ABSTRACT
Alterations in the gut microbiota composition have been associated with a range of neurodevelopmental, neurodegenerative, and neuropsychiatric disorders. The gut microbes transform and metabolize dietary- and host-derived molecules generating a diverse group of metabolites with local and systemic effects. The bi-directional communication between brain and the microbes residing in the gut, the so-called gut–brain axis, consists of a network of immunological, neuronal, and endocrine signaling pathways. Although the full variety of mechanisms of the gut–brain crosstalk is yet to be established, the existing data demonstrates that a single metabolite or its derivatives are likely among the key inductors within the gut–brain axis communication. However, more research is needed to understand the molecular mechanisms underlying how gut microbiota associated metabolites alter brain functions, and to examine if different interventional approaches targeting the gut microbiota could be used in prevention and treatment of neurological disorders, as reviewed herein.
Abbreviations:4-EPS 4-ethylphenylsulfate; 5-AVA(B) 5-aminovaleric acid (betaine); Aβ Amyloid beta protein; AhR Aryl hydrocarbon receptor; ASD Autism spectrum disorder; BBB Blood–brain barrier; BDNF Brain-derived neurotrophic factor; CNS Central nervous system; GABA ɣ-aminobutyric acid; GF Germ-free; MIA Maternal immune activation; SCFA Short-chain fatty acid; 3M-4-TMAB 3-methyl-4-(trimethylammonio)butanoate; 4-TMAP 4-(trimethylammonio)pentanoate; TMA(O) Trimethylamine(-N-oxide); TUDCA Tauroursodeoxycholic acid; ZO Zonula occludens proteins
KEYWORDS: Gut microbiota, gut-brain axis, metabolism, metabolites, short-chain fatty acids
Introduction
The circulating metabolome is influenced by factors including nutrition, health status, and the gut microbiota composition.1 Further, it is possible to predict the blood metabolome through the collection of nutritional, clinical, and microbial data highlighting the host-gut microbiota crosstalk.1–3 As the tight relationship between gut microbiota composition and circulating metabolome has become evident, it is essential to focus on the concomitant evaluation of nutritional intake and the clinical profile alongside microbiota composition.
The central nervous system (CNS) and the intestine form a multifaceted, bidirectional communication network where signals are conveyed by nervous, endocrine, and immune systems. In this so-called ‘gut–brain axis’, the gut microbiota signals the brain through the mentioned networks by activating sympathetic4 and parasympathetic5 neurons in the intestine, by educating the immune system6 and by regulating the production of several neurotransmitters7–9 and gut hormones.10–13 Furthermore, bacterial metabolites are of special interest as they include known neuromodulators,14,15 uremic toxins,16 pro-inflammatory17 and anti-inflammatory mediators18,19 and molecules providing energy for host’s cellular metabolism.20,21 Some metabolites of interest have been implicated in brain functions, such as neurodevelopment and regulation of neuroinflammation, as well as blood–brain barrier (BBB) integrity.
The analysis of the circulating metabolome reflects the crosstalk between nutrition, microbiome, and host metabolism (summarized in Figure 1). Therefore, it may reveal potential biomarker candidates of health and disease, enable understanding of related metabolic processes and can be helpful in rationalizing individual responses to preventive or therapeutic interventions (Box 1). In addition, modulating certain type of neuroactive metabolites, by acting on nutrition and gut microbiota, represents an interesting strategy to prevent and treat neurologic and neuropsychiatric diseases. In this review, our focus is on the small metabolites with a mass below 1600 Da and we describe how neuroactive metabolites produced by the gut microbiota from dietary source are linked to key features of neuronal processes and dysfunction and risk of health outcomes thereafter (Table 1).
Box 1. Gut-derived metabolites: Biomarker or effector? Friend or foe?
| Some gut-derived metabolites such as trimethylamine-N-oxide (TMAO), indoles and phenylacetylglutamine have been strongly associated with pathological outcomes including cardiovascular diseases and death. Robust studies showed that even after adjusting for several confounding factors (i.e. other cardiovascular risk factors) these metabolites can predict death from cardiovascular diseases.22–24 However, there are conflicting observations as phenylacetylglutamine has been highlighted as a biomarker of healthy aging being associated with a shift in microbiome composition toward increased uniqueness, a marker of good health in aged subjects.25 It confirmed previous data showing elevated levels of this metabolite in centernarians.26 Phenylacetylglutamine has also been associated with an increased α-diversity and abundance of ‘beneficial’ bacteria (Akkermansia, genus from Christensenellaceae) despite its potential detrimental effects on cardiovascular health.24 Regarding TMAO, a recent work highlighted that trimethylamine (TMA) is detrimental for the BBB integrity while TMAO was protective and promoted cognitive performance in mice.27 Several other preclinical studies showed that TMAO can be beneficial against atherosclerosis, nonalcoholic fatty liver disease and glucose homeostasis impairments and is required for neurodevelopment.28–31 Indole derivatives, despite their associations with cardiovascular diseases, have been shown to exert anti-inflammatory effects on liver and to be beneficial in inflammatory bowel disease or experimental autoimmune encephalomyelitis model of multiple sclerosis.32–35 The levels of these metabolites are influenced by several factors such as dietary intake of nutrients (e.g., choline, phenylalanine and tryptophan), gut microbiota composition and function, elimination including metabolization (e.g., in the liver) and renal excretion. Interestingly, studies found that TMAO levels are greatly influenced by the presence of a type-2 diabetes or a kidney disease and that elevation of this compound can be due to confounding factor or a reverse causation.36 Fish consumption, recognized for its health-promoting effect, also leads to elevated levels of circulating TMAO.37 Better understanding of the origin of metabolites and their altered levels due to dietary intake, gut microbiota production or host metabolism will help to clarify the relationship between metabolome and health on individual level. Studying the compounds’ effect, alone or in combination, at physiological dose is also important to distinguish what happens in pathological conditions (e.g., loss of renal or hepatic function) or in basal condition. Thus, future studies should gather nutritional, metabolomic, microbial and clinical data to be able to control for confounding factors. |
Figure 1.
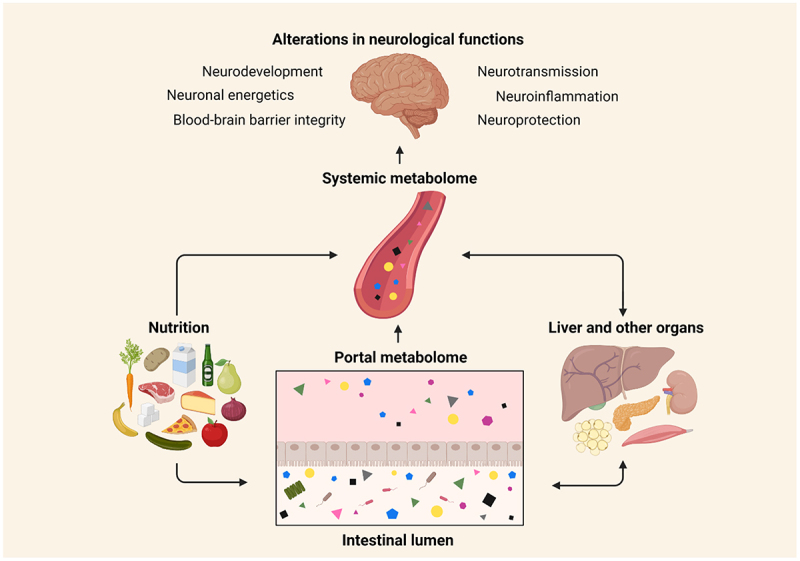
Host metabolic homeostasis and neurological impact.
The portal metabolome reflects the intestinal metabolism of dietary compounds. After processing by the liver, a pool of metabolites will reach the systemic circulation. The systemic metabolome is a dynamic metabolic signature reflecting the interaction between the diet, gut microbiota, liver, and other organs. Metabolites can influence the functioning of all organs, including the brain by modulating a range of metabolic pathways related to neurological functions. (Figure created with Biorender.com)
Table 1.
Gut microbiota-associated metabolites, their biological pathway, preclinical setups, and main outcomes and associations to clinical studies. Direction of outcomes are indicated by symbols as followed; increase (↑) and decrease (↓).
| Mechanism of action | Common name | Chemical formula | Origin | Preclinical model | Main preclinical outcomes | Clinical association | Reference |
|---|---|---|---|---|---|---|---|
| Neurodevelopment | |||||||
| 3-(3-sulfooxylphenyl) propionic acid Indoxyl sulfate Phenyl sulfate Pyrocatechol sulfate |
C9H10O6S C8H7NO4S C6H5O4S C6H6O5S |
Xenobiotic metabolism Tryptophan metabolism Xenobiotic metabolism |
SPF, GF and gnotobiotic C57BL/6 J mice | Fear extinction learning ↑ Altered learning-related neuronal activity |
n.a | 38 | |
| 4-EPS | C8H10O4S | Tyrosine metabolism | C57BL/6 N MIA mice SPF and GF C57BL/6 J mice Ex vivo brain tissue cultures |
Anxiety-like behavior ↑ Oligodendrocyte maturation ↓ CNS neuronal myelination ↓ Altered brain activity patterns |
Fecal levels in ASD children ↑ | 39–41 | |
| 5-AVAB Hippuric acid Imidazole propionic acid TMAO |
C5H11NO2 C9H9NO3 C6H8N2O3 C3H9NO |
Lysine metabolism Xenobiotic metabolism Choline and carnitine metabolism Histidine metabolism |
Ex vivo C57Bl/6 J mice thalamic, striatal and hypothalamic explants of axons | Fetal thalamocortical axonogenesis ↑ | n.a | 28 | |
| 5-AVA Taurine |
C5H11NO2 C2H7NO3S |
Lysine metabolism Bile acid and cysteine metabolism |
Humanized and GF C57BL/6 miceBTBR T+ tf/J mice | Repetitive behavior ↓ Social behavior ↑ Anxiety-like behavior ↓ |
n.a | 42 | |
| p-Cresol | C7H8O | Tyrosine and phenylalanine metabolism | NOD mice C57BL/6 mice Mouse primary oligodendrocyte cultures |
Social behavior ↓ Medial prefrontal cortex myelination ↓ |
Fecal and urinary levels in ASD children ↑ | 16,43,44 | |
| Acetic acid Propionic acid |
C2H4O2 C3H6O2 |
Dietary fiber fermentation | Obese C57BL/6 J mice dams and offspring | Social behavior ↑ Memory scores↑ Microglia maturation ↑Improved synaptic ultrastructure |
Altered fecal SCFA profiles in ASD children | 43,45–47 | |
| Indole | C8H7N | Tryptophan metabolism | GF, SPF and E. coli monocolonized C57BL/6 J mice Ex vivo neural progenitor and stem cell cultures |
Neuronal maturation ↑ Neurogenesis ↑ |
n.a | 36 | |
| Neurotransmission | |||||||
| 4-aminobenzoic acid Deoxycholic acid Tyramine |
C7H7NO2 C24H40O C8H11NO |
Folate metabolism Bile acid metabolism Tyrosine metabolism |
SPF, GF and monocolonized C57Bl/6 J, Slc6a4 KO, Swiss Webster and Rag1 KO mice RIN14B (ATCC) cell cultures |
EECs stimulation ↑ Serotonin biosynthesis by EECs ↑ |
n.a | 9 | |
| p-Cresol | C7H8O | Tyrosine and phenylalanine metabolism | Chronic (p.o.) or acute (iv, ip) administration to Wistar rats and C57BL/6 J or BTBR T+ tf/J mice | Altered brain dopamine metabolism Anxiety-like behavior ↑Social behavior ↓ |
CSF levels ↑ in Alzheimer’s and Parkinson’s disease patients | 48–51 | |
| Butyric acid Isobutyric acid Isovaleric acid Norepinephrine |
C4H8O2 C4H8O2 C5H10O2 C8H11NO3 |
Dietary fiber fermentation Tyrosine/phenylalanine metabolism |
Ex vivo C57BL/6 J mice intestinal organoids and colonic neuron cultures HEK293T (ATCC) cell cultures |
EECs are stimulated by microbial metabolites Serotonin biosynthesis by EECs ↑ EECs modulate primary afferent nerve fibers |
n.a | 52 | |
| Dopamine Norepinephrine |
C8H11NO2 C8H11NO3 |
Tyrosine and phenylalanine metabolism | GF and SPF BALB/c mice BALB/c mice monocolonized with E. coli |
Gut microbes transform luminal dopamine and norepinephrine into biologically active form | n.a | 7 | |
| GABA | C4H9NO2 | Glutamic acid metabolism | BALB/c mice treated with L. rhamnosus (JB-1) probiotics | Altered brain GABA and glutamate/glutamine levels Anxiety-like behavior ↓ Depression-like behavior ↓ Stress ↓ |
Altered GABA and glutamate in postmortem brains of persons with history of heavy alcohol use | 5,14,53,54 | |
| Histamine Phenethylamine |
C5H9N3 C8H11N |
Phenylalanine and histidine metabolism | C57Bl/6 mice monocolonized with M. morganii HEK293 cell cultures | M. morganii strains generate trace amines activating dopamine- and histamine-receptors | Microbial histidine decarboxylase genes are enriched in patients with Chron’s disease | 15 | |
| Indole | C8H7N | Tryptophan metabolism | F344 rats monocolonized with E.coli Acute (i.p.) administration to F344 rats Mouse EEC cultures |
Acute GLP-1 secretion from EECs ↑ Anxiety-like behavior ↑ Helplessness ↑ |
n.a | 55,56 | |
| Indole Indole-3-aldehyde |
C8H7N C9H7NO |
Tryptophan metabolism | HEK-293 T mammalian cell cultures Ex vivo human and mouse small intestine tissue sections |
Activation of EECs via TRP1 receptor ↑ Serotonin biosynthesis by EECs ↑ |
Indole derivatives were positively associated with memory scores | 57,58 | |
| Kynurenic acid | C10H7NO3 | Tryptophan metabolism | Kynurenin aminotransferase II KO mice Acute (i.c.v.) administration to Sprague-Dawley or Long Evans rats |
Brain glutamate levels ↑ by limiting brain kynurenic acid supply Learning ↓ Memory scores ↓ |
Decreased plasma tryptophan and kynurenic acid levels in patients with depressionCognitive functions negatively correlated to plasma kynurenine/tryptophan ratio | 59–64 | |
| Lactate | C3H6O3 | Dietary fiber fermentation | Hooded rats treated with Lactobacillus- and Bifidobacterium probiotics C57BL/6NTac mice monocolonized with Lactobacillus strains |
Plasma and brain lactate ↑ GABA expression and signaling ↑ Memory, learning and neuroplasticity ↑ |
n.a | 20,65,66 | |
| Pipecolic acid | C6H11NO2 | Lysine metabolism | GF and SPF BALB/c mice | Levels of pipecolic acid in the cerebrum ↑ in the presence of microbiota | n.a | 67 | |
| Serotonin | C10H12N2O | Tryptophan metabolism | Lactobacillus and Streptococcus cultures | In vitro production of biogenic amines, including serotonin | Circulating levels decrease in substance use disorders | 68–70 | |
| Tryptamine | C10H12N2 | Tryptophan metabolism | Humanized or B. thetaiotaomicron monocolonized Swiss Webster, 129/Sv and 5-HT4R KO mice Colonic organoids |
Activation of colonic serotonin receptor 4 ↑ Colonic secretion ↑ |
n.a | 71 | |
| Modulation of BBB integrity | |||||||
| p-Cresol glucuronide | C13H16O7 | Tyrosine metabolism | Acute (i.p.) administration to C57BI/6 mice Human CMEC/D3 cultures |
BBB permeability ↓ LPS-stimulated BBB permeability↓ |
n.a | 72 | |
| Acetic acid Propionic acid |
C2H4O2 C3H6O2 |
Dietary fiber fermentation | C57BL/6 J mice monocolonized with B. thetaiotaomicron | BBB permeability ↓ Tight junction protein expression ↑ |
n.a | 73 | |
| Butyric acid | C4H8O2 | Dietary fiber fermentation | C57BL/6 J mice monocolonized with C. tyrobutyricumGF and SPF C57BL/6 J, BALB/c and NMRI mice Acute (ip) administration to C57BL/6 TBI mice |
BBB permeability ↓ Tight junction protein expression ↑ Histone acetylation ↑ Neurological deficits ↓ |
n.a | 73,74 | |
| Deoxycholic acid | C24H40O4 | Bile acid metabolism | Acute (i.v.) administration to sham or BDL-operated Sprague Dawley rats Rat BMEC cultures |
Impairments in tight junction integrity Phosporylation of occludin ↑ |
Serum levels associated with cognitive decline and cerebrospinal fluid t-tau aggregation in Alzheimer’s disease patients | 75–77 | |
| Propionic acid | C3H6O2 | Dietary fiber fermentation | Human postmortem brain endothelium and CMEC/D3 cell cultures | Oxidative stress ↓ LPS-stimulated BBB permeability ↓ |
n.a | 78 | |
| TMA | C3H9N | Choline and carnitine metabolism | C57BL/6 J mice Human CMEC/D3 cultures |
Impairments in barrier integrity Cytoskeleton disruption and metabolic stress ↑ |
n.a | 27 | |
| TMAO | C3H9NO | Choline and carnitine metabolism | Chronic (i.p.) and acute (i.p.) administration to C57Bl/6 J mice Human CMEC/D3 cultures |
Improved barrier integrity Astrocyte and microglial function ↑ |
Cerebrospinal fluid levels ↑ in Alzheimer’s disease patients and associated with markers of Alzheimer’s disease | 27,79 | |
| Ursodeoxycholic acid | C24H40O4 | Bile acid metabolism | Human BMEC cultures | Improved barrier integrity Endothelial cell apoptosis ↓ |
Serum and postmortem brain levels ↑ in Alzheimer’s disease patients | 76,80,81 | |
| Neuroinflammation | Acetic acid | C2H4O2 | Dietary fiber fermentation | Chronic (p.o.) administration to GF and SPF C57BL/6 or 5× FAD micePrimary glia cell culturesEx vivo isolated mice microglia | Microglial metabolism ↑ Microglia maturation ↑ Aβ plaque deposition ↑ in SPF mice but not in GF mice |
n/a | 82 |
| Acetic acid Butyric acid Propionic acid |
C2H4O2 C4H8O2 C3H6O2 |
Dietary fiber fermentation | Chronic (p.o.) administration to GF C57BL/6 mice Chronic (p.o.) administration to surgically or sham-treated Sprague-Dawley rat model of CCH Primary glia cell culturesex vivo brain endothelial cell cultures Chronic (p.o.) administration to GF or SPF Thy1-αSyn and GF APPPS1 mice |
Microglial activity ↑ Microglia maturation ↑ Neuroinflammation, cognitive decline and depressive-like behavior ↓ rat model of CCHα-syn aggregation ↑ in genetically predisposed SPF mice Aβ plaque deposition ↑ in genetically predisposed GF mice |
Brain amyloid load positively associated with plasma acetic acid and inversely associated with plasma butyric acid in patients with cognitive complaints | 83–87 | |
| Dihydrocaffeic acid | C9H10O4 | Xenobiotic metabolism | Chronic (p.o.) administration to CD45.2+ C57BL/6 miceMice PBMC and neuron tissue cultures | Pro-inflammatory cytokine production ↓ | n.a | 88 | |
| Indoxyl sulfate | C8H7NO4S | Tryptophan metabolism | Acute (i.p.) administration to C57BL/6 mice Chronic (p.o.) administration to Albino Wistar rats Ex vivo rat and mice glial cell cultures |
Oxidative stress ↑ Pro-inflammatory cytokine production ↑ Locomotor activity ↑ Spatial memory ↓ Apathic behavior ↑ |
CSF levels ↑ in patients with Parkinson’s diseaseUrinary levels positively associated to recurrent depressive symptoms | 17,51,89,90 | |
| Indole Indole-3-aldehyde Indolepropionic acid Indoxyl sulfate |
C8H7NO C9H7NO C11H11NO2 C8H7NO4S |
Tryptophan metabolism | C57BL/6 J EAE and CX3CR1-AHR mice Ex vivo C57BL/6 J mice microglia and astrocyte cell cultures Primary human microglia, brain and fetal astrocyte cultures |
Type 1-interferon signaling ↓ EAE disease scores ↓ Pro-inflammatory cytokine expression ↓ |
Serum AhR activating tryptophan metabolites ↓ in patients with multiple sclerosis | 32,91 | |
|
N6- carboxymethyllysine |
C8H16N2O4 | Lysine metabolism | Chronic (i.p. or p.o.) administration to SPF and GF C57BL/6 mice Human cortical tissue and mice brain preparations Bone marrow-derived macrophage cultures |
Oxidative stress in microglia ↑ Microglial dysfunction ↑ |
Associated with oxidative stress in brain tissue of elderly and patients with Alzheimer’s disease and/or diabetes mellitus | 92–94 | |
| Propionic acid | C3H6O2 | Dietary fiber fermentation | Acute (i.c.v or i.v.) administration to Western albino, Long-Evans, seizure-prone or seizure-resistant rats Chronic (p.o.) administration to C57BL/6 EAE miceEx vivo mice Treg-cell cultures |
Astrogliosis and microglial activity ↓ Hyperactivity ↑ Social behavior ↓ Inflammatory cytokines and oxidative stress ↑ Treg-cell differentiation ↑ CNS autoimmunity ↑ |
n.a | 95–100 | |
| TMAO | C3H9NO | Choline and carnitine metabolism | Chronic (p.o.) administration to surgically or sham treated F344× BN F1 rats | Hippocampus oxidative stress ↑ Neuroinflammation ↓ Postoperative cognitive decline ↑ |
Positive and inverse associations with Parkinson’s or Alzheimer’s diseases | 101–105 | |
| Urolithin A | C13H8O4 | Xenobiotic metabolism | Chronic (p.o.) administration to C57BL/6 EAE miceEx vivo C57BL/6 mice CNS mononuclear and dendritic cell culturesEx vivo 2D2 TCR mice Th17 cell culturesMurine BV-2 microglia cell cultures SPF mice model of ischemic stroke |
Pro-inflammatory cytokine gene expression ↓ Microglia, dendritic and T-cell activation ↓ Neuroinflammation ↓ Neuronal apoptosis ↓ |
n.a | 93,94,106–108 | |
| Neuronal energy metabolism | |||||||
| 3M-4-TMAB 4-TMAP |
C8H18NO2C8H18NO2 | Carnitine metabolism | GF and SPF C57BL/6 J mice Primary murine CNS white matter cell culture |
Fatty acid oxidation in CNS white matter tissue ↓ | n.a | 109 | |
| Acetic acid Butyric acid Indolepropionic acid Propionic acid TUDCA |
C2H4O2 C4H8O2 C11H11NO2 C3H6O2 C26H45NO6S |
Dietary fiber fermentation Tryptophan metabolism Dietary fiber fermentation Bile acid metabolism |
Chronic (p.o. or i.p.) administration to db/db mice | Improved cognitive functionsImproved mitochondrial function Mitochondrial biogenesis and function ↑ |
n.a | 110 | |
| Butyric acid Propionic acid |
C4H8O2 C3H6O2 |
Dietary fiber fermentation | Chronic (i.c.v.) administration to Long-Evans rats | Locomotor activity ↑ Altered brain fatty acid profiles |
n.a | 111 | |
| Butyric acid | C4H8O2 | Dietary fiber fermentation | Neuroblastoma or lymphoplastoid cell cultures derived from ASD males | Improved mitochondrial function Mitochondrial gene expression ↑ |
n.a | 112 | |
| Ethanol | C2H5OH | Energy metabolism | SPF or humanized C57BL/6 J mice | Depression-like behavior ↑ Circulating β-hydroxybutyrate ↓ Social behavior ↓ |
Microbial ethanol ↑ together with β-hydroxybutyrate ↓ in alcohol-dependent subject with behavioral alterations | 113 | |
| Lactate | C3H6O3 | Energy metabolism | Chronic (p.o.) administration to CC042 miceC57BL/6NTac mice monocolonized with Lactobacillus species Exercised or acute (i.p.) administration to C57BL/6 mice |
Long-term memory formation ↑ Hippocampus GABA ↑ Learning ↑ Hippocampus BDNF ↑ |
n.a | 65,66,114 | |
| Indolepropionic acid | C11H11NO2 | Tryptophan metabolism | Mice neuro2a-APPsw cell cultures | Restoration of cell respiratory rate Reactive oxygen species ↓ |
n.a | 115 | |
| Neuroprotection | |||||||
| 3-(3′-hydroxyphenyl) propionic acid 3-hydroxybenzoic acid |
C9H10O3 C7H6O3 |
Xenobiotic metabolism | Chronic (p.o.) administration to Sprague-Dawley rats Synthetic Aβ preparations |
Aβ plaque deposition ↑ Neuroplasticity ↑ |
n.a | 116 | |
| Ergothioneine | C9H15N3O2S | Histidine metabolism | Chronic (p.o.) administration to stressed Sprague Dawley rats or Aβ-injected C57BL/6 mice | Intracerebral oxidative stress ↓ Aβ plaque deposition ↓ Depression-like behavior ↓ Social behavior ↑ |
Plasma levels ↓ in patients with mild cognitive impairment or Parkinson’s disease | 117,118 | |
| Ferulic acid | C10H10O4 | Xenobiotic metabolism | Rat pheochromocytoma cell culturesChronic (p.o.) administration corticosterone-treated Swiss mice, PSAPP mice or Sprague-Dawley rats with acute ischemia | Oxidative stress ↓ Aβ plaque deposition ↓ Neuronal apoptosis ↓ Depression-like behavior ↓ |
n.a | 18,119,120 | |
| Indolepropionic acid | C11H11NO2 | Tryptophan metabolism | SK-N-SH human neuroblastoma cell cultures E-18 fetal rat primary neuron cell cultures |
Neuronal apoptosis ↓ Oxidative stress ↓ |
n.a | 121 | |
| Menadione Menaquinone Phylloquinone |
C11H8O2 C31H40O2 C31H46O2 |
Energy metabolism | α-Synuclein fibril preparations | α-syn aggregation ↓ | n.a | 122 | |
| TUDCA | C26H45NO6S | Bile acid metabolism | Chronic (p.o.) administration to APPPS1 mice | Aβ plaque deposition ↓ Neuronal degeneration ↓ |
n.a | 123 | |
Abbreviations: 3 -M-4-TMAB, 3-methyl-4-(trimethylammonio)butanoate; 4-EPS, 4-ethylphenylsulfate;; 4-TMAP, 4-(trimethylammonio)pentanoate: 5-AVA(B), 5-aminovaleric acid (betaine); Aβ, amyloid beta protein; AD, Alzheimer’s disease; AhR, aryl hydrocarbon receptor; ASD, autism spectrum disorder; BDL, bile duct ligation; BMEC, brain microvascular endothelial cells; CCH, chronic cerebral hypoperfusion; CNS, central nervous system; EAE, experimental autoimmune encephalomyelitis; EEC, enteroendocrine cell; GABA, γ-aminobutyric acid; GF, germ-free; I.C.V, intracerebroventricular; I.P., intraperitoneal; I.V., intravenous; MIA, maternal immune activation; NOD, nonobese diabetic; PBMC, peripheral blood mononuclear cell; P.O., per os; SCFA, short-chain fatty acid; SPF, specific-pathogen free; TBI, traumatic brain injury; TMA(O), trimethylamine(-N-oxide); TUDCA, tauroursodeoxycholic acid.
Microbiota-related metabolites modulating brain function
Neurodevelopment
Mouse models devoid of gut microbiota, the germ-free (GF) mice, elicit characteristics supporting the view that the gut microbiota is crucial for the normal brain development and behavior.124,125 Desbonnet et al.126 showed that despite of normal gut microbiota at birth, mice treated with antibiotics in the early weeks of life display cognitive and behavioral alterations later in life. Given that the neurodevelopmental processes are initiated in the prenatal phases, maternal microbiota, and related molecules may influence fetal development as observed in the field of early immune development.127–129 The maternal immune activation (MIA) mice model of autism spectrum disorder (ASD) was used in the pivotal work by Hsiao et al.39 to study how the maternal microbiota influences offspring’s brain development. The postnatal serum metabolomic analyses revealed that the level of 4-ethylphenylsulfate (4-EPS), a tyrosine-derivative, was 46-fold higher in MIA offspring compared to control offspring. Postnatal treatment with the human commensal Bacteroides fragilis improved several features of ASD in MIA offspring and restored the 4-EPS levels which were explained by the improvements in intestinal permeability and thus decreased translocation of 4-EPS to the circulation. The microbiota modulates the production of 4-EPS as it was nearly undetectable in GF compared to specific pathogen-free mice. Additionally, administration of 4-EPS to naive wild-type mice at postnatal weeks 3–6 induced anxiety-like behavior similar to MIA offspring. The biosynthetic pathway and mechanisms behind the detrimental effects of 4-EPS were lately elucidated by Needham et al.40 showing that 4-EPS interfered with oligodendrocyte maturation, myelination, and brain activity patterns in brain areas of the limbic system. p-Cresol, a tyrosine-derivative and a metabolite differing from 4-EPS by the methyl substitution in the phenyl ring instead of ethyl, has also been directly associated with neurodevelopmental disorders. Social behavior impairments were triggered by daily gavages of vehicle but not with antibiotics and a fecal microbiota transplant from vehicle-treated mice to recipient mice transferred the behavioral traits.16 The recipients also displayed decreased medial prefrontal cortex myelination and high levels of circulating p-cresol.
Interestingly, some microbial products may also protect from ASD behavioral features as reported by Sharon et al.42 in GF mice offspring colonized by human ASD microbiota. Compared to mice with microbiota from normally developing donors, the ASD mice had significantly lower levels of 5-aminovaleric acid (5-AVA) and taurine, products of amino acid metabolism by the microbiota. Moreover, administration of these metabolites during the prenatal period or before reaching postnatal age of 4 weeks ameliorated ASD-like behavior in the offspring. Maternal obesity-induced cognitive and social deficits in the offspring were also reversed by post-weaning supplementation of short-chain fatty acids (SCFAs) acetic and propionic acid together with improvements in synaptic ultrastructure and microglial maturation in the hippocampus.45 Microbiota signaling during a critical development period was also required for fear extinction learning and related neuronal plasticity in mice.38 Moreover, multi-site metabolomics revealed four phenolic derivatives and sulfates: phenyl sulfate, pyrocatechol sulfate, 3-(3-sulfooxyphenyl)propanoic acid and indoxyl sulfate, as potential microbiota-derived signaling molecules in the context of fear learning and behavior.
In a recent work, Vuong et al.28 demonstrated that maternal microbiota has a crucial role in fetal thalamocortical axonogenesis, the axonal branching connecting thalamus to the cortical areas of the brain. Subsequently, absence or depletion of maternal microbiota impaired the neurobehavioral responses of the adult offspring. Colonization by Clostridia-dominant bacteria was sufficient to restore fetal axonogenesis, and the effect was mediated through selected bacteria-derived metabolites. TMAO, 5-AVA, 5-aminovaleric acid betaine (5-AVAB), imidazolepropionic acid, and hippuric acid were found to promote axonogenesis both in vitro and in vivo, in the absence of live bacteria.28 Although the microbiota-dependency and maternal translocation of the named metabolites to the fetus have been reported in several works,67,130–132 their contribution to host neurodevelopment is a novel finding likely to spark intensive further research. Additionally, the neurogenic properties of metabolites may not be limited to early life since indole, a tryptophan metabolite, was found to increase neurogenesis in the hippocampus of adult mice.36 The effect mediated by aryl hydrocarbon receptor (AhR) was specific to indole because another AhR ligand and also a tryptophan-derivative, kynurenine, did not induce any changes in neurogenesis ex vivo.
Studies on the relationship between human neurodevelopment and gut microbiota metabolites rely on limited number of cross-sectional and associative works done mainly in children with ASD. These children display deviations in a range of sulfates when compared to normally developing children. For instance, the elevation of 4-EPS was observed in plasma41 in line with earlier findings of the increase in urinary and fecal p-cresol sulfate43,44 in children with ASD. De Angelis et al.43 also noted elevations in the fecal indole levels among the ASD group. Results on differences between fecal SCFA concentrations, fermentation products of dietary fiber, in children diagnosed with ASD compared to normally developing children have been inconclusive as increased,46 decreased,43,47,133 and comparable134,135 concentrations have been reported.
Human studies initiated at the early stages of life with long follow-up times and application of multi-omics approaches are essential to understand how the host–microbe interactions influence neurodevelopment. Efforts such as the first pilot studies employing fecal microbiota transplantation in children136 and infants137 open new possibilities to study whether there are long-term effects on neurodevelopment or behavior in early-life interventions targeting the gut microbiota composition and functionality.
Neurotransmission
In addition to the production of neurotransmitters, gut microbiota, and its metabolites can influence host’s central metabolism of neuroactive compounds.130 The most robust examples of gut bacteria-derived neurotransmitters are aromatic amino acid derivatives dopamine and norepinephrine138 and glutamate derivative γ-aminobutyric acid (GABA).14 In the gut lumen, microbiota significantly contributes via the activity of β-glucuronidase to the levels of free dopamine and norepinephrine.7 However, this may not directly translate into increased brain levels of dopamine as GF mice had higher concentration of dopamine in cerebrum compared to conventionalized mice.67 Also, inconsistent tissue-specific variation has been observed in GABA levels as conventionalized mice exhibit substantially higher levels of GABA within the colon and the blood but not in the brain in relation to their GF counterparts.67,139
Kynurenic acid, a tryptophan metabolite, serves as a modulator of extracellular glutamate as it reduces glutamate levels that participate in the glutamatergic signaling in the hippocampus.59,60 Consequently, increasing glutamate levels through limiting hippocampal kynurenic supply enhanced cognitive abilities and memory in rats and mice.59–61 Deviations in the CNS and peripheral glutamate-glutamine-GABA metabolism were depicted by elevated glutamine and GABA and decreased glutamate in hippocampus while serum GABA remained unchanged in mice after fecal microbiota transplantation from schizophrenic patients.140 However, a 4-week treatment with Lactobacillus rhamnosus increased glutamate, GABA and N-acetyl aspartate concentrations in mice brains, returning to baseline after 4-weeks of treatment cessation.55 L. rhamnosus treatment was also shown to alter brain GABA receptor expression in a regionally dependent manner and reduce stress-induced corticosterone and anxiety- and depression-like behavior in mice.5 These effects occurred through vagal communication as vagotomy prevented such changes suggesting a peripheral, gut-centered route to modulate brain functions and behavior.
Over 90% of serotonin in the body is produced by the enterochromaffin cells of the gut synthesizing serotonin from tryptophan, a process where gut commensals have an important regulatory role.8,9 Tyramine, deoxycholic acid, and 4-aminobenzoic acid were found to stimulate serotonin synthesis both in vitro and in vivo.9 Moreover, microbiota-associated metabolites like norepinephrine, indole, indole-3-aldehyde, isovaleric acid, butyric acid, and isobutyric acid stimulate enterochromaffin cells to release serotonin, which then engages with serotonin-receptor-expressing nerve fibers.52,57 In addition, some gut bacteria can produce serotonin but the physiological significance of this is currently unknown.68,141
The presence of pipecolic acid in the CNS can be partially derived from the microbiota130 and has been associated with GABA signaling and release.142,143 Another metabolite with a plausible connection to GABA expression in the brain, hippocampus and frontal cortex more precisely, is lactate.20,65 Lactate can also induce brain-derived neurotrophic factor (BDNF) expression in the hippocampus through the upregulation of Sirtuin1 deacetylase.66 Together, these studies show that lactate affects neural plasticity and has a beneficial effect on learning and memory in mice. SCFAs, particularly butyric acid, may also have additional regulatory effects on the production of neurotrophic factors, such as BDNF, signal the brain via vagal nerve and induce biosynthesis of neurotransmitters in the CNS.21 Individual administration of SCFAs butyric, acetic, and propionic acid ameliorated stress-responsivity, anxiety- and depressive-like behavior in mice concomitant with downregulation of several hypothalamic genes involved in stress signaling.144 Metabolites may also have negative neuromodulatory activities: administration of p-cresol has been observed to modulate oxytocinergic and opioidergic systems, dopamine turnover and receptor activity in specific brain regions while eliciting negative traits in social and anxiety-like behaviors in mice or rats.48–50 Such findings may partially explain the harmful effects of p-cresol on neurodevelopment.
Selected gut microbial species can also produce neurotransmitter receptor agonists or induce local synthesis of neuroactive mediators. Phenylethylamine is a full dopamine receptor agonist produced by Morganella morganii strains and capable of crossing the BBB.15 The previously described 5-AVA and taurine may also act as weak GABAA receptor agonists.42 Tryptamine is found in low concentrations in the brain, produced by selected bacterial strains of the Clostridium sporogenes and Ruminococcus gnavus species145 and acts through epithelial serotonin-receptor (5-HT4), a G-protein coupled receptor, increasing colonic secretion and gut transit time without affecting the colonic serotonin excretion.71 Indole-derivatives accumulate in the brain;55,146 overproduction of indole, whether acute or chronic, triggered anxiety-like behavior and stimulated vagal afferent fibers in the intestinal mucosa in rats.55 However, in mice, the chronic overproduction of indole had adverse effects on behavior only when mice were subjected to chronic stress.146 Controversially, increased indoles, especially indoleacetic acid, were linked with reduced anxiety-like behavior in mice receiving fecal microbiota transplantation from patients with irritable bowel syndrome and subsequently treated by probiotic strain Saccharomyces boulardii.147 Indole also promotes short-term glucagon-like-peptide-1 release from colonic L cells,56 while SCFAs induce the secretion of both glucagon-like-peptide-1 and peptide YY from the enteroendocrine cells and leptin from adipocytes, all hormones participating in the food intake regulation signaling in the central and peripheral nervous system.10−13
These observations described above suggest that neurotransmitters, neurotransmitter receptor agonists and other neuroactive mediators produced by the gut microbiota signal the brain via the peripheral nervous system and can alter the synthesis pathways of neurotransmitters in the brain. While the findings from clinical studies are associative and focus on plasma metabolites, they support the preclinical results, to some extent. Decreased circulating levels of serotonin are a classical sign of related brain disorders like depression and alcohol use disorder.69,148,149 For example, reduced circulating serotonin levels precede future diagnosis of alcohol use-related diseases even when baseline alcohol drinking is accounted for.70 However, the regulation of circulating serotonin levels is multifaceted and influenced by external factors, such as diet.148,150 The situation of serotonin system in the brain is even more complex, since serotonin is an important modulator of neurotransmission and therefore the brain levels are controlled differently to the levels in the blood.148,149 The tryptophan metabolism, especially increased catabolism of tryptophan through the kynurenine pathway, has been linked with depression and ASD in humans.151 Arnoriaga-Rodríguez et al.58 showed that while tyrosine and tryptophan correlated positively with memory scores, the associations between their catabolites and memory scores were only evident in the obese but not in lean subjects and bacterial functions related to tryptophan and phenylalanine metabolism were negatively correlated with performance in memory tests. Cognitive functions such as learning were also negatively correlated with plasma kynurenine/tryptophan ratio in female subjects with medically diagnosed depression.62 A meta-analysis of the peripheral blood metabolites in medically diagnosed patients showed consistent reduction of tryptophan and kynurenic acid relative to healthy controls.63 Very recent data associated urinary excretion of indoxyl sulfate, the major final metabolite of indole, to recurrent depressive symptoms in female patients.89 Administration of the so-called psychobiotics – probiotic strains that could benefit mental health – reduced plasma kynurenine level while improving cognitive functions64 and increased gene expressions related to the tryptophan-serotonin metabolism in stressed adults.152 Valles-Colomer et al.141 found a positive correlation between the mental quality of life score and the microbial pathway synthesizing a dopamine metabolite 3, 4-dihydroxyphenylacetic acid. However, 3, 4-dihydroxyphenylacetic acid has also been proposed as a biomarker of neurodegenerative disorders, mainly Alzheimer’s disease.153
Blood–brain barrier integrity
The BBB is a semipermeable diffusion barrier separating circulating blood and brain regions, apart from the circumventricular organs.154 Together with astrocytes, pericytes, microglia, neurons, and extracellular matrix, the brain microvascular endothelial cells connected by tight junction proteins form a dynamic cellular system maintaining brain homeostasis. In its neuroprotective role, the barrier restricts pathogens, peripheral immune factors, and large molecules from entering the brain, also limiting the passage of gut metabolites to the CNS. Gut microbiota is vital for the normal barrier function during pre- and postnatal periods as BBB permeability was increased in GF compared to specific pathogen-free mice.73 Moreover, colonizing GF mice with butyric acid-producing C. tyrobutyricum or acetic and propionic acid-producing Bacteroides thetaiomicron or oral administration of sodium butyrate restored the BBB permeability to the status seen in the controls. The administration of sodium butyrate increased the expression of occludin in the frontal cortex and hippocampus and increased brain histone deacetylation, which was also detected in C. tyrobutyricum inoculated GF mice. Accordingly, in a mice model of traumatic brain injury characterized by severe BBB disruption, a single intraperitoneal injection of sodium butyrate reproduced the partially alleviating effects on BBB integrity through increased expression of zonula occludens-1 (ZO) protein and occludin.74
Addition of a physiologically relevant dose of propionic acid to an in vitro model of human BBB showed the modulatory effect being independent of tight junction protein expression.78 Instead, propionate promoted BBB integrity by mitigating oxidative and pro-inflammatory pathways and by reducing the expression of a specific efflux transporter low-density lipoprotein receptor-related protein 1. High affinity monocarboxylate transporters facilitate the translocation of SCFAs and are abundantly expressed on the endothelial cells, neurons, and astrocytes.21 Furthermore, SCFA receptor FFAR3 is widely expressed in the sympathetic nervous system and has been found in rat – but not in mice – brain tissue. However, contrary to the verdicts from in vitro and rat studies, the uptake of SCFAs into the CNS seems to be nominal in humans as peripheral levels are in µmolar range compared to the picomolar range observed in the brain tissue.21 This implies that the effects of SCFAs may rather result from peripheral signaling instead of direct uptake to the brain as seen in animal models.
Aside from the SCFAs, secondary bile acids deoxycholic acid and ursodeoxycholic acid may modulate BBB integrity. Injection of deoxycholic acid in rats increased BBB permeability demonstrated by colorimetric assay and albumin immunoreactivity.75 The mechanism was mediated by disruption of the tight junction proteins occludin and ZO-1 and ZO-2. On contrary, in an in vitro model mimicking the effect of severe hyperbilirubinemia on BBB, ursodeoxycholic acid partially protected endothelial cells from apoptosis and partially restored the barrier integrity.80
Lastly, recent data sustain that trimethylamine (TMA), a metabolite derived from dietary choline, betaine, and L-carnitine had a dose-dependent detrimental impact on the barrier integrity in an in vitro BBB model by impairing the actin cytoskeleton and inducing metabolic stress.27 Interestingly, the oxidized form of TMA, TMAO, improved the barrier integrity both in vitro and in vivo when physiologically relevant doses were used. Systemic administration of TMAO to wild-type male mice protected from lipopolysaccharide-induced damages to the BBB function and prevented the loss of performance in the working memory test. Similarly, a host–gut co-metabolite p-cresol glucuronide improved BBB integrity in vivo and prevented lipopolysaccharide-induced permeabilization of the BBB in vitro by acting as a Toll-like receptor 4 antagonist.72
Selectivity of the BBB restricts the passage of most metabolites to the brain although transporters, diffusion, or transcytosis could facilitate crossing of the BBB of certain compounds (Figure 2) corroborating the evidence from GF mice.28,54,131 Nevertheless, microbial metabolites such as 5-AVAB, TMAO, p-cresol sulfate, and hippuric acid among others have been found in the human brain.54 In addition to translocation to the brain, the metabolites could alter barrier function to some extent but the physiological significance of this in humans is yet to be determined. In Alzheimer’s disease patients the increase in microbially produced deoxycholic acid and its taurine or glycine conjugated forms in the serum had a strong association with cognitive impairment76 and cerebrospinal fluid t-tau aggregation.77 A gradual increase in the plasma lithocholic acid along the disease progression over follow up time of 9 years has also been recorded.155 In the brain, the concentrations of taurolithocholic, 3-dehydrochenodeoxycholic, and ursodeoxycholic acid were significantly higher in relation to controls.81 The findings from genome-scale reconstructions suggested that the potential to synthetize these molecules is limited to a small number of bacteria species156 that could be the focus of future studies to scrutinize the role of bacterial bile acid transformation and BBB integrity in neurodegenerative diseases.
Figure 2.
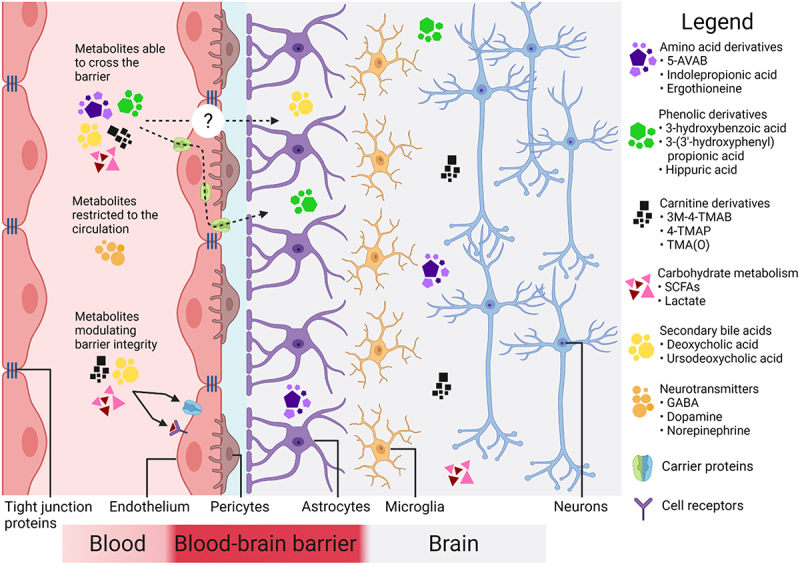
The integrity and selectivity of the blood–brain barrier in terms of microbial metabolites.
The blood–brain barrier restrains the passage of most microbially produced molecules from the circulation to the central nervous system. Only certain amino acids, carnitine, and phenolic derivatives together with secondary bile acids and products of carbohydrate metabolism may cross the blood–brain barrier. Carrier proteins such as monocarboxylate transporters, receptor-mediated or adsorptive transcytosis facilitate the metabolite’s translocation. Instead of crossing the blood–brain barrier, metabolites may alter the blood–brain barrier integrity resulting into increased permeability and translocation. Abbreviations: 5-AVAB, 5-aminovaleric acid betaine; GABA, ɣ-aminobutyric acid; SCFA, short-chain fatty acid; 3M-4-TMAB, 3-methyl-4-(trimethylammonio)butanoate; 4-TMAP, 4-(trimethylammonio)pentanoate; TMA(O), trimethylamine (-N-oxide). (Figure created with Biorender.com)
Neuroinflammation
The inflammatory responses of the CNS to infection, non-sterile inflammation (brain lesion, traumatic brain injury, etc.), pathological conditions, obesity/metabolic diseases (meta-inflammation), and aging (inflammaging) are characterized by the production of pro-inflammatory cytokines, reactive oxygen species, and chemokines by invading immune cells and/or resident brain cells.157,158 However, depending on the magnitude and extent of neuroinflammation, and the phagocytic capacity of microglia, the resident brain macrophages, the outcomes may support brain return to homeostasis or elicit neuropathological processes, such as the one associated with neurodegenerative diseases and traumatic injuries.159
Studies in GF mice have indicated that microbiota has an integral role in the modulation of host immune development, both locally in the intestine and in the CNS.83,160–162 Bacterial components are recognized by the host immune system and this constant crosstalk maintains the immune homeostasis and educates the system to discriminate pathogens from symbionts. Nevertheless, bacterial metabolites also contribute to this crosstalk and can modulate inflammation, including in the brain.162 For example, TMAO administration exacerbated postoperative cognitive dysfunction in rodents, possibly through its effect on increased microglia activation and neuroinflammation.101,102 In aged mice, the microbiota-related TMAO and N6-carboxymethyllysine were upregulated in the serum and brain tissue, but only N6-carboxymethyllysine induced microglial dysfunction by interfering with mitochondrial function and increasing oxidative stress.92 Contrary to aged mice, such effects were evident in young mice only after intraperitoneal, but not oral administration. The assessment of gut permeability revealed age-dependent changes in microbiota composition facilitating the disruption of gut barrier properties and concomitant translocation to the circulation and brain.
On the other hand, malformed microglia in the absence of microbiota could be restored by administration of gut microbial metabolites, such as SCFAs that reinstated the T cell balance in the CNS.83,95 Such findings indicate that SCFAs can participate in the immune cell maturation and homeostasis in the CNS via peripheral signaling. Recent data further indicate that SCFAs (as a mix of butyric, propionic, and acetic acid) increase microglial recruitment and reactivity.84 Also, reinstating the SCFA-producing floras or administration of SCFAs inhibited hippocampal neuroinflammation and neuronal apoptosis, thus ameliorating cognitive decline and depressive-like behavior in a rat model of chronic cerebral hypoperfusion.85 Controversially, propionic acid alone has been linked with CNS oxidative stress, astrogliosis, hyperactivity, and social behavior abnormalities in a series of rat models of ASD.96–100 Interestingly, SCFAs had no effect on the α-synuclein aggregation in vitro, but induced aggregation in selected brain regions and promoted motor deficits in an in vivo genetic model of Parkinson’s disease.86 Correspondingly, SCFAs were found to increase amyloid beta protein (Aβ) deposition and microglia derived ApoE expression in an in vivo model of Alzheimer’s disease but not in vitro.84 Acetate induced GF mice’s microglia maturation and restored defects in the microglial mitochondrial metabolism but decreased microglia phagocytosis in an mice model of Alzheimer’s disease resulting in increased Aβ deposition.82 SCFAs could be of high interest in regulating microglia activity through the maintenance of physiological neuroimmune activity and through their polarization toward a pro-resolutive phenotype in neurodegenerative diseases.
Pro-inflammatory cytokines are well-known contributors to the pathophysiology of mood disorders through their action in the brain.163,164 Dihydrocaffeic acid was identified as a potential suppressor of peripheral IL-6 in a mice model of stress-induced depression.88 This compound with antioxidant properties is a polyphenol derivative from caffeic acid and requires the biotransformation of the gut bacteria. The mechanism behind the attenuated depression symptoms after the administration of dihydrocaffeic acid was independent of changes in the brain monoaminergic pathways. Rather, it was suggested to derive from epigenetic changes in the form of DNA methylation in genes coding IL-6 resulting in decreased pro-inflammatory cytokine expression.
Tryptophan-derived metabolites are a major group of AhR activators and the significance of AhR-signaling pathway has been recently associated with intestinal immune responses and neurological signaling.165,166 Tryptophan is catabolized through several pathways, including the kynurenine pathway, that produces intermediates with both pro- and anti-inflammatory properties, such as indoles, although their overall significance remains controversial.167 For instance, indoxyl sulfate promoted oxidative stress and inflammation in mice primary astrocyte and mixed glial cell cultures and caused dose-dependent neuronal cell death.17 Furthermore, single administration in vivo was followed by histological brain alterations and increase in inflammatory markers in mice. In rats, chronic indoxyl sulfate treatment modeling patients with chronic kidney disease resulted in behavioral alterations and impairments in spatial memory and locomotor activity.90 Controversially, indoxyl sulfate, indolepropionic acid, and indole-3-aldehyde modulated astrocyte activation and suppressed CNS inflammation both in vitro and in vivo in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis.32,91 Furthermore, AhR was also activated by indoxyl sulfate in human astrocytes followed by a decrease in proinflammatory gene expression. Samples from individuals with multiple sclerosis suggested that AhR-dependent regulation was impaired together with reduced number of tryptophan-derived activators.32 Such contradicting results may spur from species- or disease-related differences, intensity, and duration of indoxyl sulfate administration or currently unspecified agonistic properties. Recently, urolithin A, a gut-derived metabolite of ellagic acid, has been shown to reduce neuroinflammatory processes and microglia activation, and alleviate symptoms of experimental multiple sclerosis, through AhR.106,107 In a mouse model of stroke, urolithin A reduced neuroinflammation and neuronal loss limiting the deficits in neurological functions.108
A number of preclinical works suggest that SCFAs are imperative bacterial metabolites in terms of immune modulation and neuroinflammation but with their limited translocation to the brain, the suppression of neuroinflammation is likely transmitted via peripheral signaling.21 Moreover, the detrimental effects of intracerebroventricular propionic acid imply that within the CNS, SCFAs might aggravate inflammatory responses.96–99 However, the number of clinical studies with SCFAs is limited and results on systemic inflammation markers are inconclusive.87,168 Bacterial metabolites that function as AhR agonists can reach the CNS and regulate inflammation, but the overall outcome is dictated by factors, such as the health status of the host and the balance between pro- and anti-inflammatory agonists.165 For instance, Sankowski et al.51 demonstrated in a pilot study that the cerebrospinal fluid/plasma ratios of indoxyl sulfate, TMAO, and p-cresol sulfate were 4–8 times higher in Parkinson’s disease patients than in healthy controls and their concentration in the cerebrospinal fluid was linked to a more advanced disease stage in the absence of decreased kidney function. In addition to Parkinson’s disease, TMAO-related microbial genetic pathways have been associated with Alzheimer’s disease153 and elevated TMAO in the cerebrospinal fluid has been recorded in patients with Alzheimer’s disease,79 in the plasma of patients with post-stroke cognitive impairment103 in addition to inverse correlation to cognitive performance102 in middle-aged and older humans. However, recent contradicting evidence did not support causality between TMAO and Alzheimer’s disease104 accompanied with findings of lower plasma TMAO in Parkinson’s patients105 compared to controls. Finally, the advanced glycation end product N6-carboxymethyllysine has been identified in brain tissue and associated with oxidative stress in elderly and patients with Alzheimer’s disease or diabetes.93,94
Neuronal energy metabolism
The neurons, principal component of the nervous tissue use up to 80% of the brain’s energy production to maintain the excitability of the synapses.169 Brain cells rely mainly on glucose, although ketone bodies and lactate can be utilized flexibly to support the energy demand of the neuronal network. Astrocytes can store glucose as glycogen or metabolize it via glycolysis yielding lactate to be oxidized in neurons and this astrocyte-neuron glycogenolytic lactate production and transporting is crucial for long-term memory formation and subsequent synaptic plasticity that is not sustained by glucose delivery alone.114 Peripherally supplied lactate can also reach the CNS since bacteria-produced lactate was identified as the source of memory enhancements in mice.20 The lactate production in astrocytes is also entangled with the glutamine-glutamate cycle, possibly linking the observed effects to the downstream production of neurotransmitters glutamate and/or GABA, both participating in learning and memory functions.170,171 Nevertheless, lactate itself augments neural activity as the primary energy source and the lactate receptor GPR81 is enriched in regions of cerebral neocortex, hippocampus, and the BBB highlighting its several functions in neural processes.172,173
Fecal microbiota transplantation from subjects with alcohol use disorders to recipient mice resulted in enrichment of ethanol-producing bacterial species, reduction in lipolysis, and the supply of ketone bodies in the circulation.113 Specifically, depletion of β-hydroxybutyrate was associated with increased inflammatory responses and decreased markers of myelination and social behavior. The latter findings were also replicated in a cohort of subjects with alcohol use disorder.113 Moreover, ketogenic meals or administration of β-hydroxybutyrate has been shown to improve memory or cognitive performance in elderly or type 2 diabetic subjects.174,175 Also, the GABAergic neurons can utilize β-hydroxybutyrate to produce neurotransmitters GABA and glutamate,176 which are altered in the postmortem brains of persons with history of heavy alcohol use.54
Recently, intermittent fasting was shown to improve cognitive function via gut–brain axis in db/db mice and simultaneously modulate brain energy metabolism in hippocampus.110 Fasting significantly increased the plasma levels of indolepropionic acid and tauroursodeoxycholic acid (TUDCA) and fecal levels of SCFAs. Individual administrations of indolepropionic acid or TUDCA or a mixture of SCFAs acetic, propionic and butyric acid were able to reproduce the effects of fasting in cognition, hippocampal mitochondrial biogenesis, and energy metabolism-related gene expression. Previously, incubating mice brain mitochondrial preparations with indolepropionic acid supported mitochondrial function by increasing membrane potential and inhibited generation of free radicals.177 Indolepropionic acid has also been shown to increase mitochondrial respiratory rates in amyloid precursor protein expressing neuroblastoma cell cultures.115 On the contrary, butyric or propionic acid treatments have been associated with disturbed mitochondrial fatty acid metabolism in a rat model of ASD111 but butyric acid alone improved mitochondrial function in lymphoblastoid cell line derived from ASD boys.112 Recently, Hulme et al. identified two novel microbial-derived structural analogues of carnitine, 3-methyl-4-(trimethylammonio)butanoate (3M-4-TMAB), and 4-(trimethylammonio)pentanoate (4-TMAP), that were found to colocalize with carnitine in brain white matter.109 The fatty acid oxidation in mitochondria is in part mediated by carnitine and in subsequent in vitro cell models of murine white matter the metabolites were observed to inhibit the rate of fatty acid oxidation, thus interfering with mitochondrial function. An isomer of the compounds, gut microbiota-associated compound 5-AVAB, has also been shown to limit fatty acid oxidation in mouse cardiomyocytes by interfering with carnitine transportation into the cell.178
Impairments in mitochondrial functions are considered as independent drivers of cognitive decline observed in the aging brain, neurodegenerative disorders, and neurodegeneration caused by external exposures like alcohol.179 Also, mitochondrial dysfunction has been associated with ASD.180 Thus, approaches targeting and supporting brain bioenergetics should be evaluated in the future. Findings connecting microbial metabolites to brain bioenergetics are preliminary but show that certain compounds are incorporated in the neuronal energy metabolism. However, the overall impact of many of these metabolites on mitochondrial output has not been investigated in humans making their contribution to brain health uncertain.
Neuroprotection
Actions resulting in preservation or recovery of the neuronal cells, their structural network or function are defined as neuroprotective. Hence, metabolites of the gut microbiota reducing oxidative stress or aggregation of neurotoxic proteins can be considered as neuroprotective agents. In this context, metabolites that reduce inflammation or promote neurodevelopment or neurotransmission are also neuroprotective.
The number of phytochemicals with suggested neuroprotective effects is considerable and here we cover only few examples of gut microbial metabolites as there are other focused articles available (for reviews, see refs.181–183). For instance, ferulic acid is a phytochemical associated to dietary fibers present in many plant products, released from the fiber matrix, and further metabolized by the gut microbes.184 Ferulic acid holds neuroprotective effects by limiting neuronal cell death and improves memory deficits in a mouse model of cerebral ischemia and reperfusion injury.18 In a mouse model of corticosterone administration-induced depression, ferulic acid ameliorated depression-like behavior and oxidative stress.119 A microbiota-borne metabolite and a downstream product of ferulic acid, dihydroferulic acid, also exhibits neuroprotective antioxidative properties in vitro.19
Phytochemicals have also shown potential neuroprotective properties in cell and murine models of Alzheimer’s disease where the aggregation of neurotoxic Aβ fragments were inhibited in the presence of polyphenol metabolites. For example, ferulic acid reduced hyperactivity and Alzheimer’s disease-related pathological changes in the brain while improving spatial working and reference memory in a murine model of cerebral amyloidosis.120 Metabolites of flavonoids and phenolic acids, 3-hydroxybenzoic acid and 3-(3′-hydroxyphenyl)propionic acid, were shown to accumulate in mice brain at µM concentration following administration of grape seed polyphenol extract or red wine and inhibit the formation of Aβ aggregates in vitro.116 In preclinical models of neurodegenerative diseases called synucleinopathies, vitamins K (phylloquinone, menaquinone, and menadione) produced by the microbiota did inhibit the aggregation of the protein α-synuclein within the neurons in vitro.122 Ergothioneine is a histidine derivative produced by gut bacteria that is translocated to the brain and protects from oxidative stress as well as Aβ-induced cellular damage in vitro and in vivo.117,118 Finally, TUDCA has been shown to reduce neuronal apoptosis in several other preclinical models of neurodegenerative disease such as Huntington’s, Alzheimer’s and Parkinson’s diseases and acute ischemia and interfere with Aβ production123,185 while addition of indolepropionic acid prevented oxidative stress and neuron apoptosis in neuroblastoma cell cultures exposed to Aβ.121
A range of gut-derived metabolites exert neuroprotective properties in in vitro and in vivo preclinical models, but convincing clinical studies are lacking. For example, administration of plant or fruit extracts to humans has improved cognitive functions and mood in various feeding trials, but these studies did not explore the effect on the metabolome.183 Nevertheless, the accumulating number of reports showing that amino acid- and polyphenol-derivatives interfere with the formation of neurotoxic proteins, oxidative, and inflammatory cascades warrant for further clinical studies targeting patients with neurodegenerative diseases.
Enteric nervous and immune systems as pathways of communication
Enteric nervous system
Without ever reaching the circulation and brain, the microbial metabolites can begin communication toward the brain through the gastrointestinal sites of nervous and immune systems (Figure 3). The enteric nervous system is a neuronal network of sensory neurons, motor neurons, interneurons, and supporting cells such as enteric glial cells embedded in the intestinal wall throughout the gastrointestinal tract.186 As the endings of the intrinsic primary afferent neurons are in the submucosa, the enteric nervous system is the first neuronal interface that could be directly or indirectly activated by the microbial metabolites.187 While it can operate functions such as local motility and secretion autonomously, this component of autonomic nervous system is connected to the CNS via sympathetic and parasympathetic pathways forming a loop of gut–brain–gut signaling. The sensory information from the gut to the brain is carried by primary afferent neurons of the vagal and spinal branches of which the former have been suggested as a key transmitter of gut microbiota stimulus. Indeed, the vagus nerve is composed of up to 90% of afferent fibers and depending on the location and type of the afferent fiber, they are stimulated by neurotransmitters, gut microbial metabolites, or gut hormones.188 Consequently, it has been shown that the vagus nerve is indirectly activated by the gut microbiota-associated metabolites such as serotonin,57 SCFAs,4 and indole.55 Moreover, the vagal pathways are also linked with the hypothalamic–pituitary–adrenal axis, which is a major regulator of stress responses. Coupled with the findings that vagotomy affects brain functions5 and vagus nerve stimulation modulates mood and behavior,188 the enteric nervous system–vagus–brain pathway is likely one of the main mechanisms of gut–brain axis communication.
Figure 3.
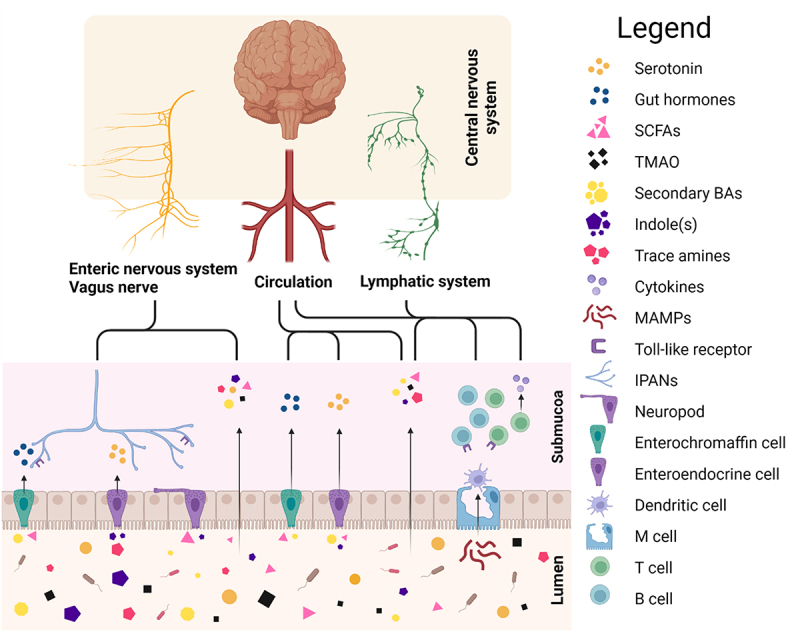
Communication routes between the gut and brain.
Schematic representation of various potential communication pathways connecting the gut to the brain. Gut microbiota-derived metabolites can interact with the enteral nervous system directly or activate it indirectly via colonic enterochromaffin or L cells’ enteroendocrine function. Alternatively, metabolites or gut hormones can translocate to the circulation or lymphatic system and eventually reach the brain. Similarly, inflammatory mediators and immune cells activated by microbiota associated molecular profiles can translocate to the brain via circulation or the lymphatic system. Abbreviations: BA, bile acid; IPAN, intrinsic primary afferent neuron; MAMP, microbiota-associated molecular profile; SCFA, short-chain fatty acid; TMAO, trimethylamine-N-oxide. (Figure created with Biorender.com)
Other forms of indirect enteric neuron activation are the enteroendocrine signaling by colonic L cells or enterochromaffin cells. The colonic enterochromaffin cells produce the majority of the circulating serotonin in the human blood.189 As discussed previously, several bacterial metabolites have been shown to influence the enterochromaffin cells’ serotonin synthesis.9 Enteric neurons in the submucosa are in close proximity of the translocated serotonin from the lumen and express serotonin receptors. In addition to neuronal stimulation, serotonin might have a role in the development, maturation, and protection of the enteric nervous system.190 Similar to enterochromaffin cells, colonic L cells are activated by microbial metabolites, such as SCFAs and secondary bile acids among others.188 The L cells produce bioactive peptides such as GLP-1 and PYY both known for their local effects on gut motility and secretion, and central effects on food intake and feeding behavior.191–193 The hormones released by L cells have multiple effectors, including enteric neurons and vagus nerve, that carry the signal toward CNS.194 However, recently Bohórquez et al.195 uncovered a neuroepithelial circuit connecting the enteroendocrine cell to sensory neurons, together referred as neuropods. This suggests that among the endocrine signaling, the enteroendocrine cells could form physical connections with the neurons as a conduit for sensory transmission activated by microbial metabolites.
In theory, the microbiota’s production of neurotransmitters, for example, histamine, serotonin, or GABA, could directly engage with the enteric neurons in a paracrine manner.194 However, it is questionable whether microbiota-derived neurotransmitters could reach enteric neurons in meaningful concentrations. The enteric neurons also express pattern recognition receptors, including the Toll-like receptors, that are activated by microbial molecules.194 The significance of the Toll-like receptors in the physiology of the enteric nervous system has been shown in mice, where the lack of these receptors reduced gut motility and produced neuronal defects, both reversed by the administration of Toll-like receptor agonist to naïve animals.196 Moreover, in the absence of gut microbiota, the maturation and function of enteric nervous system is compromised underlining the significance of microbial cues in normal gut physiology.194
Immune system
The cells of innate and adaptive immune systems in the gastrointestinal tract are the first responders to immune challenges produced by the gut microbiota.197 Reports from GF animals have suggested that the microbiota–host immunity crosstalk, especially in the early life, is imperative for the development and modulation of the immune homeostasis.6,160 The innate immune system detects microbial components through pattern-recognizing receptors, which are activated by microbe-associated molecular patterns.197 As with enteric neurons, Toll-like receptors are central pattern-recognizing receptors detecting microbe-associated molecules such as lipopolysaccharides, glycolipids, or lipopeptides. Activation of the innate immune response results into a signaling cascade, increasing the production of cytokines and chemokines and the recruitment of local macrophages and dendritic cells. These cells also act as antigen-presenting cells to further trigger local adaptive immune responses facilitating the migration of circulating T- and B-lymphocytes to the site of inflammation. Apparently, some gut-derived metabolites like the SCFAs may have a regulatory role in the intestinal T cell differentiation.129,198
As circulating lymphocytes may infiltrate peripheral tissues, similarly the cytokines or activated immune cells can be transported to the CNS and modulate the immune homeostasis.199 While the BBB restricts the passage of immune cells but not cytokines, the lymphatic system is an alternative route facilitating migration of these cells and bacterial components from the periphery to the CNS.200 The meningeal lymphatic vessels reach the CNS, exchange the cerebrospinal fluid, and drain immune cells and small compounds from the brain toward the periphery. Direct supply of microbiota-derived molecules or activated immune cells could induce a central immune response, such as the activation of microglia and T cells.197 This in turn leads to the production of pro-inflammatory cytokines promoting neuroinflammation. Cytokines may also deteriorate BBB permeability that predisposes to increased passage of harmful compounds or metabolites from the circulation through the BBB.200 Moreover, peripheral administration of pro-inflammatory cytokines has been shown to induce sickness behavior characterized by depressive-like behavior and reduced appetite in rodents.199 However, the range of mechanisms describing microbially mediated crosstalk between peripheral and central immune responses are widely uncharacterized.
Microbial metabolites and the relationship between preclinical and clinical evidence
A wealth of preclinical discoveries has clarified the extensive range of pathways through which microbiota-derived metabolites could drive the communication between gut and brain as illustrated in Figure 4. The human microbiome harbors a variety of microbial species capable of producing neurotransmitters, signaling molecules, or metabolizing their precursors into distinct compounds.145,189,201 As certain bacterium may contribute significantly to the levels of gut-derived metabolites observed in the host,138 it is compelling to speculate how much the presence, or absence, of such bacterium could modulate the gut–brain crosstalk and contribute to overall health status. For example, abundance of secondary bile acid-producing bacteria could result into increased circulation of deoxycholic acid and other secondary bile acids compromising the BBB integrity and resulting into increased translocation of microbial metabolites into the brain.81 Keeping in mind that the overall impact of these metabolites on health could depend on the genetic background or the presence of an illness, decreased liver or kidney function could exacerbate the accumulation of toxic metabolites like p-cresol and indoxyl sulfate that have been associated with neurodegenerative or neurodevelopmental diseases.16,17,48–50,90
Figure 4.
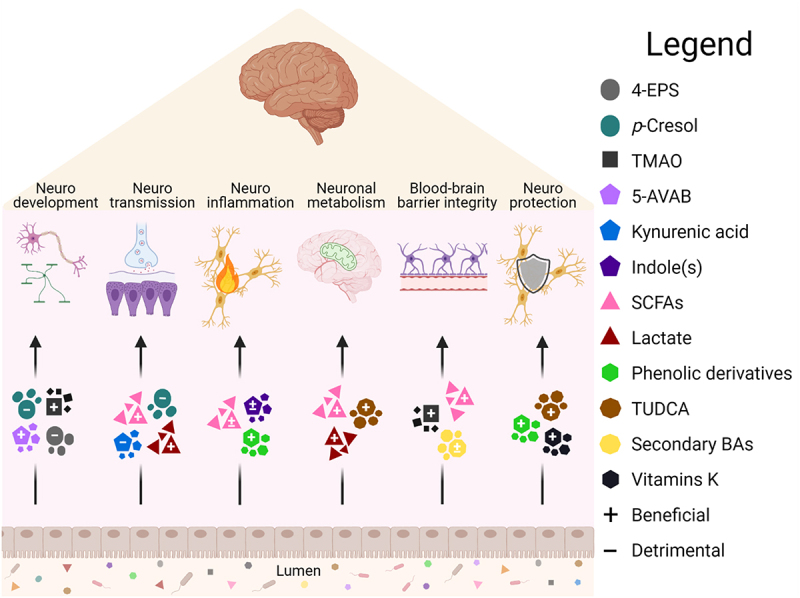
In the intersection of the gut–brain axis signaling: Selected microbiota metabolites and associated neurological functions.
Brain functions influenced by the gut microbiota metabolites. Certain metabolites like indoles or secondary bile acids have been shown to have both beneficial (+) and detrimental (-) effects depending on the pathway of action or disease model. Signaling pathways can be initiated in the gut via peripheral routes or after translocation to the central nervous system. Abbreviations: 4-EPS, 4-ethylphenylsulfate; 5-AVAB, 5-aminovaleric acid betaine; BA, bile acids; SCFA, short-chain fatty acid; TMAO, trimethylamine-N-oxide; TUDCA, tauroursodeoxycholic acid. (Figure created with Biorender.com)
Categorizing gut microbial metabolites based on their effect on neurological function reveals significant overlapping between metabolite classes and individual metabolites (Figure 5, Table 2). In preclinical studies, SCFAs have been implicated with all discussed neurological functions but human evidence is scarce and contradictory.21 Similarly, derivatives of amino acid metabolism, especially tryptophan, have been implicated in all other concepts except for neuronal energy metabolism. However, clinical findings support the notion that alterations in tryptophan-metabolism might be associated with cognitive and mental status.58,62–64,141,151,152 Although preclinical models display controversial impact of secondary bile acids, they have recently been associated with neurodegenerative diseases in humans.76,77,81,155 4-EPS, p-cresol sulfate and indoxyl sulfate are sulfates that show conflicting effects on neurodevelopment, -transmission, and -inflammation in vitro and in vivo but the clinical results, although associative, favor the harmful properties depicted by the preclinical data.41,43,44,51 However, the findings are largely obtained in a pathological context, yielding a wealth of disease-associated metabolites. Whether such associations are disease-specific or the metabolites, or microbiota, could be utilized in managing brain-related disorders, requires extensive and decisive work. Besides, the associations might reflect the altered metabolism or microbiota composition due to the disease status and confounders rather than contribute to the pathogenesis per se. For example, a recent stool metagenomics study in ASD children suggested that autistic traits promote restrictive dietary preferences in turn associated with microbiome composition, rather than a direct association between the ASD and microbiome.203
Figure 5.
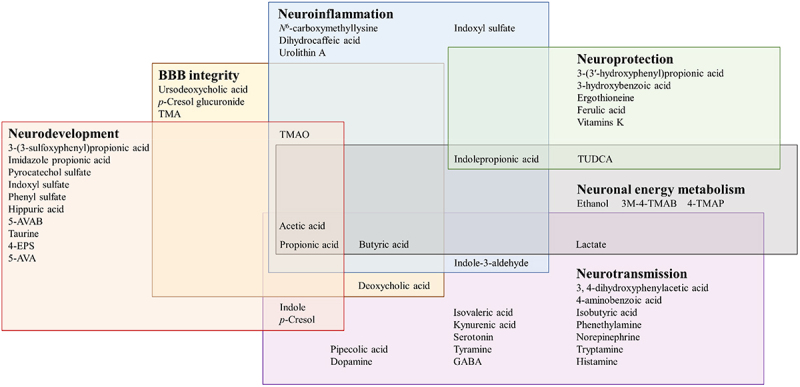
Overlapping gut microbiota associated metabolites in neurological functions.
Individual gut microbiota metabolites have been associated with single or several neurological functions involving neuronal energy metabolism, blood–brain barrier integrity, neurodevelopment, -inflammation, -transmission, and -protection. Abbreviations: 4-EPS, 4-ethylphenylsulfate; 5-AVA(B), 5-aminovaleric acid(betaine); BBB, blood–brain barrier; GABA, γ-aminobutyric acid; 3M-4-TMAB, 3-methyl-4-(trimethylammonio)butanoate; 4-TMAP, 4-(trimethylammonio)pentanoate; TMA(O), trimethylamine(-N-oxide); TUDCA, tauroursodeoxycholic acid.
Table 2.
Gut microbial metabolites associated neurological functions, methods of preclinical models and presence in the human brain.
| Metabolite | Neurological function | Preclinical methods | Measured from human brain51,54,155,202 | Reference |
|---|---|---|---|---|
| 3-(3′-hydroxyphenyl)propionic acid | Neuroprotection | in vitro, in vivo | Yes | 116 |
| 3-(3-sulfooxyphenyl)propionic acid | Neurodevelopment | in vivo | No | 38 |
| 3-hydroxybenzoic acid | Neuroprotection | in vitro, in vivo | Yes | 116 |
| 3M-4-TMAB | Neuronal energy metabolism | in vitro, in vivo | No | 109 |
| 4-aminobenzoic acid | Neurotransmission | in vitro, in vivo | No | 9 |
| 4-EPS | Neurodevelopment | in vivo | No | 39–41 |
| 4-TMAP | Neuronal energy metabolism | in vitro, in vivo | No | 109 |
| 5-AVA | Neurodevelopment | in vitro, in vivo | Yes | 42 |
| 5-AVAB | Neurodevelopment | in vivo, ex vivo | Yes | 28,131 |
| Acetic acid | Neurodevelopment Neurotransmission BBB integrity Neuroinflammation Neuronal energy metabolism |
in vitro, in vivo, ex vivo | Yes | 45,73,83–87,110,144,163 |
| Butyric acid | Neurotransmission BBB integrity Neuroinflammation Neuronal energy metabolism |
in vitro, in vivo, ex vivo | Yes | 52,73,74,83–87,110–112 |
| Deoxycholic acid | Neurotransmission BBB integrity |
in vitro, in vivo | Yes | 9,75 |
| Dihydrocaffeic acid | Neuroinflammation | in vitro, in vivo | No | 88 |
| Dopamine | Neurotransmission | in vivo | Yes | 7 |
| Ethanol | Neuronal energy metabolism | in vivo | Yes | 113 |
| Ergothioneine | Neuroprotection | in vitro, in vivo | Yes | 117,118 |
| Ferulic acid | Neuroprotection | in vitro, in vivo | No | 18,119,120 |
| GABA | Neurotransmission | in vitro, in vivo | Yes | 5,14,53 |
| Hippuric acid | Neurodevelopment | in vivo, ex vivo | Yes | 28 |
| Histamine | Neurotransmission | in vitro, in vivo | Yes | 15 |
| Imidazole propionic acid | Neurodevelopment | in vivo, ex vivo | No | 28 |
| Indole-3-aldehyde | Neurotransmission Neuroinflammation |
in vivo, ex vivo | No | 32,57,91 |
| Indole | Neurodevelopment, Neurotransmission |
in vitro, in vivo, ex vivo | Yes | 32,36,55–57,91 |
| Indolepropionic acid | Neuroinflammation Neuronal energy metabolism Neuroprotection |
in vitro, in vivo, ex vivo | No | 32,110,115,121 |
| Indoxyl sulfate | Neurodevelopment Neuroinflammation |
in vitro, in vivo | Yes | 17,32,38,51,90,91 |
| Isobutyric acid | Neurotransmission | in vitro, ex vivo | Yes | 52 |
| Isovaleric acid | Neurotransmission | in vitro, ex vivo | Yes | 52 |
| Kynurenic acid | Neurotransmission | in vitro | Yes | 59–61 |
| Lactate | Neurotransmission Neuronal energy metabolism |
in vivo | Yes | 20,65,114,144 |
| N6-carboxymethyllysine | Neuroinflammation | in vitro, in vivo | Yes | 92–94 |
| Norepinephrine | Neurotransmission | in vitro, in vivo, ex vivo | Yes | 7,52 |
| p-Cresol | Neurodevelopment Neurotransmission |
in vitro, in vivo | Yes | 16,48–51 |
| p-Cresol glucuronide | BBB integrity | in vitro, in vivo | No | 72 |
| Phenethylamine | Neurotransmission | in vivo | Yes | 15 |
| Phenylsulfate | Neurodevelopment | in vivo | No | 38 |
| Pipecolic acid | Neurotransmission | in vivo | Yes | 67 |
| Propionic acid | Neurodevelopment Neurotransmission BBB integrity Neuroinflammation Neuronal energy metabolism |
in vitro, in vivo, ex vivo | Yes | 27,45,73,83–86,95–100,111 |
| Pyrocatechol sulfate | Neurodevelopment | in vivo | No | 38 |
| Serotonin | Neurotransmission | in vivo | Yes | 9,68,141 |
| Taurine | Neurodevelopment | in vivo | Yes | 42 |
| TMA | BBB integrity | in vitro | Yes | 27 |
| TMAO | Neurodevelopment BBB integrity Neuroinflammation |
in vitro, in vivo, ex vivo | Yes | 27,28,101,102 |
| TUDCA | Neuronal energy metabolism Neuroprotection |
in vitro, in vivo | Yes | 110,123 |
| Tryptamine | Neurotransmission | in vitro, in vivo | Yes | 71 |
| Tyramine | Neurotransmission | in vitro, in vivo | Yes | 9 |
| Urolithin A | Neuroinflammation | in vitro, in vivo, ex vivo | No | 106–108,168 |
| Ursodeoxycholic acid | BBB integrity | in vitro | Yes | 80 |
| Vitamins K | Neuroprotection | in vitro | Yes | 122 |
Abbreviations: 4-EPS, 4-ethylphenylsulfate; 5-AVA(B), 5-aminovaleric acid (betaine); BBB, blood–brain barrier; GABA, γ-aminobutyric acid; 3 M-4-TMAB, 3-methyl-4-(trimethylammonio)butanoate; 4-TMAP, 4-(trimethylammonio)pentanoate; TMA(O), trimethylamine(-N-oxide); TUDCA, tauroursodeoxycholic acid
In terms of the neurological functions, metabolites linked with BBB integrity, neuronal energy metabolism, or neuroprotection, the clinical evidence are extremely limited for evaluating their significance in humans. Given the value of these functions to brain homeostasis and the range of diet-derived metabolites identified, especially amino acid- and polyphenol-derived, future interventions focusing on these pathways and compounds are strongly advocated. To date, few studies using nutritional or gut microbiome modifying strategy have shown marked beneficial effect on neurological and psychiatric disorders. Even if some studies applying nutritional, pro- or prebiotic interventions showed interesting outcomes such as decreasing anxiety levels or improving cognition, the effects are widely variable between subjects and dependent on the type of diseases.204–206
Conclusion and future prospects
An array of preclinical reports show that the gut microbial metabolites regulate brain functions through peripheral and local signaling by immunological, neuronal, and endocrinological pathways. Cell and animal models provide valuable mechanistic information about the metabolites in isolation but there is limited clinical evidence to support such effects in humans. Nevertheless, it is evident that nutritional, metabolic, and disease status modulate the overall impact of metabolites on health and disease. Thus, to catch the full scope of the relationship between diet, gut microbiota, the circulating metabolome and the brain, extensive profiling of nutritional habits and clinical features are fundamental. In order to gain a comprehensive view on the microbial species involved, metabolites they produce, and metabolic effect exerted thereafter, necessitates combining metagenomic, metabolomic, and metatranscriptomic approaches in well-defined populations, healthy and disease, to uncover the intricate communication within the gut–brain axis and pave the way for personalized care involving the role of gut microbiota in the context of prevention and treatment of neurological disorders.
Funding Statement
This work was supported by the ERA-NET NEURON 2019 Translational Biomarkers Grant no 334814 ”Gut2Behave”, by the Fonds de la Recherce Scientifique (FRD-FNRS) in Belgium (PINT-MULTI R.8013.19, NEURON, call 2019) and PDR T.0068.19 attributed to ND. KH has received support from Academy of Finland (Grant no 321716) and European Union's Horizon 2020 research and innovation programme under Marie Sklodowska-Curie (Grant no 754412. SL has received support from Chaire d'excellence Région Nouvelle Aquitaine ExoMarquAge (135059720-13062120); The Foundation for Medical Research (FRM, DEQ20170336724) and the Institut National pour la Recherche Agronomique, l'Alimentation et l'Environnement.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Contributions
H.A. V.K., O.K. and K.H. designed the outline of the review. H.A., Q.L. and S.L. wrote the manuscript. H.A. and Q.L. produced the figures and tables. All authors have participated in the manuscript review, editing and approved the final manuscript.
References
- 1.Bar N, Korem T, Weissbrod O, Slieker R, Rutters F, Beulens J, Nijpels G, Koopman A.. A reference map of potential determinants for the human serum metabolome. Nature. 2020;588(7836):135–33. doi: 10.1038/s41586-020-2896-2. [DOI] [PubMed] [Google Scholar]
- 2.Leyrolle Q, Cserjesi R, Mulders MDGH, Zamariola G, Hiel S, Gianfrancesco MA, Rodriguez J, Portheault D, Amadieu C, and Leclercq S, et al. Specific gut microbial, biological, and psychiatric profiling related to binge eating disorders: a cross-sectional study in obese patients. Clin Nutr. 2021;40(4):2035–2044. doi: 10.1016/j.clnu.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, and Bar N, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–228. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 4.Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, Del Mármol J, Castro TBR, and Furuichi M, et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 2020;583(7816):441–446. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of lactobacillus strain regulates emotional behavior and central gaba receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thion MS, Ginhoux F, Garel S. Microglia and early brain development: an intimate journey. Science. 2018;362(6411):185–189. doi: 10.1126/science.aat0474. [DOI] [PubMed] [Google Scholar]
- 7.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303(11):G1288–G1295. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- 8.Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;163(1):258. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, and Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding g protein-coupled receptor, GPR41. Proc Natl Acad Sci U S A. 2008;105(43):16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–G65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 12.Nøhr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, Møller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi: 10.1016/j.neuroscience.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett E, Ross RP, O’Toole PW, Fitzgerald GF. Stanton C.ɣ-aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113(2):411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Nwe P-K, Yang Y, Rosen CE, Bielecka AA, Kuchroo M, Cline GW, Kruse AC, Ring AM, and Crawford JM, et al. A forward chemical genetic screen reveals gut microbiota metabolites that modulate host physiology. Cell. 2019;177(5):1217–1231.e18. doi: 10.1016/j.cell.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gacias M, Gaspari S, Santos P-MG, Tamburini S, Andrade M, Zhang F, Shen N, Tolstikov V, Kiebish MA, and Dupree JL, et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLife. 2016;5:e13442. doi: 10.7554/elife.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adesso S, Magnus T, Cuzzocrea S, Campolo M, Rissiek B, Paciello O, Autore G, Pinto A, Marzocco S. Indoxyl sulfate affects glial function increasing oxidative stress and neuroinflammation in chronic kidney disease: interaction between astrocytes and microglia. Front Pharmacol. 2017;8:370. doi: 10.3389/fphar.2017.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren Z, Zhang R, Li Y, Li Y, Yang Z, Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int J Mol Med. 2017;40(5):1444–1456. doi: 10.3892/ijmm.2017.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verzelloni E, Pellacani C, Tagliazucchi D, Tagliaferri S, Calani L, Costa LG, Brighenti F, Borges G, Crozier A, Conte A, et al. Antiglycative and neuroprotective activity of colon‐derived polyphenol catabolites. Mol Nutr Food Res. 2011;55(S1):S35–S43. doi: 10.1002/mnfr.201000525. [DOI] [PubMed] [Google Scholar]
- 20.Mao J-H, Kim Y-M, Zhou Y-X, Hu D, Zhong C, Chang H, Brislawn C, Langley S, Wang Y, Peisl BYL, et al. Genetic and metabolic links between the murine microbiome and memory. Microbiome. 2020;8(1):53. doi: 10.1186/s40168-020-00817-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 22.Li XS, Wang Z, Cajka T, Buffa JA, Nemet I, Hurd AG, Gu X, Skye SM, Roberts AB, Wu Y, et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight. 2018;3(6):e99096. doi: 10.1172/jci.insight.99096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemet I, Saha PP, Gupta N, Zhu W, Romano KA, Skye SM, Cajka T, Mohan ML, Li L, Wu Y, et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180(5):862–877.e22. doi: 10.1016/j.cell.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottosson F, Brunkwall L, Smith E, Orho-Melander M, Nilsson P, Fernandez C, Melander O. The gut microbiota-related metabolite phenylacetylglutamine associates with increased risk of incident coronary artery disease. J Hypertens. 2020;38(12):2427–2434. doi: 10.1097/HJH.0000000000002569. [DOI] [PubMed] [Google Scholar]
- 25.Wilmanski T, Diener C, Rappaport N, Patwardhan S, Wiedrick J, Lapidus J, Earls JC, Zimmer A, Glusman G, Robinson M, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3(2):274–286. doi: 10.1038/s42255-021-00348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collino S, Montoliu I, Martin F-PJ, Scherer M, Mari D, Salvioli S, Bucci L, Ostan R, Monti D, Biagi E, et al. Metabolic signatures of extreme longevity in Northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PloS One. 2013;8(3):e56564. doi: 10.1371/journal.pone.0056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyles L, Pontifex MG, Rodriguez-Ramiro I, Anis-Alavi MA, Jelane KS, Snelling T, Solito E, Fonseca S, Carvalho AL, Carding SR, et al. Regulation of blood–brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome. 2021;9(1):235. doi: 10.1186/s40168-021-01181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586(7828):281–283. doi: 10.1038/s41586-020-2745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Z-H, Xin F-Z, Zhou D, Xue Y-Q, Liu X-L, Yang R-X, Pan Q, Fan J-G. Trimethylamine N-oxide attenuates high-fat high-cholesterol diet-induced steatohepatitis by reducing hepatic cholesterol overload in rats. World J Gastroenterol. 2019;25(20):2450–2462. doi: 10.3748/wjg.v25.i20.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins HL, Drazul-Schrader D, Sulpizio AC, Koster PD, Williamson Y, Adelman SJ, Owen K, Sanli T, Bellamine A. L-carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE−/− transgenic mice expressing CETP. Atherosclerosis. 2015;244:29–37. doi: 10.1016/j.atherosclerosis.2015.10.108. [DOI] [PubMed] [Google Scholar]
- 31.Dumas M-E, Rothwell AR, Hoyles L, Aranias T, Chilloux J, Calderari S, Noll EM, Péan N, Boulangé CL, Blancher C, et al. Microbial-host co-metabolites are prodromal markers predicting phenotypic heterogeneity in behavior, obesity, and impaired glucose tolerance. Cell Rep. 2017;20(1):136–148. doi: 10.1016/j.celrep.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao -C-C, Patel B, Yan R, Blain M, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and CNS inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaumont M, Neyrinck AM, Olivares M, Rodriguez J, Rocca Serra A, Roumain M, Bindels LB, Cani PD, Evenepoel P, Muccioli GG, et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J. 2018;32(12):6681–6693. doi: 10.1096/fj.201800544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD, Gerich ME, Jenkins BR, Walk ST, Kominsky DJ, et al. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am J Pathol. 2018;188(5):1183–1194. doi: 10.1016/j.ajpath.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 36.Wei GZ, Martin KA, Xing PY, Agrawal R, Whiley L, Wood TK, Hejndorf S, Ng YZ, Low JZY, Nicholson JK, et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2021;118(27):e2021091118. doi: 10.1073/pnas.2021091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin X, Gibbons H, Rundle M, Frost G, McNulty BA, Nugent AP, Walton J, Flynn A, Brennan L. The relationship between fish intake and urinary trimethylamine‐N‐oxide. Mol Nutr Food Res. 2020;64(3):e1900799. doi: 10.1002/mnfr.201900799. [DOI] [PubMed] [Google Scholar]
- 38.Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, Tsaava T, Addorisio ME, Putzel GG, Zhou L, et al. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574(7779):543–548. doi: 10.1038/s41586-019-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Needham BD, Funabashi M, Adame MD, Wang Z, Boktor JC, Haney J, Wu WL, Rabut C, Ladinsky MS, Hwang SJ, et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature. 2022;602(7898):647–653. doi: 10.1038/s41586-022-04396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Needham BD, Adame MD, Serena G, Rose DR, Preston GM, Conrad MC, Campbell AS, Donabedian DH, Fasano A, Ashwood P, et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol Psychiatry. 2021;89(5):451–462. doi: 10.1016/j.biopsych.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharon G, Cruz NJ, Kang D-W, Gandal MJ, Wang B, Kim Y-M, Zink EM, Casey CP, Taylor BC, Lane CJ, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600–1618.e17. doi: 10.1016/j.cell.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PloS One. 2013;8(10):e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altieri L, Neri C, Sacco R, Curatolo P, Benvenuto A, Muratori F, Santocchi E, Bravaccio C, Lenti C, Saccani M, et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers. 2011;16(3):252–260. doi: 10.3109/1354750X.2010.548010. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Li X, Xia B, Jin X, Zou Q, Zeng Z, Zhao W, Yan S, Li L, Yuan S, et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021;33(5):923–938.e6. doi: 10.1016/j.cmet.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57(8):2096–2102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Li E, Sun Z, Fu D, Duan G, Jiang M, Yu Y, Mei L, Yang P, Tang Y, et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep. 2019;9(1):287. doi: 10.1038/s41598-018-36430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tevzadze G, Tevzadze G, Oniani N, Oniani N, Zhuravliova E, Zhuravliova E, Darchia N, Darchia N, Eliozishvili M, Eliozishvili M, et al. Effects of a gut microbiome toxin, p-cresol, on the indices of social behavior in rats. Neurophysiology. 2018;50(5):372–377. doi: 10.1007/s11062-019-09764-1. [DOI] [Google Scholar]
- 49.Pascucci T, Colamartino M, Fiori E, Sacco R, Coviello A, Ventura R, Puglisi-Allegra S, Turriziani L, Persico AM. P-cresol alters brain dopamine metabolism and exacerbates autism-like behaviors in the btbr mouse. Brain Sci. 2020;10(4):233. doi: 10.3390/brainsci10040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bermudez-Martin P, Becker JAJ, Caramello N, Fernandez SP, Costa-Campos R, Canaguier J, Barbosa S, Martinez-Gili L, Myridakis A, Dumas M-E, et al. The microbial metabolite p-cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome. 2021;9(1):157. doi: 10.1186/s40168-021-01103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sankowski B, Księżarczyk K, Raćkowska E, Szlufik S, Koziorowski D, Giebułtowicz J. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin Chim Acta. 2020;501:165–173. doi: 10.1016/j.cca.2019.10.038. [DOI] [PubMed] [Google Scholar]
- 52.Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA, Julius D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170(1):185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janik R, Thomason LAM, Stanisz AM, Forsythe P, Bienenstock J, Stanisz GJ. Magnetic resonance spectroscopy reveals oral lactobacillus promotion of increases in brain GABA, n-acetyl aspartate and glutamate. NeuroImage. 2016;125:988–995. doi: 10.1016/j.neuroimage.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Kärkkäinen O, Kokla M, Lehtonen M, Auriola S, Martiskainen M, Tiihonen J, Karhunen PJ, Hanhineva K, Kok E. Changes in the metabolic profile of human male postmortem frontal cortex and cerebrospinal fluid samples associated with heavy alcohol use. Addict Biol. 2021;26(6):e13035. doi: 10.1111/adb.13035. [DOI] [PubMed] [Google Scholar]
- 55.Jaglin M, Rhimi M, Philippe C, Pons N, Bruneau A, Goustard B, Daugé V, Maguin E, Naudon L, Rabot S. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front Neurosci. 2018;12:216. doi: 10.3389/fnins.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chimerel C, Emery E, Summers David K, Keyser U, Gribble Fiona M, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine l cells. Cell Rep. 2014;9(4):1202–1208. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye L, Bae M, Cassilly CD, Jabba SV, Thorpe DW, Martin AM, H-y L, Wang J, Thompson JD, Lickwar CR, et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29(2):179–196.e9. doi: 10.1016/j.chom.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arnoriaga-Rodríguez M, Mayneris-Perxachs J, Burokas A, Contreras-Rodríguez O, Blasco G, Coll C, Biarnés C, Miranda-Olivos R, Latorre J, Moreno-Navarrete J-M, et al. Obesity impairs short-term and working memory through gut microbial metabolism of aromatic amino acids. Cell Metab. 2020;32(4):548–560.e7. doi: 10.1016/j.cmet.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Pocivavsek A, Wu H-Q, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36(11):2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu H-Q, Schwarcz R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35(8):1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull. 2007;33(3):797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, Chen L, Ning Y. Cross-sectional relationship between kynurenine pathway metabolites and cognitive function in major depressive disorder. Psychoneuroendocrinology. 2019;101:72–79. doi: 10.1016/j.psyneuen.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Pu J, Liu Y, Zhang H, Tian L, Gui S, Yu Y, Chen X, Chen Y, Yang L, Ran Y, et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol Psychiatry. 2020;26(8):4265–4276. doi: 10.1038/s41380-020-0645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rudzki L, Ostrowska L, Pawlak D, Małus A, Pawlak K, Waszkiewicz N, Szulc A. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 65.O’Hagan C, Li JV, Marchesi JR, Plummer S, Garaiova I, Good MA. Long-term multi-species lactobacillus and bifidobacterium dietary supplement enhances memory and changes regional brain metabolites in middle-aged rats. Neurobiol Learn Mem. 2017;144:36–47. doi: 10.1016/j.nlm.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 66.El Hayek L, Khalifeh M, Zibara V, Abi Assaad R, Emmanuel N, Karnib N, El-Ghandour R, Nasrallah P, Bilen M, Ibrahim P, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci. 2019;39(13):2369–2382. doi: 10.1523/JNEUROSCI.1661-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsumoto M, Kibe R, Ooga T, Aiba Y, Sawaki E, Koga Y, Benno Y. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci. 2013;7:9. doi: 10.3389/fnsys.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Özoǧul F, Kuley E, Özoǧul Y, Özoǧul İ. The function of lactic acid bacteria on biogenic amines production by food-borne pathogens in arginine decarboxylase broth. Food Sci Technol Res. 2012;18(6):795–804. doi: 10.3136/fstr.18.795. [DOI] [Google Scholar]
- 69.Lehikoinen AI, Karkkainen OK, Lehtonen MAS, Auriola SOK, Hanhineva KJ, Heinonen ST. Alcohol and substance use are associated with altered metabolome in the first trimester serum samples of pregnant mothers. Eur J Obstet Gynecol Reprod Biol. 2018;223:79–84. doi: 10.1016/j.ejogrb.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Karkkainen O, Klavus A, Voutilainen A, Virtanen J, Lehtonen M, Auriola S, Kauhanen J, Rysa J. Changes in circulating metabolome precede alcohol-related diseases in middle-aged men: a prospective population-based study with a 30-year follow-up. Alcohol Clin Exp Res. 2020;44(12):2457–2467. doi: 10.1111/acer.14485. [DOI] [PubMed] [Google Scholar]
- 71.Bhattarai Y, Williams BB, Battaglioli EJ, Whitaker WR, Till L, Grover M, Linden DR, Akiba Y, Kandimalla KK, Zachos NC, et al. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe. 2018;23(6):775–785.e5. doi: 10.1016/j.chom.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stachulski AV, Knausenbergrer TB-A, Shah SN, Hoyles L, McArthur S. A host–gut microbial co-metabolite of aromatic amino acids, p-cresol glucuronide, promotes blood–brain barrier integrity in vivo. bioRxiv Pre-print. 2022. doi: 10.1101/2022.01.11.475932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H, Sun J, Wang F, Ding G, Chen W, Fang R, Yao Y, Pang M, Lu Z-Q, Liu J. Sodium butyrate exerts neuroprotective effects by restoring the blood–brain barrier in traumatic brain injury mice. Brain Res. 2016;1642:70–78. doi: 10.1016/j.brainres.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 75.Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via RAC1-dependent mechanisms. Dig Liver Dis. 2014;46(6):527–534. doi: 10.1016/j.dld.2014.01.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MahmoudianDehkordi S, Arnold M, Nho K, Ahmad S, Jia W, Xie G, Louie G, Kueider-Paisley A, Moseley MA, Thompson JW, et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-an emerging role for gut microbiome. Alzheimers Dement. 2019;15(1):76–92. doi: 10.1016/j.jalz.2018.07.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nho K, Kueider-Paisley A, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G, Blach C, Baillie R, Han X, Kastenmüller G, et al. Altered bile acid profile in mild cognitive impairment and Alzheimer’s disease: relationship to neuroimaging and csf biomarkers. Alzheimers Dement. 2019;15(2):232–244. doi: 10.1016/j.jalz.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoyles L, Snelling T, Umlai U-K, Nicholson JK, Carding SR, Glen RC, McArthur S. Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome. 2018;6(1):55. doi: 10.1186/s40168-018-0439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM, Asthana S, Blennow K, Zetterberg H, Bendlin BB, et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimers Res Ther. 2018;10(1):124. doi: 10.1186/s13195-018-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palmela I, Correia L, Silva RFM, Sasaki H, Kim KS, Brites D, Brito MA. Hydrophilic bile acids protect human blood-brain barrier endothelial cells from disruption by unconjugated bilirubin: an in vitro study. Front Neurosci. 2015;9:80. doi: 10.3389/fnins.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baloni P, Funk CC, Yan J, Yurkovich JT, Kueider-Paisley A, Nho K, Heinken A, Jia W, Mahmoudiandehkordi S, Louie G, et al. Metabolic network analysis reveals altered bile acid synthesis and metabolism in Alzheimer’s disease. Cell Rep Med. 2020;1(8):100138. doi: 10.1016/j.xcrm.2020.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erny D, Dokalis N, Mezö C, Castoldi A, Mossad O, Staszewski O, Frosch M, Villa M, Fuchs V, Mayer A, et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021;33(11):2260–2276.e7. doi: 10.1016/j.cmet.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 83.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colombo AV, Sadler RK, Llovera G, Singh V, Roth S, Heindl S, Sebastian Monasor L, Verhoeven A, Peters F, Parhizkar S, et al. Microbiota-derived short chain fatty acids modulate microglia and promote aβ plaque deposition. eLife. 2021;10:e59826. doi: 10.7554/eLife.59826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao W, Su J, Gao X, Yang H, Weng R, Ni W, Gu Y. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome. 2022;10(1):62. doi: 10.1186/s40168-022-01255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marizzoni M, Cattaneo A, Mirabelli P, Festari C, Lopizzo N, Nicolosi V, Mombelli E, Mazzelli M, Luongo D, Naviglio D, et al. Short-chain fatty acids and lipopolysaccharide as mediators between gut dysbiosis and amyloid pathology in Alzheimer’s disease. J Alzheimers Dis. 2020;78(2):683–697. doi: 10.3233/JAD-200306. [DOI] [PubMed] [Google Scholar]
- 88.Wang J, Hodes GE, Zhang H, Zhang S, Zhao W, Golden SA, Bi W, Menard C, Kana V, Leboeuf M, et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun. 2018;9(1):477–14. doi: 10.1038/s41467-017-02794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Philippe C, Szabo de Edelenyi F, Naudon L, Druesne-Pecollo N, Hercberg S, Kesse-Guyot E, Latino-Martel P, Galan P, Rabot S. Relation between mood and the host-microbiome co-metabolite 3-indoxylsulfate: results from the observational prospective nutrinet-santé study. Microorganisms. 2021;9(4):716. doi: 10.3390/microorganisms9040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karbowska M, Hermanowicz JM, Tankiewicz-Kwedlo A, Kalaska B, Kaminski TW, Nosek K, Wisniewska RJ, Pawlak D. Neurobehavioral effects of uremic toxin–indoxyl sulfate in the rat model. Sci Rep. 2020;10(1):9483. doi: 10.1038/s41598-020-66421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao -C-C, Ardura-Fabregat A, de Lima KA, Gutiérrez-Vázquez C, Hewson P, Staszewski O, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557(7707):724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mossad O, Batut B, Yilmaz B, Dokalis N, Mezo C, Nent E, Nabavi LS, Mayer M, Maron FJM, Buescher JM, et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nat Neurosci. 2022;25(3):295–305. doi: 10.1038/s41593-022-01027-3. [DOI] [PubMed] [Google Scholar]
- 93.Wong A, Luth HJ, Deuther-Conrad W, Dukic-Stefanovic S, Gasic-Milenkovic J, Arendt T, Munch G. Advanced glycation endproducts co-localize with inducible nitric oxide synthase in Alzheimer’s disease. Brain Res. 2001;920(1–2):32–40. doi: 10.1016/s0006-8993(01)02872-4. [DOI] [PubMed] [Google Scholar]
- 94.Girones X, Guimera A, Cruz-Sanchez CZ, Ortega A, Sasaki N, Makita Z, Lafuente JV, Kalaria R, Cruz-Sanchez FF. Nɛ -carboxymethyllysine in brain aging, diabetes mellitus, and Alzheimer’s disease. Free Radic Biol Med. 2004;36(10):1241–1247. doi: 10.1016/j.freeradbiomed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 95.Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee D-H, May C, Wilck N, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. 2016;44(4):951–953. doi: 10.1016/j.immuni.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 96.Shultz SR, MacFabe DF, Ossenkopp K-P, Scratch S, Whelan J, Taylor R, Cain DP. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54(6):901–911. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 97.Shultz SR, Aziz NAB, Yang L, Sun M, MacFabe DF, O’Brien TJ. Intracerebroventricular injection of propionic acid, an enteric metabolite implicated in autism, induces social abnormalities that do not differ between seizure-prone (fast) and seizure-resistant (slow) rats. Behav Brain Res. 2015;278:542–548. doi: 10.1016/j.bbr.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 98.MacFabe DF, Cain NE, Boon F, Ossenkopp K-P, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res. 2011;217(1):47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 99.MacFabe D, Cain D, Rodriguez-Capote K, Franklin A, Hoffman J, Boon F, Taylor A, Kavaliers M, Ossenkopp K. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176(1):149–169. doi: 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 100.El-Ansary AK, Ben Bacha A, Kotb M. Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation. 2012;9(1):74. doi: 10.1186/1742-2094-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meng F, Li N, Li D, Song B, Li L. The presence of elevated circulating trimethylamine N-oxide exaggerates postoperative cognitive dysfunction in aged rats. Behav Brain Res. 2019;368:111902. doi: 10.1016/j.bbr.2019.111902. [DOI] [PubMed] [Google Scholar]
- 102.Brunt VE, LaRocca TJ, Bazzoni AE, Sapinsley ZJ, Miyamoto-Ditmon J, Gioscia-Ryan RA, Neilson AP, Link CD, Seals DR. The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience. 2020;43(1):377–394. doi: 10.1007/s11357-020-00257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu C, Li G, Lv Z, Li J, Wang X, Kang J, Zhan C. Association of plasma trimethylamine-N-oxide levels with post-stroke cognitive impairment: a 1-year longitudinal study. Neurol Sci. 2019;41(1):57–63. doi: 10.1007/s10072-019-04040-w. [DOI] [PubMed] [Google Scholar]
- 104.Zhuang Z, Gao M, Yang R, Liu Z, Cao W, Huang T. Causal relationships between gut metabolites and Alzheimer’s disease: a bidirectional Mendelian randomization study. Neurobiol Aging. 2021;100:119.e15–119.e18. doi: 10.1016/j.neurobiolaging.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 105.Chung SJ, Rim JH, Ji D, Lee S, Yoo HS, Jung JH, Baik K, Choi Y, Ye BS, Sohn YH, et al. Gut microbiota-derived metabolite trimethylamine N-oxide as a biomarker in early Parkinson’s disease. Nutrition. 2021;83:111090. doi: 10.1016/j.nut.2020.111090. [DOI] [PubMed] [Google Scholar]
- 106.Toney AM, Albusharif M, Works D, Polenz L, Schlange S, Chaidez V, Ramer-Tait AE, Chung S. Differential effects of whole red raspberry polyphenols and their gut metabolite urolithin A on neuroinflammation in BV-2 microglia. Int J Environ Res Public Health. 2020;18(1):68. doi: 10.3390/ijerph18010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shen P-X, Li X, Deng S-Y, Zhao L, Zhang -Y-Y, Deng X, Han B, Yu J, Li Y, Wang -Z-Z, et al. Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor. EBioMedicine. 2021;64:103227. doi: 10.1016/j.ebiom.2021.103227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin XH, Ye XJ, Li QF, Gong Z, Cao X, Li JH, Zhao ST, Sun XD, He XS, Xuan AG. Urolithin A prevents focal cerebral ischemic injury via attenuating apoptosis and neuroinflammation in mice. Neuroscience. 2020;448:94–106. doi: 10.1016/j.neuroscience.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 109.Hulme H, Meikle LM, Strittmatter N, vdJJJ H, Swales J, Bragg RA, Villar VH, Ormsby MJ, Barnes S, Brown SL, et al. Microbiome-derived carnitine mimics as previously unknown mediators of gut-brain axis communication. Sci Adv. 2020;6(11):eaax6328. doi: 10.1126/sciadv.aax6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, Zhang W, Wang L, Wang Q, Wang D, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. 2020;11(1):855. doi: 10.1038/s41467-020-14676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF. Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem. 2010;113(2):515–529. doi: 10.1111/j.1471-4159.2010.06614.x. [DOI] [PubMed] [Google Scholar]
- 112.Rose S, Bennuri SC, Davis JE, Wynne R, Slattery JC, Tippett M, Delhey L, Melnyk S, Kahler SG, MacFabe DF, et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl Psychiatry. 2018;8(1):42–17. doi: 10.1038/s41398-017-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leclercq S, Le Roy T, Furgiuele S, Coste V, Bindels LB, Leyrolle Q, Neyrinck AM, Quoilin C, Amadieu C, Petit G, et al. Gut microbiota-induced changes in β-hydroxybutyrate metabolism are linked to altered sociability and depression in alcohol use disorder. Cell Rep. 2020;33(2):108238. doi: 10.1016/j.celrep.2020.108238. [DOI] [PubMed] [Google Scholar]
- 114.Suzuki A, Stern Sarah A, Bozdagi O, Huntley George W, Walker Ruth H, Magistretti Pierre J, Alberini Cristina M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dragicevic N, Copes N, O’Neal‐Moffitt G, Jin J, Buzzeo R, Mamcarz M, Tan J, Cao C, Olcese JM, Arendash GW, et al. Melatonin treatment restores mitochondrial function in Alzheimer’s mice: a mitochondrial protective role of melatonin membrane receptor signaling. J Pineal Res. 2011;51(1):75–86. doi: 10.1111/j.1600-079X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 116.Wang D, Ho L, Faith J, Ono K, Janle EM, Lachcik PJ, Cooper BR, Jannasch AH, D’Arcy BR, Williams BA, et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol Nutr Food Res. 2015;59(6):1025–1040. doi: 10.1002/mnfr.201400544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsuda Y, Ozawa N, Shinozaki T, Wakabayashi K-I, Suzuki K, Kawano Y, Ohtsu I, Tatebayashi Y. Ergothioneine, a metabolite of the gut bacterium Lactobacillus reuteri, protects against stress-induced sleep disturbances. Transl Psychiatry. 2020;10(1):170. doi: 10.1038/s41398-020-0855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Halliwell B, Cheah IK, Tang RMY. Ergothioneine – a diet‐derived antioxidant with therapeutic potential. FEBS Lett. 2018;592(20):3357–3366. doi: 10.1002/1873-3468.13123. [DOI] [PubMed] [Google Scholar]
- 119.Zeni ALB, Camargo A, Dalmagro AP. Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids. 2017;125:131–136. doi: 10.1016/j.steroids.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 120.Mori T, Koyama N, Guillot-Sestier M-V, Tan J, Town T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and Alzheimer-like pathology in transgenic mice. PloS One. 2013;8(2):e55774. doi: 10.1371/journal.pone.0055774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chyan Y-J, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA. Potent neuroprotective properties against the Alzheimer β-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem. 1999;274(31):21937–21942. doi: 10.1074/jbc.274.31.21937. [DOI] [PubMed] [Google Scholar]
- 122.da Silva FL, Coelho Cerqueira E, de Freitas MS, Gonçalves DL, Costa LT, Follmer C. Vitamins k interact with N-terminus α-synuclein and modulate the protein fibrillization in vitro. Exploring the interaction between quinones and α-synuclein. Neurochem Int. 2013;62(1):103–112. doi: 10.1016/j.neuint.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 123.Nunes A, Amaral J, Lo A, Fonseca M, Viana R, Callaerts-Vegh Z, D’Hooge R, Rodrigues C. TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-β deposition in APP/PS1 mice. Mol Neurobiol. 2012;45(3):440–454. doi: 10.1007/s12035-012-8256-y. [DOI] [PubMed] [Google Scholar]
- 124.Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. 2004;558(1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 127.Dougherty MW, Kudin O, Mühlbauer M, Neu J, Gharaibeh RZ, Jobin C. Gut microbiota maturation during early human life induces enterocyte proliferation via microbial metabolites. BMC Microbiol. 2020;20(1):205. doi: 10.1186/s12866-020-01892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gomezde Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 129.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106(10):3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pessa-Morikawa T, Husso A, Kärkkäinen O, Koistinen V, Hanhineva K, Iivanainen A, Niku M. Maternal microbiota-derived metabolic profile in fetal murine intestine, brain and placenta. BMC Microbiol. 2021;22(1):46. doi: 10.1186/s12866-022-02457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koistinen VM, Kärkkäinen O, Borewicz K, Zarei I, Jokkala J, Micard V, Rosa-Sibakov N, Auriola S, Aura AM, Smidt H, et al. Contribution of gut microbiota to metabolism of dietary glycine betaine in mice and in vitro colonic fermentation. Microbiome. 2019;7(1):103. doi: 10.1186/s40168-019-0718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism – comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11(1):22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kang D-W, Ilhan ZE, Isern NG, Hoyt DW, Howsmon DP, Shaffer M, Lozupone CA, Hahn J, Adams JB, Krajmalnik-Brown R. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe. 2018;49(C):121–131. doi: 10.1016/j.anaerobe.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 135.Wang J, Pan J, Chen H, Li Y, Amakye WK, Liang J, Ma B, Chu X, Mao L, Zhang Z. Fecal short-chain fatty acids levels were not associated with autism spectrum disorders in Chinese children: a case-control study. Front Neurosci. 2019;13:1216. doi: 10.3389/fnins.2019.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Korpela K, Helve O, Kolho K-L, Saisto T, Skogberg K, Dikareva E, Stefanovic V, Salonen A, Andersson S, de Vos WM. Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell. 2020;183(2):324–334.e5. doi: 10.1016/j.cell.2020.08.047. [DOI] [PubMed] [Google Scholar]
- 138.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551(7682):648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matsumoto M, Kibe R, Ooga T, Aiba Y, Kurihara S, Sawaki E, Koga Y, Benno Y. Impact of intestinal microbiota on intestinal luminal metabolome. Sci Rep. 2012;2(1):233. doi: 10.1038/srep00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, Liu Y, Cheng K, Zhou C, Wang H, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-gaba cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4(4):623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 142.Bernasconi R, Jones RS, Bittiger H, Olpe HR, Heid J, Martin P, Klein M, Loo P, Braunwalder A, Schmutz M. Does pipecolic acid interact with the central GABA-ergic system? J Neural Transm. 1986;67(3–4):175. doi: 10.1007/BF01243346. [DOI] [PubMed] [Google Scholar]
- 143.Takahama K, Hashimoto T, Wang MW, Akaike N, Hitoshi T, Okano Y, Kasé Y, Miyata T. Pipecolic acid enhancement of GABA response in single neurons of rat brain. Neuropharmacology. 1986;25(3):339–342. doi: 10.1016/0028-3908(86)90263-7. [DOI] [PubMed] [Google Scholar]
- 144.van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, Clarke G, Stanton C, Dinan TG, Cryan JF. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596(20):4923–4944. doi: 10.1113/jp276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16(4):495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mir H-D, Milman A, Monnoye M, Douard V, Philippe C, Aubert A, Castanon N, Vancassel S, Guérineau NC, Naudon L, et al. The gut microbiota metabolite indole increases emotional responses and adrenal medulla activity in chronically stressed male mice. Psychoneuroendocrinology. 2020;119:104750. doi: 10.1016/j.psyneuen.2020.104750. [DOI] [PubMed] [Google Scholar]
- 147.Constante M, De Palma G, Lu J, Jury J, Rondeau L, Caminero A, Collins SM, Verdu EF, Bercik P. Saccharomyces boulardii CNCM I-745 modulates the microbiota-gut-brain axis in a humanized mouse model of irritable bowel syndrome. Neurogastroenterol Motil. 2020;33(3):e13985. doi: 10.1111/nmo.13985. [DOI] [PubMed] [Google Scholar]
- 148.Dell’Osso L, Carmassi C, Mucci F, Marazziti D. Depression, serotonin and tryptophan. Curr Pharm Des. 2016;22(8):949–954. doi: 10.2174/1381612822666151214104826. [DOI] [PubMed] [Google Scholar]
- 149.Marcinkiewcz CA, Lowery-Gionta EG, Kash TL. Serotonin’s complex role in alcoholism: implications for treatment and future research. Alcohol Clin Exp Res. 2016;40(6):1192–1201. doi: 10.1111/acer.13076. [DOI] [PubMed] [Google Scholar]
- 150.Keski-Rahkonen P, Kolehmainen M, Lappi J, Micard V, Jokkala J, Rosa-Sibakov N, Pihlajamäki J, Kirjavainen PV, Mykkänen H, Poutanen K, et al. Decreased plasma serotonin and other metabolite changes in healthy adults after consumption of wholegrain rye: an untargeted metabolomics study. Am J Clin Nutr. 2019;109(6):1630–1639. doi: 10.1093/ajcn/nqy394. [DOI] [PubMed] [Google Scholar]
- 151.Gao K, C-l M, Farzi A, W-y Z. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr. 2020;11(3):709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chong HX, Yusoff NAA, Hor YY, Lew LC, Jaafar MH, Choi SB, Yusoff MSB, Wahid N, Abdullah MFIL, Zakaria N, et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double-blind, placebo-controlled study. Benef Microbes. 2019;10(4):355–373. doi: 10.3920/BM2018.0135. [DOI] [PubMed] [Google Scholar]
- 153.Xu R, Wang Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol. 2016;10(Suppl 3):63. doi: 10.1186/s12918-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marksteiner J, Blasko I, Kemmler G, Koal T, Humpel C. Bile acid quantification of 20 plasma metabolites identifies lithocholic acid as a putative biomarker in Alzheimer’s disease. Metabolomics. 2018;14(1):1–10. doi: 10.1007/s11306-017-1297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Heinken A, Ravcheev DA, Baldini F, Heirendt L, Fleming RMT, Thiele I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome. 2019;7(1):75. doi: 10.1186/s40168-019-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 159.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 160.Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell. 2018;172(3):500–516.e16. doi: 10.1016/j.cell.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada González F, Perrin P, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353(6301):aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 162.Levy M, Thaiss Christoph A, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi Jemal A, David E, Savidor A, Korem T, Herzig Y, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163(6):1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Capuron Lmiller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Barroso A, Mahler JV, Fonseca-Castro PH, Quintana FJ. The aryl hydrocarbon receptor and the gut-brain axis. Cell Mol Immunol. 2021;18(2):259–268. doi: 10.1038/s41423-020-00585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11(4):1024–1038. doi: 10.1038/s41385-018-0019-2. [DOI] [PubMed] [Google Scholar]
- 167.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112(Pt B):399–412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 168.McLoughlin RF, Berthon BS, Jensen ME, Baines KJ, Wood LG. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106(3):930–945. doi: 10.3945/ajcn.117.156265. [DOI] [PubMed] [Google Scholar]
- 169.Magistretti Pierre J, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86(4):883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 170.Gibbs ME, Lloyd HGE, Santa T, Hertz L. Glycogen is a preferred glutamate precursor during learning in 1‐day‐old chick: biochemical and behavioral evidence. J Neurosci Res. 2007;85(15):3326–3333. doi: 10.1002/jnr.21307. [DOI] [PubMed] [Google Scholar]
- 171.Wideman CE, Jardine KH, Winters BD. Involvement of classical neurotransmitter systems in memory reconsolidation: focus on destabilization. Neurobiol Learn Mem. 2018;156:68–79. doi: 10.1016/j.nlm.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 172.Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, Bergersen LH. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex. 2014;24(10):2784–2795. doi: 10.1093/cercor/bht136. [DOI] [PubMed] [Google Scholar]
- 173.Wyss MT, Jolivet R, Buck A, Magistretti PJ, Weber B. In vivo evidence for lactate as a neuronal energy source. J Neurosci. 2011;31(20):7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ota M, Matsuo J, Ishida I, Hattori K, Teraishi T, Tonouchi H, Ashida K, Takahashi T, Kunugi H. Effect of a ketogenic meal on cognitive function in elderly adults: potential for cognitive enhancement. Psychopharmacology. 2016;233(21):3797–3802. doi: 10.1007/s00213-016-4414-7. [DOI] [PubMed] [Google Scholar]
- 175.Jensen NJ, Nilsson M, Ingerslev JS, Olsen DA, Fenger M, Svart M, Møller N, Zander M, Miskowiak KW, Rungby J. Effects of β-hydroxybutyrate on cognition in patients with type 2 diabetes. Eur J Endocrinol. 2020;182(2):233–242. doi: 10.1530/EJE-19-0710. [DOI] [PubMed] [Google Scholar]
- 176.Lund TM, Obel LF, Ø R, Sonnewald U. Β-hydroxybutyrate is the preferred substrate for gaba and glutamate synthesis while glucose is indispensable during depolarization in cultured gabaergic neurons. Neurochem Int. 2011;59(2):309–318. doi: 10.1016/j.neuint.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 177.Poeggeler B, Sambamurti K, Siedlak SL, Perry G, Smith MA, Pappolla MA. A novel endogenous indole protects rodent mitochondria and extends rotifer lifespan. PloS One. 2010;5(4):e10206. doi: 10.1371/journal.pone.0010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kärkkäinen O, Tuomainen T, Koistinen V, Tuomainen M, Leppänen J, Laitinen T, Lehtonen M, Rysä J, Auriola S, Poso A, et al. Whole grain intake associated molecule 5-aminovaleric acid betaine decreases β-oxidation of fatty acids in mouse cardiomyocytes. Sci Rep. 2018;8(1):13036–13037. doi: 10.1038/s41598-018-31484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Camandola Smattson MP. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017;36(11):1474–1492. doi: 10.15252/embj.201695810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rossignol Dafrye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012;17(3):290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mithul Aravind S, Wichienchot S, Tsao R, Ramakrishnan S, Chakkaravarthi S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res Int. 2021;142:110189. doi: 10.1016/j.foodres.2021.110189. [DOI] [PubMed] [Google Scholar]
- 182.Bakoyiannis I, Daskalopoulou A, Pergialiotis V, Perrea D. Phytochemicals and cognitive health: are flavonoids doing the trick? Biomed Pharmacother. 2019;109:1488–1497. doi: 10.1016/j.biopha.2018.10.086. [DOI] [PubMed] [Google Scholar]
- 183.Westfall S, Pasinetti GM. The gut microbiota links dietary polyphenols with management of psychiatric mood disorders. Front Neurosci. 2019;13:1196. doi: 10.3389/fnins.2019.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Russell WR, Hoyles L, Flint HJ, Dumas M-E. Colonic bacterial metabolites and human health. Curr Opin Microbiol. 2013;16(3):246–254. doi: 10.1016/j.mib.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 185.Mertens KL, Kalsbeek A, Soeters MR, Eggink HM, Wang L, Li Q, Wei M, Du Z, Fan Y. Bile acid signaling pathways from the enterohepatic circulation to the central nervous system. Front Neurosci. 2017;11:11. doi: 10.3389/fnins.2017.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. 2016;13(9):517–528. doi: 10.1038/nrgastro.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fried S, Wemelle E, Cani PD, Knauf C. Interactions between the microbiota and enteric nervous system during gut-brain disorders. Neuropharmacology. 2021;197:108721. doi: 10.1016/j.neuropharm.2021.108721. [DOI] [PubMed] [Google Scholar]
- 188.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 189.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28(8):1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.De Vadder F, Grasset E, Manneras Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Backhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018;115(25):6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 192.Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 193.Trapp S, Brierley DI. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br J Pharmacol. 2022;179(4):557–570. doi: 10.1111/bph.15638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hyland N,P, Cryan JF. Microbe-host interactions: influence of the gut microbiota on the enteric nervous system. Dev Biol. 2016;417(2):182–187. doi: 10.1016/j.ydbio.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 195.Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125(2):782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143(4):1006–16 e4. doi: 10.1053/j.gastro.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol. 2012;33(3):267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron. 2018;100(2):375–388. doi: 10.1016/j.neuron.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2019;4(3):396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, Sajed T, Johnson D, Li C, Karu N, et al. Hmdb 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608–D617. doi: 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yap CX, Henders AK, Alvares GA, Wood DLA, Krause L, Tyson GW, Restuadi R, Wallace L, McLaren T, Hansell NK, et al. Autism-related dietary preferences mediate autism-gut microbiome associations. Cell. 2021;184(24):5916–5931.e17. doi: 10.1016/j.cell.2021.10.015. [DOI] [PubMed] [Google Scholar]
- 204.Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G, Murphy E, Cryan JF, Dinan TG, Clarke G. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry. 2016;6(11):e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, Fan B, Lu C, McLntyer RS. Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry. 2019;9(1):190–199. doi: 10.1038/s41398-019-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang X, Sun G, Feng T, Zhang J, Huang X, Wang T, Xie Z, Chu X, Yang J, Wang H, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019;29(10):787–803. doi: 10.1038/s41422-019-0216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


