Abstract
The impact of COVID-19 on the burden of cardiovascular diseases (CVD) during the early pandemic remains unclear. COVID-19 has become one of the leading causes of global mortality, with a disproportionate impact on persons with CVD. Studies of health facility admissions for CVD found significant decreases during the pandemic. Studies of hospital mortality for CVD were more variable. Studies of population-level CVD mortality differed across countries, with most showing decreases, although some revealed increases in deaths. In some countries where large increases in CVD deaths were reported in vital registration systems, misclassification of COVID-19 as CVD may have occurred. Taken together, studies suggest heterogeneous effects of the COVID-19 pandemic on CVD without large increases in CVD mortality in 2020 for a number of countries. Clinical and population science research is needed to examine the ways in which the pandemic has affected CVD burden.
Key Words: burden of disease, COVID-19, hospital outcomes, population health
Abbreviations and Acronyms: CVD, cardiovascular; IHD, ischemic heart disease; OHCA, out-of-hospital cardiac arrest
Central Illustration
COVID-19 has rapidly become one of the leading causes of death in the world.1 The impact of COVID-19 on the burden of cardiovascular diseases (CVD) and other leading causes of death around the world remains an important question as policy makers grapple with both the acute and long-term effects of the pandemic on health systems. Reliable estimates of population health are an essential input for evidence-based decision making; however, data availability, quality, and timeliness as well as variation in study methods and outcomes have led to disparate conclusions regarding how the burden of CVD may have changed over the course of the pandemic. As part of the ongoing GBD (Global Burden of Disease)–National Heart, Lung, and Blood Institute–Journal of the American College of Cardiology Global Burden of CVD Collaboration, this review will consider current evidence regarding the consequences of COVID-19 for population-level changes in CVD.2
This review considers 2 sources of evidence to better understand the impact of COVID-19 on CVD. First, for countries where detailed vital registration data were available since 2020, the GBD study has analyzed the underlying cause of death reported on death certificates and compared CVD mortality with that of preceding years to understand if there have been excess CVD deaths during the COVID-19 pandemic. Second, multicenter studies of CVD health care delivery, health facility admission or visit, and hospital- and population-level mortality during the COVID-19 pandemic were identified using a structured search of the scientific literature. Changes in CVD burden may offer opportunities for innovation in how public health and health care systems address the ongoing challenges of CVD burden and cardiovascular care.
Multiple hypotheses have been proposed for the pathways by which the COVID-19 pandemic may alter trends in cardiovascular population health. Atherosclerotic cardiovascular disease risk factors and established CVD have all been associated with worse outcomes among people with COVID-19.3 COVID-19 alters vascular endothelium and cardiomyocyte function.4 Myocarditis, thrombotic events, and multisystem vascular syndromes have all been described as a result of COVID-19.5 Indirect effects of COVID-19 on CVD have also been proposed. Hospitalizations for acute cardiovascular events may have been deferred or delayed in the early phases of the pandemic.6 , 7 Changes in access to medications, diagnostic testing, and procedures may have increased CVD burden, while decreases in air pollution during lockdown periods may have decreased CVD.8 , 9 Alterations in lifestyle and physical inactivity in the setting of pandemic-related restrictions may have contributed to population-level worsening of cardiometabolic risk factor control.10 , 11
GBD Study
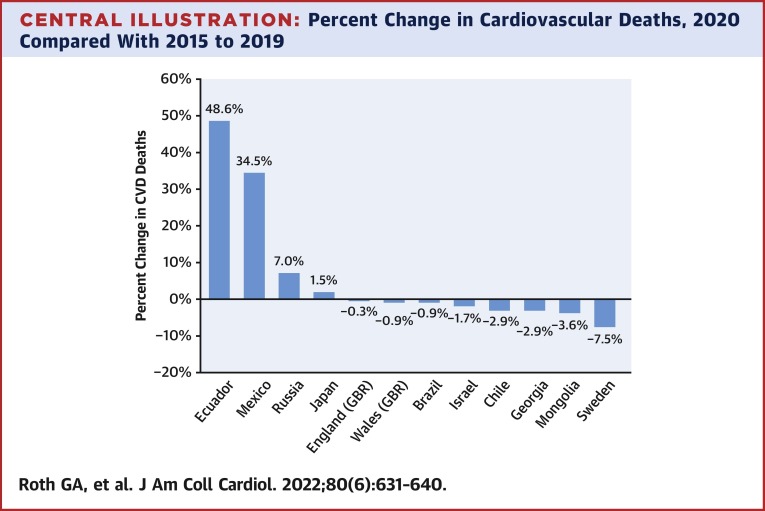
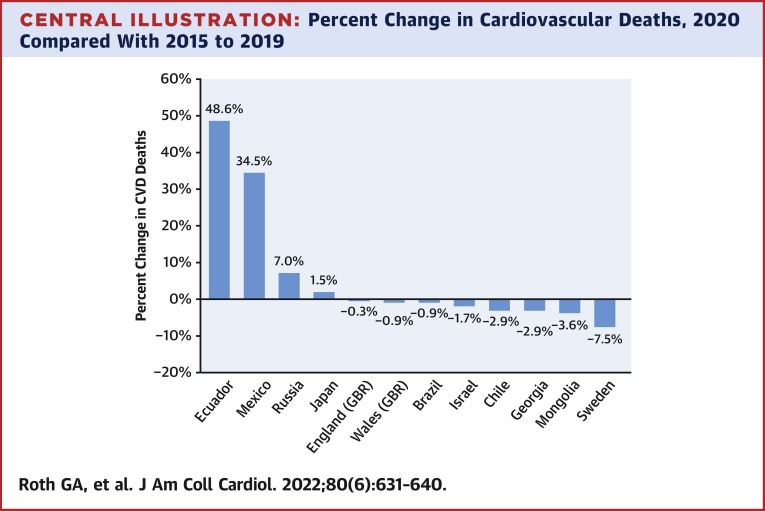
The GBD study offers one opportunity for better understanding how CVD may have changed because of the COVID-19 pandemic. The GBD study is an ongoing multinational effort to produce a consistent and comparable assessment of disease burden for all countries in the world. As we have previously reported, the global CVD burden has increased over the last 3 decades because of population growth and aging, while the age-standardized death rate of ischemic heart disease (IHD), stroke, and hypertensive heart disease have declined.12 In high-income countries, this trend has reversed itself in the last 5 years, and age-standardized mortality has begun to increase again in some locations.12 In the Central Illustration and Table 1 , we show the change in the number of CVD deaths before and during the first year of the COVID-19 pandemic.
Central Illustration.
Percent Change in Cardiovascular Deaths, 2020 Compared With 2015 to 2019
The impact of COVID-19 on the burden of cardiovascular diseases (CVD) during the early pandemic remains unclear. Studies of population-level CVD mortality differed across countries, with most showing decreases, although some revealed small to moderate increases in deaths. These differences may be explained by the misclassification of COVID-19 as CVD in some countries. Taken together, studies suggest heterogenous effects of the COVID-19 pandemic on CVD without large increases in CVD mortality in 2020 for a number of countries.
Table 1.
Change in Deaths Reported With Underlying Cause of CVD, 2020 vs 2015 to 2019, Global Burden of Disease Study
| Countries | Mean Number of CVD Deaths per Year, 2015-2019 | Number of CVD Deaths, 2020 | Change in Number of CVD Deaths From Average, 2020 vs 2015-2019 | Percent Change in CVD Deaths, 2020 vs 2015-2019 |
|---|---|---|---|---|
| Ecuador | 18,871 | 28,042 | 9,171 | 48.6 |
| Mexico | 180,526 | 242,857 | 62,332 | 34.5 |
| Russia | 986,141 | 1,054,949 | 68,808 | 7.0 |
| Japan | 345,044 | 350,088 | 5,044 | 1.5 |
| England (GBR) | 137,728 | 137,300 | −428 | −0.3 |
| Wales (GBR) | 9,246 | 9,164 | −82 | −0.9 |
| Brazil | 354,551 | 351,420 | −3,130 | −0.9 |
| Israel | 711 | 699 | −12 | −1.7 |
| Chile | 26,552 | 25,785 | −767 | −2.9 |
| Georgia | 25,327 | 24,590 | −737 | −2.9 |
| Mongolia | 5,227 | 5,038 | −189 | −3.6 |
| Sweden | 31,171 | 28,828 | −2,342 | −7.5 |
Change in deaths coded to CVD may reflect misclassification of deaths caused by COVID-19. Numbers of death have been corrected for nonspecific or nonunderlying coding using the GBD standard redistribution method.13 Chile 2019 data were not available and therefore were excluded from calculation of the mean. Mongolia 2015 and 2017 data were not available and were excluded from mean. All data were from vital registration death registration in each location. Colors represent heatmap scaled to the values in that column.
CVD = cardiovascular disease; GBR = Great Britain.
Results are estimates of deaths caused by CVD based on the underlying cause of death reported on death certificates, adjusted for nonspecific and nonunderlying causes using GBD study methods.12 Data were obtained from 12 locations where detailed cause-specific vital registration death certification data could be obtained by the GBD study for the year 2020. Deaths were classified as CVD based on the GBD cause of death hierarchy and its standardized set of International Classification of Diseases system codes. Nonspecific and nonunderlying causes of death were reclassified based on statistical models examining the co-occurrence of CVD and other codes in multiple-causes-of-death data sets, which report the intermediate and immediate causes of death alongside the underlying cause. A Bayesian noise-reduction algorithm is applied to account for the occurrence of zero counts in the data. An ensemble of geospatial time series regression models was created using covariates with causal associations to disease outcomes (the Cause of Death Ensemble Model software package, CODem, developed by the Global Burden of Disease study), to produce smooth time series estimates with uncertainty bounds.
The number of deaths coded to CVD as the underlying cause are similar or even lower in 2020 for most of these countries when compared with recent prior years, with the exceptions of Ecuador, Mexico, and Russia, where moderate to large increases were reported. In Ecuador, the number of deaths reported as attributable to CVD increased by almost 50% in 2020 compared with the average of the prior 5 years, with a 35% increase in Mexico and a 7% increase in Russia. CVD deaths in Sweden were 7.5% lower in 2020 compared with prior years. CVD deaths varied far less in the remaining countries, with fewer CVD deaths when compared with prior years in all of them other than Japan, where a 1.5% increase in CVD deaths occurred. In England, Wales, and Brazil, the percent change in CVD deaths was <1%. The degree to which deaths caused by COVID-19 may have been misclassified as CVD is unclear.1 Estimated COVID-19 death rates in 2020 in Ecuador and Mexico were 2-3 times higher than rates in the other countries in this sample, and COVID-19 diagnostic testing capacity was likely to have been limited in at least parts of these countries.14 Especially high rates of deaths caused by COVID-19 may reflect countries where cardiovascular diagnostic testing and health system performance overall were put under particular stress.
Studies of COVID-19 Pandemic Effects on CVD Population Health
For the purpose of this narrative review, the Web of Science Core Collection database was searched for studies published through December 31, 2021, in 34 cardiovascular or general medicine journals selected by the authors, using a structured search string with the terms “cardiovascular,” “mortality,” and “covid.” There were 1,953 studies that resulted initially, with 249 studies remaining after limiting the results to selected journals, of which 51 were identified as relevant based on a review of the title and abstract and then a full-text review. Of these 51 studies, we identified 19 studies meeting our inclusion criteria. We included studies that were multicenter and included any measures of health-related outcomes. Studies were found from 11 countries, and all examined data from the first 1-10 months of the pandemic. No published studies were found that included data from the year 2021; however, the U.S. National Center for Health Statistics has made available limited amounts of tabulated CVD mortality data from 2021.
Health Care Delivery
Decreases in health care delivery were observed in many countries in 2020 because of lockdown policies and health systems shifting to create capacity for COVID-19 patients (Table 2 ). In England, percutaneous coronary interventions, surgical aortic valve replacement, and transcatheter aortic valve replacement procedures decreased substantially.15 , 16 Japan saw no change in prescriptions filled for cardioprotective therapies such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.17
Table 2.
Summary of Studies on the Impact of the COVID-19 Pandemic on CVD Health Care Delivery
| Study Setting | Number of Pandemic Months Included | Comparison | Process Measure | Outcome | Effect |
|---|---|---|---|---|---|
| Japan17 | 5 | April 2018 to February 2020/March to July 2020 | ACE inhibitor/ARB prescription | No difference | No change |
| England15 | 4 | January 2017 to 2019/March to April 2020 | PCI procedures | −43% | Decrease |
| England16 | 9 | January 2018/March to November 2020 | Observed vs expected SAVR and TAVR cases | 4,989 fewer cases | Decrease |
Comparisons of CVD pre– vs post–COVID-19 pandemic that were multicenter, were population-based, and included measures of health or health-related outcomes performed through December 30, 2021. The 249 matches from a structured search in Web of Science were reviewed by title and abstract; 51 sources were identified and reviewed as full text, with 19 sources matching the inclusion criteria and extracted to this table. Effect column describes the result of the outcome reported for each study, with increase shaded red, no change shaded yellow, and decrease shaded green.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CVD = cardiovascular disease; PCI = percutaneous coronary intervention; SAVR = surgical aortic valve replacement; TAVR = transcutaneous aortic valve replacement.
Health Facility Admissions or Visits
Studies comparing health facility admissions or visits for CVD in 2020 with prior years generally found significant decreases (Table 3 ). Heart failure hospitalizations declined 3.6% per week in Japan.17 New stroke and myocardial infarction diagnoses decreased by 9,047 cases in men and 10,315 cases in women in Chile.19 In Italy, the Lazio and Rome region saw almost a 70% decline in ST-segment elevation myocardial infarction admissions, and the Bologna region saw a 58% decline in CVD-related emergency department visits, with a 10% decline in CVD admissions.20 , 21 In Austria, the Styria region saw the likelihood of an admission for MI decline by a quarter.22 Of note, in England, the risk of admission for out-of-hospital cardiac arrest (OHCA) increased more than 50%.23 Taken together, these results could reflect either decreases in CVD event rates or increased difficulty for patients accessing health care during the early pandemic because of patient or health system choices.24
Table 3.
Summary of Studies on the Impact of the COVID-19 Pandemic on CVD Facility Admission or Visit
| Study Setting | Number of Pandemic Months Included | Comparison | Process Measure | Outcome | Effect |
|---|---|---|---|---|---|
| England23 | 12 | February to May 2019/2020 | Incidence rate ratio for OHCA admissions | 1.56 95% CI: 1.39-1.74 | Increase |
| Japan17 | 5 | April 2018 to February 2020 /March to July 2020 | Heart failure hospitalization | −3.6% cases per week | Decrease |
| Chile19 | 7 | January 2017 to March 2020/March to September 2020 | Absolute reduction in myocardial infarction and stroke cases diagnosed | 19,326 fewer cases | Decrease |
| Italy, tertiary care cardiovascular centers in the Lazio region and Rome metropolitan area21 | 12 | March 2019/2020 | STEMI admissions per day | 0.55 vs 0.32 | Decrease |
| Italy, Bologna20 | 3 | December to May 2019/2020 | CVD emergency department visits | −58% | Decrease |
| Germany18 | 10 | March to December 2019/2020 | CVD admissions | −10% | Decrease |
| Germany18 | 10 | March to December 2019/2020 | CVD outpatient cases | −5% | Decrease |
| Styria, Austria22 | 2 | March to April 2016 to 2019/2020 | Relative risk for admission with myocardial infarction | 0.77 | Decrease |
Effect column describes the result of the outcome reported for each study, with increase shaded red, no change shaded yellow, and decrease shaded green.
CVD = cardiovascular disease; OHCA = out-of-hospital cardiac arrest; STEMI = ST-segment elevation myocardial infarction.
Hospital Mortality
Studies comparing changes in CVD outcomes in 2020 were more variable when restricted to observations among patients who were admitted to the hospital (Table 4 ).25 Studies of hospitalized patients with heart failure in Japan and the United States as well as of patients undergoing percutaneous coronary intervention in the United States and England showed no change in in-hospital mortality.15 , 17 , 26 In a comprehensive study including all citizens with a diagnosis of CVD in Denmark, inpatient mortality decreased by 8%. In-hospital mortality following CVD events increased by 5%-20% in Germany, Austria, and China.22 , 27, 28, 29 In Germany, in-hospital mortality among patients admitted with heart failure increased from 5.5% to 7%.27 More severe effects were observed in the United States in the New York area in the first 3 months of the pandemic, when inpatient CVD mortality more than doubled.30 These results may be related to selective hospitalization of high-risk patients or potentially higher case fatality rates caused by coexisting COVID-19 illness.
Table 4.
Summary of Studies on the Impact of the COVID-19 Pandemic on CVD In-Hospital or After-Hospital Mortality
| Study Setting | Number of Pandemic Months Included | Comparison | Outcome Measure | Outcome | Effect |
|---|---|---|---|---|---|
| Lithuania, 2 hospitals25 | 2 | March to April 2019/2020 | 6-month adverse CVD events following CVD admission | 13.6% vs 30.8% | Increase |
| England23 | 1 | May 2019/2020 | Adjusted probability of in-hospital mortality following OHCA admission | 35.8% vs 29.8% | Increase |
| Germany18 | 10 | March to December 2019/2020 | Odds ratio for in-hospital mortality in CVD cases | 1.10 | Increase |
| Germany27 | 2 | March to April 2019/2020 | In-hospital mortality among heart failure admissions | 5.5% vs 7% | Increase |
| Styria, Austria22 | 1 | March to April 2016 to 2019/2021 | Odds ratio for in-hospital mortality following myocardial infarction | 1.8 | Increase |
| China29 | 3 | January vs February 2020 | Odds ratio for in-hospital mortality following STEMI | 1.21 | Increase |
| United States, 13 hospitals in New York30 | 3 | March to May 2019 vs 2020 | Inpatient cardiovascular mortality | 111.1% | Increase |
| Japan17 | 5 | April 2018 to February 2020/March to July 2020 | In-hospital heart failure mortality | No difference | No change |
| United States, 2 health systems in 6 western states26 | 3 | March to April/April to June 2020 | Risk-adjusted in-hospital mortality for patients undergoing PCI, CABG, TAVR, or SAVR | No difference | No change |
| United States, Boston health system6 | 1 | January to March 2019/2020 | In-hospital mortality among acute CVD admissions | 6.2% vs 4.4% P = 0.30 | No change |
| England15 | 2 | January 2017 to 2019/March to April 2020 | Odds ratio for in-hospital deaths among patients undergoing PCI | 0.87 | No change |
| England15 | 1 | January 2017 to 2019/March to April 2020 | Odds ratio for in-hospital MACE among patients undergoing PCI | 0.71 | No change |
| England16 | 9 | January 2017 to 2019/March to November 2020 | Isolated SAVR 30-day adjusted mortality HR | 1.02 | No change |
| England16 | 9 | January 2017 to 2019/March to November 2020 | SAVR + CABG 30-day adjusted mortality HR | 1.41 | No change |
| England16 | 9 | January 2017 to 2019/March to November 2020 | SAVR + other surgery 30-day adjusted mortality HR | 0.94 | No change |
| England16 | 9 | January 2017 to 2019/March to November 2020 | TAVR 30-day adjusted mortality HR | 0.86 | No change |
| Denmark28 | 10 | January to October 2019/2020 | Adjusted incident rate ratio for inpatient mortality among citizens with CVD | 0.92 | Decrease |
Population Mortality
Studies of population-level changes in CVD mortality provide the broadest lens into understanding the overall impact of COVID-19 on cardiovascular health (Table 5 ). These analyses show widely varying results, ranging from decreases to large increases in CVD mortality during the first year of the pandemic. During the first 10 months of the pandemic in the United States, IHD and hypertensive disease deaths rose more rapidly than in the prior year, while there was no change in heart failure, cerebrovascular, or other circulatory deaths.34 In England and Wales, there were an estimated 12 per 100,000 excess CVD and diabetes deaths during the first 8 months of the pandemic, and in England, there was an estimated 8% increase in acute CVD deaths.35 CVD mortality was 7.5% greater in the Bologna, Italy, region during the pandemic’s first 6 months compared with the prior year, while OHCA deaths increased.20 CVD deaths rose almost 30% in Wuhan, China, in the first 3 months of 2020.36 In Brazil, CVD deaths increased in selected provincial capital cities in 2020 while decreasing in Rio de Janeiro, with deaths coded to unspecified CVD accounting for the majority of observed differences.37 The magnitude of these increases varied widely, from 7% in Recife to almost 46% in Manaus. A study in Denmark made use of national data that included all citizens to examine all-cause mortality among the entire population with a diagnosis of CVD and found no change in overall mortality but a small increase in out-of-hospital deaths and a small decrease in in-hospital deaths.28
Table 5.
Summary of Published Studies on the Impact of the COVID-19 Pandemic on CVD Population Mortality
| Study Setting | Number of Pandemic Months Included | Comparison | Outcome Measure | Outcome | Effect |
|---|---|---|---|---|---|
| Brazil, Fortaleza37 | 3 | April to June 2019/2020 | Percent excess in total CVD deaths | 12.6% | Increase |
| Denmark28 | 10 | January to October 2019/2020 | Adjusted incident rate ratio for out-of-hospital mortality among citizens with CVD | 1.04 | Increase |
| Wuhan, China36 | 3 | January to March 2020 vs predicted | CVD deaths | 1.29 | Increase |
| England31 | 4 | January 2014 to 2019/March to June 2020 | Acute CVD deaths compared with regression model of expected deaths | 8% | Increase |
| Germany32 | 1 | March to April 2019 vs 2020 | Percent increase in CVD deaths | 7.6% | Increase |
| Brazil, Sao Paulo37 | 3 | April to June 2019/2020 | Percent excess in total CVD deaths | 10.1% | Increase |
| United States34 | 10 | March to December 2019/2020 | Ratio of relative change in IHD death rates in 2020 vs 2019 | 1.11 | Increase |
| United States34 | 6 | January to June 2019/2020 | Ratio of relative change in hypertensive diseases Death rates in 2020 vs 2019 | 1.17 | Increase |
| England and Wales35 | 8 | 2014 to 2019/March to October 2020 | CVD and diabetes deaths compared with regression model of expected deaths | 12 per 100,000 excess deaths | Increase |
| Italy, Bologna20 | 6 | December to May 2019/2020 | CVD mortality | 7.5% | Increase |
| Italy, Emilia-Romagna region33 | 6 | Before the pandemic/January to June 2020 | Excess OOH cardiac death | 17% | Increase |
| Brazil, Belém37 | 3 | April to June 2019/2020 | Percent excess in total CVD deaths | 43.6% | Increase |
| Brazil, Manaus37 | 3 | April to June 2019/2020 | Percent excess in total CVD deaths | 46.1% | Increase |
| United States34 | 6 | January to June 2019/2020 | Ratio of relative change in cerebrovascular death rates in 2020 vs 2019 | 1.03 | No change |
| United States34 | 6 | January to June 2019/2020 | Ratio of relative change in other circulatory death rates in 2020 vs 2019 | 1.99 | No change |
| United States34 | 6 | January to June 2019/2020 | Ratio of relative change in heart failure death rates in 2020 vs 2019 | 0.97 | No change |
| Denmark28 | 10 | January to October 2019/2020 | Adjusted incident rate ratio for overall mortality among citizens with CVD | 0.99 | No change |
| Brazil, Recife37 | 3 | April to June 2019/2020 | Percent excess in total CVD deaths | 6.6% | No change |
| Brazil, Rio de Janeiro37 | 3 | April to June 2019/2020 | Percent excess in total CVD deaths | −7.1% | Decrease |
Effect column describes the result of the outcome reported for each study, with increase shaded red, no change shaded yellow, and decrease shaded green.
CVD = cardiovascular disease; IHD = ischemic heart disease; OOH = out-of-hospital.
Unanswered Questions and the Risk of Misclassification
Taken together, these studies suggest heterogenous effects of the COVID-19 pandemic on CVD burden (Central Illustration). Numerous factors may explain this variable effect, including the local incidence and severity of COVID-19 disease, health system capacity and resilience during the pandemic, social distancing and lockdown policies, age structure of populations, and prior burden of CVD and its risk factors. Variation in study data and methods may explain some of the observed differences, as could publication bias if there were a systematic bias in decisions not to write or publish papers showing an increase or decrease in CVD during the pandemic.
Findings from the multinational GBD analysis described here, where consistent methods were used across countries to allow for the comparability of estimates, suggest that the effect of the pandemic on CVD across locations is relatively small and may be limited by data quality in some locations. Large increases in CVD mortality >30% in Mexico and Ecuador are of particular concern and may reflect the misclassification of many deaths that were caused by COVID-19. Assigning an underlying cause of death is a task that may be particularly difficult for physicians given the degree to which COVID-19 can induce myocardial injury. Lack of access to diagnostic testing in settings with limited resources and the resulting misclassification of the underlying cause of death is a significant limitation in the interpretation of vital registration data, especially during a global pandemic. Given these challenges, it is especially striking that CVD mortality was minimally changed in a number of countries analyzed by the GBD study.
Similar increases are also seen for some weeks in data released by the U.S. National Center for Health Statistics for 2021, where hypertensive deaths increased up to 50% per week compared with the average of the 5 years prior, stroke deaths increased from 10%-19%, and IHD deaths increased from 8%-11% in weeks 34, 35, 36, and 38 of 2021, coinciding with a rise in COVID-19 deaths caused by the delta variant, but not in other weeks. These U.S. data suggest that changes in CVD mortality will persist as the pandemic continues.38 The decline in severity observed with the omicron variant has led to the observation that many patients could now be admitted to the hospital with COVID-19 but not because of COVID-19, leading to additional possibilities for misclassification of disease in hospital-based studies.
Population data on CVD during the pandemic remain unavailable or unanalyzed for many parts of the world, and it is not known the degree to which the reported patterns in CVD mortality are generalizable to other countries.
Implications for Research
The changes observed in CVD burden during the COVID-19 pandemic provide unique opportunities for basic, clinical, and population science research to address the possibility of direct or indirect COVID-19– and SARS-CoV-2–related changes in CVD event rates. At the same time, the CVD burden changes necessitate an in-depth exploration of the accessibility of cardiovascular care and innovations in cardiovascular care delivery and implementation science during this and future pandemics.
Clinical research
At the clinical level, the observed increases in hypertensive, IHD, stroke, and other circulatory deaths require further exploration of the possible underlying mechanisms. There also remain significant uncertainty and controversy regarding angiotensin-converting enzyme 2–mediated SARS-CoV-2 cell entry, infection, and COVID-19 and the roles played by key moieties in the renin-angiotensin-aldosterone system, especially in patients with hypertension, hypertensive heart disease, and heart failure.39, 40, 41, 42 Mechanistic clinical investigations and prospective, controlled clinical studies of an appropriate sample size would be invaluable in shedding more light on the observed increased mortality in patients with IHD and hypertension.
Population science
At the population science level, a major lesson learned during the COVID-19 pandemic is that countries with sophisticated data linkage capabilities for almost the entire population, such as England, where substantial linkages of electronic health records and vital registration data exist, have been able to rapidly produce evidence to answer key population health questions. For example, Wood et al43 describe an England-wide electronic health record resource on 54.4 million people (more than 96% of the English population) that enables whole-population research on COVID-19 and CVD while ensuring data security, privacy, and public trust. Resources like this could be developed and adapted to support research beyond COVID-19 and cardiovascular diseases to inform clinical and public health practice.
Several important challenges and unanswered questions persist. The misclassification of CVD deaths, especially in elderly individuals, is a perennial epidemiologic challenge that warrants attention in the COVID-19 era.44 In a study in one community in the United States, a panel of physicians retrospectively reviewing medical records after death assigned an underlying cause of coronary heart disease for only two-thirds of the death certificates reporting that cause, a difference that increased for the oldest age group of >85 years.45 Considering that older persons who also have underlying coronary disease and other CVD are among those at highest risk for COVID-19–related mortality, the issue of misclassification takes on additional importance. Novel strategies that help reduce misclassification are needed, especially in areas where disproportionately high COVID-19–related CVD deaths are recorded. Best practices for improved death certification have been studied across a range of settings. Strategies that include the training of trainers involved in health statistics, direct training of physicians, and online trainings have all shown efficacy.46
Other unanswered questions would benefit from longitudinal cohort population studies. The long-term effects of SARS-CoV-2 infection remain unclear. The recently described postacute sequelae of SARS-CoV-2 infection has myriad manifestations that include cardiopulmonary and neurologic symptoms such as fatigue; palpitations; chest pain; breathlessness; brain fog; and dysautonomia, including postural orthostatic tachycardia syndrome.47 , 48 The underlying mechanisms for these manifestations and their long-term courses need to be explored. There appears to be marked variability of symptomatology from patient to patient. It is unknown whether this clinical course can be prevented or ameliorated. The National Institutes of Health is currently committed to exploring these scientific questions.49
Health services and implementation science
From a health services research and implementation science perspective, it would be valuable to understand the extent to which the changes in CVD burden were attributable to pandemic-related reductions in the accessibility of cardiovascular care and changes in health care–seeking behavior vs changes in disease rates. Additionally, it would be important to understand the impact of several cardiovascular care delivery adaptations and modifications.50 For example, how has technology been used to deliver cardiac rehabilitation during the COVID-19 pandemic,51 and what innovations have been made in the remote monitoring of vital signs and telemetry in isolation wards during the COVID-19 pandemic that may have applicability in routine cardiovascular care postpandemic?52 Exploring these questions using validated implementation science theories, models and frameworks as well as health services research can contribute substantively to our current knowledge of how recurring stressors such as influenza, violence, and climate may affect health system performance.
Conclusions
COVID-19 is now one of the leading causes of global mortality, with a disproportionate impact on persons with underlying CVD. Its impact on the global burden of CVD and the delivery of cardiovascular care remains incompletely understood. In several countries, when compared with the most recent prior years, CVD deaths are similar to or even lower than in 2020. However, Ecuador, Mexico, Russia, and the United States report increases in deaths ascribed to CVD. The degree to which misclassification of COVID-19 deaths may explain these increases is unclear. Studies comparing hospitalizations for CVD during the pandemic with prior years generally found significant decreases, suggesting either decreases in CVD event rates, potential avoidance of hospital settings to limit exposure to COVID-19, or challenges in patients accessing health care during the early pandemic. Studies of CVD outcomes were more variable when restricted to observations among patients who were admitted to the hospital. There is a great need for basic, clinical, and population science research as well as health services research and implementation science to provide a deeper understanding of the direct and indirect effects of SARS-CoV-2 infection and COVID-19 on changes in CVD burden.
Funding Support and Author Disclosures
Funded by the Bill and Melinda Gates Foundation. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Listen to this manuscript's audio summary by Editor-in-Chief Dr Valentin Fuster onwww.jacc.org/journal/jacc.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Wang H., Paulson K.R., Pease S.A., et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet. 2022;399(10334):1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth G., Mensah G., Fuster V., et al. The global burden of cardiovascular diseases and risks. J Am Coll Cardiol. 2020;76(25):2980–2981. doi: 10.1016/j.jacc.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 4.Giustino G., Pinney S.P., Lala A., et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J Am Coll Cardiol. 2020;76(17):2011–2023. doi: 10.1016/j.jacc.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt A.S., Moscone A., McElrath E.E., et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280–288. doi: 10.1016/j.jacc.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia S., Albaghdadi M.S., Meraj P.M., et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the united states during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siedner M.J., Kraemer J.D., Meyer M.J., et al. Access to primary healthcare during lockdown measures for COVID-19 in rural South Africa: an interrupted time series analysis. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-043763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venter Z.S., Aunan K., Chowdhury S., Lelieveld J. COVID-19 lockdowns cause global air pollution declines. Proc Natl Acad Sci U S A. 2020;117(32):18984–18990. doi: 10.1073/pnas.2006853117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laffin L.J., Kaufman H.W., Chen Z., et al. Rise in blood pressure observed among US adults during the COVID-19 pandemic. Circulation. 2022;145(3):235–237. doi: 10.1161/CIRCULATIONAHA.121.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin A.L., Vittinghoff E., Olgin J.E., Pletcher M.J., Marcus G.M. Body weight changes during pandemic-related shelter-in-place in a longitudinal cohort study. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth G.A., Mensah G.A., Johnson C.O., et al. global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos T., Lim S.S., Abbafati C., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute for Health Metrics and Evaluation, University of Washington COVID-19 Projections. 2020. https://covid19.healthdata.org/projections
- 15.Kwok C.S., Gale C.P., Kinnaird T., et al. Impact of COVID-19 on percutaneous coronary intervention for ST-elevation myocardial infarction. Heart. 2020;106(23):1805–1811. doi: 10.1136/heartjnl-2020-317650. [DOI] [PubMed] [Google Scholar]
- 16.Martin G.P., Curzen N., Goodwin A.T., et al. Indirect impact of the COVID-19 pandemic on activity and outcomes of transcatheter and surgical treatment of aortic stenosis in England. Circ Cardiovasc Interv. 2021;14(5):532–543. doi: 10.1161/CIRCINTERVENTIONS.120.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morishita T., Takada D., Shin J.-H., et al. Effects of the COVID-19 pandemic on heart failure hospitalizations in Japan: interrupted time series analysis. ESC Heart Fail. 2022;9(1):31–38. doi: 10.1002/ehf2.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bollmann A., Hohenstein S., Pellissier V., et al. Utilization of in- and outpatient hospital care in Germany during the Covid-19 pandemic insights from the German-wide Helios hospital network. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0249251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacheco J., Crispi F., Alfaro T., Martinez M.S., Cuadrado C. Gender disparities in access to care for time-sensitive conditions during COVID-19 pandemic in Chile. BMC Public Health. 2021;21(1):1802. doi: 10.1186/s12889-021-11838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santi L., Golinelli D., Tampieri A., et al. Non-COVID-19 patients in times of pandemic: emergency department visits, hospitalizations and cause-specific mortality in Northern Italy. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Versaci F., Gaspardone A., Danesi A., et al. Interplay between COVID-19, pollution, and weather features on changes in the incidence of acute coronary syndromes in early 2020. Int J Cardiol. 2021;329:251–259. doi: 10.1016/j.ijcard.2020.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bugger H., Gollmer J., Pregartner G., et al. Complications and mortality of cardiovascular emergency admissions during COVID-19 associated restrictive measures. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rashid M., Gale C.P., Curzen N., et al. Impact of coronavirus disease 2019 pandemic on the incidence and management of out-of-hospital cardiac arrest in patients presenting with acute myocardial infarction in England. J Am Heart Assoc. 2020;9(22) doi: 10.1161/JAHA.120.018379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Czeisler M.É., Marynak K., Clarke K.E.N., et al. Delay or avoidance of medical care because of COVID-19–related concerns—United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250–1257. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aldujeli A., Hamadeh A., Tecson K.M., et al. Six-month outcomes for COVID-19 negative patients with acute myocardial infarction before versus during the COVID-19 pandemic. Am J Cardiol. 2021;147:16–22. doi: 10.1016/j.amjcard.2021.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yong C.M., Spinelli K.J., Chiu S.T., et al. Cardiovascular procedural deferral and outcomes over COVID-19 pandemic phases: a multi-center study. Am Heart J. 2021;241:14–25. doi: 10.1016/j.ahj.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bollmann A., Hohenstein S., Koenig S., Meier-Hellmann A., Kuhlen R., Hindricks G. In-hospital mortality in heart failure in Germany during the Covid-19 pandemic. ESC Heart Fail. 2020;7(6):4416–4419. doi: 10.1002/ehf2.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butt J.H., Fosbol E.L., Gerds T.A., et al. All-cause mortality and location of death in patients with established cardiovascular disease before, during, and after the COVID-19 lockdown: a Danish Nationwide Cohort Study. Eur Heart J. 2021;42(15):1516–1523. doi: 10.1093/eurheartj/ehab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang D., Xiang X., Zhang W., et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318–1324. doi: 10.1016/j.jacc.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mountantonakis S.E., Makker P., Saleh M., et al. Increased inpatient mortality for cardiovascular patients during the first wave of the COVID-19 epidemic in New York. J Am Heart Assoc. 2021;10(16) doi: 10.1161/JAHA.120.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J., Mamas M.A., Mohamed M.O., et al. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart. 2021;107(2):113–119. doi: 10.1136/heartjnl-2020-317912. [DOI] [PubMed] [Google Scholar]
- 32.Nef H.M., Elsässer A., Möllmann H., et al. Impact of the COVID-19 pandemic on cardiovascular mortality and catherization activity during the lockdown in central Germany: an observational study. Clin Res Cardiol. 2021;110(2):292–301. doi: 10.1007/s00392-020-01780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Pasquale G., De Palma R., Fortuna D., Berti E., Campo G., Casella G. Indirect effects of the COVID-19 pandemic on cardiovascular mortality. G Ital Cardiol (Rome) 2021;22(3):188–192. doi: 10.1714/3557.35336. [DOI] [PubMed] [Google Scholar]
- 34.Wadhera R.K., Shen C., Gondi S., Chen S., Kazi D.S., Yeh R.W. Cardiovascular deaths during the COVID-19 pandemic in the United States. J Am Coll Cardiol. 2021;77(2):159–169. doi: 10.1016/j.jacc.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kontopantelis E., Mamas M.A., Webb R.T., et al. Excess deaths from COVID-19 and other causes by region, neighbourhood deprivation level and place of death during the first 30 weeks of the pandemic in England and Wales: a retrospective registry study. Lancet Reg Health Eur. 2021;7 doi: 10.1016/j.lanepe.2021.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Zhang L., Yan Y., et al. Excess mortality in Wuhan city and other parts of China during the three months of the covid-19 outbreak: findings from nationwide mortality registries. BMJ. 2021;372:n415. doi: 10.1136/bmj.n415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brant L.C.C., Nascimento B.R., Teixeira R.A., et al. Excess of cardiovascular deaths during the COVID-19 pandemic in Brazilian capital cities. Heart. 2020;106(24):1898–1905. doi: 10.1136/heartjnl-2020-317663. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention Excess deaths associated with COVID-19. January 5, 2022. https://www.cdc.gov/nchs/nvss/vsrr/covid19/excess_deaths.htm
- 39.An J., Zhou H., Luong T.Q., et al. Risk of hospitalization and mortality associated with uncontrolled blood pressure in patients with hypertension and COVID-19. Int J Cardiol Cardiovasc Risk Prev. 2021;11 doi: 10.1016/j.ijcrp.2021.200117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan S.H., Zaidi S.K. Review of evidence on using ACEi and ARBs in patients with hypertension and COVID-19. Drugs Ther Perspect. 2020;36(8):347–350. doi: 10.1007/s40267-020-00750-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swamy S., Koch C.A., Hannah-Shmouni F., Schiffrin E.L., Klubo-Gwiezdzinska J., Gubbi S. Hypertension and COVID-19: updates from the era of vaccines and variants. J Clin Transl Endocrinol. 2022;27 doi: 10.1016/j.jcte.2021.100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wood A., Denholm R., Hollings S., et al. Linked electronic health records for research on a nationwide cohort of more than 54 million people in England: data resource. BMJ. 2021;373:n826. doi: 10.1136/bmj.n826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naghavi M., Makela S., Foreman K., O’Brien J., Pourmalek F., Lozano R. Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr. 2010;8(1):9. doi: 10.1186/1478-7954-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lloyd-Jones D.M., Martin D.O., Larson M.G., Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129(12):1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 46.Hart J.D., Sorchik R., Bo K.S., et al. Improving medical certification of cause of death: effective strategies and approaches based on experiences from the Data for Health Initiative. BMC Med. 2020;18(1):74. doi: 10.1186/s12916-020-01519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chilazi M., Duffy E.Y., Thakkar A., Michos E.D. COVID and cardiovascular disease: what we know in 2021. Curr Atheroscler Rep. 2021;23(7):37. doi: 10.1007/s11883-021-00935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.RECOVER Continuing the search for answers. 2022. https://recovercovid.org/funding
- 50.Laur C.V., Agarwal P., Mukerji G., et al. Correction: building health services in a rapidly changing landscape: lessons in adaptive leadership and pivots in a COVID-19 remote monitoring program. J Med Internet Res. 2021;23(6) doi: 10.2196/31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Doherty A.F., Humphreys H., Dawkes S., et al. How has technology been used to deliver cardiac rehabilitation during the COVID-19 pandemic? An international cross-sectional survey of healthcare professionals conducted by the BACPR. BMJ Open. 2021;11(4) doi: 10.1136/bmjopen-2020-046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J., Han C., Yang F., Xu S., Hu H., Chen E. Application value of vital signs telemetry system for 2019 novel coronavirus disease suspected cases in isolation wards. Infect Drug Resist. 2020;13:2971–2977. doi: 10.2147/IDR.S256803. [DOI] [PMC free article] [PubMed] [Google Scholar]