytes following chronic cold exposure. Adipocyte-specific deletion of SERCA2b was also used to show that SERCA is necessary for adrenergic-induced beiging in inguinal adipose tissue. Interestingly, this work also suggests the involvement of both β- and α-adrenergic receptors for UCP1-independent oxygen consumption in beige adipocytes, but, in contrast to the previous work, they showed full potentiation of oxygen consumption by β3-adrenergic receptor stimulation alone. To extend the focus of thermogenic SR-calcium cycling beyond SERCA, it was also demonstrated that expression of RyR is sufficient to increase oxygen consumption. More impressively, in vivo treatment with the RyR stabilizer S107 was able to confer thermogenic resistance to cold exposure in UCP1-null mice.
Proposed Heat Generating Mechanisms of SR Calcium Transport in AT
It is important to note that calcium signaling appears to contribute to thermogenesis through mechanisms both involving and independent of UCP1. Calcium has been shown to promote the transcription of UCP1 through activation of cAMP response element-binding protein (CREB) signaling by PKA or calcium/calmodulin-dependent protein kinase II (CAMKII) (Sun et al., 1994; Robidoux et al., 2005), however, studies on UCP1−/− mice provide insight on SR-calcium cycling as an alternative heat-generating mediator in adipose tissue. UCP1−/− mice were shown to have increased mRNA and protein expression of several SR-calcium genes in inguinal WAT, including PLN and SERCA2, yet no significant changes in expression of calcium-handling proteins in skeletal muscle were observed (Ukropec et al., 2006), likely representing a compensatory response of increased beiging within the WAT. Consideration of the existing literature and the established dynamics of SR calcium uptake and release suggest two possible mechanisms for SR calcium cycling–mediated thermogenesis. The first mechanism is through direct uncoupling of SERCA ATPase activity from calcium transport, potentially by regulin binding, resulting in less SR calcium uptake per ATP molecule consumed. The second potential mechanism is a futile substrate cycling by which SERCA-mediated SR calcium influx is offset by RyR/IP3 receptor–mediated calcium efflux, resulting in SERCA-mediated ATP consumption without a net change in SR calcium localization. While SR calcium cycling may regulate mitochondrial ATP synthesis in this way, the relationship between SR calcium cycling, mitochondrial calcium transport, and thermogenesis remains unclear.
The mechanistic understanding of regulin-mediated uncoupling of SERCA has been driven largely by the skeletal muscle and cardiac field. As discussed above, regulin binding of SERCA reduces calcium transport efficiency through the distortion of SERCA calcium- binding sites, subsequently decreasing SERCA’s affinity for calcium. Thus, not every available calcium binding site is occupied in each catalytic cycle, yet the ATP hydrolysis activity of SERCA is maintained. This uncoupling results in inefficient SERCA-mediated calcium transport per ATP molecule hydrolyzed, yielding greater ATP usage to transport the same amount of calcium into the SR. This regulin-mediated inhibition of SERCA calcium transport is reversable through their phosphorylation by PKA or CaMKII, both of which are activated by stimulation of β-adrenergic receptors on the cell membrane. However, exploration into the structural and functional properties of newly identified regulins raises the possibility of distinct mechanisms of SERCA modulation by isoform, thus suggesting that each regulin may operate differently to modulate calcium affinity and transport activity of SERCA (Rathod et al., 2021) (Table 1). Although regulin-mediated ATPase activity has been extensively studied in myocytes, to date there has been no investigation into the expression or activity of SERCA regulins in BAT.
Table 1.
Overview of the identified regulins describing their primary tissue expression profile, SERCA target interaction, and biologic properties
| Regulin | Primary Tissue Expression | Primary Target | Properties | References |
|---|---|---|---|---|
| SLN | Fast-twitch skeletal muscle, slow twitch skeletal muscle, atria | SERCA1a | • 31 amino acid peptides • Involved in atrial fibrillation, arrythmias, cardiac remodeling, and muscle NST • Activated by CAMKII and STK16 • Reduces calcium affinity and Vmax of SERCA |
Odermatt et al., 1997; Xie et al., 2012; Bal et al., 2012; Bhupathy et al., 2009; Rathod et al., 2021 |
| PLN | Cardiac Ventricles, atria, slow-twitch skeletal muscle, smooth muscle | SERCA2a | • 52 amino acid peptides • Mutations linked to various cardiomyopathies • Activated by PKA or CAMKII • Reduces calcium affinity of SERCA |
Tada et al.,1975; Davis et al., 1983; Gorski et al., 2015; Verboomen et al., 1992; Rathod et al., 2021; Schmitt et al., 2003; Simmerman et al., 1986 |
| MLN | Skeletal muscle | Unknown | • 46 amino acid peptides • Suggested role in skeletal muscle performance and exercise fatigue • Inhibits SERCA by reducing Vmax without affecting calcium affinity |
Nelson et al., 2016; Anderson et al., 2016; Rathod et al., 2021; Anderson et al., 2015 |
| ELN | Endothelial and epithelial tissue | SERCA3a | • 62 amino acid peptides • Lowers Vmax of SERCA without affecting calcium affinity |
Anderson et al., 2016; Rathod et al., 2021 |
| ALN | Ubiquitous, detected in skeletal and cardiac muscles | SERCA2b | • 66 amino acid peptides • Affects both calcium affinity and Vmax of SERCA |
Anderson et al., 2016; Rathod et al., 2021 |
Brown and Beige Adipocytes Are Distinct Cell Types
When considering the regulation and relative thermogenic contribution of these pathways in beige and brown adipocytes, it is important to keep in mind that these two cell types arise from distinct development lineages and should thus be investigated independently in the context of thermogenic mechanisms (Wu et al., 2012; Long et al., 2014). Beige adipocytes uniquely express thermogenic components only upon chronic stimulation, suggesting that the energy-expending phenotype of beige adipose tissue is reversible and requires sustained adrenergic signaling for the maintenance of thermogenesis (Wu et al., 2012; Ramseyer and Granneman, 2016). Conversely, activation of BAT occurs rapidly in response to cold, exercise, or feeding, and depends on sympathetic activation of adrenergic receptors, which have been reviewed extensively in terms of their role in BAT activation (Sanchez-Delgado et al., 2015; Dewal and Stanford, 2019; Peres Valgas da Silva et al., 2019; Saito et al., 2020). Thus, while the complete functional thermogenic contribution of SR calcium uncoupling in each remains to be worked out, it is possible, if not likely, that the underlying mechanisms are unique within each cell type.
In light of this, our laboratory has recently demonstrated that adipocyte-specific deletion of the RNA-binding protein Human antigen R (HuR) impairs acute adaptive thermogenesis in mice independently of UCP1 expression (Anthony et al., 2020). As an RNA-binding protein, the mechanistic effects of HuR would be expected to be exerted at the level of post-transcriptional gene regulation. RNA sequencing analysis from our knockout mice revealed a BAT-specific decrease in the expression of many genes responsible for SR calcium cycling, including SERCA1a, RyR1, RyR2, and additional RyR complex proteins such as triadin, junctin, and CASQ. Work exploring the mechanistic and functional consequence of HuR-mediated expression of SR calcium cycling genes in BAT is ongoing.
Pharmacological Manipulation of Calcium Cycling
Because of the ubiquitous nature of SR calcium sequestration across cell types and the importance of this regulation to maintenance of physiological homeostasis, several pharmacological manipulations targeting calcium cycling through SERCA or RyR expression or activity have been pursued in various pathological settings (Peterkova et al., 2020; Frank et al., 2002; Haghighi et al., 2014; Andersson and Marks, 2010). These investigations have not yet been broadly extended to adipose tissue. For example, therapeutic targeting of calcium cycling has been extensively studied in cardiac muscle due to the sensitivity of calcium regulation on a beat-to-beat basis in the heart and the demonstrated disruption of calcium homeostasis in cardiovascular disease (Kranias and Hajjar, 2012). Pathological cardiac hypertrophy and fibrosis are marked by an increase in cytosolic calcium and decreased SR calcium load, which are exacerbated in the progression to heart failure. A primary mediator of decreased calcium load in heart failure is decreased expression of SERCA2a, and preliminary gene therapy trials yielded promising results with the reintroduction of SERCA expression in the heart to improve cardiac function (Jessup et al., 2011). In addition to decreased expression and activity of SERCA, SR calcium leak through RyR is suspected to play a key mechanistic role in the pathogenesis of cardiac arrhythmias (Haghighi et al., 2014). Interestingly, SERCA activity is associated with evasion of apoptosis and tumorigenesis in cancer, and thus the inhibition of SERCA is of therapeutic interest in the cancer field (Tadini-Buoninsegni et al., 2018; Peterkova et al., 2020; Pagliaro et al., 2021). Unfortunately, changes in the function or expression of these proteins in adipose tissue in the setting of pathology or changes in metabolic homeostasis, or their therapeutic manipulation, have not been sufficiently studied.
Numerous SERCA inhibitors have been identified, including those with reported specificity for SERCA and other clinically used compounds with potential selective inhibition activity toward SERCA (Michelangeli and East, 2011; Peterkova et al., 2020). Thapsigargin is one of the most abundantly used SERCA inhibitor in research models, though derivations of this drug, such as mipsagargin (G-202), have been used in clinical trials as cancer therapeutics (Mahalingam et al., 2016; Mahalingam et al., 2019).
Like SERCA, numerous pharmacological agents exist that are known to modulate RyR activity, though RyR targeting appears to be less pursued therapeutically compared with its SERCA pump counterparts, most likely due to its involvement in several diseases. In addition to its role in SR calcium leak in heart failure mentioned above, dysfunctional RyRs have been linked to neurodegenerative disorders (Sun and Wei, 2021) and RyR mutations have been linked to multiple myopathies, including cardiac ventricular tachycardia and central core disease (Betzenhauser and Marks, 2010). Stabilizing point mutations in RyR has also been demonstrated to induce malignant hyperthermia through increased SR calcium cycling activity in skeletal muscle, representing a potential limitation for the use of RyR-activating or -stabilizing agents to mediate thermogenesis (Lawal et al., 2020). Dantrolene (Dantrium) is a clinically approved RyR antagonist used to treat malignant hyperthermia that also acts as a potent muscle relaxant through its ability to reduce RyR-dependent calcium release from the SR. Small molecule drugs derived from 1,4-benzothiazepine (termed “Rycals” due to their RyR-calcium release regulation) are a class of compounds that stabilize the inactive state of RyR through maintenance of the calstabin-RyR interaction, presenting a therapeutic tool for several cardiac and skeletal muscle pathologies, including Duchenne muscular dystrophy, catecholaminergic polymorphic ventricular tachycardia, and heart failure (Lehnart et al., 2008; Bellinger et al., 2009; Fauconnier et al., 2010). One such Rycal compound, JTV519, has been shown to enhance RyR1-calstabin1 binding in skeletal muscle and decrease muscle fatigue in mice after myocardial infarction (Wehrens et al., 2005). S107, a more specific RyR stabilizer, has also been shown to enhance RyR1-calstabin binding activity and improve skeletal muscle fatigue in exercised mice (Bellinger et al., 2008). S107 also reduced muscle damage and improved muscle function in a murine model of Duchenne Muscular Dystrophy (Bellinger et al., 2009), and prevented arrhythmias by inhibiting calcium leak in these mice (Fauconnier et al., 2010). S107 was also used in the aforementioned work by Ikeda et al. to show that RyR stabilization is sufficient to increase cold tolerance through thermogenic metabolism via SR calcium cycling (Ikeda et al., 2017).
While potential limitations of targeting increased SR-mediated calcium cycling may exist, due to the ubiquitous and critical nature of calcium transport in physiological homeostasis, pharmacological manipulation of SERCA and RyR expression and activity in adipose tissue to increase thermogenic metabolism through the mechanisms discussed herein deserves further experimentation.
Conclusions and Future Outlook
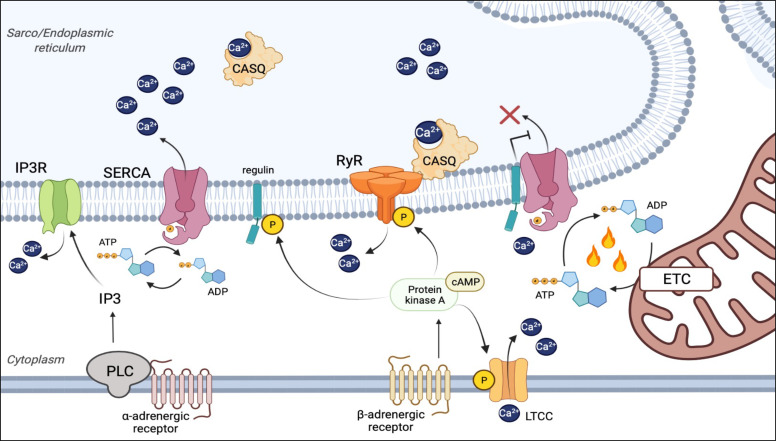
Activation of BAT as a therapeutic strategy for weight loss was suggested as early as the 1930s and has progressed with agents such as glucocorticoids, capsinoids, and β3-adrenergic receptor agonists (Yoneshiro et al., 2013; Rosen and Spiegelman, 2014; Ramage et al., 2016; Cypess et al., 2015). However, targeting UCP1 activation as a therapeutic strategy for weight loss has been unsuccessful in achieving sustained weight reduction without undesired side effects. It is now generally accepted that uncoupling of the calcium transport and ATPase functions of SERCA can be adapted by multiple cell types as a significant means to drive thermogenic energy expenditure. This most likely occurs by directly uncoupling the energy gained via ATP hydrolysis from calcium transport by SERCA, thereby reducing the efficiency of SR calcium uptake and releasing free energy as heat in the process. Another potential heat-generating step is the uncoupling of the SR-calcium gradient via RyR gating in an analogous manner to that which UCP1 uncouples the proton gradient across the inner mitochondrial membrane (Fig. 2). Although, as discussed, RyR may be capable of directly mediating thermogenesis as both SERCA (SR Ca2+ uptake) and RyR (SR Ca2+ release) have been shown to be both necessary and sufficient for SR calcium-mediated thermogenesis.
Fig. 2.
Thermogenesis through uncoupling of SR calcium transport. Under normal physiological conditions of SR calcium cycling, SERCA utilizes the energy of ATP hydrolysis to transport calcium against its concentration gradient into the SR (Left). The uncoupling of calcium transport from ATP hydrolysis occurs through structural changes to the calcium binding domains of SERCA induced by binding of unphosphorylated regulins (PLN, SLN, MLN, ELN, and ALN) (Right), and renders SERCA less efficient at calcium transport without affecting ATP hydrolysis activity. In this regulin- bound state, the ATPase-mediated calcium pump efficiency of SERCA is reduced and the excess free energy of ATP hydrolysis is released as heat. Under both conditions, release of calcium from the SER is primarily mediated by RyRs, as well as IP3 receptors. β-adrenergic activation of these pathways potentiates calcium cycling via phosphorylation of RyRs and regulins, which reduces their interaction with SERCA through a PKA/cAMP-dependent mechanism. α-adrenergic signaling can also induce SER calcium release through a PLC/IP3-dependent activation of IP3 receptors. As the SERCA-mediated calcium pump is inherently inefficient, a second potential thermogenic mechanism is futile SR calcium transport by SERCA that is offset by equal RyR- or IP3-mediated SR calcium efflux.
Many of the basic regulatory mechanisms that govern SR calcium cycling in adipose tissue during homeostasis and pathological conditions remain largely unexplored. The interactome of calcium handling proteins in adipose tissue has yet to be fully identified, including the tantalizing possibility of an adrenergic-sensitive, SERCA-modulating regulin, analogous to SLN, PLN, or ALN, in adipose tissue. Many questions also remain regarding the transcriptional, post-transcriptional, and post-translational regulation of SR calcium-cycling genes in both beige and brown adipocytes. In addition, it remains to be seen to what extent SR calcium cycling may mediate thermogenesis in BAT in vivo or how well conserved these mechanisms are to human adipose tissue. Together, an increased understanding of these regulatory networks and how they’re altered in pathophysiology will be critical to understanding how compartmentalization, transport, and metabolic regulation of calcium contribute to human health and disease. Furthermore, mechanistic delineation of these pathways in adipose tissue will be necessary for potential therapeutic manipulation of calcium cycling in the setting of obesity and metabolic syndrome.
Abbreviations
- ALN
another-regulin
- BAT
brown adipose tissue
- CASQ
calsequestrin
- ELN
endoregulin
- IP3
inositol triphosphate
- MLN
myoregulin
- NST
nonshivering thermogenesis
- PLN
phospholamban
- RyR
ryanodine receptor
- SERCA
sarco/endoplasmic reticulum calcium ATPase
- SLN
sarcolipin
- SR
sarcoplasmic reticulum
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Guarnieri, Benson, Tranter.
Footnotes
This work was supported by the American Heart Association (AHA) [Grant TPA34910086] (to M.T.), the AHA Predoctoral Fellowship [Award 16812] (to A.R.G.), and the National Institutes of Health [Grant T32-HL125204] (to A.R.G. and T.W.B.).
The authors declare that they have no actual or perceived conflicts of interest with the contents of this article.
References
- Al-Shaikhaly MH, Nedergaard J, Cannon B (1979) Sodium-induced calcium release from mitochondria in brown adipose tissue. Proc Natl Acad Sci USA 76:2350–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Makarewich CA, Anderson KM, Shelton JM, Bezprozvannaya S, Bassel-Duby R, Olson EN (2016) Widespread control of calcium signaling by a family of SERCA-inhibiting micropeptides. Sci Signal 9:ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DMAnderson KMChang CLMakarewich CANelson BRMcAnally JRKasaragod PShelton JMLiou JBassel-Duby R, et al. (2015) A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 160:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DC, Marks AR (2010) Fixing ryanodine receptor Ca leak - a novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today Dis Mech 7:e151–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SR, Guarnieri AR, Gozdiff A, Helsley RN, Phillip Owens A, Tranter M (2019) Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin Sci (Lond) 133:2329–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony SR, Guarnieri A, Lanzillotta L, Gozdiff A, Green LC, O’Grady K, Helsley RN, Owens Iii AP, Tranter M (2020) HuR expression in adipose tissue mediates energy expenditure and acute thermogenesis independent of UCP1 expression. Adipocyte 9:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry JM, Thomas DD, Espinoza-Fonseca LM (2016) Sarcolipin Promotes Uncoupling of the SERCA Ca2+ Pump by Inducing a Structural Rearrangement in the Energy-Transduction Domain. Biochemistry 55:6083–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal NC, Maurya SK, Singh S, Wehrens XH, Periasamy M (2016) Increased Reliance on Muscle-based Thermogenesis upon Acute Minimization of Brown Adipose Tissue Function. J Biol Chem 291:17247–17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal NC, Periasamy M (2020) Uncoupling of sarcoendoplasmic reticulum calcium ATPase pump activity by sarcolipin as the basis for muscle non-shivering thermogenesis. Philos Trans R Soc London B Biol Sci 375:20190135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal NCMaurya SKSopariwala DHSahoo SKGupta SCShaikh SAPant MRowland LABombardier EGoonasekera SA, et al. (2012) Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med 18:1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher TPalanisamy SKramer DJEljalby MMarx SJWibmer AGButler SDJiang CSVaughan RSchöder H, et al. (2021) Brown adipose tissue is associated with cardiometabolic health. Nat Med 27:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR (2009) Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger AMReiken SDura MMurphy PWDeng SXLandry DWNieman DLehnart SESamaru MLaCampagne A, et al. (2008) Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA 105:2198–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzenhauser MJ, Marks AR (2010) Ryanodine receptor channelopathies. Pflugers Arch 460:467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhupathy P, Babu GJ, Periasamy M (2007) Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca2+ ATPase. J Mol Cell Cardiol 42:903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhupathy P, Babu GJ, Ito M, Periasamy M (2009) Threonine-5 at the N-terminus can modulate sarcolipin function in cardiac myocytes. J Mol Cell Cardiol 47:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BA (1994) Thermogenesis in muscle. Annu Rev Physiol 56:535–577. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Bultynck G (2020) Fundamentals of Cellular Calcium Signaling: A Primer. Cold Spring Harb Perspect Biol 12:a038802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359. [DOI] [PubMed] [Google Scholar]
- Cardenas CLovy ASilva-Pavez EUrra FMizzoni CAhumada-Castro UBustos GJaňa FCruz PFarias P, et al. (2020) Cancer cells with defective oxidative phosphorylation require endoplasmic reticulum-to-mitochondria Ca2+ transfer for survival. Sci Signal 13:eaay1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE(2007) Calcium signaling. Cell 131:1047–1058. [DOI] [PubMed] [Google Scholar]
- Clapham DE (1995) Calcium signaling. Cell 80:259–268. [DOI] [PubMed] [Google Scholar]
- Curran J, Hinton MJ, Ríos E, Bers DM, Shannon TR (2007) Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res 100:391–398. [DOI] [PubMed] [Google Scholar]
- Cypess AMLehman SWilliams GTal IRodman DGoldfine ABKuo FCPalmer ELTseng YHDoria A, et al. (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AMWeiner LSRoberts-Toler CFranquet Elía EKessler SHKahn PAEnglish JChatman KTrauger SADoria A, et al. (2015) Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AM, Harris DA (1990) Control of mitochondrial ATP synthase in heart cells: inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc Res 24:411–417. [DOI] [PubMed] [Google Scholar]
- Davis BA, Schwartz A, Samaha FJ, Kranias EG (1983) Regulation of cardiac sarcoplasmic reticulum calcium transport by calcium-calmodulin-dependent phosphorylation. J Biol Chem 258:13587–13591. [PubMed] [Google Scholar]
- de Meis L, Bianconi ML, Suzano VA (1997) Control of energy fluxes by the sarcoplasmic reticulum Ca2+-ATPase: ATP hydrolysis, ATP synthesis and heat production. FEBS Lett 406:201–204. [DOI] [PubMed] [Google Scholar]
- de Meis L (2003) Brown adipose tissue Ca2+-ATPase: uncoupled ATP hydrolysis and thermogenic activity. J Biol Chem 278:41856–41861. [DOI] [PubMed] [Google Scholar]
- de Meis L, Arruda AP, Carvalho DP (2005) Role of sarco/endoplasmic reticulum Ca(2+)-ATPase in thermogenesis. Biosci Rep 25:181–190. [DOI] [PubMed] [Google Scholar]
- de Meis L, Arruda AP, da Costa RM, Benchimol M (2006) Identification of a Ca2+-ATPase in brown adipose tissue mitochondria: regulation of thermogenesis by ATP and Ca2+. J Biol Chem 281:16384–16390. [DOI] [PubMed] [Google Scholar]
- de Meis L, Ketzer LA, da Costa RM, de Andrade IR, Benchimol M (2010) Fusion of the endoplasmic reticulum and mitochondrial outer membrane in rats brown adipose tissue: activation of thermogenesis by Ca2+. PLoS One 5:e9439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejos C, Gkika D, Cantelmo AR (2020) The two-way relationship between calcium and metabolism in cancer. Front Cell Dev Biol 8:573747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM (2009) Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787:1309–1316. [DOI] [PubMed] [Google Scholar]
- Dewal RS, Stanford KI (2019) Effects of exercise on brown and beige adipocytes. Biochim Biophys Acta Mol Cell Biol Lipids 1864:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387:90–94. [DOI] [PubMed] [Google Scholar]
- Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, Marks AR, Lacampagne A (2010) Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 107:1559–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Wagenknecht T, McEwen BF, Marko M, Hsieh CE, Mannella CA (2002) Three-dimensional imaging of biological complexity. J Struct Biol 138:85–91. [DOI] [PubMed] [Google Scholar]
- Fuller-Jackson JP, Henry BA (2018) Adipose and skeletal muscle thermogenesis: studies from large animals. J Endocrinol 237:R99–R115. [DOI] [PubMed] [Google Scholar]
- Golozoubova V, Cannon B, Nedergaard J (2006) UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab 291:E350–E357. [DOI] [PubMed] [Google Scholar]
- Gorski PA, Trieber CA, Ashrafi G, Young HS (2015) Regulation of the sarcoplasmic reticulum calcium pump by divergent phospholamban isoforms in zebrafish. J Biol Chem 290:6777–6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramolini AO, Trivieri MG, Oudit GY, Kislinger T, Li W, Patel MM, Emili A, Kranias EG, Backx PH, Maclennan DH (2006) Cardiac-specific overexpression of sarcolipin in phospholamban null mice impairs myocyte function that is restored by phosphorylation. Proc Natl Acad Sci USA 103:2446–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke I, Hester N, Jones LR, Györke S (2004) The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J 86:2121–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi K, Bidwell P, Kranias EG (2014) Phospholamban interactome in cardiac contractility and survival: A new vision of an old friend. J Mol Cell Cardiol 77:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi KKolokathis FPater LLynch RAAsahi MGramolini AOFan GCTsiapras DHahn HSAdamopoulos S, et al. (2003) Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J Clin Invest 111:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper ME, Green K, Brand MD (2008) The efficiency of cellular energy transduction and its implications for obesity. Annu Rev Nutr 28:13–33. [DOI] [PubMed] [Google Scholar]
- Hayato R, Higure Y, Kuba M, Nagai H, Yamashita H, Kuba K (2011) β3-Adrenergic activation of sequential Ca(2+) release from mitochondria and the endoplasmic reticulum and the subsequent Ca(2+) entry in rodent brown adipocytes. Cell Calcium 49:400–414. [DOI] [PubMed] [Google Scholar]
- Hofmann WE, Liu X, Bearden CM, Harper ME, Kozak LP (2001) Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J Biol Chem 276:12460–12465. [DOI] [PubMed] [Google Scholar]
- Ikeda KKang QYoneshiro TCamporez JPMaki HHomma MShinoda KChen YLu XMaretich P, et al. (2017) UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med 23:1454–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Yamada T (2020) UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front Endocrinol (Lausanne) 11:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang ZM, Ross R (2000) Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol 89:81–88. [DOI] [PubMed] [Google Scholar]
- Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ; Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators (2011) Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 124:304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima Y, Ogawa H, Nakajima R, Toyoshima C (2020) What ATP binding does to the Ca2+ pump and how nonproductive phosphoryl transfer is prevented in the absence of Ca2. Proc Natl Acad Sci USA 117:18448–18458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak LChouchani ETJedrychowski MPErickson BKShinoda KCohen PVetrivelan RLu GZLaznik-Bogoslavski DHasenfuss SC, et al. (2015) A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163:643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho C, Lee A, Hajjar RJ (2012) Altered sarcoplasmic reticulum calcium cycling--targets for heart failure therapy. Nat Rev Cardiol 9:717–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranias EG, Hajjar RJ (2012) Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res 110:1646–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Joshi AD, Hamilton SL (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2:a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal TA, Wires ES, Terry NL, Dowling JJ, Todd JJ (2020) Preclinical model systems of ryanodine receptor 1-related myopathies and malignant hyperthermia: a comprehensive scoping review of works published 1990-2019. Orphanet J Rare Dis 15:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver EV, Pappone PA (2002) Beta-adrenergic potentiation of endoplasmic reticulum Ca(2+) release in brown fat cells. Am J Physiol Cell Physiol 282:C1016–C1024. [DOI] [PubMed] [Google Scholar]
- Lehnart SEMongillo MBellinger ALindegger NChen BXHsueh WReiken SWronska ADrew LJWard CW, et al. (2008) Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 118:2230–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang C, Li L, Li L (2021) Skeletal muscle non-shivering thermogenesis as an attractive strategy to combat obesity. Life Sci 269:119024. [DOI] [PubMed] [Google Scholar]
- Long JZSvensson KJTsai LZeng XRoh HCKong XRao RRLou JLokurkar IBaur W, et al. (2014) A smooth muscle-like origin for beige adipocytes. Cell Metab 19:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam DWilding GDenmeade SSarantopoulas JCosgrove DCetnar JAzad NBruce JKurman MAllgood VE, et al. (2016) Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br J Cancer 114:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam D, Peguero J, Cen P, Arora SP, Sarantopoulos J, Rowe J, Allgood V, Tubb B, Campos L (2019) A phase II, multicenter, single-arm study of mipsagargin (G-202) as a second-line therapy following sorafenib for adult patients with progressive advanced hepatocellular carcinoma. Cancers (Basel) 11:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarewich CA (2020) The hidden world of membrane microproteins. Exp Cell Res 388:111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman KCárdenas CDoonan PJChandramoorthy HCIrrinki KMGolenár TCsordás GMadireddi PYang JMüller M, et al. (2012) MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 14:1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101:365–376. [DOI] [PubMed] [Google Scholar]
- Michelangeli F, East JM (2011) A diversity of SERCA Ca2+ pump inhibitors. Biochem Soc Trans 39:789–797. [DOI] [PubMed] [Google Scholar]
- Møller JV, Olesen C, Winther AM, Nissen P (2010) The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q Rev Biophys 43:501–566. [DOI] [PubMed] [Google Scholar]
- Morigny P, Boucher J, Arner P, Langin D (2021) Lipid and glucose metabolism in white adipocytes: pathways, dysfunction and therapeutics. Nat Rev Endocrinol 17:276–295. [DOI] [PubMed] [Google Scholar]
- Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG (2014) Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J Lipid Res 55:2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BRMakarewich CAAnderson DMWinders BRTroupes CDWu FReese ALMcAnally JRChen XKavalali ET, et al. (2016) A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 351:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt ATaschner PEScherer SWBeatty BKhanna VKCornblath DRChaudhry VYee WCSchrank BKarpati G, et al. (1997) Characterization of the gene encoding human sarcolipin (SLN), a proteolipid associated with SERCA1: absence of structural mutations in five patients with Brody disease. Genomics 45:541–553. [DOI] [PubMed] [Google Scholar]
- Pagliaro L, Marchesini M, Roti G (2021) Targeting oncogenic Notch signaling with SERCA inhibitors. J Hematol Oncol 14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres Valgas da Silva C, Hernández-Saavedra D, White JD, Stanford KI (2019) Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology (Basel) 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterková L, Kmoníčková E, Ruml T, Rimpelová S (2020) Sarco/endoplasmic reticulum calcium ATPase inhibitors: Beyond anticancer perspective. J Med Chem 63:1937–1963. [DOI] [PubMed] [Google Scholar]
- Primeau JO, Armanious GP, Fisher ME, Young HS (2018) The sarcoendoplasmic reticulum calcium ATPase. Subcell Biochem 87:229–258. [DOI] [PubMed] [Google Scholar]
- Ramage LE, Akyol M, Fletcher AM, Forsythe J, Nixon M, Carter RN, van Beek EJ, Morton NM, Walker BR, Stimson RH (2016) Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metab 24:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseyer VD, Granneman JG (2016) Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues. Adipocyte 5:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod N, Bak JJ, Primeau JO, Fisher ME, Espinoza-Fonseca LM, Lemieux MJ, Young HS (2021) Nothing Regular about the Regulins: Distinct Functional Properties of SERCA Transmembrane Peptide Regulatory Subunits. Int J Mol Sci 22:8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robidoux J, Cao W, Quan H, Daniel KW, Moukdar F, Bai X, Floering LM, Collins S (2005) Selective activation of mitogen-activated protein (MAP) kinase kinase 3 and p38α MAP kinase is essential for cyclic AMP-dependent UCP1 expression in adipocytes. Mol Cell Biol 25:5466–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM (2014) What we talk about when we talk about fat. Cell 156:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H, Pollock N, Schiemann A, Bulger T, Stowell K (2015) Malignant hyperthermia: a review. Orphanet J Rare Dis 10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LA, Bal NC, Periasamy M (2015) The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev Camb Philos Soc 90:1279–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Bruhn DS, Kopec W, Khandelia H, Periasamy M(2015) The N terminus of sarcolipin plays an important role in uncoupling sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) ATP hydrolysis from Ca2+ transport. J Biol Chem 290:14057–14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Periasamy M (2013) Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J Biol Chem 288:6881–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Matsushita M, Yoneshiro T, Okamatsu-Ogura Y (2020) Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: from mice to men. Front Endocrinol (Lausanne) 11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Delgado G, Martinez-Tellez B, Olza J, Aguilera CM, Gil Á, Ruiz JR (2015) Role of exercise in the activation of brown adipose tissue. Ann Nutr Metab 67:21–32. [DOI] [PubMed] [Google Scholar]
- Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE (2003) Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299:1410–1413. [DOI] [PubMed] [Google Scholar]
- Shanmugam M, Molina CE, Gao S, Severac-Bastide R, Fischmeister R, Babu GJ (2011) Decreased sarcolipin protein expression and enhanced sarco(endo)plasmic reticulum Ca2+ uptake in human atrial fibrillation. Biochem Biophys Res Commun 410:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR (1986) Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem 261:13333–13341. [PubMed] [Google Scholar]
- Solinas G, Summermatter S, Mainieri D, Gubler M, Pirola L, Wymann MP, Rusconi S, Montani JP, Seydoux J, Dulloo AG (2004) The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. FEBS Lett 577:539–544. [DOI] [PubMed] [Google Scholar]
- Sun L, Wei H (2021) Ryanodine receptors: a potential treatment target in various neurodegenerative disease. Cell Mol Neurobiol 41:1613–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung PS, Maurer RA (1994) Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev 8:2527–2539. [DOI] [PubMed] [Google Scholar]
- Tada M, Kirchberger MA, Katz AM (1975) Phosphorylation of a 22,000-dalton component of the cardiac sarcoplasmic reticulum by adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem 250:2640–2647. [PubMed] [Google Scholar]
- Tadini-Buoninsegni F, Smeazzetto S, Gualdani R, Moncelli MR (2018) Drug Interactions With the Ca2+-ATPase From Sarco (Endo)Plasmic Reticulum (SERCA). Front Mol Biosci 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoonen R, Hindle AG, Scherrer-Crosbie M (2016) Brown adipose tissue: The heat is on the heart. Am J Physiol Heart Circ Physiol 310:H1592–H1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend K, Tseng YH (2012) Brown adipose tissue: Recent insights into development, metabolic function and therapeutic potential. Adipocyte 1:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP (2006) UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J Biol Chem 281:31894–31908. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508. [DOI] [PubMed] [Google Scholar]
- Van Petegem F (2012) Ryanodine receptors: structure and function. J Biol Chem 287:31624–31632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandecaetsbeek I, Vangheluwe P, Raeymaekers L, Wuytack F, Vanoevelen J (2011) The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb Perspect Biol 3:a004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangheluwe P, Raeymaekers L, Dode L, Wuytack F (2005) Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium 38:291–302. [DOI] [PubMed] [Google Scholar]
- Verboomen H, Wuytack F, De Smedt H, Himpens B, Casteels R (1992) Functional difference between SERCA2a and SERCA2b Ca2+ pumps and their modulation by phospholamban. Biochem J 286:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya J, Cereijo R, Villarroya F (2013) An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab 305:E567–E572. [DOI] [PubMed] [Google Scholar]
- Virtanen KALidell MEOrava JHeglind MWestergren RNiemi TTaittonen MLaine JSavisto NJEnerbäck S, et al. (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525. [DOI] [PubMed] [Google Scholar]
- Wang Q, Michalak M (2020) Calsequestrin. Structure, function, and evolution. Cell Calcium 90:102242. [DOI] [PubMed] [Google Scholar]
- Wehrens XHLehnart SEReiken Svan der Nagel RMorales RSun JCheng ZDeng SXde Windt LJLandry DW, et al. (2005) Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci USA 102:9607–9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JBoström PSparks LMYe LChoi JHGiang AHKhandekar MVirtanen KANuutila PSchaart G, et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LH, Shanmugam M, Park JY, Zhao Z, Wen H, Tian B, Periasamy M, Babu GJ (2012) Ablation of sarcolipin results in atrial remodeling. Am J Physiol Cell Physiol 302:C1762–C1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M (2013) Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 123:3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Cannon B, Nedergaard J (1997) alpha1-Adrenergic stimulation potentiates the thermogenic action of beta3-adrenoreceptor-generated cAMP in brown fat cells. J Biol Chem 272:32847–32856. [DOI] [PubMed] [Google Scholar]
- Zhou P, Zhao YT, Guo YB, Xu SM, Bai SH, Lakatta EG, Cheng H, Hao XM, Wang SQ (2009) Beta-adrenergic signaling accelerates and synchronizes cardiac ryanodine receptor response to a single L-type Ca2+ channel. Proc Natl Acad Sci USA 106:18028–18033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurlo F, Larson K, Bogardus C, Ravussin E (1990) Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 86:1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurlo F, Nemeth PM, Choksi RM, Sesodia S, Ravussin E (1994) Whole-body energy metabolism and skeletal muscle biochemical characteristics. Metabolism 43:481–486. [DOI] [PubMed] [Google Scholar]