Abstract
Purpose:
To provide a clinical framework for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome.
Materials and Methods:
A systematic review of the literature using the MEDLINE® database (search dates 1/1/83–7/22/09) was conducted to identify peer reviewed publications relevant to the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. Insufficient evidence-based data were retrieved regarding diagnosis and, therefore, this portion of the Guideline is based on Clinical Principles and Expert Opinion statements. The review yielded an evidence base of 86 treatment articles after application of inclusion/exclusion criteria. These publications were used to create the majority of the treatment portion of the Guideline. When sufficient evidence existed, the body of evidence for a particular treatment was assigned a strength rating of A (high), B (moderate) or C (low). Additional treatment information is provided as Clinical Principles and Expert Opinion when insufficient evidence existed. See text and algorithm for definitions, and detailed diagnostic management, and treatment frameworks.
Results:
The evidence-based guideline statements are provided for diagnosis and overall management of interstitial cystitis/bladder pain syndrome as well as for various treatments. The panel identified first through sixth line treatments as well as developed guideline statements on treatments that should not be offered.
Conclusions:
Interstitial cystitis/bladder pain syndrome is best identified and managed through use of a logical algorithm such as is presented in this Guideline. In the algorithm the panel identifies an overall management strategy for the interstitial cystitis/bladder pain syndrome patient. Diagnosis and treatment methodologies can be expected to change as the evidence base grows in the future.
Keywords: cystitis, interstitial
The purpose of this guideline is to provide direction to clinicians and patients regarding how to: recognize interstitial cystitis/bladder pain syndrome, conduct a valid diagnostic process, and approach treatment with the goals of maximizing symptom control and patient quality of life while minimizing adverse events and patient burden. The strategies and approaches set forth in this document were derived from evidence-based and consensus-based processes. IC/BPS nomenclature is a controversial issue; for the purpose of clarity the Panel decided to refer to the syndrome as IC/BPS and to consider these terms synonymous. The Panel notes that this document constitutes a clinical strategy and is not intended to be interpreted rigidly. The most effective approach for a particular patient is best determined by the clinician together with the patient. As the science relevant to IC/BPS evolves and improves, the strategies presented here will require amendment in order to remain consistent with the highest standards of clinical care.
METHODOLOGY
A systematic review was conducted to identify published articles relevant to the diagnosis and treatment of IC/BPS. Using the MEDLINE database, literature searches were performed on English language publications from January 1, 1983 to July 22, 2009 using the following search strategy: “cystitis, interstitial”[MeSH] OR “interstitial cystitis”[TIAB] OR “painful bladder syndrome”[TIAB] OR “bladder pain syndrome”[TIAB] OR “chronic pelvic pain” [TIAB] OR dysuria[TIAB] OR “Pelvic floor dysfunction”[TIAB] OR “nonbacterial cystitis”[TIAB] OR “hypersensitive bladder syndrome”[TIAB] OR “detrusor mastocytosis”[TIAB] OR “urgency-frequency syndrome”[TIAB] OR “urgency/frequency syndrome”[TIAB] OR “Pain, Pelvic”[TIAB] OR PBS/IC[TIAB] OR IC/PBS[TIAB] AND ((“2009/07/01”[PDAT]: “2009/10/09”[PDAT]) AND English[lang]) NOT “case reports”[ptyp] NOT comment[ptyp] NOT editorial[PTYP] NOT letter[PTYP].
Studies published after July 22, 2009 were not included as part of the evidence base considered by the Panel from which evidence-based guideline statements (Standards, Recommendations, Options) were derived. A few studies published after this cut-off date provided relevant information and are cited in the text as background material. Data from studies published after the literature search cut-off will be incorporated into the next revision of this Guideline. Review article references were checked to ensure inclusion of all possibly relevant studies. Studies using treatments not available in the United States, studies using herbal or supplement treatments, or studies that reported outcomes information collapsed across multiple interventions also were excluded. Studies on mixed patient groups (ie some patients did not have IC/BPS) were retained as long as more than 50% of patients were IC/BPS patients. Multiple reports on the same patient group were carefully examined to ensure inclusion of only non-redundant information. In a few cases individual studies constituted the only report on a particular treatment. Because sample sizes in individual studies were small, single studies were not considered a sufficient and reliable evidence base from which to construct a guideline statement (ie a Standard, Recommendation, or Option). These studies were used to support Clinical Principles as appropriate.
IC/BPS Diagnosis and Overall Management
The systematic review revealed insufficient publications to address IC/BPS diagnosis and overall management from an evidence basis; the diagnosis and management portions of the algorithm (see figure), therefore, are provided as Clinical Principles or as Expert Opinion with consensus achieved using a modified Delphi technique if differences of opinion emerged.1 A Clinical Principle is a statement about a component of clinical care that is widely agreed upon by urologists or other clinicians for which there may or may not be evidence in the medical literature. Expert Opinion refers to a statement, achieved by consensus of the Panel, that is based on members’ clinical training, experience, knowledge and judgment for which there is no evidence.
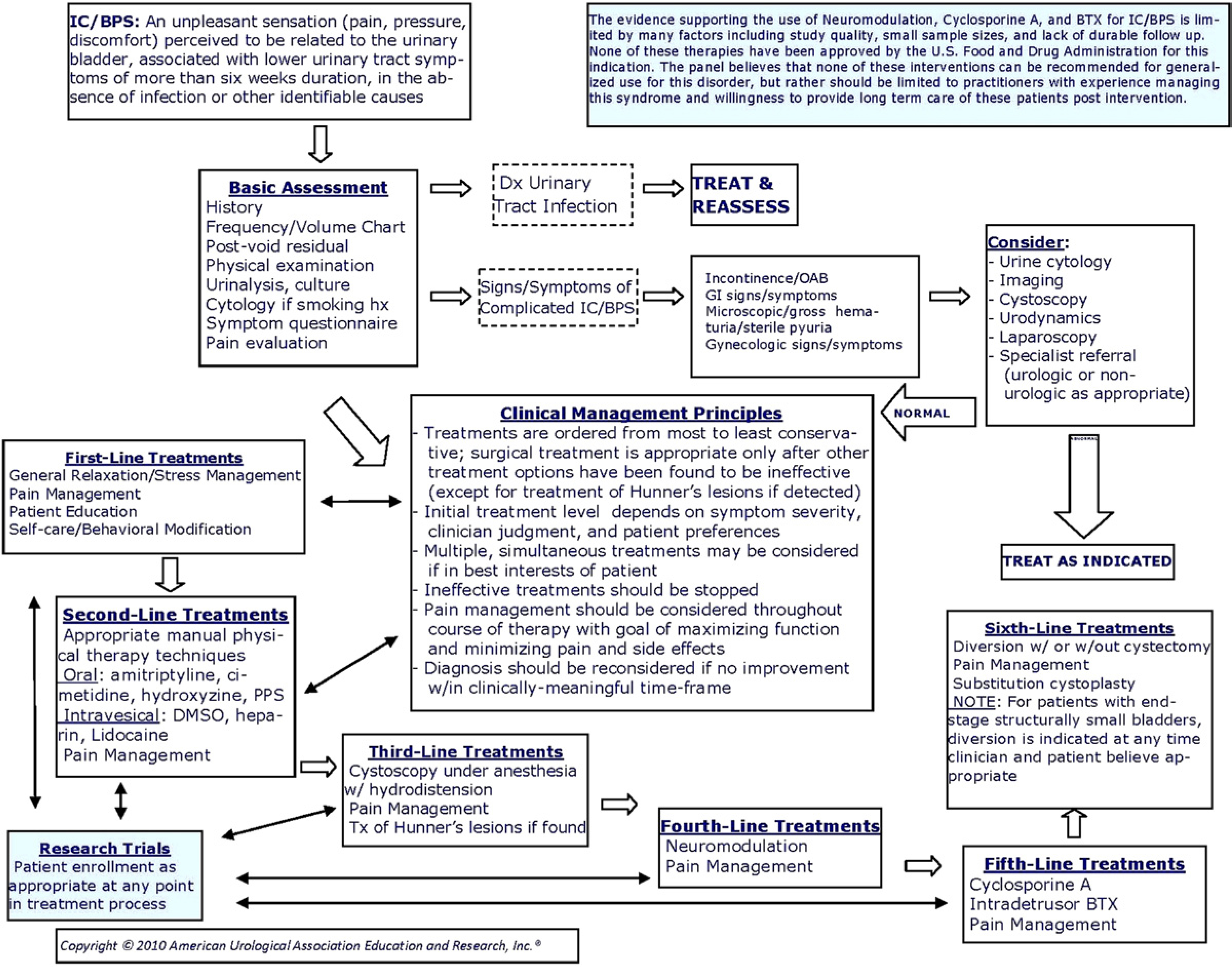
IC/BPS Treatment
With regard to treatment, a total of 86 articles met the inclusion criteria; the Panel judged that these formed a sufficient evidence base from which to construct the majority of the treatment portion of the algorithm. Data on study type (eg randomized controlled trial, randomized crossover trial, observational study), treatment parameters (eg dose, administration protocols, followup durations), patient characteristics (ie age, gender, symptom duration), adverse events and primary outcomes (as defined by study authors) were extracted. The primary outcome measure for most studies was some form of patient-rated improvement scale. For studies that did not use this type of measure, other outcomes were extracted (eg Interstitial Cystitis Symptom Index, Interstital Cystitis Problem Index, visual analog scales).
Quality of Individual Studies and Determination of Evidence Strength
Quality of individual studies that were randomized controlled trials or crossover trials was assessed using the Cochrane Risk of Bias tool.2 Because placebo effects are common in controlled trials conducted with IC/BPS patients, any apparent procedural deviations that could compromise the integrity of randomization or blinding resulted in a rating of increased risk of bias for that particular trial. Because there is no widely agreed upon quality assessment tool for observational studies, the quality of individual observational studies was not assessed.
The categorization of evidence strength is conceptually distinct from the quality of individual studies. Evidence strength refers to the body of evidence available for a particular question and includes consideration of study design, individual study quality, consistency of findings across studies, adequacy of sample sizes, and generalizability of samples, settings and treatments for the purposes of the Guideline. The AUA categorizes body of evidence strength as Grade A (well-conducted RCTs or exceptionally strong observational studies), Grade B (RCTs with some weaknesses of procedure or generalizability or generally strong observational studies) or Grade C (observational studies that are inconsistent, have small sample sizes or have other problems that potentially confound interpretation of data). Because treatment data for this condition are difficult to interpret in the absence of a placebo control, bodies of evidence comprised entirely of studies that lacked placebo control groups (ie observational studies) were assigned a strength rating of Grade C.
AUA Nomenclature: Linking Statement Type to Evidence Strength
The AUA nomenclature system explicitly links statement type to body of evidence strength and the Panel’s judgment regarding the balance between benefits and risks/burdens.3 Standards are directive statements that an action should (benefits outweigh risks/burdens) or should not (risks/burdens outweigh benefits) be undertaken based on Grade A or Grade B evidence. Recommendations are directive statements that an action should (benefits outweigh risks/burdens) or should not (risks/burdens outweigh benefits) be undertaken based on Grade C evidence. Options are nondirective statements that leave the decision to take an action up to the individual clinician and patient because the balance between benefits and risks/burdens appears relatively equal or appears unclear; Options may be supported by Grade A, B or C evidence. In the treatment portion of this guideline most statements are Options because most treatments demonstrate limited efficacy in a subset of patients that is not readily identifiable a priori. The Panel interpreted these data to indicate that for a particular patient, the balance between benefits and risks/burdens is uncertain or relatively equal, and whether to use a particular treatment is a decision best made by the clinician who knows the patient with full consideration of the patient’s treatment history, current quality of life, preferences and values.
Definition
The bladder symptom complex includes a large group of patients with bladder and/or urethral and/or pelvic pain, lower urinary tract symptoms and sterile urine cultures, many with specific identifiable causes. IC/BPS comprises a part of this complex. The Panel used the IC/BPS definition agreed upon by the Society for Urodynamics and Female Urology:
“An unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder, associated with lower urinary tract symptoms of more than six weeks duration, in the absence of infection or other identifiable causes.”4
This definition was selected because it allows treatment to begin after a relatively short symptomatic period, minimizing the delay in initiation of treatment which could occur with definitions that require longer symptom durations (ie 6 months). Definitions used in research or clinical trials should be avoided in clinical practice; many patients may be misdiagnosed or have delays in diagnosis and treatment if these criteria are used.5
PATIENT PRESENTATION
Symptoms
Pain (including sensations of pressure and discomfort) is the hallmark symptom of IC/BPS. Typical IC/BPS patients report not only suprapubic pain (or pressure, discomfort) related to bladder filling, but pain throughout the pelvis—in the urethra, vulva, vagina, rectum—and in extragenital locations such as the lower abdomen and back.6–8 Warren et al found that by using “pelvic pain” as the key descriptor that 100% of their population fit the case definition.9 It is important that the term “pain” encompass a broad array of descriptors. Many patients use other words to describe symptoms, especially “pressure” and may actually deny pain.7,10 Finally, pain that worsened with specific foods or drinks and/or worsened with bladder filling or improved with urination contributed to a sensitive case definition of IC/BPS.8
The prototypical IC/BPS patient may also present with marked urinary urgency and frequency but because these symptoms may indicate other disorders, they do not exclusively indicate the presence of IC/BPS. Voiding frequency is almost universal (92% of one population6) but does not distinguish the IC/BPS patient from other lower urinary tract disorders. Change in urinary frequency is valuable to evaluate response to therapy but is of little help in diagnosis. Urinary urgency is also extremely common (84% of the same population6) but urgency is considered to be the characteristic symptom of overactive bladder and thus it can actually confound the diagnosis.
There may, however, be qualitative differences in the urgency experienced by IC/BPS patients compared to patients with overactive bladder; IC/BPS patients may experience a more constant urge to void as opposed to the classic International Continence Society definition of a, “compelling need to urinate which is difficult to postpone.”11,12 Typically IC/BPS patients void to avoid or to relieve pain; patients with overactive bladder, however, void to avoid incontinence. Symptoms of urinary urgency and frequency may precede symptoms of pain.13 Median time to the development of a full symptom complex of frequency, urgency and pain was reported to be two years in one study.13
Male IC/BPS vs. Chronic Prostatitis
Chronic prostatitis/chronic pelvic pain syndrome, or NIH (National Institutes of Health) type III prostatitis,14 is characterized by pain in the perineum, suprapubic region, testicles or tip of the penis.15 The pain is often exacerbated by urination or ejaculation. Voiding symptoms such as sense of incomplete bladder emptying and urinary frequency are also commonly reported but pain is the primary defining characteristic of CP/CPPS. It is clear that the clinical characteristics that define CP/CPPS are similar to those previously described for IC/BPS. In general, the Panel believes that the diagnosis of IC/BPS should be strongly considered in men with pain, pressure or discomfort perceived to be related to the bladder and associated with urinary frequency, nocturia or an urgent desire to void. However, it is also quite clear that certain men have symptoms that meet criteria for both conditions (IC/BPS and CP/CPPS). In such cases the treatment approach can include established IC/BPS therapies as well as other therapies that are more specific to CP/CPPS. Empiric IC/BPS strategies have led to clinical symptomatic improvement in some CP/CPPS patients.15–17 Consistent with this idea, some patients with CP/CPPS demonstrate bladder glomerulations after distention under anesthesia,15,16 although the significance of this finding is unclear.
DIAGNOSIS
The diagnosis of IC/BPS can be challenging. Patients present with a wide spectrum of symptoms, physical exam findings and clinical test responses. This complexity causes significant misdiagnosis, under diagnosis and delayed diagnosis.18 Insufficient literature was identified to constitute an evidence base for diagnosis of IC/BPS in clinical practice. The lack of evidence is not surprising given the various definitions of the disorder used and the focus of most trials on National Institute of Diabetes and Digestive and Kidney Diseases diagnostic criteria (note that this diagnostic criteria are not appropriate for use outside of clinical trials).19,20 For this reason, diagnosis statements are based on Clinical Principles or Expert Opinion with consensus achieved using a modified Delphi technique when differences of opinion emerged. This section is intended to provide clinicians and patients with a framework for determining whether a diagnosis of IC/BPS is appropriate; it is not intended to replace the judgment and experience of the individual clinician faced with a particular patient.
GUIDELINE STATEMENTS
1. The basic assessment should include a careful history, physical examination and laboratory examination to document symptoms and signs that characterize IC/BPS and exclude other disorders commonly associated with IC/BPS in the differential diagnosis. Clinical Principle
The basic laboratory testing includes a urinalysis and urine culture. If the patient reports a history of smoking and/or presents with unevaluated microhematuria, then urine cytology may be considered given the risk of bladder cancer. Urine culture may be indicated even in patients with a negative urinalysis in order to detect lower levels of bacteria that are clinically significant but not readily identifiable with a dipstick or on microscopic exam. The potassium sensitivity test has neither the specificity nor sensitivity to change clinical decision-making and is not recommended.
2. Baseline voiding symptoms and pain levels should be obtained in order to measure subsequent treatment effects. Clinical Principle
The O’Leary-Sant Interstitial Cystitis Symptom Index/Interstitial Cystitis Problem Index is useful to gather comprehensive symptom information, including symptoms in addition to those of pain or discomfort.21 A 0 to 10 Likert-style scale and a visual analog scale are simple, easily administered instruments that can capture pain intensity. Pain body maps can be used with patients whose presentation suggests a more global pain syndrome.
3. Cystoscopy and/or urodynamics should be considered when the diagnosis is in doubt; these tests are not necessary for making the diagnosis in uncomplicated presentations. Expert Opinion
There are no agreed upon cystoscopic findings diagnostic for IC/BPS. The only consistent cystoscopic finding that leads to a diagnosis of IC/BPS is one or more inflammatory appearing lesions or ulcerations (as initially described by Hunner).22 Glomerulations (pinpoint petechial hemorrhages) may be detected on cystoscopy and can be consistent with IC/BPS but these lesions are commonly seen in other conditions which may coexist with or be misdiagnosed as IC/BPS such as chronic undifferentiated pelvic pain or endometriosis.23,24 Glomerulations may also be present in asymptomatic patients undergoing cystoscopy for other conditions.25
There are no agreed upon urodynamic diagnostic criteria for IC/BPS. Urodynamic evaluation may provide information regarding concomitant lower urinary tract symptoms.
TREATMENT GUIDELINE STATEMENTS
Overall Management
4. Treatment strategies should proceed using more conservative therapies first with less conservative therapies used if symptom control is inadequate for acceptable quality of life; because of their irreversibility, surgical treatments (other than fulguration of Hunner’s lesions) are appropriate only after other treatment alternatives have been exhausted, or at any time in the rare instance when an end stage small, fibrotic bladder has been confirmed and the patient’s quality of life suggests a positive risk-benefit ratio for major surgery. Clinical Principle
5. Initial treatment type and level should depend on symptom severity, clinician judgment and patient preferences; appropriate entry points into the treatment portion of the algorithm depend on these factors. Clinical Principle
6. Multiple, simultaneous treatments may be considered if it is in the best interests of the patient; baseline symptom assessment and regular symptom level reassessment are essential to document efficacy of single and combined treatments. Clinical Principle
7. Ineffective treatments should be stopped once a clinically meaningful interval has elapsed. Clinical Principle
8. Pain management should be continually assessed for effectiveness because of its importance to quality of life. If pain management is inadequate, then consideration should be given to a multidisciplinary approach and the patient referred appropriately. Clinical Principle
9. The IC/BPS diagnosis should be reconsidered if no improvement occurs after multiple treatment approaches. Clinical Principle
Treatments That May Be Offered
Treatments that may be offered are divided into first, second, third, fourth, fifth and sixth line groups based on the balance between potential benefits to the patient, potential severity of adverse events and the reversibility of the treatment. See body of Guideline for protocols, study details and rationales (www.auanet.org/guidelines).
First line treatments.
First line treatments should be performed on all patients.
10. Patients should be educated about normal bladder function, what is known and not known about IC/BPS, benefits vs risks/burdens of the available treatment alternatives, the fact that no single treatment has been found effective for the majority of patients and the fact that acceptable symptom control may require trials of multiple therapeutic options (including combination therapy) before it is achieved. Clinical Principle
11. Self-care practices and behavioral modifications that can improve symptoms should be discussed and implemented as feasible. Clinical Principle
Clinical experience and a limited literature suggest that modifying certain behaviors can improve symptoms in some IC/BPS patients.26 Suggesting that patients become aware of and avoid specific behaviors that worsen symptoms reproducibly for a particular patient is appropriate and can provide some sense of control in a disease process that can be a devastating ordeal. Behavioral modification strategies may include altering the concentration and/or volume of urine either by fluid restriction or additional hydration, application of local heat or cold over the bladder or perineum, avoidance of certain foods known to be common bladder irritants for IC/BPS patients such as coffee or citrus products, use of an elimination diet to determine which foods or fluids may contribute to symptoms, techniques applied to trigger points and areas of hypersensitivity (eg application of heat or cold), strategies to manage IC/BPS flare-ups (eg meditation, imagery27), pelvic floor muscle relaxation and bladder training with urge suppression.28–30 Other controllable behaviors or conditions that may worsen symptoms in some patients include certain types of exercise (eg pelvic floor muscle exercises, see Physical Therapy), sexual intercourse, wearing of tight-fitting clothing and constipation. A trial of over-the-counter products (eg quercitin, calcium glycerophosphates, pyridium) is commonly initiated by patients themselves and, although data in the literature are limited, individual patients may find some to be worthwhile in alleviating symptoms.
12. Patients should be encouraged to implement stress management practices to improve coping techniques and manage stress-induced symptom exacerbations. Clinical Principle
Second line treatments.
13. Appropriate manual physical therapy techniques (eg maneuvers that resolve pelvic, abdominal and/or hip muscular trigger points, lengthen muscle contractures, and release painful scars and other connective tissue restrictions), if appropriately trained clinicians are available, should be offered. Pelvic floor strengthening exercises (eg Kegel exercises) should be avoided. Clinical Principle
14. Multimodal pain management approaches (eg pharmacological, stress management, manual therapy if available) should be initiated. Expert Opinion
Whether pain management is best accomplished by the primary treating clinician and/or by a multidisciplinary team or other pain specialists should be determined by the clinician in consultation with the patient. Patients with intractable pain and/or complex presentations may require referral to other specialists to achieve satisfactory pain control. It is important to note that pain management alone does not constitute sufficient treatment for IC/BPS; pain management is one component of treatment. To the extent possible, it is essential that patients also are treated for the underlying bladder related symptoms.
15. Amitriptyline, cimetidine, hydroxyzine or pentosan polysulfate may be administered as second line oral medications (listed in alphabetical order; no hierarchy is implied). Options
The body of evidence strength for each medication was Grade B or Grade C (see complete Guideline for detailed evidence description). These medications are grouped together as second line treatments because their administration is associated with minor adverse events and efficacy for any individual is unpredictable.
16. Dimethyl sulfoxide, heparin or lidocaine may be administered as second line intravesical treatments (listed in alphabetical order; no hierarchy is implied). Option
The body of evidence strength for each intravesical treatment was Grade B or Grade C (see complete Guideline for detailed evidence description). These treatments are grouped together as second line treatments because their administration is associated with minor adverse events and efficacy for any individual is unpredictable.
Third line treatments.
17. Cystoscopy under anesthesia with short duration, low pressure hydrodistension may be undertaken if first and second line treatments have not provided acceptable symptom control and quality of life or if the patient’s presenting symptoms suggest a more invasive approach is appropriate. Option (Evidence Strength—Grade C)
Cystoscopy is intended to serve three purposes. First, before distension, the bladder is inspected for other potential symptom causes (eg stones, tumors) and for Hunner’s lesions. If these are found, then the causes are treated appropriately (see below for treatment of Hunner’s lesions). Second, if no bladder abnormalities or ulcers are found, then the distension may proceed and serve as a treatment. Hunner’s lesions can be easier to identify after distention when cracking and mucosal bleeding become evident. Third, distension allows for disease “staging” by determining anatomic as opposed to functional bladder capacity and identifying the subset of patients who suffer reduced capacity as a result of fibrosis.
18. If Hunner’s lesions are present, then fulguration (with laser or electrocautery) and/or injection of triamcinolone should be performed. Recommendation (Evidence Strength—Grade C)
Fourth line treatment.
19. A trial of neurostimulation may be performed and, if successful, implantation of permanent neurostimulation devices may be undertaken if other treatments have not provided adequate symptom control and quality of life, or if the clinician and patient agree that symptoms require this approach. Option (Evidence Strength—Grade C)
Fifth line treatment.
20. Cyclosporine A may be administered as an oral medication if other treatments have not provided adequate symptom control and quality of life or if the clinician and patient agree that symptoms require this approach. Option (Evidence Strength—Grade C)
21. Intradetrusor botulinum toxin A may be administered if other treatments have not provided adequate symptom control and quality of life or if the clinician and patient agree that symptoms require this approach. Patients must be willing to accept the possibility that intermittent self-catheterization may be necessary after treatment. Option (Evidence Strength—Grade C)
The evidence supporting the use of neuromodulation, cyclosporine A and botulinum toxin for IC/BPS is limited by many factors including study quality, small sample sizes and lack of durable follow up. None of these therapies has been approved by the U.S. Food and Drug Administration for IC/BPS. The Panel believes that none of these interventions can be recommended for general use for this disorder, but rather should be limited to practitioners with experience managing this syndrome and willingness to provide long-term care of these patients post intervention.
Sixth line treatment.
22. Major surgery (substitution cystoplasty, urinary diversion with or without cystectomy) may be undertaken in carefully selected patients for whom all other therapies have failed to provide adequate symptom control and quality of life (see caveat in Guideline statement 4). Option (Evidence Strength—Grade C)
In the properly selected refractory patient urinary diversion will relieve frequency and nocturia, and sometimes can relieve pain. If frequency is perceived as a major problem, then diversion can almost certainly improve quality of life in select patients who have failed to respond to standard and investigational interventions. However, patients must understand that symptom relief is not guaranteed. Pain can persist even after cystectomy, especially in nonulcer IC/BPS.31
Treatments That Should Not Be Offered
The treatments below appear to lack efficacy and/or appear to be accompanied by unacceptable adverse event profiles. See complete Guideline for study details and rationales at www.auanet.org/guidelines .
23. Long-term oral antibiotic administration should not be offered. Standard (Evidence Strength—Grade B)
One RCT reported that an 18-week protocol of sequential antibiotic administration resulted in 20% of the treatment group reporting 50% or greater symptom improvement compared to 16% of the placebo group, a nonsignificant difference.32 Adverse events were typical of long-term antibiotic administration (eg gastrointestinal disturbances, vaginal infections, nausea, dizziness). Given the potential hazards associated with long-term antibiotic administration in general (eg fostering of antibiotic resistant organisms), the Panel judged that antibiotic treatment is contraindicated in patients who have previously been administered antibiotics without efficacy and who present with a negative urine culture.
24. Intravesical instillation of bacillus Calmette-Guérin should not be offered outside of investigational study settings. Standard (Evidence Strength—Grade B)
Intravesical instillation of bacillus Calmette-Guérin is associated with no significant efficacy compared to placebo in the context of potentially life threatening adverse events detailed in the bladder cancer literature, with long-term followup data indicating no differences between bacillus Calmette-Guérin and placebo treated patients.
25. Intravesical instillation of resiniferatoxin should not be offered. Standard (Evidence Strength—Grade A)
This Standard is based on the findings from two high quality RCTs, both of which demonstrated no statistically significant differences between treatment and placebo groups or between different resiniferatoxin dose groups.33,34 Adverse event rates were high (eg ranging from 52% to 89%) although generally not serious.
26. High pressure, long duration hydrodistension should not be offered. Recommendation (Evidence Strength—Grade C)
High pressure (eg greater than 80 to 100 cm H2O), long duration (greater than 10 minutes) hydrodistension is associated with increased frequency of serious adverse events (eg bladder rupture, sepsis) without a consistent increase in benefit. This Recommendation is based on results of three observational studies that used high pressure (eg systolic blood pressure, mean arterial pressure) and/or long duration (eg repeated intervals of 30 minutes, 3 hours continuously).35–37 The efficacy rates from these studies ranged from 22% to 67% and all reported at least one case of ruptured bladder.
27. Systemic (oral) long-term glucocorticoid administration should not be offered. Recommendation (Evidence Strength—Grade C)
This Recommendation is based on the findings from two observational studies.38,39 Although high rates of efficacy were reported (47% to 64%), given the extremely small combined sample size of fewer than 30 patients, the relatively serious adverse events (eg new diabetes onset, exacerbation of existing diabetes, pneumonia with septic shock, increased blood pressure) and the known risks of systemic long-term glucorticoid use, risks/burdens clearly outweigh benefits. This Recommendation does not preclude the use of short-term glucocorticoid therapy to manage symptom flares.
ACKNOWLEDGMENTS AND DISCLAIMERS
This document was written by the Interstitial Cystitis Guidelines Panel of the American Urological Association Education and Research, Inc., which was created in 2008. The Practice Guidelines Committee of the AUA selected the committee chair. Panel members were selected by the chair. Membership of the committee included urologists, nurses, and other clinicians with specific expertise on this disorder. The mission of the committee was to develop recommendations that are analysis-based or consensus-based, depending on Panel processes and available data, for optimal clinical practices in the diagnosis and treatment of IC. This document was submitted for peer review to 84 urologists and other health care professionals.
Funding of the committee was provided by the AUA. Committee members received no remuneration for their work. Each member of the committee provided a Conflict of Interest disclosure to the AUA.
AUA Guidelines provide guidance only, and do not establish a fixed set of rules or define the legal standard of care. The Guidelines do not preempt physician judgment in individual cases; conformance with AUA guidelines cannot guarantee a successful outcome. The text may include information or recommendations about certain drug uses (off-label) that are not approved by the FDA or about medications or substances not subject to the FDA approval process. AUA urges strict compliance with all government regulations and protocols for prescription and use of these substances.
Abbreviations and Acronyms
- BPS
bladder pain syndrome
- CP
chronic prostatitis
- CPPS
chronic pelvic pain syndrome
- IC
interstitial cystitis
- RCT
randomized clinical trial
Footnotes
CONFLICT OF INTEREST DISCLOSURES
All panel members completed conflict of interest disclosures. Those marked with (C) indicate that compensation was received; relationships designated by (U) indicate no compensation was received.
Consultant/Advisor: Philip M. Hanno, Astellas (C), Lilly(C), Watson(C), Taris (C), Trillium Therapeutics Inc.(C), Allergan(C), Pfizer(C); J. Quentin Clemens, Afferent Pharmaceuticals, Inc.(C), Lilly (C), Medtronic (C), United Biosource Corporation(C), Pfizer(C); Roger R. Dmochowski, Allergen(C), Antrares (C), Astellas (C), Medtronic (C), Merck(C), Pfizer (C), Johnson/Johnson (C), Serenity (C); Deborah R. Erickson, Trillium Therapeutics Inc.(C), NeurAxon (C); Mary P FitzGerald, Astellas (C), Robert Mayer, Lipella (C), Bioform(C), AbbyMoore Medical (C), Taris Biomedical (C), Pfizer (C), Allergan(C); Diane K. Newman, Astellas (C), Pfizer(C), SCA Personal Products(C), Verathon Medical (C), Watson Pharma (C), Hollister(C), GlaxoSmithKline (C); Christopher K. Payne, Afferent Pharma (C), Allergan (C), AMS(C), Astellas (C), Eli Lilly (C)
Investigator: J. Quentin Clemens, Pfizer (U); Roger R. Dmochowski, Allergan (C), Christopher K. Payne, Celgene (U), Coloplast (U), Medtronic (U)
Investor/Advisor: J. Quentin Clemens, Merck (U); Christopher K. Payne, Curant (C)
Lecturer/Consultant/Advisor: David A. Burks, Astellas Pharma (C), GlaxoSmithKline Pharma (C), Bayer Corporation(C)
Meeting Participant or Lecturer: David Allen Burks, Astellas Pharma, US(C), Bayer HealthCare Corporation (C), GlaxoSmithKline Pharma, US (C); Mary P FitzGerald, Ferring, Inc.; Mikel L. Gray, Pfizer (C); Diane K. Newman, Allergan (C), Astellas (C), GlaxoSmithKline Pharma (C), Watson Pharma (C), Pfizer(C)
Researcher/Scientific Study or Trial: Diane K. Newman, Allergan (C), GTX (C), Contura(C); Robert D. Mayer, M.D., Allergan (C), Pfizer (C)
Speaker Honorarium: Mary P FitzGerald, Astellas (C), Ferring, Inc (C)
Other: Roger R. Dmochowski, Contura (C)
REFERENCES
- 1.Hsu C and Sandford BA: The Delphi Technique: making sense of consensus. Practical Assessment, Research & Evaluation 2007; 12: 1. [Google Scholar]
- 2.Higgins JDA: Assessing quality of included studies in Cochrane Reviews. The Cochrane Collaboration Methods Groups Newsletter 2007; 11. [Google Scholar]
- 3.Faraday M, Hubbard H, Kosiak B et al. : Staying at the Cutting Edge: a review and analysis of evidence reporting and grading: the recommendations of the American Urological Association. British Journal of Urology—International 2009; 104: 294. [DOI] [PubMed] [Google Scholar]
- 4.Hanno P and Dmochowski R: Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourology and Urodynamics 2009; 28: 274. [DOI] [PubMed] [Google Scholar]
- 5.Hanno PM, Landis JR, Matthews-Cook Y et al. : The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol 1999; 161: 553. [DOI] [PubMed] [Google Scholar]
- 6.Tincello DG and Walker AC: Interstitial cystitis in the UK: results of a questionnaire survey of members of the Interstitial Cystitis Support Group. Eur J Obstet Gynecol Reprod Biol 2005; 118: 91. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald MP, Koch D and Senka J: Visceral and cutaneous sensory testing in patients with painful bladder syndrome. Neurourol Urodyn 2005; 24: 627. [DOI] [PubMed] [Google Scholar]
- 8.Warren JW, Brown J, Tracy JK et al. : Evidence-based criteria for pain of interstitial cystitis/painful bladder syndrome in women. Urology 2008; 71: 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren JW, Meyer WA, Greenberg P et al. : Using the International Continence Society’s definition of painful bladder syndrome. Urology 2006; 67: 1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirinian E and Payne CK: Correlation of symptoms between 2 instruments among interstitial cystitis patients. Urology 2001; 57: 124. [DOI] [PubMed] [Google Scholar]
- 11.Diggs C, Meyer WA, Langenberg P et al. : Assessing urgency in interstitial cystitis/painful bladder syndrome. Urology 2007; 69: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg P, Tracy JK, Meyer WA et al. : Short interval between symptom onset and medical care as an indication of rapid onset of interstitial cystitis/painful bladder syndrome. BJU Int 2007; 100: 599. [DOI] [PubMed] [Google Scholar]
- 13.Driscoll A and Teichman JM: How do patients with interstitial cystitis present? J Urol 2001; 166: 2118. [PubMed] [Google Scholar]
- 14.Krieger JN, Nyberg L Jr. and Nickel JC: NIH consensus definition and classification of prostatitis. JAMA 1999; 282: 236. [DOI] [PubMed] [Google Scholar]
- 15.Litwin MS, McNaughton-Collins M, Fowler FJ et al. : The National Institutes of Health chronic prostatitis symptom Index: development and vadlidation of a new outcome measure. J Urol 1999; 162: 369. [DOI] [PubMed] [Google Scholar]
- 16.Forrest JB and Vo Q: Observations on the presentation, diagnosis, and treatment of interstitial cystitis in men. Urology 2001; 57: 26. [DOI] [PubMed] [Google Scholar]
- 17.Miller JL, Rothman I, Bavendam TG et al. : Prostatodynia and interstitial cystitis: one and the same? Urology 1995; 45: 587. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JE and Johnson KE: Ambiguous chronic illness in women: community health nursing concern. J Community Health Nurs 2006; 23: 159. [DOI] [PubMed] [Google Scholar]
- 19.Wein AJ, Hanno PM and Gillenwater JY: Interstitial Cystitis: an introduction to the problem. In: Interstitial Cystitis. Edited by Hanno PM, Staskin DR, Krane RJ et al. London: Springer-Verlag, pp. 3–15, 1990. [Google Scholar]
- 20.Striker GE: KUH notes. J Urol 1989; 142: 139. [DOI] [PubMed] [Google Scholar]
- 21.O’Leary MP, Sant GR, Fowler FJ Jr. et al. : The interstitial cystitis symptom index and problem index. Urology 1997; 49: 58. [DOI] [PubMed] [Google Scholar]
- 22.Hunner G: A rare type of bladder ulcer. Further notes, with a report of eighteen cases. JAMA 1918; 70: 203. [Google Scholar]
- 23.Paulson JD and Delgado M: Chronic pelvic pain: the occurrence of interstitial cystitis in a gynecological population. JSLS 2005; 9: 426. [PMC free article] [PubMed] [Google Scholar]
- 24.Chung MK, Chung RP and Gordon D: Interstitial cystitis and endometriosis in patients with chronic pelvic pain: The ‘Evil Twins’ syndrome. JSLS 2005; 9: 25. [PMC free article] [PubMed] [Google Scholar]
- 25.Waxman JA, Sulak PJ and Kuehl TJ: Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol 1998; 160: 1663. [PubMed] [Google Scholar]
- 26.Rovner E, Propert KJ, Brensinger C et al. : Treatments used in women with interstitial cystitis: the interstitial cystitis database (ICDB) study experience. The Interstitial Cystitis Data Base Study Group. Urology 2000; 56: 940. [DOI] [PubMed] [Google Scholar]
- 27.Carrico DJ, Peters KM and Diokno AC: Guided imagery for women with interstitial cystitis: results of a prospective, randomized controlled pilot study. J Altern Complement Med 2008; 14: 53. [DOI] [PubMed] [Google Scholar]
- 28.Shorter B, Lesser M, Moldwin RM et al. : Effect of comestibles on symptoms of interstitial cystitis. J Urol 2007; 178: 145. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh TF, Yu KJ and Lin SY: Possible application of Raman microspectroscopy to verify the interstitial cystitis diagnosis after potassium sensitivity test: phenylalanine or tryptophan as a biomarker. Dis Markers 2007; 23: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster HE, Kreder K, Fitzgerald MP et al. : Effect of amitriptyline on symptoms in newly diagnosed patients with interstitial cystitis/painful bladder syndrome. J Urol 2010; 183: 1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossberger J, Fall M, Jonsson O et al. : Long-term results of reconstructive surgery in patients with bladder pain syndrome/interstitial cystitis: subtyping is imperative. Urology 2007; 70: 638. [DOI] [PubMed] [Google Scholar]
- 32.Warren JW, Horne LM, Hebel JR et al. : Pilot study of sequential oral antibiotics for the treatment of interstitial cystitis. J Urol 2000; 163: 1685. [PubMed] [Google Scholar]
- 33.Chen TY, Corcos J, Camel M et al. : Prospective, randomized, double-blind study of safety and tolerability of intravesical resiniferatoxin (RTX) in interstitial cystitis (IC). Int Urogynecol J Pelvic Floor Dysfunct 2005; 16: 293. [DOI] [PubMed] [Google Scholar]
- 34.Payne CK, Mosbaugh PG, Forrest JB et al. : Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J Urol 2005; 173: 1590. [DOI] [PubMed] [Google Scholar]
- 35.McCahy PJ and Styles RA: Prolonged bladder distension: experience in the treatment of detrusor overactivity and interstitial cystitis. Eur Urol 1995; 28: 325. [DOI] [PubMed] [Google Scholar]
- 36.Glemain P, Riviere C, Lenormand L et al. : Prolonged hydrodistention of the bladder for symptomatic treatment of interstitial cystitis: efficacy at 6 months and 1 year. Eur Urol 2002; 41: 79. [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Murayama T and Andoh M: Adjuvant hydrodistension under epidural anesthesia for interstitial cystitis. Int J Urol 2003; 10: 463. [DOI] [PubMed] [Google Scholar]
- 38.Hosseini A, Ehren I and Wiklund NP: Nitric oxide as an objective marker for evaluation of treatment response in patients with classic interstitial cystitis. J Urol 2004; 172: 2261. [DOI] [PubMed] [Google Scholar]
- 39.Soucy F and Gregoire M: Efficacy of prednisone for severe refractory ulcerative interstitial cystitis. J Urol 2005; 173: 841. [DOI] [PubMed] [Google Scholar]


