Abstract
Capsule (cps) gene expression in Escherichia coli is controlled by a complex network of regulators. Transcription of the cps operon is controlled by at least two positive regulators, RcsA and RcsB. We show here that RcsA functions to activate its own expression, as seen by the 100-fold-increased expression of a rcsA::lacZ transcriptional fusion in strains with high levels of RcsA protein, either due to a mutation in lon or due to overexpression of RcsA from a multicopy plasmid. Expression of the rcsA::lacZ fusion is increased by but not dependent on the presence of RcsB. In addition, the effects of H-NS and RcsB on the expression of rcsA are independent of each other. A sequence motif, conserved between the E. coli cps promoter and the Erwinia amylovora ams promoter and previously shown to be the RcsA-RcsB binding site, was identified in the rcsA promoter region and shown to be required for high-level expression of rcsA.
Colanic acid capsular polysaccharide (cps) gene expression in Escherichia coli is governed by a complex network of regulators. At least two pathways which can lead to the activation of cps expression have been identified. The first pathway appears to be activated in response to an environmental stimulus, such as osmotic shock (26), which impacts the levels of the membrane-bound protein MdoH, involved in the biosynthesis of membrane-derived oligosaccharides (MDOs) (15). The change in levels of MDOs, in response to changes in the osmolarity of the environment, appears to be the signal (8) that a proposed sensor, RcsC, senses and then relays as an internal signal either directly or indirectly to an activator of cps expression, RcsB (10). The second pathway leading to the activation of cps expression involves the other cps activator, RcsA. RcsA is degraded in a Lon-dependent fashion, with a half-life of approximately 1 min in lon+ cells (29). RcsA is the limiting factor for the activation of cps expression, and stabilization of RcsA in Δlon cells or overproduction of RcsA from a multicopy plasmid in lon+ cells leads to high-level expression of the cps operon (29). RcsB apparently is essential for cps expression (3). cps expression in rcsB strains is low, and cps expression cannot be activated by RcsA in the absence of RcsB, suggesting an auxiliary role for RcsA in cps expression (3, 31). The primary amino acid sequence of RcsA contains a putative helix-turn-helix motif which has been hypothesized to be the DNA binding site of RcsA; however, no in vitro data demonstrating RcsA binding to the cps promoter region exists (29). RcsA protein cannot be detected in cells mutant in RcsB and Lon protease activity yet can be detected in lon rcsB mutant cells if multiple copies of rcsA, controlled by its native promoter, are present in the cell, suggesting that expression of RcsA is not absolutely dependent on RcsB (6). Recently, Sledjeski and Gottesman have identified H-NS, a histone-like protein, as a negative regulator of rcsA expression, as well as a small, stable RNA, the product of the dsrA gene, which can overcome H-NS silencing when expressed in multicopy (25). Beyond the silencing of rcsA expression by H-NS and the multicopy effect of DsrA on H-NS, little is known about the regulation of rcsA expression. In this study, we report that RcsA functions to activate its own expression. We have identified a putative RcsA binding site in both the rcsA and cps promoter regions which appears to be required for high-level expression of both the rcsA gene and the cps operon.
MATERIALS AND METHODS
Materials.
All restriction enzymes were obtained from New England Biolabs (Beverly, Mass.), chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise noted, and Taq DNA polymerase was purchased from Promega Corp. (Madison, Wis.).
Strains, media, and growth conditions.
Strains, plasmids, and phages used in this study are listed in Table 1. Cells were grown at 37°C in Luria-Bertani (LB) broth (21) containing the appropriate antibiotics (ampicillin at 100 μg/ml, kanamycin at 25 μg/ml, tetracycline at 25 μg/ml, and chloramphenicol at 25 μg/ml). LB agar, M63 glucose B1 agar (23), or MacConkey’s lactose agar (23) was supplemented with the appropriate antibiotics after autoclaving whenever needed.
TABLE 1.
Bacterial strains, plasmids, and phages used in this study
| Strain,a plasmid or bacteriophage | Relevant genotype | Construction, source, or reference |
|---|---|---|
| Bacterial strains | ||
| DH5αb | F−endA1 hsdR17 (rK− mK+) supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169(φ80dlacΔ(lacZ)M15) | Gibco BRL |
| DDS90 | lon+ rcsA+ rcsB+ rcsA90::lacZ | D. Sledjeski; 19, 24a |
| JT2029 | proCYA221 zaj-403::ΔTn10 rcsA90::lacZ | DDS90 + P1 (SG1030) |
| JT2046 | Δlon-510 rcsA+ rcsB+ rcsA90::lacZ | JT2029 + P1 (SG4144) |
| JT2055 | Δlon-510 rcsA72::ΔTn10 rcsB+ rcsA90::lacZ | JT2046 + P1 (SG23001) |
| JT2056 | Δlon-510 rcsA+ rcsB62::ΔKan rcsA90::lacZ | JT2046 + P1 (SG23002) |
| JT2059 | Δlon-510 rcsA72::ΔTn10 rcsB62::ΔKan rcsA90::lacZ | JT2056 + P1 (SG20806) |
| JT4000 | Δlon-510 | SG1030 + P1 (SG4144) |
| SG1030 | F− Δlac araD proCYA221 zaj-403::ΔTn10 | 33 |
| SG4144c | N99 Δlon-510 | 16 |
| SG20250 | lon+ Δlac | 11 |
| SG20250-λi434 | This study | |
| SG20780 | Δlon-510 cpsB10::lacZ | 3 |
| SG20781 | lon+ cpsB10::lacZ | 3 |
| SG20806 | rcsA72::ΔTn10 | 3 |
| SG21081 | lon+ cps+ rcsA72::ΔTn | 3 |
| SG23002 | rcsB62::ΔKan | 29 |
| WE10 | hns::ΔKan rcsA90::lacZ | DDS90 + P1 (DDS1398) |
| WE12 | Δlon-510 hns::ΔKan rcsA90::lacZ | JT2046 + P1 (DDS1398) |
| WE20 | hns::ΔTn10 | This study |
| WE28 | lon+ rcsA+ rcsB+ hns::ΔTn10 rcsA90::lacZ | DDS90 + P1 (WE20) |
| WE29 | Δlon-510 rcsA+ rcsB+ hns::ΔTn10 rcsA90::lacZ | JT2046 + P1 (WE20) |
| WE30 | lon+ rcsA+ rcsB62::ΔKan hns::ΔTn10 rcsA90::lacZ | JT2058 + P1 (WE20) |
| WE31 | Δlon-510 rcsA+ rcsB62::ΔKan hns::ΔTn10 rcsA90::lacZ | JT2056 + P1 (WE20) |
| WE1002 | lon+ rcsA+ rcsA109::lacZ | SG20250 + λRS45-rcsA109::lacZ |
| WE1003 | lon+ rcsA+ cps103::lacZ | SG20250 + λRS45-cps103::lacZ |
| WE1004 | lon+ rcsA+ cps41::lacZ | SG20250 + λRS45-cps41::lacZ |
| WE1012 | lon+ proCYA221 zaj-403::ΔTn10 rcsA109::lacZ | WE1002 + P1 (SG1030) |
| WE1013 | lon+ proCYA221 zaj-403::ΔTn10 cps103::lacZ | WE1003 + P1 (SG1030) |
| WE1014 | lon+ proCYA221 zaj-403::ΔTn10 cps41::lacZ | WE1004 + P1 (SG1030) |
| WE1022 | Δlon-510 rcsA+ rcsA109::lacZ | WE1012 + P1 (SG4144) |
| WE1023 | Δlon-510 rcsA+ cps103::lacZ | WE1013 + P1 (SG4144) |
| WE1024 | Δlon-510 rcsA+ cps41::lacZ | WE1014 + P1 (SG4144) |
| WE1052 | Δlon-510 rcsA72::ΔTn10 rcsA109::lacZ | WE1022 + P1 (SG21081) |
| WE1053 | Δlon-510 rcsA72::ΔTn10 cps103::lacZ | WE1023 + P1 (SG21081) |
| WE1054 | Δlon-510 rcsA72::ΔTn10 cps41::lacZ | WE1024 + P1 (SG21081) |
| WE1062 | Δlon-510 rcsB62::ΔKan rcsA109::lacZ | WE1022 + P1 (SG23002) |
| WE1063 | Δlon-510 rcsB62::ΔKan cps103::lacZ | WE1023 + P1 (SG23002) |
| WE1064 | Δlon-510 rcsB62::ΔKan cps41::lacZ | WE1024 + P1 (SG23002) |
| WE1082 | Δlon-510 rcsA72::ΔTn10 rcsB62::ΔKan rcsA109::lacZ | WE1052 + P1 (SG23002) |
| WE1083 | Δlon-510 rcsA72::ΔTn10 rcsB62::ΔKan cps103::lacZ | WE1053 + P1 (SG23002) |
| WE1084 | Δlon-510 rcsA72::ΔTn10 rcsB62::ΔKan cps41::lacZ | WE1054 + P1 (SG23002) |
| Plasmids | ||
| pATC400 | pBR322-rcsA+ | 31 |
| pRS415 | bla-T14EcoRI-SmaI-BamHI lacZ | 24 |
| pRS415-cpsBox | pRS415 plus 194-bp cps promoter fragment | This study |
| pRS415-cpsNoBox | pRS415 plus 132-bp cps promoter fragment | This study |
| pRS415-rcsANoBox | pRS415 plus 121-bp rcsA promoter fragment | This study |
| pACYC184-lon::ΔKan | pACYC184-lon plus 1.5-kb Kanr cassette from pUC-4K | This study |
| Bacteriophages | ||
| λRS45 | imm21 ind+ bla′-lacZSC | 24 |
| λcI−imm434 | cI−imm434 | N. Trun |
| λ imm434 | imm434 | N. Trun |
All strains are derived from MC4100 (ΔlacU169 araD flbB rel) unless otherwise noted.
Commercial strain.
National Institutes of Health strain.
P1vir and λ lysates, as well as transductions, were prepared as described by Silhavy et al. (23).
Detection of RcsA.
Strains were grown in LB broth (containing the appropriate antibiotics) to an optical density at 600 nm of approximately 0.6. One-milliliter samples were removed, washed in 10 mM MgSO4, resuspended in 100 μl sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer (21), and boiled for 10 min. Protein concentrations were determined for all samples by the bicinchoninic acid protein assay method (Pierce, Rockford, Ill.). Equal amounts of total cellular protein (30 μg) were fractionated on a 14% Tricine-SDS-polyacrylamide gel (22). Proteins were transferred to a polyvinylidene difluoride membrane (NEF-1000; Dupont) in 10 mM CAPS [3-(cyclohexylamino)-1-propanesulfonic acid] buffer (pH 11) supplemented with 20% methanol (30). After transfer, membranes were briefly air dried and subsequently blocked in Tris-buffered saline (20 mM Tris [pH 7.4], 125 mM NaCl) containing 0.1% Tween 20 and 1% nonfat dry milk (TBSTM). Membranes were incubated in TBSTM with preabsorbed polyclonal antiserum specific to E. coli RcsA, washed three times in TBSTM, and incubated with an appropriate dilution of monoclonal goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (American Qualex, La Mirada, Calif.) in TBSTM. After three washes with TBST, immunoreactive proteins were visualized on autoradiographic film (Hyperfilm; Amersham, Arlington Heights, Ill.) by enhanced chemiluminescence (Amersham), according to the manufacturer’s instructions.
β-Galactosidase assays.
β-Galactosidase activity was assayed as described by Miller (17). Values presented are averages of three independent assays.
Identification of a putative Rcs box and construction of rcsA::lacZ and cps::lacZ promoter fusions.
Alignments of the region upstream of the rcsA transcriptional start site (25) and the region upstream of the cps transcriptional start site (28) were carried out with the Bestfit program of the Genetics Computer Group software package (5). rcsA and cps promoter fragments, either with or without the putative RcsA box, were amplified by PCR with SG20250 chromosomal DNA as a template and the following primers: rcsANoBoxF, 5′ CCG AAA AAG AAT TCC TAC GA 3′; rcsAR, 5′ GGC GGA CTT AGG ATC CCG TA 3′; cpsBoxF, 5′ CAA CCT AAA GGA ATT CCT AA 3′; cpsNoBoxF, 5′ GCC AAT TAC CGA ATT CTT AT 3′; cpsR, 5′ CCG TCT CAG GAT CCA GTC GT 3′. All forward primers were designed to include an EcoRI restriction site, while the reverse primers contained a BamHI restriction site. Reaction mixtures (100 μl) containing 30 ng of DNA template, 0.5 mM concentrations of each primer, 1.5 mM MgCl2, 5% acetamide, 200 mM (each) deoxynucleoside triphosphates, and 2.5 U of Taq DNA polymerase were incubated for 35 cycles (1 min at 94°C, 1 min at 55°C, and 3 min at 72°C) following a hot-start cycle (5 min at 94°C followed by 2 min at 80°C). Amplification products were digested with EcoRI and BamHI, purified with Qiagen (Santa Clarita, Calif.) PCR purification kits, and ligated into pUC18 digested with EcoRI and BamHI. Recombinant plasmids were isolated, inserts were verified by restriction digests followed by agarose gel electrophoresis, and the inserts were sequenced on an ABI377 automated sequencer with the M13 forward and reverse primers. (Sequencing reactions were carried out at the Center for Gene Research and Biotechnology, Central Services Laboratory, Oregon State University.) Promoter fragments with the correct sequence were subcloned from pUC18 into pRS415 (24). The resulting lacZ operon fusions (pRS415-rcsANoBox, pRS415-cpsBox, and pRS415-cpsNoBox) were crossed onto λRS45 as follows. Fifty microliters of a JT4000 culture, transformed with the recombinant plasmids, was infected with λRS45 at multiplicity of infection of approximately 1.0 and incubated at room temperature for 5 min. Two milliliters of LBMM (LB plus 0.2% maltose–10 mM MgSO4) were added, and the culture was incubated at 37°C until complete lysis of the bacteria was evident. Remaining cells were lysed by the addition of 100 μl of chloroform and brief vortexing, followed by a 5-min centrifugation step. To isolate recombinant phages, 1, 10, and 100 μl of the lysate was mixed with 100 μl of JT4000 culture, 100 μl of 20 mg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) per ml, and 3 ml of melted top agar (LB broth plus 0.7% agar); the mixture was then plated on LB agar and incubated at 37°C overnight. Blue plaques were picked and purified by preparing a lysate, which was subsequently plated out as described above. Once a pure lysate, resulting from a single blue plaque, was obtained, the lysate was titered and the presence of the correctly sized insert was determined by PCR with the following primers specific to regions upstream (RS45F) and downstream (RS45R) from the promoter regions of the fusions: RS45F, 5′ GGA ATT GGG GAT CGG AAT TC 3′; RS45R, 5′ CGA CGG CCA GTG AAT CCG GT 3′. PCRs were carried out as described above with the following modifications: 10 μl of a 1:100 dilution of the phage lysates (approximately 109 PFU/ml) was used as the template, and the annealing temperature was raised to 65°C. Once the inserts were verified, the fusions were introduced into the chromosome of SG20250 at the λatt site according to the procedure described by Simons et al. (24). One hundred microliters of a fresh SG20250 overnight culture in LBMM was mixed with 100 μl of phage stock (>106 PFU/ml) and incubated at room temperature for 20 min; 2 ml of LBMM was added, and the culture was incubated for 2 h at 37°C before dilutions of 10−3 to 10−8 were plated on LB plus X-Gal (3 ml of a 20-mg/ml stock per liter of agar) plates and incubated overnight at 32°C. Individual blue colonies were purified, and the presence of the correctly sized fusion in the chromosome was verified by PCR with chromosomal DNA (30 ng) from each strain as the template under the PCR conditions described above. The prophage copy number was determined by using the Ter test (18, 24); single-copy lysogens were used for all subsequent experiments.
RESULTS
rcsA expression increases in Δlon mutant cells.
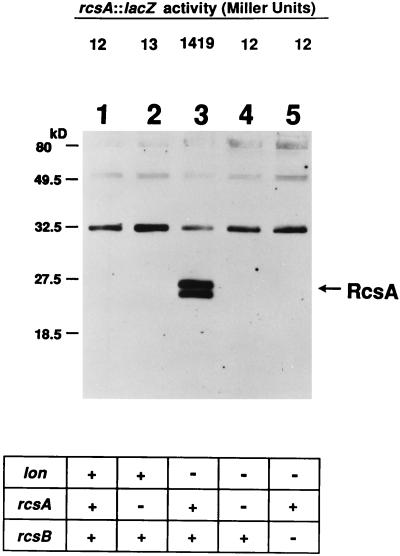
Dierksen and Trempy demonstrated the absence of RcsA in cells mutant for RcsB and Lon protease (6). This led to the hypothesis that rcsA expression might be regulated by other members of the regulatory network controlling cps expression, including Lon, RcsB, and RcsA itself. To test this hypothesis, a reporter gene fusion, consisting of the wild-type rcsA regulatory region fused to a promoterless lacZ gene (designated rcsA90::lacZ [19, 24a]) was used to assess the expression levels of this fusion in various strain backgrounds. The fusion consists of a 2-kb fragment from pATC352 (31) inserted into the EcoRI/SmaI sites of the lacZ operon fusion vector pRS415 (24). The fusion was subsequently crossed onto bacteriophage λRS45 (24), which was used to lysogenize E. coli SG20250, resulting in strain DDS90 (19, 24a). Strain DDS90 is a partial diploid, carrying a wild-type copy of the rcsA gene, directing the synthesis of RcsA protein, as well as the rcsA90::lacZ operon fusion inserted at the λatt site, directing the synthesis of β-galactosidase. Levels of RcsA protein and levels of β-galactosidase can be assessed from the same sample by splitting the sample and using one half to determine levels of RcsA protein with a Western blot approach and the other half to determine the activity of the rcsA90::lacZ operon fusion with a β-galactosidase enzyme assay. This approach allowed the effects of a number of individual mutations and combinations of mutations on the expression level of rcsA to be tested and an examination of the levels of RcsA protein present in the cells to be conducted. The data collected from these experiments is shown in Fig. 1. In a wild-type strain (DDS90 lon+ rcsA+ rcsB+), steady-state levels of RcsA protein are below detection limits, presumably due to Lon-dependent degradation of RcsA, and the activity of the rcsA90::lacZ fusion is low (Fig. 1, lane 1). No RcsA protein can be detected in a lon+ strain carrying a mutation in the wild-type copy of the rcsA gene (JT2057 rcsA72::ΔTn10), and the expression level of the rcsA90::lacZ fusion is similar to that seen in a lon+ rcsA+ strain (Fig. 1, lane 2). In a Δlon rcsA+ rcsB+ rcsA90::lacZ strain (JT2046), the steady-state level of RcsA protein is high and, correspondingly, the rcsA90::lacZ fusion is expressed at a level 100-fold higher than that seen in the isogenic lon+ strain (Fig. 1, lane 3). These results suggest that a high level of RcsA accompanies high-level rcsA expression. In a strain which carries a null mutation in lon, as well as a null mutation in the rcsA gene (JT2055 rcsA72::ΔTn10), the expression level of the rcsA90::lacZ fusion drops back to the level seen in a wild-type strain (Fig. 1, lane 4), thus further supporting the hypothesis that RcsA regulates its expression. Finally, Fig. 1, lane 5, depicts the results obtained with a Δlon rcsA+ rcsB rcsA90::lacZ strain (JT2056). In agreement with previously published data, no RcsA protein was detectable in this strain (6). Furthermore, rcsA90::lacZ expression is similar to that seen in the wild-type strain (lon+ rcsA+ rcsB+).
FIG. 1.
rcsA expression increases in the absence of Lon protease. rcsA expression was assessed at the transcriptional level with a rcsA90::lacZ fusion and at the protein level by Western blotting. β-Galactosidase assays were carried out as described by Miller (17). Specific activity is expressed in Miller units. Values are means of three assays. To assess RcsA protein levels, equal amounts of protein (30 μg) from whole-cell extracts were boiled in sample buffer, fractionated on a 14% Tricine-SDS-polyacrylamide gel, and analyzed by Western blotting with preabsorbed polyclonal anti-RcsA (E. coli) antiserum (the preabsorbed antiserum cross-reacts with a 32-kDa protein which is used as an internal control for equal protein loading). Consistent with data published by Dierksen and Trempy (6), two separate forms of RcsA can be detected. Immunoreactive proteins were visualized by enhanced chemiluminescence. Lanes: 1, DDS90 (lon+ rcsA+ rcsB+); 2, JT2057 (lon+ rcsA72::ΔTn10 rcsB+); 3, JT2046 (Δlon rcsA+ rcsB+); 4, JT2055 (Δlon rcsA72::ΔTn10 rcsB+); 5, JT2056 (Δlon rcsA+ rcsB62::ΔKan). Genotypes of strains are indicated at the bottom. +, wild-type; −, null mutation. All strains are MC4100 derivatives carrying an rcsA90::lacZ fusion integrated at the λatt site.
Sledjeski and Gottesman identified the histone-like protein H-NS as a regulator of rcsA expression (25). In light of our observations, it seemed reasonable to further investigate the impact of Lon, RcsB, and H-NS on rcsA expression. Levels of the rcsA90::lacZ fusion were measured and a visual assessment of the mucoid phenotype was made in a series of rcsA+ strains carrying mutations in lon, rcsB, and hns individually or in combination. The data obtained from these experiments is summarized in Table 2. In a wild-type strain (DDS90 lon+ rcsA+ rcsB+ hns+), the expression level of the rcsA90::lacZ fusion is low (Table 2, line 1) and the cells are nonmucoid. In a lon+ strain carrying a mutation in rcsB (JT2058) the expression level of the rcsA90::lacZ fusion remains low and the cells are nonmucoid (Table 2, line 2). Expression of the rcsA90::lacZ fusion increases 10-fold in an hns strain (WE28) (Table 2, line 3), and the cells are mucoid. This increase is in agreement with the increase in rcsA expression observed by Sledjeski and Gottesman (25) in an hns strain. Even though this strain is lon+, the mutation in hns and the resulting 10-fold increase in rcsA expression result in a mucoid phenotype (Table 2, line 3). A strain mutant in both rcsB and hns (WE30) (Table 2, line 4) still expresses the rcsA90::lacZ fusion at a level 10-fold higher than the DDS90 lon+ rcsA+ rcsB+ hns+ wild-type strain (Table 2, line 1). This result indicates that the effects of RcsB and H-NS on rcsA expression are independent of each other, because a mutation in rcsB does not impact the expression of the rcsA90::lacZ fusion in a lon+ hns strain. Furthermore, this observation indicates that the effect of RcsB on rcsA expression is dependent on the presence of RcsA protein. As expected, the WE30 lon+ rcsA+ rcsB hns strain does not produce capsule, presumably due to the absolute requirement of RcsB for capsule gene expression (3). In a strain mutant in Lon protease (JT2046) (Table 2, line 5), expression of the rcsA90::lacZ fusion increases to a level approximately 100-fold higher than that seen in the DDS90 lon+ rcsA+ rcsB+ hns+ wild-type strain (Table 2, lane 1), and this is consistent with results shown in Fig. 1. In a Δlon rcsB double mutant (JT2056) (Table 2, line 6), expression of the rcsA90::lacZ fusion drops back to the level observed in the DDS90 lon+ rcsA+ rcsB+ hns+ strain (Table 2, line 1), consistent with previous observations (6) and the results shown in Fig. 1. The JT2056 Δlon rcsB strain (Table 2, line 6) is nonmucoid, in agreement with the observation that RcsB is required for cps expression. rcsA90::lacZ expression in a Δlon hns rcsB+ double mutant (WE29) (Table 2, line 7) does not significantly increase compared to the JT2046 Δlon hns+ rcsB+ strain (Table 2, line 5). Finally, in a Δlon rcsB hns triple mutant (WE31), expression of the rcsA90::lacZ fusion is increased approximately 10-fold (Table 2, line 8) compared to the isogenic JT2056 hns+ strain. Expression of rcsA90::lacZ in the WE31 strain background seems to be representative of the true basal level of rcsA expression in the absence of all regulators.
TABLE 2.
Effects of mutations in lon, rcsB, and hns on expression of the rcsA90::lacZ transcriptional fusion and expression of capsule
| Straina | Relevant genotype | β-Galactosidase activityb | Mucoidyc |
|---|---|---|---|
| DDS90 | lon+ rcsB+ hns+ | 23 | − |
| JT2058 | lon+ rcsB hns+ | 27 | − |
| WE28 | lon+ rcsB+ hns | 264 | + |
| WE30 | lon+ rcsB hns | 242 | − |
| JT2046 | Δlon rcsB+ hns+ | 2,415 | + |
| JT2056 | Δlon rcsB hns+ | 21 | − |
| WE29 | Δlon rcsB+ hns | 2,664 | + |
| WE31 | Δlon rcsB hns | 197 | − |
All strains are MC4100 derivatives carrying an rcsA90::lacZ fusion integrated at the λatt site.
β-Galactosidase assays were carried out as described by Miller (17). Values (in Miller units) are averages of three independent assays.
Mucoidy was assessed visually. −, nonmucoid; +, mucoid.
Identification of a putative “Rcs box” in the rcsA promoter region.
The data obtained from the experiments described above indicate the involvement of RcsA protein in regulating its own expression. Previous work by Stout et al. (29) identified RcsA as a positive activator of cps gene expression. If RcsA acts as a transcriptional activator of both cps and rcsA expression, by specifically interacting with the regulatory regions of these genes, then a prediction can be made that a sequence motif which represents the site of RcsA binding would be identified. In support of this prediction, Kelm et al. (14) have localized a putative RcsA-RcsB binding site to a 40-bp region of the Erwinia amylovora ams (amylovoran biosynthesis) regulatory region by demonstrating the binding of E. amylovora RcsB and RcsA-RcsB, as well as E. coli RcsB and RcsA-RcsB, to a fragment representing the putative ams RcsA-RcsB binding site. Neither E. coli RcsA nor E. amylovora RcsA alone could bind to this region (14). Binding of either E. coli RcsA or RcsB has not been demonstrated for the proposed E. coli cps RcsA-RcsB binding region. Alignment of the region upstream of the E. coli rcsA transcriptional start site (Fig. 2b) and the putative RcsA-RcsB binding sites of the E. coli cps operon (Fig. 2a) by using the Bestfit program of the Genetics Computer Group software package (5) identified a 25-bp region of 80% identity between the rcsA and cps promoter regions which we have termed the “Rcs box” (Fig. 2c). This region lies within the region identified by Kelm et al. (14) as a putative RcsA-RcsB binding site of the E. amylovora ams operon (Fig. 2c). Similar to the E. amylovora ams region, the putative E. coli Rcs box is AT rich (80%), but, in contrast to the E. amylovora ams region, the 13 bases at the 3′ end of the box constitute a perfect, for cps, and nearly perfect, for rcsA, 6-bp inverted repeat separated by a single base (CTTAAT-A-TAATTC). The Rcs box is located 30 bp upstream from the −35 signal of the cps promoter (Fig. 2a) and 117 bp upstream from the −35 signal of the rcsA promoter (Fig. 2b). The E. amylovora RcsA-RcsB binding site identified by Kelm et al. does not have the high degree of homology as do the E. coli cps and rcsA regions. In particular, the 13-bp region, which is virtually identical between the E. coli rcsA and cps promoter regions, is not well conserved in the E. amylovora ams promoter region. The region of highest conservation between the cps and ams promoter regions lies upstream of the 13-bp region.
FIG. 2.
Identification of a putative Rcs box. cps (a) and rcsA (b) promoter regions are shown; −10 and −35 regions, the transcriptional start site (+1), and the putative Rcs box are underlined. (c) Clustal multiple sequence alignment of E. coli cps and rcsA promoter regions and the E. amylovora ams promoter region. Residues conserved only between rcsA and cps are marked by vertical lines; residues conserved between all three promoter regions are marked with plus (+) signs. The putative Rcs box is underlined. Accession numbers: E. coli cps, U52666; E. coli rcsA, U17137; E. amylovora ams, X77921.
Effects of the Rcs box on expression of rcsA and cps.
To assess the effects of the putative Rcs box on rcsA and cps expression, a series of strains containing single copies (as determined by the Ter test) of operon fusions of the rcsA or cps promoter region, with or without the putative Rcs box, to a promoterless lacZ gene was constructed as described in Materials and Methods. A prediction can be made that if the putative Rcs box is the site of RcsA binding to the promoter regions of rcsA and cps, the respective constructs lacking the putative Rcs box should not respond to a mutation in lon, since stabilized RcsA would not have a site to bind to in front of the rcsA or cps promoter. In contrast, the constructs containing the putative Rcs box should respond to a mutation in lon, since stabilized RcsA can bind to the putative Rcs box and activate rcsA or cps expression. β-Galactosidase assays were carried out for all promoter fusion constructs in strains carrying mutations in lon, rcsA, rcsB, and combinations of these mutations. The results of these experiments are shown in Table 3. As observed before, in a lon+ strain the expression level of the rcsA90::lacZ fusion is low (Table 3, column 1, line 1) and the introduction of mutations in rcsA, rcsB, or rcsA and rcsB does not affect expression of the rcsA90::lacZ fusion (data not shown). A mutation in lon increases the expression level of the rcsA90::lacZ fusion 300-fold (Table 3, column 1, line 2). Introduction of mutations in rcsA, rcsB, or rcsA and rcsB in lon mutant cells returns the activity of the rcsA90::lacZ fusion to the level seen in a lon+ strain (Table 3, column 1, lines 3, 4, and 5). The rcsA fusion lacking the putative Rcs box, designated rcsA109::lacZ, expresses levels of β-galactosidase in a lon+ strain (Table 3, column 2, line 1) that are similar to levels expressed in lon+ strains containing mutations in rcsA, rcsB, or rcsA and rcsB (data not shown) and which represent levels previously described as basal for other short rcsA::lacZ fusions which are nonresponsive to H-NS silencing (25). A Δlon strain carrying the Rcs box-less fusion, rcsA109::lacZ (Table 3, column 2, line 2), does not show the greater-than-100-fold increase in β-galactosidase levels compared to its lon+ counterpart and as observed in a Δlon strain carrying the rcsA90::lacZ fusion. These results demonstrate that rcsA lacking an Rcs box does not respond to a mutation in lon, suggesting that in addition to the loss of H-NS regulation, stabilized RcsA does not have a site to bind to in front of the rcsA promoter.
TABLE 3.
Effects of the putative Rcs box on expression of rcsA::lacZ and cps::lacZ fusions
| Relevant genotype | β-Galactosidase activitya
|
|||
|---|---|---|---|---|
| rcsA90::lacZb | rcsA109::lacZc | cps103::lacZb | cps41::lacZc | |
| lon+ rcsA+ rcsB+ | 4 | 220 | 7 | 17 |
| Δlon rcsA+ rcsB+ | 1,189 | 176 | 139 | 20 |
| Δlon rcsA rcsB+ | 5 | 219 | 6 | 8 |
| Δlon rcsA+ rcsB | 5 | 203 | 3 | 8 |
| Δlon rcsA rcsB | 5 | 234 | 12 | 24 |
β-Galactosidase assays were carried out as described by Miller (17). Values (in Miller units) are averages of three independent assays. All strains are MC4100 derivatives carrying the described fusion integrated at the λatt site.
These fusions contain the putative Rcs box: DDS90 (lon+ rcsA+ rcsB+ rcsA90::lacZ), WE1003 (lon+ rcsA+ rcsB+ cps103::lacZ), JT2046 (Δlon-510 rcsA+ rcsB+ rcsA90::lacZ), WE1023 (Δlon-510 rcsA+ rcsB+ cps103::lacZ), JT2055 (Δlon-510 rcsA72::ΔTn10 rcsB+ rcsA90::lacZ), WE1053 (Δlon-510 rcsA72::ΔTn10 rcsB+ cps103::lacZ), JT2058 (lon+ rcsA+ rcsB62::ΔKan rcsA90::lacZ), WE1063 (Δlon-510 rcsA+ rcsB62::ΔKan cps103::lacZ), JT2059 (Δlon-510 rcsA72::ΔTn10 rcsB62::ΔKan rcsA90::lacZ), and WE1083 (Δlon-510 rcsA72::ΔTn10 rcsB62::ΔKan cps103::lacZ).
These fusions do not contain the putative Rcs box: WE1002 (lon+ rcsA+ rcsB+ rcsA109::lacZ), WE1004 (lon+ rcsA+ rcsB+ cps41::lacZ), WE1022 (Δlon-510 rcsA+ rcsB+ rcsA109::lacZ), WE1024 (Δlon-510 rcsA+ rcsB+ cps41::lacZ); WE1052 (Δlon-510 rcsA72::ΔTn10 rcsB+ rcsA109::lacZ), WE1054 (Δlon-510 rcsA72::ΔTn10 rcsB+ cps41::lacZ), WE1062 (Δlon-510 rcsA+ rcsB62::ΔKan rcsA109::lacZ), WE1064 (Δlon-510 rcsA+ rcsB62::ΔKan cps41::lacZ), WE1082 (Δlon-510 rcsA72::ΔTn10 rcsB62::ΔKan rcsA109::lacZ), and WE1084 (Δlon-510 rcsA72::ΔTn10 rcsB62::ΔKan cps41::lacZ).
The impact of the Rcs box is further illustrated with the cps fusions, where H-NS regulation does not appear to be a factor. Baseline expression of the cps103::lacZ (contains Rcs box) and cps41::lacZ (lacks Rcs box) fusions is low in a lon+ strain (Table 3, columns 3 and 4, line 1). The level seen with both of these fusions is similar to that seen with other wild-type cps fusions (i.e. cpsB10::lacZ [33]) when RcsA is degraded in a Lon-dependent fashion. Mutations in rcsA, rcsB, or both rcsA and rcsB do not change the expression level of either cps fusion in a lon+ strain (data not shown). This observation is in agreement with the model for the regulation of cps expression, since both RcsA and RcsB are required for optimal cps expression. The introduction of a mutation in lon leads to a 20-fold increase in cps103::lacZ expression compared to the corresponding lon+ strain yet has no impact on cps41::lacZ expression (Table 3, columns 3 and 4, line 2). Furthermore, in comparing the expression of cps103::lacZ to the expression of cps41::lacZ, there is a sevenfold difference of expression in the corresponding Δlon strains (Table 3, columns 3 and 4, line 2), suggesting that regardless of the high levels of RcsA protein in a Δlon strain, the missing putative Rcs box impacts cps expression. The expression levels of both cps fusions in Δlon strains return to the baseline levels seen in the wild-type strains with the introduction of mutations in rcsA, rcsB, or rcsA and rcsB.
DISCUSSION
These studies constitute the first report of an involvement of RcsA protein in regulating its own expression. The observation that RcsA could not be detected in a Δlon rcsB strain (6) and the identification of an H-NS silencing mechanism (25) suggested a multilayer regulatory mechanism for rcsA expression. In the course of these studies, it was noted that expression of the rcsA90::lacZ transcriptional fusion used to assess the levels of rcsA expression was dramatically increased in strains carrying a mutation in lon, and the increased expression of the rcsA90::lacZ fusion was paralleled at the protein level. What might account for the increased rcsA90::lacZ expression in lon mutant derivatives of an rcsA90::lacZ diploid strain? In the absence of Lon protease, RcsA protein is stabilized, as reflected by the mucoid phenotype of lon mutant cells. If expression of the rcsA90::lacZ fusion is increased in lon mutant cells, then a prediction can be made that the stabilized RcsA protein is involved in activating its own expression. Assessment of the levels of rcsA90::lacZ expression in a Δlon rcsA double mutant support this hypothesis: in the absence of Lon and a functional RcsA gene, the activity of the rcsA90::lacZ fusion is low. In contrast, if RcsA is produced from a multicopy plasmid in the presence of Lon, the level of rcsA90::lacZ expression increases (data not shown). Thus, it appears that RcsA is involved in activating its own expression: (i) expression levels of the rcsA90::lacZ fusion increase in response to increased levels of RcsA, and (ii) rcsA90::lacZ expression levels are equally low in lon+ rcsA+ and Δlon rcsA cells under conditions where no RcsA is present in the cells. In support of these conclusions, two other Lon substrates, CcdA in E. coli (20) and ςG in Bacillus subtilis (3) have been shown to be involved in regulating their own expression. Additionally, Gervais et al. (9) have demonstrated that RcsB is an activator of its own expression; therefore, this mechanism of selfactivation might be a conserved feature of the regulators of cps expression in E. coli.
The selfactivation of rcsA expression in the absence of Lon protease was observed initially in Δlon strains expressing wild-type RcsA protein. If this observation is correct, then presumably an increase in the expression levels of the rcsA90::lacZ fusion should be observed in a lon+ strain expressing a mutant RcsA protein (RcsA* [7]) which is stable in the presence of Lon protease. Strains such as these were constructed (7), and indeed, rcsA90::lacZ expression increases in such a strain, indicating that expression of the rcsA90::lacZ fusion is increased whenever the levels of RcsA protein are increased, either through increased synthesis or through increased stability with respect to Lon-dependent degradation (data not shown). Additional support for the hypothesis presented here comes from studies on the Lon-dependent degradation of subunits of the HU protein. Overexpression of either subunit of the HU protein (HUα or HUβ) in E. coli lon+ cells induces expression of the cps genes (19). This activation of cps expression was shown to be due to activation of rcsA expression (19). Since individual HU subunits represent substrates for Lon-dependent degradation (2), the increase in rcsA expression can be explained with a stabilization of RcsA due to saturation of Lon with either HU subunit, leading to increased rcsA expression.
If RcsA binds to the regulatory region upstream of rcsA and cps, then a conserved nucleotide sequence should be present. Such a region was identified by comparing the promoter regions identified for both the rcsA gene (25) and the cps genes (28). The putative Rcs box identified is 25 bp long and is 80% identical between the rcsA and the cps promoter. Comparing the sequence of the putative Rcs box to the complete genome of E. coli with the FASTA search engine did not identify any other putative Rcs box locations on the E. coli chromosome. The Rcs box shows the longest stretch of identity on the 3′ side, where a stretch of 12 bp is 100% conserved. This stretch consists of an inverted repeat of 6 bp, which might represent the binding site for RcsA. In the cps promoter region, the putative Rcs box is located between positions −91 and −68 with respect to the cps transcriptional start site (28), while it is located between positions −180 and −164 with respect to the rcsA transcriptional start site (25). The Rcs box identified in the rcsA promoter region coincides with a region identified by Kelm et al. as the putative RcsA-RcsB binding site within the promoter regions of the E. amylovora ams and the E. coli cps operons. RcsB and RcsA-RcsB binding to the ams promoter region was demonstrated by Kelm et al. (14); however, direct interaction of RcsB and/or RcsA with either the E. coli cps or the rcsA promoter region remains to be shown. The region of highest conservation between the E. coli rcsA and the cps promoter regions is not well conserved in the E. amylovora ams promoter region; thus, this region might constitute an RcsA binding site, whereas the region of highest conservation between the cps and ams promoter regions might represent an RcsB binding site. Binding of RcsA and RcsB to the cps promoter remains to be shown, but the observation that RcsB binds to the ams promoter constitutes strong indirect evidence for RcsB binding to the cps promoter. Furthermore, the sequence motif conserved between the rcsA and the cps promoter regions strongly suggests a potential binding site for RcsA. Interestingly, there are no sequence motifs resembling the Rcs box present in the rcsB promoter region or in the promoter regions of rcsA genes identified in other organisms (e.g., Salmonella typhi, E. amylovora, Erwinia stewartii, and Klebsiella aerogenes).
The positive effect of RcsB on rcsA expression appears to be dependent on the presence of RcsA: overproduction of RcsB from a multicopy plasmid in a lon+ strain does not lead to increased rcsA90::lacZ expression, possibly due to the absence of RcsA (data not shown). In support of this, Dierksen and Trempy demonstrated that RcsA protein could not be detected in a lon rcsB double mutant strain unless rcsA was expressed from a high-copy-number plasmid (6). Therefore, it appears that the RcsB effect on rcsA expression can be overcome by excess RcsA, indicating that RcsB functions as an auxilliary factor in rcsA expression. This is in contrast to cps expression, where multicopy RcsB can overcome the absence of RcsA to activate cps expression. These studies have also demonstrated that the effect of H-NS on rcsA expression is independent of RcsB: a mutation in hns leads to a 10-fold increase in rcsA90::lacZ expression in the presence or absence of RcsB.
If both regulatory mechanisms (H-NS silencing and Lon-dependent degradation) for rcsA expression are removed, one might expect the expression of rcsA to increase continuously. However, rcsA expression in a Δlon hns strain does not increase beyond approximately 100-fold above wild-type levels. What factors might explain this observation? If RcsA is involved in activating its own expression, a mechanism might exist to limit rcsA expression, thus providing the means to limit expression of rcsA to levels adequate under the given circumstances. RcsA has been shown to aggregate into inclusion bodies when present at high levels (12) and thus presumably would not be functionally available beyond a certain level in the cells. RcsA and RcsB are proposed to form heterodimers in order to be functional in the activation of cps expression (14, 27). Thus, another possibility is the degradation of free, unpartnered RcsA by alternative proteases with substrate specificities overlapping that of Lon protease. The existence of such proteases was shown by several laboratories (4, 32), and RcsA has been shown to have a half-life of approximately 30 min in a Δlon strain, indicating that RcsA is not completely stable even in the absence of Lon. These observations suggest that alternative proteases which might degrade unpartnered RcsA in the absence of Lon exist. This Lon-independent degradation of RcsA may constitute the limiting factor in the selfactivation of RcsA. Alternatively, high levels of RcsA might be inhibitory to rcsA expression, leading to a selflimiting effect of the autoregulation. Such a negative effect on selfactivation has been observed with RcsB (9) and presumably would ensure balanced expression of RcsA.
What might explain the complex regulatory network governing rcsA expression and ultimately cps expression? The production of the colanic acid capsule in E. coli has been implicated in protection from desiccation and osmotic shock (26). An increase in cps expression in response to osmotic shock has been shown (26). According to the current model, activation of cps expression is accomplished through interactions, either directly or indirectly, between MDOs, RcsC (a sensor), and RcsB (a positive regulator). RcsA, the other known positive regulator, is effectively degraded in a Lon-dependent fashion, and in wild-type cells, RcsA protein would not be available to participate in the activation of cps expression. How can a cell accomplish maximal expression of the cps genes in the presence of Lon? Many genes under the negative control of H-NS are regulated in response to changes in the environment (1, 34). Changes in the pH or the osmolarity of the medium, cold shock, entry into stationary phase, and other factors have been shown to activate genes silenced by H-NS. The exact mechanism by which H-NS functions in the regulation of many of these genes remains unknown. Increased expression of the cps genes has been demonstrated in response to osmotic shock (26), and this increase was shown to be dependent on RcsA, RcsB, RcsC, and MdoH (8, 26). One can envision a mechanism in which the silencing of rcsA by H-NS is removed, leading to increased expression of rcsA. The level of RcsA in the cell increases, by a selfactivation mechanism, to a point at which the level of RcsA synthesis exceeds the level of Lon-dependent RcsA degradation, allowing for the induction of cps expression, cps genes are expressed as long as the environmental stimulus persists. Once H-NS silencing is reestablished, Lon can clear RcsA from the system and cps expression is turned off. This regulatory pattern is analogous to the regulation of SulA activity, another protein susceptible to Lon-dependent degradation. sulA expression is derepressed upon SOS induction, and levels of SulA protein increase to the point where the protein cannot be completely eliminated from the cell. This allows SulA to carry out its function, inhibition of cell division, until sulA expression is again repressed; SulA is then cleared in a Lon-dependent fashion from the cell and cell division resumes.
The regulatory mechanism proposed in this study allows for the fine tuning of cps expression through two, possibly independent, pathways. A potential stimulus for the induction of rcsA expression has not yet been identified, but it can be envisioned that maximum capsule expression might be in response to a stimulus that activates both pathways simultaneously.
ACKNOWLEDGMENTS
We are grateful to S. Gottesman, V. Stout, and D. Sledjeski for generous gifts of strains.
W.E. was partially supported by a predoctoral fellowship from the N. L. Tartar Foundation. This work was supported by grant DCB-9016809 from the National Science Foundation and by a grant from the Medical Research Foundation of Oregon to J.E.T.
REFERENCES
- 1.Atlung T, Ingmer H. H-NS: a modulator of environmentally regulated gene expression. Mol Microbiol. 1997;24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonnefoy E, Almeida A, Rouviere-Yaniv J. Lon-dependent regulation of the DNA binding protein HU in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:7691–7695. doi: 10.1073/pnas.86.20.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brill J A, Quinlan-Walshe C, Gottesman S. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli K-12. J Bacteriol. 1988;170:2599–2611. doi: 10.1128/jb.170.6.2599-2611.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canceill D, Dervyn E, Huisman O. Proteolysis and modulation of the activity of the cell division inhibitor SulA in Escherichia coli lon mutants. J Bacteriol. 1990;172:7297–7300. doi: 10.1128/jb.172.12.7297-7300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dierksen K P, Trempy J E. Identification of a second RcsA protein, a positive regulator of colanic acid capsular polysaccharide genes, in Escherichia coli. J Bacteriol. 1996;178:5053–5056. doi: 10.1128/jb.178.16.5053-5056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebel W, Claycomb J L, Trempy J E. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Defining substrate parameters for the Escherichia coli Lon protease, abstr. H-95; p. 244. [Google Scholar]
- 8.Ebel W, Vaughn G J, Peters H K R, Trempy J E. Inactivation of mdoH leads to increased expression of colanic acid capsular polysaccharide in Escherichia coli. J Bacteriol. 1997;179:6858–6861. doi: 10.1128/jb.179.21.6858-6861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gervais F G, Phoenix P, Drapeau G R. The rcsB gene, a positive regulator of colanic acid biosynthesis in Escherichia coli, is also an activator of ftsZ expression. J Bacteriol. 1992;174:3964–3971. doi: 10.1128/jb.174.12.3964-3971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottesman S. Regulation of capsule synthesis: modification of the two-component paradigm by an accessory unstable regulator. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: American Society for Microbiology; 1995. pp. 253–262. [Google Scholar]
- 11.Gottesman S, Trisler P, Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985;162:1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jubete Y, Maurizi M R, Gottesman S. Role of the heat shock protein DnaJ in the lon-dependent degradation of naturally unstable proteins. J Biol Chem. 1996;271:30798–30803. doi: 10.1074/jbc.271.48.30798. [DOI] [PubMed] [Google Scholar]
- 13.Karmazyn-Campelli C, Bonamy C, Savelli B, Straiger P. Tandem genes encoding ς-factors for consecutive steps of development in Bacillus subtilis. Genes Dev. 1989;3:150–157. doi: 10.1101/gad.3.2.150. [DOI] [PubMed] [Google Scholar]
- 14.Kelm O, Kiecker C, Geider K, Bernhard F. Interaction of the regulator proteins RcsA and RcsB with the promoter of the operon for amylovoran biosynthesis in Erwinia amylovora. Mol Gen Genet. 1997;256:72–83. doi: 10.1007/s004380050547. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix J M, Loubens I, Tempete M, Menichi B, Bohin J P. The mdoA locus of Escherichia coli consists of an operon under osmotic control. Mol Microbiol. 1991;5:1745–1753. doi: 10.1111/j.1365-2958.1991.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 16.Maurizi M R, Trisler P, Gottesman S. Insertional mutagenesis of the lon gene in Escherichia coli: lon is dispensable. J Bacteriol. 1985;164:1124–1135. doi: 10.1128/jb.164.3.1124-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 18.Mousset S, Thomas R. Ter, a function which generates the ends of the mature λ chromosome. Nature. 1969;221:242–244. doi: 10.1038/221242a0. [DOI] [PubMed] [Google Scholar]
- 19.Painbeni E, Mouray E, Gottesman S, Rouviere-Yaniv J. An imbalance of HU synthesis induces mucoidy in Escherichia coli. J Mol Biol. 1993;234:1021–1037. doi: 10.1006/jmbi.1993.1656. [DOI] [PubMed] [Google Scholar]
- 20.Salmon M A, Van Melderen L, Bernard P, Couturier M. The antidote and autoregulatory functions of the F plasmid CcdA protein: a genetic and biochemical survey. Mol Gen Genet. 1994;244:530–538. doi: 10.1007/BF00583904. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 22.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 23.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 24.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 24a.Sledjeski, D. Personal communication.
- 25.Sledjeski D, Gottesman S. A small RNA acts as an antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:2003–2007. doi: 10.1073/pnas.92.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sledjeski D D, Gottesman S. Osmotic shock induction of capsule synthesis in Escherichia coli K-12. J Bacteriol. 1996;178:1204–1206. doi: 10.1128/jb.178.4.1204-1206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stout V. Regulation of capsule synthesis includes interactions of the RcsC/RcsB regulatory pair. Res Microbiol. 1994;145:389–392. doi: 10.1016/0923-2508(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 28.Stout V. Identification of the promoter region for the colanic acid polysaccharide biosynthetic genes in Escherichia coli K-12. J Bacteriol. 1996;178:4273–4280. doi: 10.1128/jb.178.14.4273-4280.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stout V, Torres-Cabassa A, Maurizi M R, Gutnick D, Gottesman S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J Bacteriol. 1991;173:1738–1747. doi: 10.1128/jb.173.5.1738-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szewczyk B, Kozloff L M. A method for the efficient blotting of strongly basic proteins from sodium dodecyl sulfate-polyacrylamide gels to nitrocellulose. Anal Biochem. 1985;150:403–407. doi: 10.1016/0003-2697(85)90528-7. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Cabassa A S, Gottesman S. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J Bacteriol. 1987;169:981–989. doi: 10.1128/jb.169.3.981-989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trempy J E, Gottesman S. Alp, a suppressor of lon protease mutants in Escherichia coli. J Bacteriol. 1989;171:3348–3353. doi: 10.1128/jb.171.6.3348-3353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trisler P, Gottesman S. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1984;160:184–191. doi: 10.1128/jb.160.1.184-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ussery D W, Hinton J C, Jordi B J, Granum P E, Seirafi A, Stephen R J, Tupper A E, Berridge G, Sidebotham J M, Higgins C F. The chromatin-associated protein H-NS. Biochimie. 1994;76:968–980. doi: 10.1016/0300-9084(94)90022-1. [DOI] [PubMed] [Google Scholar]