Abstract
Polyhydroxyalkanoic acids (PHA) are carbon and energy storage polymers that accumulate in inclusion bodies in many bacteria and archaea in response to environmental conditions. This work presents the results of a study of PHA inclusion body-associated proteins and an analysis of their coding region in Bacillus megaterium 11561. A 7,917-bp fragment of DNA was cloned and shown to carry a 4,104-bp cluster of 5 pha genes, phaP, -Q, -R, -B, and -C. The phaP and -Q genes were shown to be transcribed in one orientation, each from a separate promoter, while immediately upstream, phaR, -B, and -C were divergently transcribed as a tricistronic operon. Transfer of this gene cluster to Escherichia coli and to a PhaC− mutant of Pseudomonas putida gave a Pha+ phenotype in both strains. Translational fusions to the green fluorescent protein localized PhaP and PhaC to the PHA inclusion bodies in living cells. The data presented are consistent with the hypothesis that the extremely hydrophilic protein PhaP is a storage protein and suggests that PHA inclusion bodies are not only a source of carbon, energy, and reducing equivalents but are also a source of amino acids.
Polyhydroxyalkanoic acids (PHA) are a class of aliphatic polyesters that accumulate in inclusion bodies in many bacteria and archaea (2, 41). Their physiological role in the cell is that of carbon and energy reserves and that of a sink for reducing power. The most-studied PHA have repeating subunits of –[O–CH(R)(CH2)xCO]–, where the most common form is polyhydroxybutyrate, for which R is CH3 and x is 1 (45). The PHA biosynthetic pathway has been worked out for Alcaligenes eutrophus (17, 18, 44). In this organism two molecules of acetyl coenzyme A (acetyl CoA) are condensed by β-ketothiolase (PhaA), followed by a stereo-specific reduction catalyzed by an NADPH-dependent reductase (PhaB) to produce the monomer D-(−)-β-hydroxybutyryl CoA, which is polymerized by PHA synthase (PhaC). These three pha genes are encoded on the phaCAB operon, which is constitutively expressed, but PHA is not constitutively synthesized. Alternative pathways for synthesis of the monomer in other organisms have been suggested, most notably in the Pseudomonas species where the side chain, R, is longer than CH3 and its composition is influenced by carbon substrates in the growth medium (7, 45). In addition to being cloned from A. eutrophus, phaC has been cloned from more than 20 different bacteria (26, 43). Other genes associated with PHA synthesis, phaA, phaB, phaZ (PHA depolymerase), and genes for inclusion body-associated proteins and other low-molecular-weight proteins of unknown function, have also been cloned from some of these bacteria, in many cases by virtue of the fact that they are clustered with phaC.
PHA inclusion bodies are 0.2 to 0.5 μm in diameter, but their structural details are largely unknown. They were described originally for some species of Bacillus (6, 8, 15, 30, 47) and later for many more bacteria, including Pseudomonas, Alcaligenes, and Rhodococcus species (5, 11, 12, 25, 42). Those from Bacillus megaterium were shown to contain 97.7% PHA, 1.87% protein, and 0.46% lipid, with protein and lipid forming an outer layer (15). More recent reports show the presence of a 14-kDa protein (GA14) on PHA inclusion bodies of Rhodococcus ruber (36, 37) and a 24-kDa protein (GA24) with similarities to GA14 on the inclusion bodies of A. eutrophus (48). These proteins are not essential for PHA accumulation but have been shown to influence the size of PHA inclusion bodies and the rate of PHA accumulation (37, 48). GA14 and GA24 have been named phasins due to some similarities with oleosins, which are proteins on the surface of oil bodies in plant seeds (21). Proteins with similarity to GA24 are widespread in PHA-accumulating bacteria (49).
We have previously described the pattern of PHA inclusion body growth and proliferation throughout the growth cycle of B. megaterium (32). We now present the results of a study of PHA inclusion body-associated proteins from B. megaterium and the cloning and analysis of their coding region. The transcription starts were identified, the functional expression of some of the genes was confirmed in Escherichia coli and in a PHA-negative mutant of Pseudomonas putida, and PhaP and PhaC were localized to PHA inclusion bodies throughout growth.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | deoR endA1 gyrA96 hsdR17 (rK− mK+) recA1 relA1 supE44 thi-1 Δ(lacZYA-argFV169) φ80lacZΔM15 F−λ−; cloning host and for expression of pha genes | Clontech |
| B. megaterium 11561 | Wild type; used to clone pha genes | ATCCb |
| P. oleovorans 29347 | PHA-positive control | ATCC |
| P. putida GPp104 | PHA-negative mutant; phaC deletion mutant | 22 |
| Plasmids | ||
| pBluescriptIISK | Cloning vector; ColE1 oriVc Ampr | Stratagene |
| pGFPuv | Source of gfp gene; ColE1 oriV Ampr | Clontech |
| pHPS9 | Bacillus-E. coli shuttle vector; ColE1 and pTA1060 oriV Emr Cmr | 16 |
| pSUP104 | Pseudomonas-E. coli shuttle vector; Q-type and mini15 oriV Emr Tcr | 40 |
| pGM1 | EcoRI in phaP to HindIII in phaC, cloned into the EcoRI-HindIII sites of pBluescriptIISK; Ampr | This study |
| pGM6 | PstI in phaB to EcoRI in ykrM, cloned into the PstI-EcoRI sites of pBluescriptIISK; Ampr | This study |
| pGM7 | EcoRI in phaP to EcoRI in ykrM, cloned into the EcoRI site of pBluescriptIISK; Ampr | This study |
| pGM9 | HindIII upstream of ykoY to PstI in phaB, cloned into the HindIII-PstI sites of pBluescriptIISK; Ampr | This study |
| pGM10 | HindIII upstream of ykoY to EcoRI in ykrM, cloned into the HindIII-EcoRI sites of pBluescriptIISK; Ampr | This study |
| pGM7H | EcoRI in phaP to EcoRI in ykrM, cloned into the EcoRI site of pHPS9; Cmr | This study |
| pC/GFP2 | PhaC::GFP out-of-frame fusion plasmid; fragment shown in Fig. 4A cloned in pBluescriptIISK; Ampr | This study |
| pC/GFP3 | PhaC::GFP in-frame fusion plasmid; fragment shown in Fig. 4B cloned in pBluescriptIISK; Ampr | This study |
| pGM13 | PhaC::GFP in-frame fusion plasmid; fragment shown in Fig. 4C cloned in pHPS9; Emr Lmr | This study |
| pGM13C | GFP localization control plasmid; part of phaB and phaC deleted; fragment shown in Fig. 4D cloned in pHPS9; Emr Lmr | This study |
| pP/GFP3 | PhaP::GFP in-frame fusion plasmid; fragment shown in Fig. 4E cloned in pBluescriptIISK; Ampr | This study |
| pGM16.2 | PhaP::GFP in-frame fusion plasmid; fragment shown in Fig. 4F cloned in pHPS9; Emr Lmr | This study |
| pGM107 | EcoRI in phaP to EcoRI in ykrM, cloned as a BamHI-SalI fragment from pGM7, into the BamHI and SalI sites of pSUP104; Cmr | This study |
| pDR1 | PstI in phaB to EcoRI in ykrM, cloned as a SmaI-EcoRV fragment from pGM6 into the two DraI sites of pSUP104 in same orientation as the Cm gene, with phaC expressed from the Cm promoter; Tcr | This study |
Emr, EM resistant; Lmr, LM resistant; Cmr, CM resistant; Ampr, AMP resistant; Tcr, TC resistant.
ATCC, American Type Culture Collection.
Origin of replication.
Media and growth conditions.
Cultures were grown at 37°C (unless otherwise stated) in liquid media, aerated by rotation at 250 rpm in either Luria-Bertani broth (LB) (33) or M9 minimal salts (Life Technologies) with 1% (wt/vol) glucose. For growth on plates, the above media with 1.5% agar (Sigma catalog no. A4550) were used. For plasmid selections, the appropriate antibiotics were included in the media: ampicillin (200 μg/ml [AMP200]), chloramphenicol (25 μg/ml [CM25]), erythromycin (200 μg/ml [EM200]), or tetracycline (12.5 μg/ml [TC12.5]) for plasmid selection in E. coli; CM12 alone or EM1 plus lincomycin (25 μg/ml [LM25]) for plasmid selection in B. megaterium; and CM160 or TC30 for selection in Pseudomonas.
Separation of polypeptides associated with PHA inclusion bodies.
Inclusion bodies were purified as previously described (32) followed by suspension in TE (10 mM Tris-HCl [pH 8], 1 mM EDTA) with 2% sodium dodecyl sulfate (SDS). An equal volume of 2× sample buffer was added prior to boiling for 5 min, and samples were centrifuged for 3 min to pellet PHA; the supernatant was loaded on an SDS–12% polyacrylamide gel and run at 8 mA overnight at 4°C to separate the proteins. The gel was stained with Coomassie blue for 5 min prior to transfer of proteins to a polyvinylidene difluoride (PVDF) membrane by using a semidry electroblotter at 400 mA for 45 min. The membrane carrying the proteins of interest was cut for use in N-terminal amino acid sequence determination by Edman degradation using a minimum of 200 pmol of each protein.
Transformations.
E. coli and P. putida were transformed by electroporation of competent cells using an electroporator (Eppendorf) and following the manufacturer’s instructions. B. megaterium was transformed by a biolistic transformation procedure (39).
Cloning the pha region.
Purification of genomic and plasmid DNA, Southern blotting, hybridization, and cloning were performed by standard procedures (38). To clone the DNA sequences that coded for the two most abundant proteins on purified PHA inclusion bodies, degenerate oligonucleotide probes based on their N-terminal amino acid sequences were used. They were AAYACRGTNAAATAYNNNACRGTNATYNNNGCDATGATG (n2) and GCDATYCCDTAYGTNCARGAAGGHTTYAAA (n5) for the 20- and 41-kDa proteins, respectively (see Fig. 1). The restriction fragments indicated by the probes, when separately hybridized at 38°C in Southern blotting experiments to various restriction enzyme digests of B. megaterium genomic DNA, were purified from agarose following electrophoresis and cloned into pBluescriptIISK. Positive clones were identified by hybridization to the same degenerate probes, thus yielding pGM1. Sequences contiguous with and overlapping this primary cloned fragment were cloned in a similar manner, except that probes based on the ends of the sequenced DNA fragment were used and hybridization was at 55°C. The probes used were GCTTCATGCGTGCGGTTTG (bmp) and GGACCGTTCGGAAAATCAGCGG (bmc), yielding pGM9 and pGM6 (see Fig. 2), respectively.
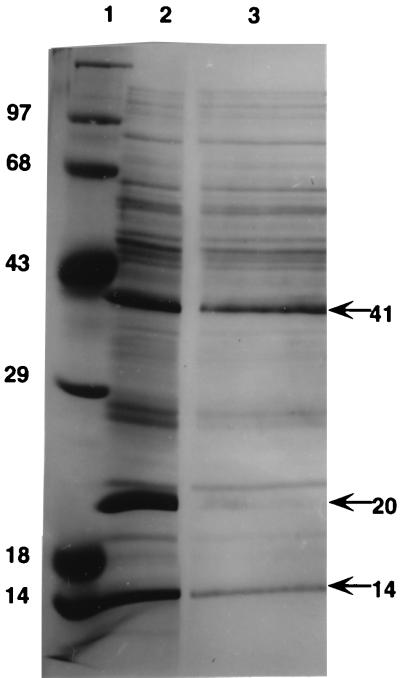
FIG. 1.
PHA inclusion body-associated proteins. Shown are the results of SDS-polyacrylamide gel electrophoresis of proteins released from purified PHA inclusion bodies. The bands were visualized by staining with Coomassie blue. Lane 1, molecular mass markers (14, 18, 29, 43, 68, and 97 kDa); lane 2, proteins from inclusion bodies of cells harvested in late exponential growth phase; lane 3, same as lane 2 except this part of the gel was stained following 45 min transfer of proteins (seen in lane 2) to PVDF membrane.
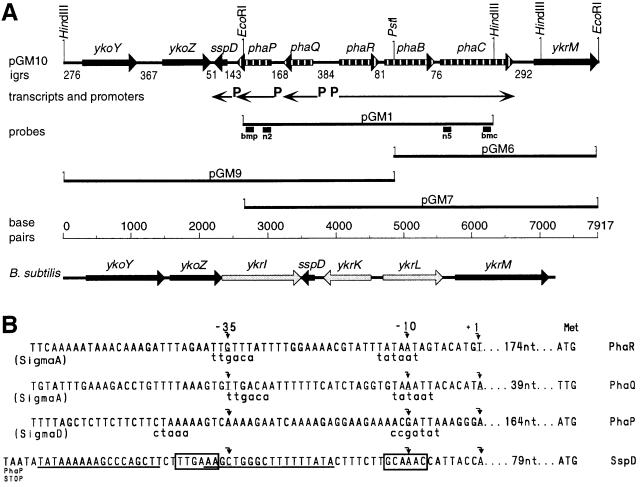
FIG. 2.
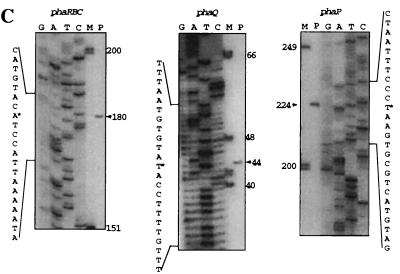
(A) The pha gene cluster and flanking sequences. A map of the cloned fragment in pGM10 carrying the pha genes (striped arrows), intergenic regions (igrs), and flanking genes (thick black arrows) from B. megaterium is shown. The thin arrows indicate the locations and directions of transcripts; P indicates promoter positions. pGM1, pGM6, pGM9, and pGM7 indicate the cloned DNA fragments in these plasmids (Table 1). Probes used to identify and clone the pha cluster are indicated by thick short lines under pGM1; n2 and n5 are degenerate probes; bmp and bmc are probes homologous to the ends of the pGM1 fragment. A ruler of sequence in base pairs is given for B. megaterium and B. subtilis. Below this, a map of the yko, sspD, and ykr region in the B. subtilis genome is shown; genes with homology to those of B. megaterium in this region are indicated by thick black arrows; nonhomologous genes are indicated by thick gray arrows. Gene annotations are given above each gene symbol. Relevant restriction enzyme sites are shown vertically. (B) Putative promoter regions for phaRBC, -Q, and -P, and sspD. Curved arrows indicate the transcription start (+1) and nucleotides (nt) −10 and −35. The closest resemblance to known −10 and −35 promoter sequences are given in lowercase letters below putative pha promoter sequences. Immediately downstream from the PhaP stop codon, the previously described (9) sspD putative promoter is boxed, and a putative hairpin structure is underlined. (C) Mapping of the 5′ ends of the phaRBC, -Q, and -P transcripts (see Materials and Methods). Lanes G, A, T, and C show the dideoxy sequencing ladders obtained with the same primers used in primer extension analysis; nucleotide sequences are complementary to the transcripts. Lane P contains the primer extension product. Lane M contains a DNA molecular size marker measured in nucleotides. The primer extension product is indicated by an arrowhead, and the 5′ end of the transcript within the sequence is indicated by a star. Only regions of the gel containing extension product bands are shown.
Sequencing the pha region.
DNA fragments of pGM1, pGM6, and pGM9 were subcloned into pBluescriptIISK and sequenced from both ends, using universal primers, and internally, by primer walking on both strands, by using dye terminator chemistry, cycle sequencing, and an ABI Prism 377 sequencer (Applied Biosystems). Sequence assembly and analysis was performed by using Lasergene (DNAStar, Inc.), Gapped BLAST, and PSI BLAST (1).
Mapping transcriptions starts.
The transcription start points were mapped in the region from the EcoRI site in phaP to the HindIII site in ykrM by primer extension analysis, using the Promega system for primer extension on RNA templates. DNA oligonucleotide primers, 17 to 20 nucleotides long, were synthesized to match target sequences, initially at approximately 500-bp intervals and subsequently at about 50 to 250 nucleotides downstream from the predicted transcription start points. The 32P 5′-end-labeled primers were extended with reverse transcriptase using total RNA (10 μg per reaction mixture) purified from B. megaterium (31). The fragment length, initially, and transcription start nucleotides, subsequently, were determined by running the cDNA on an 8% denaturing polyacrylamide gel alongside the products of sequencing reactions, which were generated with the same 5′-end-labeled primers. The primers used to identify the transcription start nucleotides for the phaP, -Q, and -RBC promoters were, respectively, CCCCTTTGTCCATTGTTCCC, CCATGTAGATTCCACCCTC, and CTCCATCTCCTTTCTTGTG.
Microscopy.
For phase-contrast microscopy, wet mounts of cultures were visualized at a ×1,000 magnification in a light microscope with phase-contrast attachments (Labophot-2 Microscope, Nikon, Inc.). To view PHA inclusion bodies, samples were heat fixed, stained with 1% (wt/vol) Nile blue A (Sigma) for 15 min at 55°C, destained for 30 s in 8% (vol/vol) acetic acid, water washed, air dried, and viewed at a ×1,000 magnification under fluorescence using filters (excitation, 446 nm; barrier filter, 590 nm; dichroic mirror, 580 nm). To view green fluorescent protein (GFP), wet mounts of cultures with or without 1% (wt/vol) agarose were viewed at a ×1,000 magnification under fluorescence using filters (excitation, 390 to 450 nm; barrier filter, 480 to 520 nm; dichroic mirror, 470 nm).
pha gene expression in E. coli and Pseudomonas.
Triplicate 500-ml cultures, were grown in 2-liter flasks at 30°C, rotating at 250, using 1% inocula of 16-h cultures, which had been grown in LB, centrifuged, and resuspended in equal volumes of 0.9% saline. At 48 h samples were removed for microscopy and cells were harvested, washed once in distilled H2O, and lyophilized. For PHA extraction, lyophilized cells were suspended in 10 volumes of 5% (wt/vol) NaOCl, shaken at 65°C for 1 h, and centrifuged. The pellet was resuspended in 10 volumes of 5% NaOCl and centrifuged, followed by sequential washing in water and 95% ethanol. The percentage of PHA is expressed as the weight vacuum-dried PHA per dry weight of cells.
Nucleotide sequence accession number.
The nucleotide sequence of the 7,917-bp fragment described in this work is available in the DDBJ, EMBL, and GenBank nucleotide databases under accession number AF109909.
RESULTS AND DISCUSSION
PHA inclusion body-associated proteins.
In an attempt to determine their relevance, proteins that copurify with PHA inclusion bodies were separated by electrophoresis on an SDS-polyacrylamide gel (Fig. 1). There were at least 13 such proteins present in various quantities. Some or all of these proteins could be intrinsic structural components of PHA inclusion bodies, enzymes involved with PHA metabolism, or possibly scaffolding components involved in inclusion body assembly. Alternatively, they could have been acquired by the inclusion bodies during the purification procedure. The three most abundant proteins had molecular masses of approximately 14, 20, and 41 kDa. The N-terminal amino acid sequence of the 14-kDa protein was KVFGRXELAAAMKRXGL, that of the 20-kDa protein was NTVKYXTVIXAMXXQ, and that of the 41-kDa protein was AIPYVQEXEKL. A BLASTp search revealed that the 14-kDa protein was lysozyme and the other two N-terminal sequences were unknown. It was concluded that the presence of lysozyme resulted from its use in cell lysis during the inclusion body purification procedure. This result confirms that not necessarily all of the proteins that copurify with PHA inclusion bodies are associated with them in vivo, as was also shown for Chromatium vinosum (27).
The 20- and 41-kDa proteins were the two most abundant proteins on the surface of purified PHA inclusion bodies. For the purpose of determining their relevance, if any, to PHA accumulation, their N-terminal amino acid sequences were used to design degenerative oligonucleotide probes for use in cloning their DNA coding regions. Both probes, used in separate hybridization experiments, identified a 6.4-kb HindIII fragment a 5.2-kb EcoRI fragment, and a 3.7-kb HindIII-to-EcoRI DNA fragment of DNA, indicating that the 5′ ends of the genes coding for both of these proteins were located less than 3.7 kb apart in the genome. This 3.7-kb fragment was cloned, sequenced, and shown to contain five open reading frames (ORFs) (Fig. 2) whose predicted amino acid sequences code for PhaP (20-kDa protein), PhaQ, PhaR, PhaB, and PhaC (41-kDa protein) (an explanation of this nomenclature is given below). The 20- and 41-kDa proteins were identified by their N-terminal amino acid sequences. Since the C terminus for each of these two proteins extended beyond the boundaries of pGM1, contiguous DNA sequences from both ends were cloned.
pha locus.
The 7,917-bp region carrying pha genes from B. megaterium was cloned, sequenced, and characterized. It was shown to carry eight complete ORFs and one incomplete ORF (Fig. 2; Table 2). Genes in this region were assigned on the basis of homology to known sequences, N-terminal amino acid sequences, putative ribosome binding sites, and operon location. The complement and arrangement of genes flanking the pha genes in B. megaterium are very similar to those in a region of Bacillus subtilis 168 (Fig. 2). This strain is negative for PHA, and no known pha genes or sequences occur in its genome, for which the complete sequence is available (24). In place of pha genes in this region of B. subtilis are ykrI, ykrK, and ykrL, which, respectively, code for putative proteins similar to two unknown proteins and a probable heat shock protein.
TABLE 2.
Sequence analysis results
| Gene | No. of amino acids | Mol mass (Da) | Isoelectric point | Homologous known or putative gene (accession no.)a | Identity (%) | Similarity (%) | Function or putative function (reference[s]) |
|---|---|---|---|---|---|---|---|
| ykoY | 271 | 29,996 | 6.89 | YkoY of B. subtilis (Z99110) | 64 | 73 | Toxic anion resistance protein (24) |
| ykoZ | 236 | 27,662 | 9.36 | YkoZ of B. subtilis (Z99111) | 57 | 74 | RNA polymerase sigma factor (24) |
| sspD | 65 | 7,027 | 8.58 | SspD of B. megaterium (P10572) | 100 | Spore-specific, DNA binding protein (10) | |
| SspD of B. subtilis (P04833) | 73 | 87 | Spore-specific, DNA binding protein (4) | ||||
| phaP | 170 | 19,906 | 5.29 | Nonec | PHA inclusion body structure, shape, and size (49) | ||
| phaQ | 146 | 16,686 | 5.09 | None | Unknown | ||
| phaR | 202 | 23,321 | 5.16 | None | Unknown | ||
| phaB | 247 | 26,098 | 7.39 | FabG of Synechocystis (D90907) | 50 | 66 | Fatty acid biosynthesis (23) |
| PhaB of C. vinosum D (P45375) | 48 | 64 | Acyl-CoA reductase (28) | ||||
| FabG of B. subtilis (P51831) | 47 | 67 | Fatty acid biosynthesis (35) | ||||
| phaC | 362 | 41,463 | 8.31 | PhaC of T. violacea (P45366) | 38 | 59 | PHA synthase (29) |
| PhaC of Synechocystis (D90906) | 37 | 56 | PHA synthase (23) | ||||
| PhaC of C. vinosum (P45370) | 35 | 55 | PHA synthase (28) | ||||
| ykrM | 318b | NDd | ND | YkrM of B. subtilis (Z99111) | 55 | 71 | Na+-transporting ATP synthase (24) |
Accession numbers are from SWISS-PROT, EMBL, or DDBJ databases.
Partial protein.
No discernible similarity to known sequences.
ND, not determined.
pha promoters.
The transcription start nucleotides in the pha region were determined. Primer extension products run on 8% denaturing polyacrylamide gels showed a single band from each reaction mixture, indicating one transcript, while control reaction mixtures in which RNA was omitted showed no bands. The extension products run alongside sequencing reaction products obtained with the same primer (Fig. 2C) identified the 5′ ends of the transcripts, thus allowing the putative promoter sequences at approximately −10 and −35 bp for phaP, -Q, and -R to be identified. The arrangement of genes in the pha cluster of B. megaterium is unique among those already published, and phaA is notably absent. The phaP, -Q, -R, -B, and -C genes were shown to be in a 4,104-bp region, with phaP and -Q transcribed in one orientation, each from a separate promoter, while phaR, -B, and -C were divergently transcribed from a promoter in front of phaR. The putative promoters responsible for transcription of phaQ and phaR, -B, and -C show strong similarity to both B. subtilis sigma A type (34) and E. coli sigma 70 type (14) promoters, which can express constitutively. This is in keeping with previous data for A. eutrophus showing that phaC is constitutively synthesized, but PHA is not constitutively accumulated (19). The third putative promoter in this region, the phaP promoter, resembles a sigma D type promoter known to control the expression of a regulon of genes associated with flagellar assembly, chemotaxis, and motility (13, 20, 46). In B. subtilis sigma D is expressed in the exponential phase and peaks in late exponential phase of growth. This parallels the pattern of PHA accumulation previously described for B. megaterium 11561 (32). However, further experiments are required to test the hypothesis that PHA accumulation is regulated by sigma D or products of its resulting transcripts. The phaP gene has 18-bp duplicate sequences that could base pair to form a rho-independent terminator close to its translational stop codon (Fig. 2B). The fact that the −35 promoter region of sspD is within this putative hairpin structure suggests that transcription of phaP and sspD could be mutually exclusive, thus allowing the expression of phaP to play a regulatory role in the expression of sspD (which codes for spore specific storage protein).
pha genes and their products.
The deduced amino acid sequence of PhaP shows a 20-kDa extremely hydrophilic product with no obvious similarity to known sequences. Inclusion body- associated low-molecular-weight proteins (phasins) have been described in many bacteria (49), but for those for which sequences were available we found no similarities of identifiable significance with PhaP of B. megaterium. PhaP does not have an obvious membrane anchoring domain, nor can it be described as an oleosin-like protein as was described for that of R. ruber (36). Low-molecular-weight, PHA inclusion body-abundant proteins obviously play an important role in PHA-producing cells, since they are involved in determining inclusion body size and shape and are present in quantities up to 5% of total protein in the case of PHA-producing A. eutrophus (48). It is interesting that the amino acid sequences of phasin proteins are so dissimilar, even in closely related bacteria. Some similarity between such proteins would be expected in closely related bacteria, were they to have a role in inclusion body biogenesis; however, conservation of sequence would be entirely unnecessary should they have a role as storage proteins.
The deduced amino acid sequences of PhaQ and PhaR also revealed small hydrophilic proteins with no significant identifiable similarity to known proteins. Figure 1 (lane 2) shows that purified inclusion bodies have proteins represented by bands of the approximate sizes of PhaQ (17 kDa) and -R (23 kDa), but the roles of these proteins are unknown. They may be nonorthologous replacements for the small putative gene products, whose roles are also unknown, encoded in known pha gene clusters. The deduced amino acid sequence of PhaB is very similar in size and amino acid sequence to known phaB and fabG gene products (Table 2). The deduced amino acid sequence of PhaC shows that while it has low homology overall to known PhaC proteins, it is most similar to that of Thiocystis violacea, Synechocystis sp., and C. vinosum. PhaC proteins from these three bacterial strains, respectively, have 355, 378, and 355 amino acids, while PhaC from B. megaterium has 362 amino acids. All other PhaC proteins studied are larger in size and range from 559 amino acids for that of Pseudomonas oleovorans (22) to 636 amino acids for that of Rhizobium etli (3). Alignment studies of sequences of all known PhaC proteins show that each of the four small PhaC proteins is missing approximately 150 amino acids from the N terminus and 50 amino acids from the C terminus; this is demonstrated for PhaC from B. megaterium and P. oleovorans in Fig. 3.
FIG. 3.
Pairwise alignment of PhaC from B. megaterium (B.meg.) (this study) and P. oleovorans (P.ole.) (SWISS-PROT accession no. P26494); amino acid identities are boxed in black. The Clustal method with a PAM250 residue weight table was used.
Expression of B. megaterium pha genes in E. coli and P. putida.
Functionality of the B. megaterium putative pha gene cluster was tested in E. coli, which is naturally PHA negative, and P. putida GPp104, a PhaC− mutant. Plasmids carrying one or more of these genes were introduced, and the resulting transformants were tested for PHA accumulation following growth on LB or M9 medium with various carbon sources and the appropriate antibiotic for plasmid selection. E. coli carrying pGM7 or pGM10 accumulated low levels of PHA while E. coli carrying pGM1 or pGM6 accumulated no PHA. Fluorescence microscopy of Nile blue A-stained cells showed that ∼1 cell in 20 had one or a few inclusion bodies and the quantity of PHA produced was ∼5% of cell dry weight. Since E. coli does not have PhaA, a low level of PHA or no PHA is the expected result. However, in Pseudomonas, in which PhaA is not known to be required, P. putida GPp104(pGM107) accumulated PHA on rich as well as minimal medium with various carbon sources to >50% of cell dry weight, and 90 to 100% of cells appeared full of PHA (Table 3). The positive control P. oleovorans, (equivalent to wild-type P. putida) accumulated PHA only when grown on longer-chain carbon sources, and not on LB. No PHA was accumulated by the negative control or by P. putida carrying phaC alone (pDR1). These results showed that this B. megaterium gene cluster is functional in both E. coli and P. putida. PhaC alone was insufficient to complement PhaC− P. putida or to synthesize PHA in E. coli. Furthermore, since Pseudomonas cannot synthesize PHA when grown in LB, these data indicate that PhaB can function to supply substrate to PhaC in P. putida. However, these data do not exclude the possibility that PhaP, -Q, or -R is necessary for PHA accumulation.
TABLE 3.
Cells with PHA as a percenta of total cells following growth on different carbon sources
| Substrate(s) (no. of C atoms) | % Cells with PHA
|
||||
|---|---|---|---|---|---|
| B. megaterium | P. oleovoransb | P. putida GPp104(pSUP104)c | P. putida GPp104(pGM107)d | P. putida GPp104(pDR1)e | |
| LB | 100 | 0 | 0 | 90 | 0 |
| LB–1% glucose | 100 | 0 | 0 | 92 | 0 |
| M9–12 mM caproate (6) | No growth | 88 | 0 | 100f | 0 |
| M9–12 mM octanoate (8) | No growth | 90 | 0 | 92 | 0 |
100%, PHA in all cells; 0%, no PHA in any cell; data averaged from more than five fields of each of three different cultures; error <5%.
Positive control.
Negative control, vector only.
phaPnQRBC (n indicates presence of N terminus only).
phaC.
Cell shape distorted by large quantity of PHA.
Localization of PhaP and PhaC.
Proteins associated with purified PHA inclusion bodies may not accurately reflect the localization of these proteins within the growing cell. Visualization of pha::gfp gene product fusion proteins in living cells throughout culture growth is a useful method for determining both the localization of the pha gene products and their comparative levels in growing cells. PhaP and PhaC, as fusion proteins (Fig. 4), localized to PHA inclusion bodies at all time points tested throughout growth of B. megaterium 11561. The negative control (pHPS9) showed no fluorescence at any time point. The localization control (pGM13C) showed nonlocalized green fluorescence at all time points. The profiles of PHA accumulation in these two control strains were similar to that of the wild-type, where the quantity of PHA decreased during the lag phase, increased during the exponential phase, and continued to increase at a lower steady-state rate in the stationary phase growth (32).
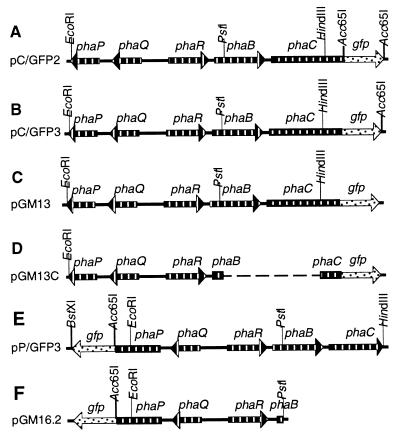
FIG. 4.
pha::gfp fusion plasmids and precursors. Only relevant restriction sites are shown. Annotations are described in the legend to Fig. 2. In all fusions the C-terminus, excluding the stop codon, of either phaC or phaP is fused to the gfp gene by the pGFPuv polylinker. For more details, see Table 1.
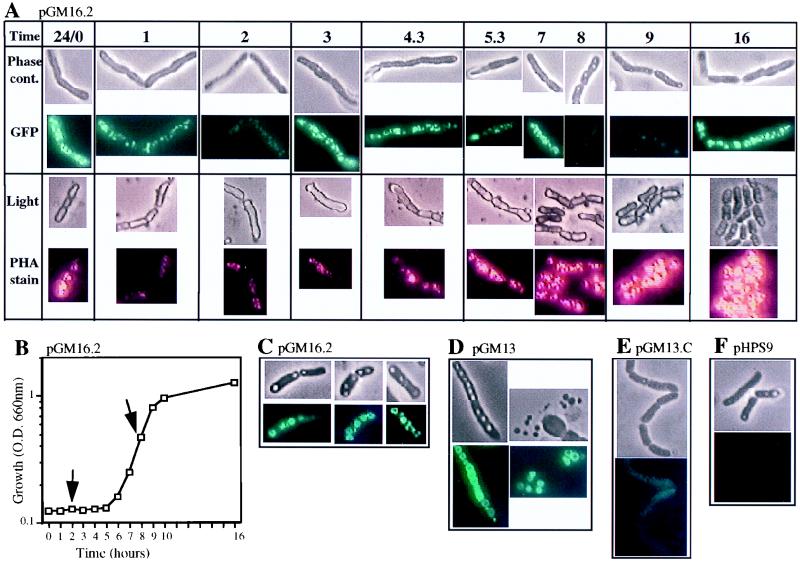
PhaP, monitored as a PhaP::GFP fusion protein in pGM16.2 (Fig. 5A and B), decreased significantly during the first half (2 h) of lag phase growth, increased during late lag phase and early to mid-exponential phase, decreased in mid- to late exponential phase, and increased during stationary phase growth. The rapid decrease of PhaP in lag phase is consistent with PhaP being a storage protein that is degraded as a source of amino acids. The profile of PHA accumulation in these cells (carrying pGM16.2) followed a pattern similar to that of PhaP except that PHA decreased only in the lag phase and continued to accumulate throughout other phases of culture growth. This data is consistent with PHA inclusion bodies’ being a source of carbon, reducing equivalents and amino acids when the organism is first provided with fresh medium. Possible explanations as to why the level of PhaP and not PHA decreased at mid- to late exponential phase are that either PhaP was synthesized at a lower rate than PHA was or PhaP was used as a source of amino acids at this phase of growth, or both scenarios may apply.
FIG. 5.
Localization of PhaP and PhaC. At time zero, cultures of B. megaterium carrying pGM16.2, pGM13, pGM13C, or pHPS9, grown in LB with LM25 EM1 for 24 h at 35°C, were inoculated (5% vol/vol) into 75 ml of fresh medium of the same composition, in 300-ml Naphelco flasks, and growth was continued at 27°C with shaking at 250 rpm. Optical densities (O.D.) of cultures were monitored and samples were removed for microscopy at time points from time zero to 24 h. One part of each sample was immediately observed for green fluorescence by embedding in 1% low-melting-point agarose for viewing by phase-contrast microscopy and under fluorescence for GFP (magnification, ×870). Another part of each sample was stained for PHA and viewed by light microscopy and by fluorescence for PHA inclusion bodies (magnification, ×870). Images were recorded by using identical parameters for all samples to allow comparison of fluorescence and light intensities (f-stop, 1/15; brightness, 0.6; sharpness, 1.0; contrast, 0.8; color, 0.3; see also Materials and Methods). (A) Time course for B. megaterium (pGM16.2). Time 24/0 was a sample taken at inoculation (time zero) using a 24-h culture; sampling times were at hours postinoculation as indicated; phase-contrast (Phase cont.) and GFP fluorescence images were of the same living cells; light and PHA fluorescence images were of the same heat-fixed cells. Images of living cells and heat-fixed cells were taken from the same sample at each sampling time. (B) Growth curve for cells shown in panel A; arrows indicate a decrease in PhaP::GFP fluorescence. (C) B. megaterium (pGM16.2) sampled at 2 days postinoculation. (D) B. megaterium (pGM13) sampled at 2 days postinoculation, (right and left panels show whole and lysed cells, respectively). (E) B. megaterium (pGM13C) sampled at 9 h postinoculation. (F) B. megaterium (pHPS9) showed no fluorescence at any time point. For panels C through F, top images are by phase-contrast microscopy and bottom images are by GFP fluorescence.
PhaC, monitored as a PhaC::GFP fusion protein in pGM13 (data not shown), showed a profile of expression similar to that of PhaP with one exception: PhaC did not reduce in level during lag phase growth. It did, however, reduce in level in mid- to late exponential phase growth, as did PhaP. The profile of PHA accumulation in these cells carrying PhaC::GFP was similar to that of cells carrying PhaP::GFP, except that the PHA level did not reduce during lag phase growth. It is reasonable to assume that the increased quantity of PhaC in the cell is a likely explanation since PhaC remained functional in the fusion protein PhaC::GFP. This was indicated by the fact that E. coli DH5α(pC/GFP3) and E. coli DH5α(pGM7) accumulated PHA to equivalent low levels, while the host strain alone, or carrying pGFPuv, accumulated no PHA, as visualized by fluorescence microscopy of Nile blue A-stained cells. The reduction in level of PhaC in mid- to late exponential phase, as was also seen with PhaP, is consistent with both PhaC’s and PhaP’s being synthesized at a lower rate than PHA is.
In cells of all growth phases, inclusion bodies were rarely visible under light in stained heat-fixed cells while larger inclusion bodies were visible by phase-contrast microscopy of living cells (Fig. 5C to F). In older cultures (2 days and older) some cells were lysed and showed PhaP::GFP and PhaC::GFP localized to free PHA inclusion bodies (Fig. 5D). Both free and intracellular inclusion bodies had doughnut-shaped localization of GFP at some focal planes while at other focal planes the same inclusion bodies appeared completely covered in GFP. We interpret this data as a difference in quantity of GFP that is visible when viewed through the edge or the center of the inclusion bodies.
Concluding remarks.
This is the first report of PHA inclusion body-associated proteins and their coding region in B. megaterium. The phaP, -Q, -R, -B, and -C genes can be positioned in the B region of the B. megaterium map due to linkage to the sspD gene (46), provided that strains 11561 and QM B1551 have similar genetic maps. PhaP, -Q, and R are extremely hydrophilic proteins, with no discernible sequence similarities to known proteins. PhaP localized to PHA inclusion bodies in living cells. In addition to a possible role in inclusion body biogenesis, the data are consistent with PhaP’s being a storage protein. PhaB and -C have homology to known PHA proteins. The sequence of PhaB is more like that of FabG than like other PhaB proteins, and our data suggest that it can function to provide substrate to the B. megaterium PhaC in a PhaC− mutant of P. putida, thus allowing PHA accumulation. PhaC is among the smallest of the known PhaC proteins and localizes to PHA inclusion bodies in living cells. The significance of PhaC and PhaP localization in the mechanism of PHA inclusion body biogenesis and PHA accumulation is being further investigated. The analysis of the pha locus of B. megaterium not only provides further comparative data on pha genes and their products but also allows a comparison of this locus with its counterpart in B. subtilis.
ACKNOWLEDGMENTS
We thank Frank Cannon for discussions and critically reading the manuscript, Tom Redlinger for help with polypeptide separations, and Tania Fernandez, Doreen Campbell, and Dennis Ryan for excellent technical assistance.
This research was supported in part by a grant from the National Science Foundation (MCB 97-28066).
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson A, Dawes E A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cevallos M A, Encarnacion S, Leija A, Mora Y, Mora J. Genetic and physiological characterization of a Rhizobium etli mutant strain unable to synthesize poly-beta-hydroxybutyrate. J Bacteriol. 1996;178:1646–1654. doi: 10.1128/jb.178.6.1646-1654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connors M J, Mason J M, Setlow P. Cloning and nucleotide sequencing of genes for three small, acid-soluble proteins Bacillus subtilis spores. J Bacteriol. 1986;166:417–425. doi: 10.1128/jb.166.2.417-425.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.deSmet M J, Eggink G, Witholt B, Kingma J, Wynberg H. Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J Bacteriol. 1983;154:870–878. doi: 10.1128/jb.154.2.870-878.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunlop W, Robards A W. Ultrastructural study of poly-β-hydroxybutyrate granules from Bacillus cereus. J Bacteriol. 1973;114:1271–1280. doi: 10.1128/jb.114.3.1271-1280.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eggink G, de Waard P, Huijberts G N M. The role of fatty acid biosynthesis and degradation in the supply of substrates for poly(3-hydroxyalkanoate) formation in P. putida. FEMS Microbiol Rev. 1992;103:159–164. [Google Scholar]
- 8.Ellar D, Lundgren D G, Okamura K, Marchessault R H. Morphology of poly-β-hydroxybutyrate granules. J Mol Biol. 1968;35:489–502. doi: 10.1016/s0022-2836(68)80009-9. [DOI] [PubMed] [Google Scholar]
- 9.Fliss E R, Loshon A C, Setlow P. Genes for Bacillus megaterium small, acid-soluble spore proteins: Cloning and nucleotide sequence of three additional genes from this multigene family. J Bacteriol. 1986;165:467–473. doi: 10.1128/jb.165.2.467-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fliss E R, Setlow P. Bacillus megaterium spore protein C-3: nucleotide sequence of its gene and the amino acid sequence at its spore cleavage site. Gene. 1984;30:167–172. doi: 10.1016/0378-1119(84)90117-3. [DOI] [PubMed] [Google Scholar]
- 11.Fuller R C, O’Donnell J P, Saulnier J, Redlinger T E, Foster J, Lenz R W. The supramolecular architecture of the polyhydroxyalkanoate inclusions in Pseudomonas oleovorans. FEMS Microbiol Rev. 1992;103:279–288. [Google Scholar]
- 12.Gerngross T U, Reilly P, Stubbe J, Sinskey A J, Peoples O P. Immunocytochemical analysis of poly-β-hydroxybutyrate (PHB) synthase enzyme at the surface of PHB granules. J Bacteriol. 1993;175:5289–5293. doi: 10.1128/jb.175.16.5289-5293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilman M Z, Wings J L, Chamberlin M J. Nucleotide sequence of two Bacillus subtilis promoters used by Bacillus subtilis sigma-28 RNA polymerase. Nucleic Acids Res. 1981;9:5991–6000. doi: 10.1093/nar/9.22.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gitt M A, Wang L F, Doi R H. A strong sequence homology exists between RNA polymerase sigma factors of Bacillus subtilis and Escherichia coli. J Biol Chem. 1985;260:7178–7185. [PubMed] [Google Scholar]
- 15.Griebel R, Smith Z, Merrick M. Metabolism of poly-β-hydroxybutyrate. 1. Purification, composition, and properties of native poly-β-hydroxyburyrate granules from Bacillus megaterium. Biochemistry. 1968;7:3676–3681. doi: 10.1021/bi00850a047. [DOI] [PubMed] [Google Scholar]
- 16.Haima P, van Sinderen D, Scholting H, Bron S, Venema G. Development of β-galactosidase α-complementation system for molecular cloning in Bacillus subtilis. Gene. 1990;86:63–69. doi: 10.1016/0378-1119(90)90114-7. [DOI] [PubMed] [Google Scholar]
- 17.Haywood G W, Anderson A J, Chu L, Dawes E A. The role of NADH- and NADPH-linked acetoacetyl-CoA reductases in the poly-3-hydroxybutyrate synthesizing organism Alcaligenes eutrophus. FEMS Microbiol Lett. 1988;52:259–264. [Google Scholar]
- 18.Haywood G W, Anderson A J, Chu L, Dawes E A. Characterization of two 3-ketothiolases in the polyhydroxyalkanoate synthesizing organism Alcaligenes eutrophus. FEMS Microbiol Lett. 1988;52:91–96. [Google Scholar]
- 19.Haywood G W, Anderson A J, Dawes E A. The importance of PHB-synthase substrate specificity in polyhydroxyalkanoate synthesis by Alcaligenes eutrophus. FEMS Microbiol Lett. 1989;57:1–6. [Google Scholar]
- 20.Helmann J D. Alternative sigma factors and the regulation of flagellar gene expression. Mol Microbiol. 1991;5:2875–2882. doi: 10.1111/j.1365-2958.1991.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang A H C. Oil bodies and oleosins in seeds. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:177–200. [Google Scholar]
- 22.Huisman G W, Wonink E, Meima R, Kazemier B, Terpstra P, Witholt B. Metabolism of poly(3-hydroxyalkanoates) (PHAs) by Pseudomonas oleovorans. J Biol Chem. 1991;266:2191–2198. [PubMed] [Google Scholar]
- 23.Kaneko T, et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 24.Kunst N, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 25.Lauzier C, Marchessault R H, Smith P, Chanzy H. Structural study of isolated poly(β-hydroxybutyrate) granules. Polymer. 1992;33:823–827. [Google Scholar]
- 26.Lee S Y. Bacterial polyhydroxyalkanoates. Biotechnol Eng. 1995;49:1–14. doi: 10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Liebergesell M, Schmidt B, Steinbüchel A. Isolation and identification of granule-associated proteins relevant for poly(hydroxyalkanoic acid) biosynthesis in Chromatium vinosum D. FEMS Microbiol Lett. 1992;99:227–232. doi: 10.1016/0378-1097(92)90031-i. [DOI] [PubMed] [Google Scholar]
- 28.Liebergesell M, Steinbüchel A. Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. Eur J Biochem. 1992;209:135–150. doi: 10.1111/j.1432-1033.1992.tb17270.x. [DOI] [PubMed] [Google Scholar]
- 29.Liebergesell M, Steinbüchel A. Cloning and molecular analysis of the poly (3-hydroxybutyric acid) biosynthetic genes of Thiocystis violacea. Appl Microbiol Biotechnol. 1993;38:493–501. doi: 10.1007/BF00242944. [DOI] [PubMed] [Google Scholar]
- 30.Lundgren D G, Pfister R M, Merrick J M. Structure of poly-β-hydroxybutyric acid granules. J Gen Microbiol. 1964;34:441–446. doi: 10.1099/00221287-34-3-441. [DOI] [PubMed] [Google Scholar]
- 31.Magni C, Marini P, de Mendoza D. Extraction of RNA from gram-positive bacteria. BioTechniques. 1995;19:882–884. [PubMed] [Google Scholar]
- 32.McCool G J, Fernandez T, Li N, Cannon M C. Polyhydroxyalkanoate inclusion-body growth and proliferation in Bacillus megaterium. FEMS Microbiol Lett. 1996;137:41–48. [Google Scholar]
- 33.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 34.Moran C P, Jr, Lang N, LeGrice S F J, Lee G, Stephens M, Sonenshein A L, Pero J, Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186:339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- 35.Morbidoni H R, de Mendoza D, Cronan J E. Bacillus subtilis acyl carrier protein is encoded in a cluster of lipid biosynthesis genes. J Bacteriol. 1996;178:4794–4800. doi: 10.1128/jb.178.16.4794-4800.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pieper-Fürst U, Madkour M H, Mayer F, Steinbüchel A. Purification and characterization of a 14-kilodalton protein that is bound to the surface of polyhydroxyalkanoic acid granules in Rhodococcus ruber. J Bacteriol. 1994;176:4328–4337. doi: 10.1128/jb.176.14.4328-4337.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pieper-Fürst U, Madkour M H, Mayer F, Steinbüchel A. Identification of the region of a 14-kilodalton protein of Rhodococcus ruber that is responsible for the binding of this phasin to polyhydroxyalkanoic acid granules. J Bacteriol. 1995;177:2513–2523. doi: 10.1128/jb.177.9.2513-2523.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Shark K B, Smith F D, Harpending P R, Rasmussen J L. Biolistic transformation of a procaryote, Bacillus megaterium. Appl Environ Microbiol. 1991;57:480–485. doi: 10.1128/aem.57.2.480-485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon R, Priefer U, Puhler A. In: Molecular genetics of the bacteria-plant interaction. Puhler A, editor. Berlin, Germany: Springer; 1983. pp. 98–106. [Google Scholar]
- 41.Steinbüchel A. Polyhydroxyalkanoic acids. In: Byrom D, editor. Biomaterials, novel materials from biological sources. Basingstoke, England: Macmillan Publishers Ltd.; 1991. pp. 123–213. [Google Scholar]
- 42.Steinbüchel A, Aerts K, Babel W, Follner C, Liebergesell M, Madkour M H, Mayer F, Pieper-Furst U, Pries A, Valentin H E, Wieczorek R. Considerations on the structure and biochemistry of bacterial polyhydroxyalkanoic acid inclusions. Can J Microbiol. 1995;41:94–105. doi: 10.1139/m95-175. [DOI] [PubMed] [Google Scholar]
- 43.Steinbüchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H. Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev. 1992;103:217–230. doi: 10.1111/j.1574-6968.1992.tb05841.x. [DOI] [PubMed] [Google Scholar]
- 44.Steinbüchel A, Schlegel H G. Physiology and molecular genetics of poly(β-hydroxyalkanoic acid) synthesis in Alcaligenes eutrophus. Mol Microbiol. 1991;5:535–542. doi: 10.1111/j.1365-2958.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 45.Steinbuchel A, Valentin H E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett. 1995;128:219–228. [Google Scholar]
- 46.Vary P. The genetic map of Bacillus megaterium. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 475–481. [Google Scholar]
- 47.Wang W S, Lundgren D G. Poly-β-hydroxybutyrate in the chemolithotrophic bacterium Ferrobacillus ferrooxidans. J Bacteriol. 1969;97:947–950. doi: 10.1128/jb.97.2.947-950.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wieczorek R, Pries A, Steinbüchel A, Mayer F. Analysis of a 24-kilodalton protein associated with the polyhydroxyalkanoic acid granules in Alcaligenes eutrophus. J Bacteriol. 1995;177:2425–2435. doi: 10.1128/jb.177.9.2425-2435.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wieczorek R, Steinbüchel A, Schmidt B. Occurrence of polyhydroxyalkanoic acid granule-associated proteins related to the Alcaligenes eutrophus H16 GA24 protein in other bacteria. FEMS Microbiol Lett. 1996;135:23–30. doi: 10.1111/j.1574-6968.1996.tb07961.x. [DOI] [PubMed] [Google Scholar]