Abstract
The swimming motions of cells within Bacillus subtilis colonies, as well as the associated fluid flows, were analyzed from video films produced during colony growth and expansion on wet agar surfaces. Individual cells in very wet dense populations moved at rates between 76 and 116 μm/s. Swimming cells were organized into patterns of whirls, each approximately 1,000 μm2, and jets of about 95 by 12 μm. Whirls and jets were short-lived, lasting only about 0.25 s. Patterns within given areas constantly repeated with a periodicity of approximately 1 s. Whirls of a given direction became disorganized and then re-formed, usually into whirls moving in the opposite direction. Pattern elements were also organized with respect to one another in the colony. Neighboring whirls usually turned in opposite directions. This correlation decreased as a function of distance between whirls. Fluid flows associated with whirls and jets were measured by observing the movement of marker latex spheres added to colonies. The average velocity of markers traveling in whirls was 19 μm/s, whereas those traveling in jets moved at 27 μm/s. The paths followed by markers were aligned with the direction of cell motion, suggesting that cells create flows moving with them into whirls and along jets. When colonies became dry, swimming motions ceased except in regions close to the periphery and in isolated islands where cells traveled in slow whirls at about 4 μm/s. The addition of water resulted in immediate though transient rapid swimming (> 80 μm/s) in characteristic whirl and jet patterns. The rate of swimming decreased to 13 μm/s within 2 min, however, as the water diffused into the agar. Organized swimming patterns were nevertheless preserved throughout this period. These findings show that cell swimming in colonies is highly organized.
Motile strains of Bacillus subtilis develop complex colony forms when grown on agar surfaces that contain particular combinations of nutrients and moisture (3, 10, 11). We previously described one of these forms in which colony expansion was restricted to finger-like projections that moved outwards from the periphery of the colony. Our studies suggested that this unusual growth habit provides a means for cells to move over a surface too dry for individual cell swimming but sufficiently wet for organized cell groups to be able to force the colony boundary outward at specific places (11). To learn more details about how cells do this and ultimately the factors that govern colony shape, we examined the swimming behavior of cells within colonies. We studied cell motions at the colony periphery, in the region of expanding fingers, and elsewhere in colonies where conditions were wet or dry and cell densities varied. We also examined fluid flows in colonies by introducing marker particles and tracing their paths.
The results described here reveal that cells move in B. subtilis colonies as organized groups that travel in whirls and jets. Whirls and jets were organized with respect to one another forming a larger pattern that persisted even though the individual subelements were in a constant state of change. A series of areas surrounding whirls were defined, and the changes in motion patterns within them were examined. All went through the same temporal changes: whirls became disorganized, frequently into opposing jets, and then reorganized, usually into a whirl with the direction opposite that initially present (Fig. 1). Jets also interacted with whirls and either disorganized or reinforced them, depending upon the geometry of the interaction. Fluid flows revealed by the addition of marker particles moved in the same direction as cells in either whirls or jets. Flows traversed large areas of the colony pattern, suggesting that cells can translocate over large distances by changing the cell groups with which they associate. Sessile cells in dry colonies swam instantly when water was added to them and quickly organized themselves into typical patterns. Motion patterns appear to be governed therefore by swimming itself in high cell density populations. The proximity of whirls and jets to the colony peripheral boundary suggests that expansion of the boundary is influenced by the whirls and jets, rather than simply by individual cell swimming motions. The development of complex colony form may therefore be dependent upon such organization.
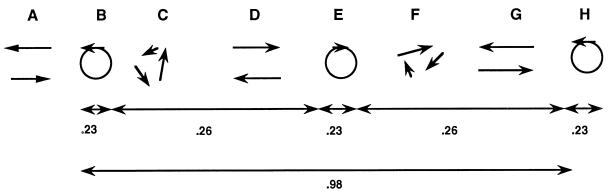
FIG. 1.
Diagram of whirl and jet interconversions. The letters A through H represent sequential times at a fixed location in a wet colony of strain M8. Two opposing jets (A) organize into a CCW whirl (B). After 0.23 s, whirl B becomes chaotic (C). A new pair of jets emerge (D) with an orientation opposite those present at time A. The jets at time D merge into a clockwise whirl (E). The whirl at time E lasts only 0.23 s before it too becomes chaotic (F). Recovery from chaos yields another pair of jets (G) that soon converge into another CCW whirl (H). An entire cycle from a whirl going in one direction to the reappearance of a whirl at the same location going in the same direction as the initial one takes just under 1 s.
MATERIALS AND METHODS
Strains.
B. subtilis 168 strain M8, a motile strain, was used in the experiments described here. M8 was produced by using M22 (purA leu ilv metB) as a recipient in transformation with DNA from strain 5:7 as previously described (11). M8 carries all of the recipient’s markers plus Tn917 (erm lacZ) and is resistant to 1 μg of erythromycin per ml and 25 μg of lincomycin per ml. The phase diagram of colony forms produced by M8 contains a particular region where colonies grow as deep-branched structures with finger-like projections. Such colonies contain highly motile cells. The “wet” conditions used in the experiments described here correspond to those in the phase diagram where deep-branched colonies are produced.
Media and growth conditions.
Strain M8 was maintained as streaks on tryptose blood agar base (TBAB) medium (Difco), the composition of which has been described previously (14). A soft agar version of TBAB that contains only 0.6% rather than the standard 1.5% agar was made as follows. The nutrients tryptose (10 g), beef extract (3 g), and NaCl (5 g) were dissolved in 1 liter of deionized water, and 6 g of agar was added. After autoclaving at 121°C for 20 min, the solution was cooled to 48°C and kept at that temperature for 1 h before pouring. Portions of the solution (62-ml volumes) were dispensed into 150-mm-diameter plastic petri plates, and the agar was allowed to solidify at 23°C in 50% relative humidity chambers. The plates were cured in the same chamber prior to being used. Plates were inoculated by toothpick transfer from colonies of different ages that had been grown on the same medium. Colonies were grown at temperatures ranging from 20 to 30°C. Specific details are given in each experiment.
Video film production and analysis.
Video films of the cultures described above were produced with a Cohu (San Diego, Calif.) charge-coupled device camera fitted to a Nikon inverted phase-contrast microscope (Nikon, Inc., Garden City, N.Y.). Images were recorded with a GYYR (Odetics, Inc., Anaheim, Calif.) time-lapse VHS tape deck. During recording, the time (in seconds, minutes, and hours) was written automatically on each video frame. The minimum time that could be resolved was 1/30 s. Images were measured either directly on plastic sheet overlays placed on the video monitor or from individual frames transferred into a personal computer with Image Pro Plus software (Media Cybernetics, Silver Spring, Md.). The Adobe Photoshop program (Adobe Systems Inc. Mountain View, Calif.) was used to produce false-colored overlays from which individual tracks were measured. Graphs were produced and analyzed with Cricket Graph (Philadelphia, Pa.).
Addition of water and marker particles to colonies.
Approximately 0.05 ml of sterile deionized distilled water was added in a single drop to a region within the interior of a large M8 colony that had grown for 72 h at 24°C and 28% relative humidity on a 150-mm-diameter dish containing soft TBAB agar. The fluid was introduced several millimeters away from the region of the colony visible in the microscope field during filming. In the experiment analyzed here, the fluid diffused into the field from the lower right as a broad front that swept through the field but did not carry cells away in its flow. Within 1 min of its addition, most of the added water had percolated into the agar, again leaving the surface too dry to support vigorous cell swimming.
Latex spheres with a diameter of 1.06 μm were used to measure fluid flows in M8 colonies grown under wet conditions. The particles were supplied as 0.1% solids (Ted Pella, Inc., Redding, Calif.). They were diluted 1:50 in sterile deionized distilled water and agitated vigorously with a cell resuspender for several minutes to disrupt clumps before use. A 3-μl volume of the diluted suspension was added directly to the interior of large colonies. Controls consisted of beads deposited on agar surfaces rather than in a colony and of beads added to colonies that had been exposed to formaldehyde fumes until no further cell motions could be detected prior to their addition.
Statistical methods.
Comparisons were made between the behavior of cells in seven equal-sized areas clustered in a region of an M8 colony where cell swimming was vigorous. The motions within each area were scored over a 15-s interval. The motions in the ith area during the tth time interval, denoted by Xi(t), are given one of three scores: −1 for movement in a counterclockwise (CCW) whirl, 0 for disorganized movement, and +1 for movement in a clockwise (CW) whirl. We compare the direction in the areas by taking the correlation of the time series as follows:
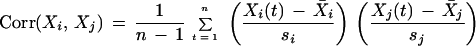 |
where
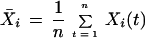 |
and
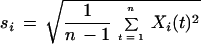 |
are the sample mean and standard deviation of the motion scores for the ith area. Some data are missing. Thus, these statistics were computed by summing over those times in the series having data for both the ith and jth areas.
The significance of these correlations were examined using a z test of the hypothesis that the correlation is zero. The correlations for the scores for each pair of areas, the corresponding z scores, and the P values of a null hypothesis of correlation 0 for a two-sided test are tabulated in Table 3.
TABLE 3.
Pairwise correlations between cell motion patterns in seven neighboring areas of colony 1142a
| Colony area | Colony area
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 1 | ||||||
| 2 | −0.052 | 1 | |||||
| −1.07 | |||||||
| 0.285 | |||||||
| 3 | −0.017 | −0.104 | 1 | ||||
| −0.35 | −2.19 | ||||||
| 0.72 | 0.029 | ||||||
| 4 | 0.153 | 0.036 | −0.176 | 1 | |||
| 3.27 | 0.74 | −3.71 | |||||
| 0.001 | 0.461 | 0.000 | |||||
| 5 | −0.069 | 0.066 | −0.152 | −0.071 | 1 | ||
| −1.43 | 1.36 | −3.14 | −1.46 | ||||
| 0.154 | 0.174 | 0.002 | 0.145 | ||||
| 6 | 0.000 | −0.070 | −0.048 | −0.052 | 0.102 | 1 | |
| 0.01 | −1.17 | −1.02 | −1.09 | 2.10 | |||
| 0.993 | 0.241 | 0.309 | 0.277 | 0.036 | |||
| 7 | 0.121 | −0.055 | −0.199 | 0.007 | 0.160 | 0.042 | 1 |
| 2.57 | −1.15 | −2.55 | 0.15 | 3.32 | 0.90 | ||
| 0.011 | 0.252 | 0.011 | 0.881 | 0.001 | 0.369 | ||
The cell motions within each area were classified into one of three states: (i) a CW whirl, coded as +1, (ii) a CCW whirl, coded as −1, or (iii) a disorganized state, coded as 0. The 15-s time series gives the classification of each state every 1/30 s. For each pair of areas, line one gives the correlation of states between areas, line two gives the z statistic for a null hypothesis of correlation 0, and line three gives the P value for a two-sided test of this hypothesis. The P value for a one-sided test is half the tabulated value.
RESULTS
Rates of motion in dense populations.
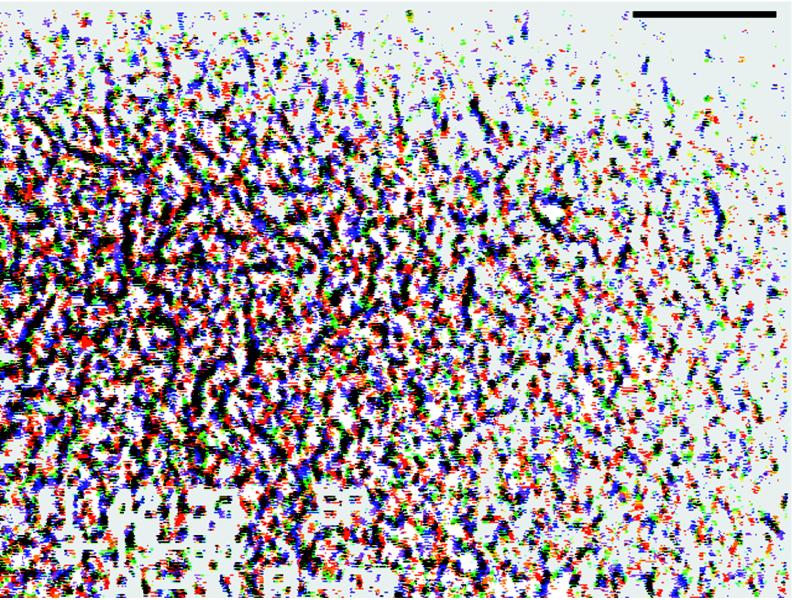
A single large colony of strain M8 (colony 1142; diameter, >50 mm) was produced by 48 h of growth at 24°C on a 150-mm-diameter petri dish containing soft (0.6%) TBAB agar. The plate was transferred to the stage of an inverted phase-contrast microscope, and a video film (30 frames per s) that shows chaotic motions of cells over broad areas of the colony throughout a 47-s interval was produced. (see http://research.biology.arizona.edu/mendelson/jbvol181). Individual frames were transferred to a computer and processed so that single-cell trajectories could be found within the populations of moving cells. By filtering, sharpening, and adjusting image brightness, contrast, and gamma values, individual cells were resolved as single white dots on a black background. Transparencies were printed from each image, and when they were layered upon one another, it was possible to find cell paths by sliding the layers and matching up dots. To measure the distances moved as a function of time, each image was inverted to black dots on a white background and false colored with a different color for each time point. Layers were superimposed in the computer, and distances between points measured in pixels were subsequently converted to microns. The three longest tracks found persisted for approximately 0.3 s. Figure 2 illustrates the multicolor eight-frame overlay from which these tracks were obtained. A plot of distance traveled as a function of time revealed that the rates at which these three cells moved were 76, 98, and 116 μm/s. The distances traversed ranged from 22 to 35 μm.
FIG. 2.
Combined image overlay illustrating how the rates of cell movement were measured in dense cell populations. Nine sequential frames from a video film of B. subtilis M8 colony 1142 were transferred to a computer and processed to give images of white dots (individual cells) on a black background. The colors were inverted, and the dots were then false colored so that each frame displayed a different color. When frames were superimposed, the tracks of individual cells could be found by locating dots that lined up with one another in the proper time (color) sequence. Motion rates were derived from plots of the distances between dots as a function of time. Vigorous cell motions were present throughout the entire area of this figure. The large dark structures reveal regions of high cell density moving in jets and whirls. Bar = 100 μm.
Organization of cell motions in whirls and jets.
A distinct granular organization of motile cells within M8 colonies can be seen by viewing films such as that used to obtain Fig. 2. Even after processing and superimposition of false-colored points, some of this organization can still be discerned. Detailed studies of the organization were performed by developing a grid overlay for the video display and examining the time-dependent changes taking place within grid squares. The first thing noted was that granular structure changed in an orderly way: local patterns repeated with a duration of about 1 to 1.8 s. Local patterns consisted of groups of cell moving either in a whirl (both CW and CCW varieties were found in about equal numbers) or in a jet. The structure and dynamics of these pattern elements were measured. Table 1 shows that both whirls and jets lasted only a fraction of a second before they became disorganized. Their properties in two M8 colonies produced in different cultures were similar. In colonies 1142 and 1442, the areas occupied by whirls on average were nearly identical. Whirls found in other colonies were also approximately the same dimensions, judged by their containment within squares of the same grid overlay. Jets were also found to be structured similarly in different colonies. The two measured examples shown were both about eightfold longer than wide and 2.3 times the diameter of their neighboring whirls. Although any given whirl or jet decayed in less than half a second, new whirls and jets formed on a similar time scale. Therefore, the dynamics of pattern reorganization was characterized to determine how the continuity of pattern was achieved.
TABLE 1.
Properties of whirls and jets in two colonies of B. subtilis M8a
| Property | Colony 1142 | Colony 1442 |
|---|---|---|
| Whirl duration (s) | 0.23b | 0.39c |
| Jet duration (s) | 0.35d | 0.47e |
| Whirl area (μm2) | 1,100f | 1,200g |
| Maximum jet length (μm) | 98.4h | 91i |
| Maximum jet width (μm) | 12.7j | 11.6k |
| Cell velocity in jets (μm/s) | 124l | 76m |
Each colony was grown at 24°C in the center of an individual 150-mm-diameter petri dish containing soft (0.6% agar) TBAB. Values in the table are means.
Standard deviation (SD) = 0.068; n = 16.
SD = 0.103; n = 10.
SD = 0.018; n = 2.
SD = 0; n = 3.
SD = 530; range = 400 to 2,000.
SD = 356; range = 600 to 1,900.
SD = 13.8; range = 76 to 114.
SD = 7.9; range = 83 to 102.
SD = 2.5; range = 9.5 to 15.9.
SD = 1.49; range = 9.5 to 12.6.
SD = 23.1; range = 95 to 158.
SD = 2.35; range = 74.6 to 79.6.
Repeating cycle of whirls in M8 colonies.
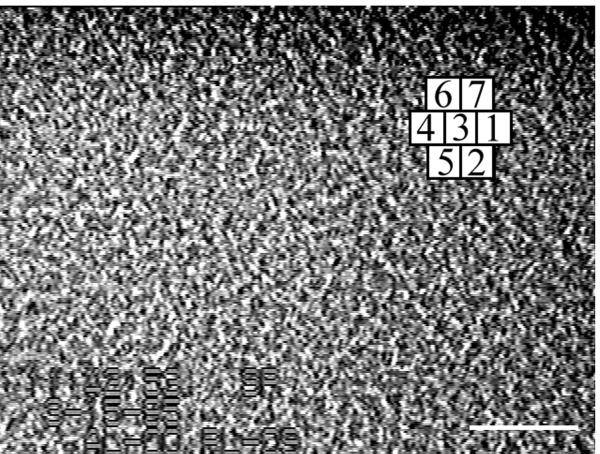
Time-dependent changes in motion patterns were examined in seven neighboring areas defined by the grid square overlay, as shown in Fig. 3. The offset alignment of squares used in the grid overlay was derived by trial and error. Boxes were drawn to contain whirls centered within them and aligned to encompass neighboring whirls. The properties of the chosen grid are such that the center of each square has a high probability of containing a whirl at any time, whereas the further away from the center one moves, the greater the chances are that a jet will be located at that position. This relationship holds over a broad area of colony 1142 and for other M8 colonies as well. There is therefore a definite large-scale pattern consisting of aligned neighboring smaller patterns of cell motions. The superpattern can be seen on the internet at http://research.arizona.edu /mendelson/jbvol181. Changes in pattern appear to be coordinated over the entire field of view represented by the frame shown in Fig. 3. The basis for these changes became evident when the details of events taking place within each grid square were characterized.
FIG. 3.
Phase-contrast microscope image of M8 colony 1142 used to measure the dimensions and dynamics of whirls and jets. An inverted microscope was used to obtain images of cell motions within a colony growing on the surface of soft TBAB. Images were recorded on video film. A single frame is shown. The grid pattern on the upper right shows the locations of seven areas analyzed in detail. The directions of whirl turning and the timing of events were taken directly from a monitor screen upon which an overlay of the same grid was superimposed. Bar = 100 μm. (The film sequence can be viewed on the internet at http://research.biology.arizona.edu/mendelson/jbvol181.)
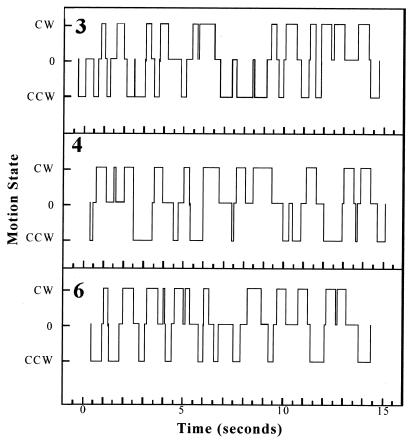
Cell motions within each square were classified as being in one of three states: a CW whirl, a CCW whirl, or motions not organized into a whirl. The progression of states was then determined for each grid area. Three examples representing a cluster of neighboring grid square areas are shown in Fig. 4. All three are aligned with respect to time. Transitions between each of the three states were observed. Whirls constantly decayed and re-formed. A total of 147 examples were recorded in the seven grid areas examined. Of these examples, 77% involved a whirl of one direction becoming disorganized and re-forming into a whirl of the opposite direction. The frequency of switching from CCW to CW direction and vice versa were virtually identical (39 and 38%, respectively). There was a slightly greater chance that CCW whirls would re-form again into CCW whirls than was the case for CW whirls (14 versus 8%), but the total numbers of cases observed, 21 and 13, respectively, was small. All four categories of changes (CW to CCW, CCW to CW, CW to CW, and CCW to CCW) were found in each of the seven areas examined.
FIG. 4.
Motion states observed in grid square areas of M8 colony 1142. Motions were classified as being either a CW whirl, a CCW whirl, or a disorganized state (0). The transitions between states throughout a 15-s period were located by advancing the video film one frame at a time and determining the times at which motions changed. The time sequences shown for grid square areas 3, 4, and 6 (as on Fig. 3) are aligned with respect to one another.
Table 2 summarizes information concerning the duration of time cells spent in each of the three motion states for all of the seven grid areas and for an area in another colony (colony 1442) equal to that of each one of the seven grid areas. In colony 1442, the global pattern was much more disorganized and drifting. In the more stable colony (colony 1142), cells spent equal amounts of time moving in either a CW or CCW whirl (31.9 and 30.2%, respectively) and slightly longer in a disorganized state (37.7%). Comparison of the number of periods cells in each area spent traveling in a whirl or in a disordered state shows that virtually all transitions from one whirl to another in colony 1142 passed through a disorganized state sufficiently long to be recognized as such and therefore classified accordingly. The cells in colony 1442 behaved similarly to those in 1142 in terms of switching between whirls moving in opposite directions but they remained in each state longer (9 versus 4.75 s in CW motion, 6.6 versus 4.5 s in CCW motion, and 44.2 versus 5.6 s in disorganized motion). Differences in the time scales of events in the two colonies can be seen also by the fact that the number of events completed in colony 1142 within 15 s required 60 s in colony 1442. The proportion of time spent in a disorganized state was much greater in colony 1442 than in colony 1142. Nevertheless, the global nature of motion patterns over a broad area of the colony is still clearly evident when viewing films of colony 1442.
TABLE 2.
Pattern dynamics of motions in neighboring areas within a single colony
| Area | Cell motions
|
% of time spent in motion:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CW
|
CCW
|
Disorganized
|
CW | CCW | Disorganized | ||||
| Aa | Bb | A | B | A | B | ||||
| 1 | 13 | 5.6 | 12 | 5.0 | 21 | 5.2 | 33.3 | 32.7 | 34 |
| 2 | 11 | 4.0 | 10 | 4.8 | 20 | 5.8 | 27.3 | 33 | 39.8 |
| 3 | 12 | 4.9 | 14 | 5.5 | 25 | 4.6 | 32.7 | 36.6 | 30.7 |
| 4 | 11 | 5.7 | 10 | 4.2 | 20 | 4.9 | 38.4 | 28.3 | 33.3 |
| 5 | 12 | 4.9 | 10 | 4.13 | 21 | 4.96 | 35.2 | 29.5 | 35.4 |
| 6 | 9 | 4.2 | 9 | 3.7 | 17 | 7.1 | 28.3 | 24.5 | 47.2 |
| 7 | 12 | 4.4 | 10 | 4.2 | 21 | 6.8 | 28.5 | 27.4 | 44.1 |
| Mean | 4.8 | 4.5 | 5.6 | 31.9 | 30.2 | 37.7 | |||
| 1442c | 11 | 9.2 | 9 | 6.6 | 21 | 44.2 | 15 | 11 | 74 |
A is the number of periods observed for each motion as shown in Fig. 3.
B is the total time (in seconds) spent in each motion obtained from the sum of the duration of each period.
These data were obtained from an area in colony 1442 with a size comparable to that of each of the other seven areas shown above. Areas 1 through 7 were located in colony 1142.
The sequential behavior of motions at a fixed place as shown in Fig. 4 was determined for all seven grid square areas of colony 1142 diagrammed in Fig. 3. A statistical comparison was made between the pattern of motions in each square and those in all other squares to determine how local patterns were organized over a greater area with respect to one another. To do so, each time line was partitioned into 510 intervals and the motion state for each interval was assigned as either CW, CCW, or disorganized. The entire sequence from each grid square time line was then compared in two-by-two pairs with those of every other grid square. The results are shown in Table 3. Negative values in the table indicate inverse correlations that imply whirls turning in opposite directions, whereas positive values correspond to whirls turning in the same direction at the same time.
A two-sided z test was performed on all of the pairs of correlations obtained from the seven grid areas that were studied. The z statistic values for a null hypothesis of correlation 0 are shown in Table 3 (second line for each pair). The corresponding P values are shown immediately below them (third line for each pair) in the table. P values of the z statistic below 0.05 are taken to indicate the strongest correlations between grid area pairs that were either positively or negatively correlated. P values of the z statistic of 0.15 are considered only weakly correlated. The spatial relationships of grid pairs that were strongly correlated with one another (either positively, indicating their motion patterns are in phase with one another, or negatively, indicating that motions in one are opposite those in the other) can be found by examining the P values of the z scores (third line for each pair in Table 3). Grid pairs with the strongest positive correlations are colony areas 5 and 7 (P = 0.001), 1 and 4 (P = 0.001), 1 and 7 (P = 0.011), and 5 and 6 (P = 0.036). Three of these four pairs consist of areas that are distant from one another. Grid pairs with the strongest negative correlations are colony pairs 3 and 4 (P = 0), 3 and 5 (P = 0.002), 3 and 7 (P = 0.011), and 3 and 2 (P = 0.029). Three of these pairs consist of neighboring areas that lie diagonally to one another. The remaining pair consists of neighbors aligned with one another.
Swimming after addition of water to dry regions of M8 colonies.
Large M8 colonies were produced by growth following inoculation by toothpick transfer to the center of 150-mm-diameter soft agar TBAB plates. After 72 h of incubation at 24°C and 28% relative humidity, the colonies contained cells that were largely sessile. Some local regions, however, contained cells moving slowly in whirls and jets. These islands and their surrounding cells were found to respond rapidly when water was added several millimeters away and allowed to diffuse into them. As soon as the region became wet, cells swam vigorously and formed typical whirl and jet patterns. An advancing front of water percolating through one such dry colony can be seen in Fig. 5 and at http://research.arizona.edu/mendelson/jbvol181. The front moved from the lower right towards the upper left of the figure. The water flow did not carry cells with it but instead enabled cells to swim in their local regions for a period of several minutes until the added water moved into the dry agar below. The shape of the advancing water front through the packed cells in the colony had the appearance of wave cusps. Once the front passed a particular region, all the cells behind it in the wet region were motile. Therefore, sessile cells in dry regions appear not to have lost flagella or the ability to swim.
FIG. 5.
Phase-contrast micrograph showing the movement of water into a dry region of an M8 colony that contained a single slow-moving whirl. The boundary between wet and dry regions can be seen at the juncture between light and dark cells that lie along a curvy diagonal line running from the lower left to the upper right of the figure. Water moved into the colony from the lower right in the field shown. The dense population of cells remaining in place after water diffused into the colony can be seen in the lower right. These are the cells that became motile and quickly established a pattern of whirls and jets. Bar = 100 μm. (The film sequence can be viewed on the internet at http://research.biology.arizona.edu/mendelson/jbvol181.)
The sizes and life spans of whirls and jets as well as the rate at which cells moved were measured from the wet region produced by the addition of water to an M8 colony. The results are summarized in Table 4. The whirl present in the dry M8 colony was slightly smaller than those found in typical wet M8 colonies (Table 1) but swelled quickly after the addition of water to the characteristic wet dimensions. The swollen whirl shrank again as water moved from the colony into the agar. Under dry conditions, whirls persisted about 10 times longer than they did when they were wet (2 versus 0.2 s). Comparison of the size and duration of whirls following the addition of water and subsequent drying reveals that the degree of moisture available strongly influenced the cooperative swimming behavior of the cell population. Similar behavior was observed for jets present in the same colony. Table 4 shows that although jet width swelled only slightly when water was added, jet velocity rose about fourfold and jet life span decreased about 10-fold during the same period when whirl life span decreased 10-fold. Also, as in the case of whirls, jets progressively regained their longer life span as conditions became drier. The coordinate changes in the kinetics of motions in whirls and jets subjected to the same environmental variations in wetness suggests that both are driven by individual cell swimming and constrained by the amount of water in the colony.
TABLE 4.
Behavior of whirls and jets following the addition of water to a dry colonya
| Time (s) after water added | Whirls
|
Jets
|
|||
|---|---|---|---|---|---|
| Size (μm2) | Duration (s) | Width (μm) | Velocity (μm/s) | Duration (s) | |
| 0 to 5 | 965a | 2A | 10.3g | 23.82 | 2.75G |
| 9.66 | 1,180b | 0.26B | 11.1h | 105.93 | 0.031H |
| 15 | 970c | 0.28C | 11.1i | 84.64 | 0.51I |
| 23 | 890d | 0.4D | 10.6j | 59.55 | 0.65J |
| 38.5 | 860e | 0.79E | 10.3k | 41.66 | 1.75K |
| 55.75 | 860f | 2.1F | 9.5l | 23.87 | 2.20L |
Water was added to a large M8 colony produced by 72 h of growth on a 150-mm-diameter petri dish containing soft TBAB. The data were obtained from a video film showing cell swimming in the colony prior to, during, and following addition of water. Ranges of values are indicated by superscript letters or numbers as follows: for whirl area size and jet width values, a = 800 to 1,130, b = 1,130 to 1,290, c = 800 to 1,050, d = 800 to 970, e = 730 to 970, f = 650 to 1,130, g = 9.5 to 11.1, h = 11.1, i = 9.5 to 12.7, j = 9.5 to 11.1, k = 9.5 to 11.1, and l = 7.9 to 11.1; for motion duration values, A = 2, B = 0.2 to 0.3, C = 0.23 to 0.3, D = 0.37 to 0.43, E = 0.67 to 0.97, F = 2 to 2.2, G = 2.5 to 3, H = 0.27 to 0.33, I = 0.37 to 0.67, J = 0.5 to 0.73, K = 55.6 to 60.3, and L = 54 to 57.2; for velocities, 2 = 20.4 to 27.2, 3 = 79.4 to 143, 4 = 63.5 to 95.2, 5 = 47.6 to 71.4, 6 = 35.7 to 47.6, and 7 = 20.4 to 27.2.
Motions of marker beads in whirls and jets.
Latex spheres with a diameter of 1 μm were added to colonies as markers to track fluid flows. The particles were easily resolved on video film images (http://research.arizona.edu/mendelson/jbvol181). Several methods were used to introduce the markers, including allowing colonies to grow into small fluid reservoirs containing latex spheres that were deposited on the agar surface in the path of advancing colony fingers. Direct addition of markers to the colony interior proved to be the least disruptive. The results shown in Table 5 and Fig. 6 and 7 were obtained in this manner.
TABLE 5.
Marker particle movement in colonies of B. subtilis M8a
| Type of motion | Sequence no.b | Rate of travelc (μm/s) | Goodness of fitd (R) |
|---|---|---|---|
| CW whirl | 1 | 22, 21 | 1.0, 0.96 |
| 2 | 14 | 0.99 | |
| 3 | 19 | 0.97 | |
| 4 | 19 | 0.99 | |
| 5 | 23 | 1.0 | |
| CCW whirl | 6 | 5, 15 | 0.99, 1.0 |
| 7 | 12 | 0.99 | |
| 8 | 19 | 0.99 | |
| 9 | 24 | 0.99 | |
| 10 | 18, 14 | 0.99, 0.97 | |
| 11 | 24 | 1.0 | |
| 12 | 26 | 1.0 | |
| Jet | 1 | 17 | 0.99 |
| 2 | 32 | 1.0 | |
| 3 | 24 | 0.99 | |
| 4 | 28 | 1.0 | |
| 5 | 34 | 1.0 | |
| 6 | 29 | 1.0 |
Latex spheres (diameter of 1 μm) were added as markers directly to colonies of M8 grown on soft TBAB, and their motions were recorded on video film.
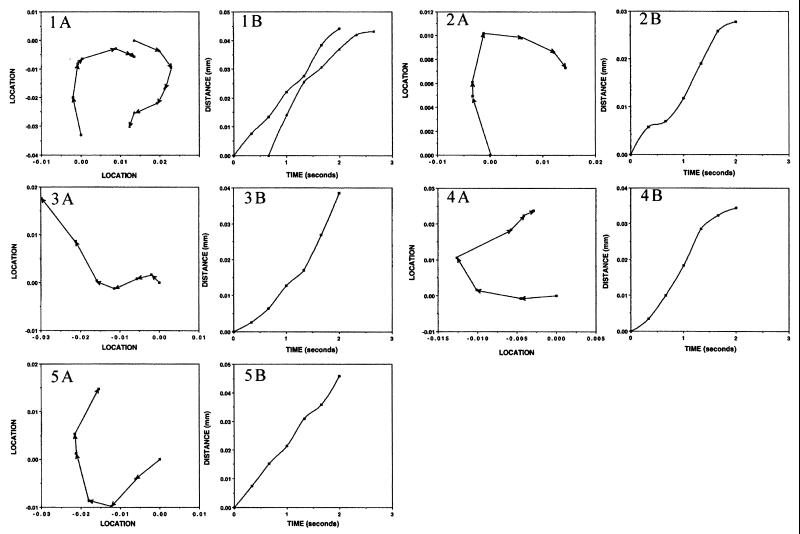
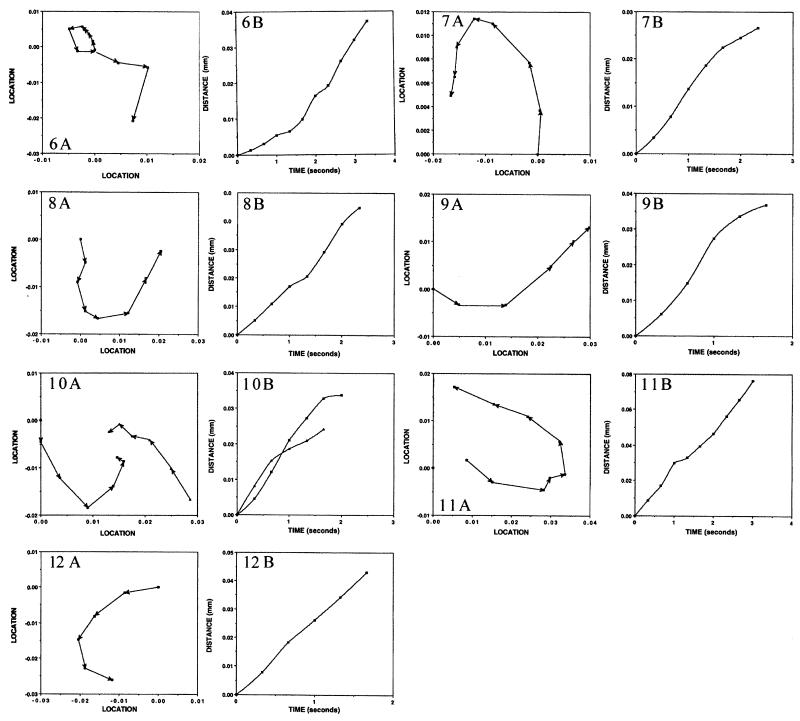
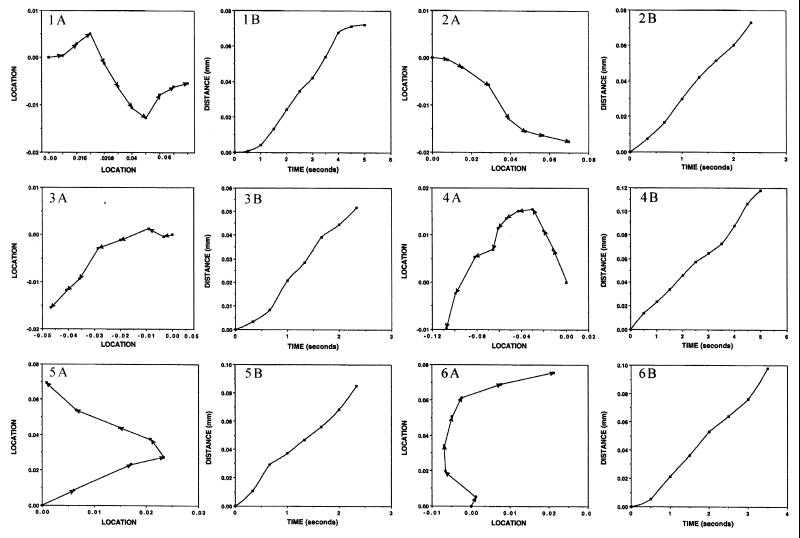
FIG. 6.
Paths taken and rates of travel in whirls of marker particles added to colonies of M8. Video films were produced with a phase-contrast microscope showing cell motions in M8 colonies prior to and following the addition of latex spheres. Individual frames were transferred to a computer, and the locations of marker particles were measured. Panels labeled A show the paths followed by individual marker spheres. Panels labeled B show the rates of movement of the corresponding markers. The x and y coordinates of markers in each time frame were determined from the digital image and used to construct the paths shown and to measure the rates of travel. Panels 1 through 5 are from CW whirls, and panels 6 through 12 are from CCW whirls. Details are given in Table 5. (The film sequence can be viewed on the internet at http://research.biology.arizona.edu/mendelson/jbvol181.)
FIG. 7.
Paths taken and rates of travel in jets of marker particles added to colonies of M8. Details are given in Fig. 6 and Table 5.
The paths that markers traveled and their rates of movement were measured from video films. Markers were observed to move into CW and CCW whirls and along jets. In all cases, the direction of marker travel was the same as that of the cells. Numerous examples were found in which markers were carried over much longer distances than that traversed by an individual jet or whirl. In these cases, the latex spheres were transferred from one pattern element to another. Clumps of marker spheres were also observed moving in flows. Occasionally, clumps dissociated, liberating individual spheres that continued on different paths. These cases suggest that flows interact with one another as pattern elements do.
The rates of marker travel show in Table 5 were derived from the data in the B panels of Fig. 6 and 7. Markers moved on average at about the same rate in CW (19.6 μm/s) and CCW (19 μm/s) whirls (excluding the one slow-moving CCW whirl that was located in a dry region of an M8 colony). Markers traveling along jets moved more rapidly than those in whirls (27.3 μm/s, on average). The paths taken by markers are shown in the A panels of Fig. 6 and 7. Marker paths leading into and out of whirls are evident. Changes in the direction of markers moving in jets are also shown. However, markers have never been observed to reverse direction and retrace an earlier route back to a starting position, even though whirls of opposite direction can arise in a given location at different times. Markers that were placed in drops on agar surfaces similar to those upon which colonies were grown but in the absence of cells showed characteristic Brownian motions. They did not move along paths similar to those observed in whirls or jets. Another control in which markers were added to colonies that had been exposed to formaldehyde vapor until all cell motions had ceased also gave negative results: only Brownian motions were detected.
DISCUSSION
The results described here show that motile cells in colonies of B. subtilis M8 grown on moist agar surfaces became organized into groups of cells moving, in local regions, either in whirls (going CW or CCW) or in jets. Individual whirls and jets occupied only small areas of the colony. When colonies were very wet, however, extensive areas were filled with whirls and jets, creating a dynamic interacting system involving three hierarchical levels of organization: (i) the movement of individual cells, (ii) the motions of cell groups forming whirls and jets, and (iii) the organization of whirls and jets into a superpattern that persisted over a time frame during which the individual pattern elements were constantly being reorganized. Our findings illustrate the interconnectedness of local regions within a colony extending to global dimensions and suggest that control processes in addition to those dealing with individual cell motility and chemotaxis may operate in colonies.
Individual cells traveling in groups in wet colonies moved at rates ranging from 75 to 100 μm/s, covering distances of 25 or more microns. These rates are five- to 10-fold greater than the rate at which B. subtilis cells swarm over moist agar surfaces (1) and more than twofold faster than the standard swimming rate of bacterial cells (approximately 10 times the cell length per second [2, 9]). In contrast, colonies that were grown initially under wet conditions on a 0.6% agar surface and then were allowed to dry contained cells in isolated whirls that moved at only <10 μm/s. The addition of water to these regions caused an immediate though transient increase in swimming rate to over 80 μm/s, swimming of neighboring cells that were initially sessile, and the rapid formation of typical whirl and jet patterns (see http://research.arizona.edu/mendelson/jbvol181). Whirls and jets in M8 colonies appear therefore to be produced by swimming in high cell density populations, not by classical swarming over an agar surface.
Whirls and jets were found with characteristic dimensions in different colonies and in different regions of the same colony, suggesting that there may be a fundamental number of cells that constitute the population that moves together. The diameter of whirls was approximately 40 μm (slightly less in dry colonies). Bacterial cells moving in whirl-like fashion and along circular routes have been described previously (7, 8, 12). Various Bacillus species can produce aggregates of cells, small and even full-size colonies that rotate and migrate over agar surfaces. A bias towards rotation in the CCW direction was observed in several cases (12). Attempts to obtain isolates restricted to rotation in only one direction were not successful (12). Single-cell filaments of Bacillus alvei were also reported to move in a circular path, but no details of direction, duration, or rate were given (12). These examples illustrate that groups of cells traveling in whirls on an agar surface are not an unusual phenomenon. A theoretical model of colony rotation has even been developed (4) based upon the idea that cells undergo chemotactic responses towards self-emitted attractants. Its relevance to our findings remains to be determined.
Swimming in circular paths is not restricted to the gram-positive bacilli. Both wild-type and a smooth swimming mutant of Escherichia coli were shown to move along a CW circular path when they swam close to a glass surface (6, 7). The diameter of the circle traversed by the mutant was about 48 μm. It took approximately 7 s for a cell to move once around the circumference. A theoretical mathematical model dealing with the movement of a sphere pushed from behind by a single flagellum showed that similar behavior would occur when the sphere moved close to a surface (13). It appears therefore that traveling in a circular path does not necessarily require a chemotactic process but rather can be caused solely by the physics of swimming.
Whirls in B. subtilis colonies underwent constant changes of state, switching to disorganized motion and then to a whirl moving in the direction opposite to the initial direction (Fig. 1). In a given region, cells spent about 62% of their time moving in whirls, divided about equally between CW and CCW directions. The remaining time was spent in disorganized chaotic motion. The fact that newly organized whirls usually turned in the direction opposite that of their predecessors (77% of cases), were located at the same place as the initial whirl, and were approximately the same size as the initial whirl illustrates that the direction cells travel in whirls is not randomly chosen. The direction of travel is influenced by the motion that it replaces. Some form of switching appears to operate at the level of individual whirls involving the change of swimming direction of an entire population of cells.
The spatial relationship of whirls to one another and a comparison of the motions taking place in neighboring whirls both reveal that a robust superorganization of pattern elements is present in the colonies. The universal grid pattern shown in Fig. 3 represents the layout of whirls with respect to one another and their approximate dimensions. The correlations of whirl direction going on in neighboring locations shown in Table 3 and Fig. 4 strongly suggest that the motions in a whirl are somehow influenced by the behavior of motions in proximity to it. A change in the state of one whirl is coordinated with changes in nearby whirls. However, the correlation (inverse) between pairs of motion states falls off with distance, indicating that although there is much order in the motions taking place in a colony, a colony is not a perfectly synchronized or structured system.
Although classical swimming and chemotactic behavior (9, 15) must govern the motions of individual cells at some level, the factors responsible for the behavior of groups of cells (whirls and jets) and for the superorganization of these dynamic populations over larger areas in colonies remain to be elucidated. Given the information described here, it is clear that the time scale of switching from movement in one direction to another, the coordinated behavior of motions in a local region with those in neighboring regions, and the nonrandom paths taken when whirls become reorganized are not behaviors easily explained on the basis of control of the direction of flagellum rotation. There must be additional factors at play that influence the directions cells travel when swimming in the dense populations within colonies.
The vigorous motions found in whirls and jets have associated fluid flows presumably set into play by cell swimming. The paths taken by marker particles suggest that cells could also become trapped in flows and be transported by the flow even without swimming. If so, cells could be passively transported hundreds of microns from their initial location. Flows are able to bring fluid as well as cells to the colony periphery and thus influence the expansion of the colony. Whirls and jets frequently strike the boundary at the periphery, become disorganized, and deposit cells there that push the edge of the colony out. Complex colony shape appears to be governed by the locations on the colony boundary where strikes occur and the behavior of the cells remaining at the boundary following the strike.
Our view of bacterial colonies has changed considerably as a result of the findings reported here. Seemingly chaotic motions in wet colonies are in fact ordered and strongly controlled on short time scales (less than a second). Motions govern structure. Three levels of organization are involved: individual cells, groups of cells (whirls and jets), and a larger scale pattern involving the locations and interactions of cell groups with one another. The stability of pattern over longer time scales (minutes) is a statistical process governed by constantly changing short-term phenomena. Understanding the control of these complex events and their relationship to known aspects of bacterial swimming and taxis presents a new challenge to both microbiologists and physicists.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the National Center for Research Resources (NIH) to N.H.M., an undergraduate Flinn Foundation Scholarship award to K.W., and a Graduate Flinn Foundation Fellowship in Biomathematics to K.A.
We are indebted to S. D. Whitworth for excellent technical assistance and to K. M. Williams and D. K. Warren for help in making the video film sequences available on the internet.
REFERENCES
- 1.Allison C, Hughes C. Bacterial swarming: an example of prokaryotic differentiation and multicellular behaviour. Sci Prog Edinburgh. 1991;75:403–422. [PubMed] [Google Scholar]
- 2.Amsler C D, Cho M, Matsumura P. Multiple factors underlying the maximum motility of Escherichia coli as cultures enter postexponential growth. J Bacteriol. 1993;175:6238–6244. doi: 10.1128/jb.175.19.6238-6244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Jacob E. From snowflake formation to growth of bacterial colonies: cooperative formation of complex colonial patterns. Contemp Phys. 1997;38:205–241. [Google Scholar]
- 4.Ben-Jacob E, Cohen I, Czirok A, Vicsek T, Gutnick D L. Chemomodulation of cellular movement, collective formation of vortices by swarming bacteria, and colonial development. Physica A. 1997;238:181–197. [Google Scholar]
- 5.Berg H C, Brown D A. Chemotaxis in Escherichia coli analyzed by three-dimensional tracking. Nature. 1972;239:500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- 6.Berg H C, Turner L. Chemotaxis of bacteria in glass capillary arrays. Biophys J. 1990;58:919–930. doi: 10.1016/S0006-3495(90)82436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frymier P D, Ford R M, Berg H C, Cummings P T. Three-dimensional tracking of motile bacteria near a solid planar surface. Proc Natl Acad Sci USA. 1995;92:6195–6199. doi: 10.1073/pnas.92.13.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972;36:478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macnab R M. Flagella and chemotaxis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 123–145. [Google Scholar]
- 10.Matsushita M. Formation of colony patterns by a bacterial cell population. In: Shapiro J A, Dworkin M, editors. Bacteria as multicellular organisms. New York, N.Y: Oxford University Press; 1997. pp. 366–393. [Google Scholar]
- 11.Mendelson N H, Salhi B. Patterns of reporter gene expression in the phase diagram of Bacillus subtilis colony forms. J Bacteriol. 1996;178:1980–1989. doi: 10.1128/jb.178.7.1980-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray R G E, Elder R H. The predominance of counterclockwise rotations during swarming of bacillus species. J Bacteriol. 1949;58:351–359. doi: 10.1128/jb.58.3.351-359.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramia M, Tullock D L, Phan-Thien N. The role of hydrodynamic interaction in the locomotion of microorganisms. Biophys J. 1993;65:755–778. doi: 10.1016/S0006-3495(93)81129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salhi B, Mendelson N H. Patterns of gene expression in Bacillus subtilis colonies. J Bacteriol. 1993;175:5000–5008. doi: 10.1128/jb.175.16.5000-5008.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stock J B, Surette M G. Chemotaxis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1103–1129. [Google Scholar]