Abstract
Background
Cardiovascular (CV) diseases are a major cause of the disease burden worldwide and contribute substantially to health care costs, in particular in people with diabetes. Their incidence can be reduced by multi-factorial interventions. This study intends to describe the occurrence of CV risk and protective/preventive factors in the adult population resident in Italy, to better target public health interventions.
Methods
Data collected in 2016–19 from adults aged 18–69 years, participating in the Italian Behavioural Risk Factor Surveillance System (PASSI) based on a cross-sectional design, were used. The frequency of CV risk/protective factors was estimated in people with and without diabetes. The contribution of socioeconomic level (SEL) to CV risk was also explored.
Results
Among 129 989 respondents, 4.7% received a diagnosis of diabetes. Many CV risk factors were significantly more frequent in people with diabetes, who often presented multiple risk factors. At the same time, they adopted protective behaviours and received treatments and preventive interventions more often than those without diabetes. Relevant disparities were observed between SEL groups in diabetic people, with the least advantaged showing a worse risk profile.
Conclusions
Adults resident in Italy with diabetes are exposed to CV risk factors more often than those without diabetes. However, they show an increased attention to control these factors and receive more frequent health care, although less than ideal in absolute terms. There is an opportunity to reduce the important CV disease burden in the population through preventive/health promotion targeted interventions, prioritizing people with diabetes and of lower SEL.
Introduction
Cardiovascular diseases (CVDs)—comprising coronary heart disease (myocardial infarction and angina), heart failure, stroke and transient ischaemic attack—have an increased incidence in people with diabetes and are a major cause of death and disability in this population.1 Indeed, diabetes is in itself a major CVD risk factor particularly if glycaemic values are not well controlled.2,3
Other important modifiable risk factors of cardiovascular (CV) outcomes and death both in people with and without diabetes are tobacco smoking, systolic blood pressure, LDL cholesterol, low physical activity, overweight/obesity and insufficient consumption of fruit and vegetables.
These conditions are also risk factors for the development of type 2 diabetes (and can worsen its metabolic control) or are associated with diabetes in the context of the dysmetabolic syndrome.4
Therefore, not unexpectedly the occurrence of multiple CVD risk factors is often observed in people with diabetes, further contributing to the emergence of CV complications.5,6
Multi-factorial interventions, such as appropriate treatments and lifestyle improvements, can substantially reduce the risk of important CV outcomes in this population.5,7 It has been estimated that having all major risk-factor variables within therapeutic guideline levels could eliminate the excess risk of acute myocardial infarction in type 2 diabetes.3
Marked reductions in CVD morbidity and mortality have occurred over the last few decades in many countries; however, the proportion of CVDs attributable to diabetes appears to have increased in comparison with other risk factors.8
Average health care costs for diabetic patients are significantly higher than those for the general population: CV complications determine a sizeable proportion of these costs, with a relevant economic burden, both for individuals and health care systems.5,9
Inequalities of socioeconomic status (SES) are an important determinant of health outcomes. SES, a reflection of social position, is a multidimensional construct, usually measured by income, education and occupation.10
People of lower SES sustain a greater disease burden, and CVDs are responsible of the major part of this burden. In particular, a low educational level is related to a higher prevalence of diabetes and a higher mortality from diabetes.11 More evidence on this topic is reported in the online Supplementary box S1.
The main objective of this study is to assess the exposure to risk/protective factors for CVD of the adult population (18–69 years) resident in Italy with and without diabetes, utilizing data from the Italian Behavioural Risk Factor Surveillance System (BRFSS), Progressi delle Aziende Sanitarie per la Salute in Italia (PASSI).
The specific objectives are (i) to estimate the occurrence and distribution in the population of modifiable CVD risk factors, protective individual behaviours and preventive-care practices, in people with and without diabetes, and (ii) to describe the differences in risk-factor distribution and preventive interventions according to SES in people with diabetes.
Methods
PASSI is an ongoing cross-sectional BRFSS. The sample for the survey is selected from the adult population (18–69 years old) resident in Italy, which comprised 40 972 682 and 40 387 805 individuals, respectively, on 1 January 2016 and 1 January 2019.
The unit of data collection for PASSI is the local health unit (LHU). Each of the 20 Italian Regions comprises 1–22 LHUs in charge of providing universal health care, including prevention and treatment services for populations ranging from 30 000 to over 1 million.
The survey population includes residents in the LHU area (18–69 years) who have a telephone number available (landline or cell phone) and are capable of being interviewed; a sample of potential interviewees is randomly drawn from the enrolment list of residents in each LHU on a monthly basis. People who have moved away or deceased when a contact is attempted are not considered in the eligible population; other criteria of exclusion are inability to understand Italian, inability to participate in the interview, hospitalization or institutionalization and being out of the age range (18–69). The sample is stratified by gender and age (18–34, 35–49 and 50–69 years) proportionally to the size of the respective strata in the general population. Public health practitioners administer telephone interviews through a standardized questionnaire, gathering information on a wide variety of health-related behavioural and preventive topics along with socio-demographic data. Informed verbal consent is obtained from all the participants. The data are anonymized and electronically recorded in a national database. Interviews collected during a calendar year are aggregated in an annual dataset. The LHUs’ data are merged and analysed to obtain regional and national estimates. The protocol of PASSI surveillance was approved by the ethics committee of the National Institute of Public Health (CE-ISS 06/158-08/03/2007). Details about methodological issues have been described elsewhere.12
Indicator definitions
Information on diabetes was collected by asking whether the participant had been diagnosed by a physician for diabetes mellitus (type 1 or type 2). Information on socio-demographic characteristics was collected and categorized, too: sex, age (18–34, 35–49 or 50–69 years), geographic area of residence (North, Centre or South and major islands, according to the criteria of the Italian National Institute of Statistics), completed education (none or primary school, middle school, high school and university) and economic difficulties (many, some or no difficulties in getting to the end of the month with the available household income). To define the educational level, we adopted the categories of the International System of Classification of Educations: primary or no education; lower secondary education; higher secondary education; and tertiary education.13
According to the questions on education and economic condition, a proxy indicator of SES was created, socioeconomic level (SEL), with four categories: SEL1—low educational attainment (primary/middle school) and some/many economic difficulties; SEL2—low educational attainment (primary/middle school) and no economic difficulties; SEL3—high educational attainment (high school/university) and some/many economic difficulties; and SEL4—high educational attainment (high school/university) and no economic difficulties. Given the low prevalence of diabetes in young people, we merged the two younger age groups into one (18–49 years) in order to obtain more precise estimates of the indicators. Sex, age and residence were objectively confirmed with the LHUs’ lists. All indicators are described in the Supplementary material.
Statistical analysis
Percentage estimates were weighted, assigning each record a probability weight equal to the inverse of the sampling fraction in each LHU stratum. Complex survey design analyses were conducted, using the Taylor series method for variance estimation.
The composition of the population under study was described through a frequency distribution of the main socio-demographic characteristics. The frequency of risk/protective factors for CVD was estimated in people with and without diabetes, in the whole population and according to gender and the other major socio-demographic variables. The frequency of exposure to multiple risk factors was determined using a dichotomous indicator (0–3 vs.4–6 factors).
For analyses of small subsamples, in order to achieve sufficient numbers, we used the educational component of SEL as an indicator for SES, combining SEL1–SEL2 (lower education) and SEL3–SEL4 (higher education).
The difference in prevalence between subgroups regarding the chosen indicators was tested with Pearson chi-squared statistic corrected for the survey design; where appropriate, it was expressed as Prevalence Ratios using an unadjusted Poisson regression model with robust variance.14
Statistical analyses were performed using the statistical package Stata 13 (StataCorp LP, TX, USA).
Results
Annual datasets for the years 2016–19 were combined to ensure sufficient sample size for exploring population subgroups. During the study period, 129 989 people were interviewed in 110 out of 121 (91%) Italian LHUs. The survey participants represented 90% of the population resident in Italy.
The outcome rates were calculated following the guidelines of the American Association for Public Opinion Research (AAPOR).15 The response rate (RR) adjusted for ineligible cases (AAPOR RR4) was 80% in the cumulative 2016–19 sample (81% in 2016, 81% in 2017, 80% in 2018 and 79% in 2019). Item non-response for the questions on diabetes was negligible (0.1%), as well as for the other variables (never higher than 0.4%). The participants’ flow diagram is shown in the Supplementary material.
Composition of the study population: socio-demographic characteristics
The relative proportions of the four sex-and-age strata in the collected sample (2016–19) were closely similar to those of the Italian population of reference in the 4-year period, according to the official demographic figures of the Italian National Statistics Institute, with a maximum difference of 0.4%.
Men and women were almost equally represented in the sample (table 1). About two-third of participants (66.2%) had a high educational level (SEL 3 & 4), while more than half (51.3%) reported some/many economic difficulties (SEL 1 & 3). People with diabetes were older (13 years on average) than people without diabetes, with a predominance of men (56.4%).
Table 1.
Composition of the study population (adults, 18–69 years, resident in Italy), overall and according to gender and reported diagnosis of diabetes, stratified by socio-demographic characteristics
| Adults resident in Italy |
Men |
Women |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W a | D b | No D | W | D | No D | W | D | No D | |||||||||||
| Mean age | 44.8 | 57.4 | 44.2 | 44.5 | 58.0 | 43.8 | 45.1 | 56.5 | 44.6 | ||||||||||
| Characteristics | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | |
| Overall | 100 | 129 989 | 4.7 | 5766 | 95.3 | 124 204 | 100 | 63 487 | 5.3 | 3204 | 94.7 | 60 274 | 100 | 66 502 | 4.0 | 2562 | 96.0 | 63 930 | |
| Gender | Men | 49.5 | 63 487 | 56.4 | 3204 | 49.2 | 60 274 | ||||||||||||
| Women | 50.5 | 66 502 | 43.6 | 2562 | 50.8 | 63 930 | |||||||||||||
| Age group | 18–49 | 59.6 | 77 293 | 19.4 | 1165 | 61.6 | 76 118 | 60.6 | 38 170 | 16.8 | 529 | 63.1 | 37 635 | 58.7 | 39 123 | 22.9 | 636 | 60.2 | 38 483 |
| 50–69 | 40.4 | 52 696 | 80.6 | 4601 | 38.4 | 48 086 | 39.4 | 25 317 | 83.2 | 2675 | 36.9 | 22 639 | 41.3 | 27 379 | 77.1 | 1926 | 39.8 | 25 447 | |
| Socioeconomic levelc | SEL 1 | 22.6 | 27 142 | 43.3 | 2306 | 21.6 | 24 835 | 22.1 | 13 056 | 39.9 | 1192 | 21.1 | 11 863 | 23.1 | 14 086 | 47.8 | 1114 | 22.0 | 12 972 |
| SEL 2 | 11.2 | 16 464 | 15.1 | 989 | 11.1 | 15 475 | 12.5 | 9047 | 16.0 | 586 | 12.3 | 8461 | 10.0 | 7417 | 13.8 | 403 | 9.8 | 7014 | |
| SEL 3 | 28.7 | 34 514 | 21.1 | 1162 | 29.0 | 33 350 | 26.8 | 15 545 | 20.9 | 633 | 27.1 | 14 912 | 30.5 | 18 969 | 21.3 | 529 | 30.9 | 18 438 | |
| SEL 4 | 37.5 | 51 237 | 20.5 | 1289 | 38.3 | 49 946 | 38.6 | 25 538 | 23.2 | 779 | 39.5 | 24 757 | 36.4 | 25 699 | 17.1 | 510 | 37.3 | 25 189 | |
| Geographic area of residenced | North | 36.0 | 55 706 | 28.9 | 2103 | 36.4 | 53 589 | 36.3 | 27 388 | 30.2 | 1210 | 36.6 | 26 173 | 35.8 | 28 318 | 27.2 | 893 | 36.2 | 27 416 |
| Centre | 23.2 | 33 455 | 21.1 | 1439 | 23.3 | 32 015 | 22.9 | 16 219 | 20.7 | 784 | 23.1 | 15 435 | 23.4 | 17 236 | 21.6 | 655 | 23.4 | 16 580 | |
| South | 40.8 | 40 828 | 50.0 | 2224 | 40.3 | 38 600 | 40.8 | 19 880 | 49.1 | 1210 | 40.3 | 18 666 | 40.8 | 20 948 | 51.2 | 1014 | 40.4 | 19 934 | |
Percentages and absolute frequencies. PASSI 2016–19 (n = 129 989).
The estimates are weighted. For each stratifying variable, the percentages of the different categories sum up to 100%, while the sum of the absolute frequencies may be lower than the total number of the interviewees, both for the whole sample and for the studied population groups, due to missing values.
W, whole population/sub-population.
D/No D, people with/without reported diabetes.
SEL 1, low educational attainment (primary/middle school) and some/many economic difficulties; SEL 2, low educational attainment (primary/middle school) and no economic difficulties; SEL 3, high educational attainment (high school/university) and some/many economic difficulties; SEL 4, high educational attainment (high school/university) and no economic difficulties.
Defined according to the census criteria of the Italian National Institute of Statistics; Southern Italy comprises the two Italian major islands (Sardinia and Sicily).
The mean age and distribution in age classes of the four SEL groups were markedly different (Supplementary table S1). Those with low educational attainment (SEL 1 and 2) were older (almost a decade on average) than people belonging to SEL 3 and 4. This fact is linked with the increase of the educational level, which occurred in Italy in the last decades.
Another relevant fact is the different composition of the SEL subpopulations according to the geographic area of residence: SEL 1 & 3 were disproportionately represented in Southern Italy, reflecting the less advantaged economic background of the area.
Overall prevalence of diabetes was 4.7% (N 5766), with ample differences among the demographic groups and evident socioeconomic gradients (Supplementary table S2).
Behaviour-related CV risk factors, protective behaviours and preventive-care practices
The frequencies of risk/protective factors in adults resident in Italy with and without diabetes—overall and stratified by gender and age—are reported in table 2.
Table 2.
Prevalence (percent) of behaviour-related CV risk factors, protective behaviours and preventive-care practices in adults (18–69 years) resident in Italy with and without diabetes, stratified by gender and age
| Indicators | Whole population |
Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18–49 years |
50–69 years |
18–49 years |
50–69 years |
||||||||
| D a | No D | D | No D | D | No D | D | No D | D | No D | ||
| Cardiovascular risk factors | Current smokerb | 22.2 | 24.4 | 35.7 | 31.6 | 24.2 | 23.3 | 22.7 | 21.1 | 15.4 | 19.4 |
| BMI≥25c | 71.4 | 40.9 | 67.3 | 42.4 | 75.0 | 61.7 | 55.6 | 24.9 | 72.2 | 44.2 | |
| Physical activity—inactived | 51.7 | 39.5 | 46.9 | 37.1 | 51.7 | 44.6 | 41.8 | 37.2 | 56.0 | 41.9 | |
| <5 portions—fruit/vegetables | 89.4 | 90.3 | 92.4 | 93.6 | 90.3 | 89.9 | 88.1 | 90.3 | 87.7 | 85.5 | |
| Hypertension | 52.1 | 18.1 | 32.0 | 8.9 | 55.6 | 35.9 | 22.9 | 6.1 | 61.0 | 31.6 | |
| Hypercholesterolemia | 43.1 | 21.3 | 26.4 | 14.4 | 44.1 | 30.1 | 25.1 | 10.7 | 51.0 | 34.5 | |
| Protective behaviours and preventive-care practices | Attempt to quit smokinge | 36.0 | 30.8 | 38.3 | 30.2 | 35.5 | 27.0 | 35.6 | 34.5 | 35.6 | 30.1 |
| Diet to lose weightf | 42.1 | 24.1 | 44.1 | 18.5 | 38.2 | 19.3 | 52.9 | 32.9 | 44.7 | 30.7 | |
| Drug—hypertensiong | 91.1 | 78.4 | 78.2 | 53.8 | 93.2 | 85.6 | 72.7 | 54.7 | 92.2 | 87.1 | |
| Drug—hypercholesterolaemiah | 67.1 | 32.3 | 54.6 | 16.6 | 68.6 | 42.2 | 43.1 | 11.4 | 70.2 | 40.4 | |
| Advice to quit smokinge | 73.6 | 50.5 | 65.3 | 44.7 | 79.5 | 60.1 | 60.1 | 47.7 | 71.5 | 57.7 | |
| Advice—physical activityi | 44.1 | 25.0 | 53.7 | 21.6 | 45.4 | 27.3 | 45.2 | 23.1 | 39.9 | 29.4 | |
| Advice to lose weightf | 69.9 | 44.7 | 69.8 | 36.3 | 66.7 | 42.2 | 76.1 | 52.3 | 73.2 | 52.8 | |
| HbA1C—past 12 months | 64.0 | – | 65.3 | – | 65.1 | – | 60.1 | – | 65.1 | – | |
PASSI 2016–19 (n = 129 989).
The estimates are weighted. The difference between values in people with and without diabetes was tested with Pearson chi-squared statistic corrected for the survey design. A significant difference (P < 0.05) is indicated by a grey shade of the corresponding cells.
D/No D, people with/without diabetes.
Reporting smoking on every day or some days when interviewed.
Body mass index ≥25.
Not engaging in moderate (vacuuming, gardening, brisk walking or bicycling) or vigorous (running, aerobics and heavy yard work) physical activity in leisure time, for at least 10 min per week, in the previous 30 days. Daily PA bouts <10 min duration do not concur to the calculation of the weekly minutes. PA, physical activity.
Among cigarette smokers.
Among overweight/obese.
Among people with hypertension.
Among people with hypercholesterolaemia.
Among inactive people.
The differences between people with and without diabetes are relevant for most factors, with some variation among the strata; in particular the discrepancies are more marked in younger people (18–49 years). This pattern is more easily appreciated through Prevalence Ratios presented as a graph (Supplementary figure S1).
Major CVD risk factors were rather frequent in our sample, and more so in people with diabetes: over half (52.1%) were affected by hypertension and 43.1% by hypercholesterolaemia; 71.4% were overweight/obese and 51.7% sedentary.
Only one risk factor (current smoking), in a subgroup of people with diabetes (women 50–69 years), had a prevalence significantly lower than in those without diabetes: respectively 15.4% vs. 19.4%.
An insufficient intake (<5 portions/day) of fruit and vegetables was the risk factor with the highest prevalence (about 90%), substantially equal in people without and with diabetes.
On the other hand, in general protective behaviours and preventive-care practices were more prevalent in all the age/gender groups of people with diabetes, who showed an increased attention to control these factors: e.g. for following a diet to lose excess weight (42.1% vs. 24.1%) and for assuming therapy for hypercholesterolaemia (67.1% vs. 32.3%). Similarly, a greater proportion of people with diabetes received their doctors’ advice to lose weight (69.9% vs. 44.7%) and to do regular physical activity (44.1% vs. 25.0%).
However, in absolute terms, the implementation of many protective behaviours and preventive-care practices was generally less than ideal, even in people with diabetes: while three-fourth of diabetic current smokers received advice to quit smoking, only about one-third reported having attempted to quit in the past 12 months. More than half reportedly did not receive advice to do regular physical activity, and about one-third did not have a HbA1c test in the past 12 months.
The association of risk/protective factors with diabetes was calculated also for each SEL sub-population (Supplementary table S3). The pattern observed in the general population was visible also in the various SEL groups.
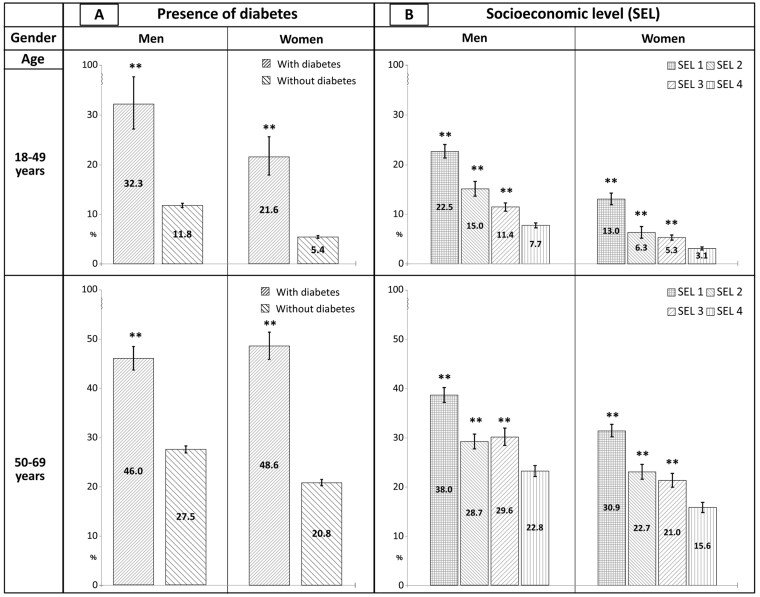
About three-fourth of the general adult population presented more than one CVD risk factor (Supplementary table S4). Using the prevalence of people reporting 4–6 risk factors as an indicator of exposure to multiple factors, a marked, highly significant difference between people with and without diabetes was found in all the demographic strata. Thus, people with diabetes—besides the relevant CVD risk entailed by their condition—present a burden of other major risk factors greater than those without diabetes. Ample differences in the frequency of multiple CVD risk factors were observed also according to SEL in all the demographic strata, with a visible gradient from the lowest to the highest level (figure 1). Detailed data can be found in the Supplementary table S5.
Figure 1.
Prevalence (percent - 95% C.I.) of people reporting 4-6 cardiovascular risk factors in the four gender/age strata, according to reported diagnosis of diabetes and socioeconomic level. Adults (18-69 years) resident in Italy. PASSI 2016-19 (n=129,989). The CV risk factors included in the analysis are: current smoking, overweight/obesity, low physical activity, insufficient consumption of fruit and vegetables, hypertension and hypercholesterolaemia (as defined in the Supplementary material). SEL, socioeconomic level; SEL 1, low education—economic difficulties; SEL 2, low education—no economic difficulties; SEL 3, high education—economic difficulties; SEL 4, high education—no economic difficulties. The estimates are weighted. The difference between groups was tested with unadjusted Poisson regression. Referent group: people without diabetes (Diagram A)—SEL 4 (Diagram B). **P < 0.01
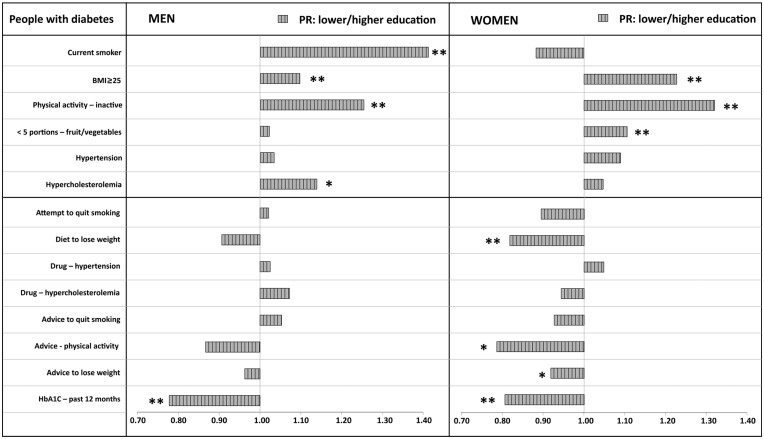
Focusing the analysis on the stratum of the diabetic population with the highest CVD risk (men and women, 50–69 years), the association of risk and protective/preventive factors with the socioeconomic condition was evaluated more thoroughly. To obtain more precise estimates, as explained in the Methods section, we adopted the educational level as a proxy of SES.
For each variable the unadjusted Prevalence Ratio was calculated, using people with higher education as referent group. The results are graphically represented in figure 2; the relative detailed data can be found in the Supplementary table S6.
Figure 2.
Prevalence ratio (PR) of risk and protective/preventive factors between educational levels in people with diabetes resident in Italy, 50-69 years old. PASSI 2016-19 (n=4,601). Lower educational level: SEL 1 and 2. Higher educational level: SEL 3 and 4. SEL, socioeconomic level; SEL 1: low education—economic difficulties; SEL 2, low education—no economic difficulties; SEL 3, high education—economic difficulties; SEL 4, high education—no economic difficulties. Prevalence ratios calculated with unadjusted Poisson regression. Referent: higher educational level (SEL 3 and 4). *P < 0.05; **P < 0.01
A significant positive association with a lower educational level was observed for overweight/obesity and no leisure-time physical activity, both in men and in women; for current smoking and hypercholesterolaemia, only in men; and for insufficient intake of fruit and vegetables, only in women. Many protective behaviours and preventive interventions were significantly less frequent in the lower education group, especially in women.
Discussion
The results here presented outline a composite epidemiological picture.
Major CVD risk factors were rather frequent in the general population of adults (18–69 years) resident in Italy and more so in those with diabetes, compounding a condition at high risk per se. A positive aspect is that in general people with diabetes showed an increased disposition to adopt protective behaviours and received appropriate health care from their doctors more frequently than people without diabetes.
That is important because there is evidence to support the efficacy of counselling by physicians in modifying behavioural CVD risks, including smoking cessation, physical activity and healthy diets.16 However, in absolute terms, the implementation of many preventive/protective factors was generally found to be lacking, both in people with and without diabetes.
A point worth mentioning is the insufficient consumption of fruit and vegetables, which is responsible of a sizeable part of the burden of disease, especially of CVD17,18 (Supplementary box S2).
Regarding health inequalities, we found that in all the demographic strata multiple risk factors were more frequent in people of lower SELs than in those of higher SELs.
Moreover, in older diabetics, most risk factors presented a significant positive association with a low educational level. At the same time, many preventive/protective factors were significantly less frequent in this disadvantaged subgroup, especially in women, including HbA1c, an essential test for monitoring the metabolic control.
Thus, within the sub-population with the highest CVD risk, those belonging to a lower socioeconomic condition were even more exposed to risk factors and received preventive interventions less frequently than the most advantaged.
It is generally acknowledged that SES shapes individuals’ health through a variety of pathways at different levels, and proximal determinants, such as the behaviour-related risk factors we studied, do not explain all its negative health effects. Therefore, policies aimed at tackling distal determinants, improving education, employment and income are in any case a priority in health strategies.10
Behaviour-related CVD risk factors can be prevented and effectively treated, and their adequate control is able to avoid the great majority of CV complications. To reach those goals, in the literature evidence-based suggestions are presented, regarding different lines of action which can be deployed in an integrated manner. Many people present multiple risk factors, which are linked to each other, such as unhealthy diets, too little physical exercise and obesity; people with type-2 diabetes often have other associated conditions, such as hypertension and hypercholesterolaemia, which compound their CV risk.5 Thus, a multi-factorial approach is required by means of behaviour modification and pharmacologic therapy.3 In addition to similar interventions, pertaining to the health system, appropriate strategies should be implemented through intersectoral governance actions (as outlined in the ‘Health in all policies’ strategy).19
However, reducing the prevalence of risk factors in people of lower socioeconomic condition is not sufficient, because improvements in this area do not invariably lead to reductions of inequalities in risk. In Europe, wide inequalities in health between people with higher and lower educational level, occupational class and income level have been found. The increasing concentration of risk factors for CVD in the lower socioeconomic groups is leading to a widening gap in future health outcomes. Where health does improve, people of higher SES gain more than those of lower status. For example, the mortality rates among people with higher SES decline proportionally more rapidly than among those less well off, particularly for CVDs.20 A similar pattern has been observed in the USA with worsening disparities for smoking and diabetes.
Therefore, to reduce health inequalities, health promotion and prevention programmes need to specifically target persons of lower income and education.21
Limitations
The results here presented are based on self-reported data, which are subject to various biases that can lead to over- or underestimate the variables of interest.
The reliability and validity of data collected in Health Interview Surveys have been evaluated through comparisons with gold standards. In general, the reliability of most health indicators, such as those we examined, is high, while the validity is moderate to high. Self-reported BMIs tend to underestimate real values. Also chronic conditions, such as diabetes, hypertension and high cholesterol are often underestimated.22 An important point is that self-reports do not include unrecognized cases, which are ascertained by objective measurements also in people who are not aware of their condition. This fact explains part of the discrepancy between the results of interview and examination surveys.23
The specificity of the above-mentioned self-reported data is high, minimizing the probability of labelling as affected by a condition people who do not have it, and besides, such estimates are reproducible in different contexts, allowing to describe reliably the variations between different areas and in different moments.22
In any case, indicators based on diagnosed conditions may be adequate for studies aimed at judging the appropriateness of health interventions and gathering evidence for allocating resources for clinical care. Similarly, recommendations by health professionals may be incompletely understood, especially by people with low education, leading to underestimate the relative indicators. However, the reported data allow to evaluate the overall effect of communicative actions, a relevant aspect for public health.
Questions concerning the classification of diabetes (type 1 and type 2) are not asked in our surveillance. Since the recommendations for primary prevention of CVD in people with diabetes appear appropriate both for patients with type 2 and type 1 diabetes, the data were analysed in all the sub-population with self-reported diabetes.24
The degree of control of diagnosed conditions (e.g. measures of blood pressure and cholesterol levels) was not explored: the reported pharmacotherapy of these conditions was interpreted as an indicator of compliance with appropriate preventive-care practices.
In people with diabetes, a diet high in fruits and vegetables is recommended and the suggested overall daily intake corresponds to that of people without diabetes. The more suitable quantity and quality of the two components can vary among persons with diabetes.25 We could not differentiate between fruits and vegetables and considered the total intake as an indicator of appropriate consumption.
Regarding glycated haemoglobin, the frequency of tests in the past 4 and 12 months is asked in our surveillance system. These intervals do not match with the most recent guidelines of the American Diabetes Association,25 which recommend to perform the test at least twice a year in patients with stable glycaemic control, and quarterly in patients not meeting glycaemic goals. In our research, having at least a HbA1c test in the past 12 months was assumed as an indicator of appropriate preventive-care practice. This assumption is in agreement with studies finding a higher CVD risk in people not having HbA1c measured.1
Conclusions
People with diabetes, especially those of lower SEL groups, present a high CVD risk, and should receive a great attention in health strategies, inspiring proactive health promotion and prevention policies, both at individual and social levels.
Since several CVD risk factors are also risk factors for diabetes, their cumulative burden on the general population can be reduced through appropriate interventions both in people with and without diabetes.
Adopting strategies that support the most vulnerable people and overall address the health gradients across the spectrum of socioeconomic groups can achieve actual health benefits even within resource-constrained settings.20 Indeed, health promotion and preventive strategies can be cost-effective and possibly cost-saving, considering the substantial contribute to treatment costs due to CVDs.9,26
We believe that our study can contribute data to outline an updated map of the situation regarding CVD risk and preventive/protective factors in the European region and provide factual evidence to support appropriately targeted health interventions.
Supplementary data
Supplementary data are available at EURPUB online.
Supplementary Material
Acknowledgements
We are grateful to Giuliano Carrozzi for his valuable comments and suggestions, to the PASSI coordinating group and to all the regional and local coordinators and interviewers of PASSI surveillance, who contributed to the data collection, for their competence and commitment.
Funding
This research received no specific grant. PASSI Surveillance is coordinated by the Italian National Institute of Health and it is supported by the Italian Ministry of Health/National Centre for Disease Prevention and Control.
Conflicts of interest: The authors of this article declare no conflict of interest that could affect the conclusions, implications or opinions reported in the paper.
Key points.
Cardiovascular (CV) diseases are a major cause of the disease burden worldwide and contribute substantially to health care costs, in particular in people with diabetes.
Among adults (18–69 years) resident in Italy, people with diabetes present multiple CV risk factors much more frequently than those without diabetes: virtually all of them have—besides their condition—at least one additional risk factor and about 70% present four or more factors.
At the same time, people with diabetes adopt protective behaviours and receive appropriate health care more frequently than those without diabetes, although the implementation of many preventive interventions appears to be lacking.
People with diabetes of low socioeconomic level (SEL) have a particularly high CV risk: they are more frequently exposed to CV risk factors and their increased risk is inadequately tackled by protective/preventive interventions.
To relieve the relevant CV disease burden in the population primary targets of appropriate health promotion and prevention strategies should be people with diabetes and those of lower SELs.
Contributor Information
Sandro Baldissera, Former Member of the PASSI Coordinating Group, National Centre for Disease Prevention and Health Promotion, National Institute of Public Health, Rome, Italy.
Valentina Minardi, National Institute of Public Health, National Centre for Disease Prevention and Health Promotion, Rome, Italy.
Maria Masocco, National Institute of Public Health, National Centre for Disease Prevention and Health Promotion, Rome, Italy.
Gianluigi Ferrante, National Institute of Public Health, National Center for Drug Research and Evaluation, Rome, Italy.
References
- 1. Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol 2015;3:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993;16:434–44. [DOI] [PubMed] [Google Scholar]
- 3. Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018;379:633–44. [DOI] [PubMed] [Google Scholar]
- 4. Groop L, Orho-Melander M.. The dysmetabolic syndrome. J Intern Med 2001;250:105–20. [DOI] [PubMed] [Google Scholar]
- 5.OECD. Cardiovascular Disease and Diabetes: Policies for Better Health and Quality of Care. 2015. Paris: OECD Publishing. [Google Scholar]
- 6. Gunton JE, Davies L, Wilmshurst E, et al. Cigarette smoking affects glycemic control in diabetes. Diabetes Care 2002;25:796–7. [DOI] [PubMed] [Google Scholar]
- 7. Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation 2007;115:1544–50. [DOI] [PubMed] [Google Scholar]
- 8. Rawshani A, Rawshani A, Franzén S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–18. [DOI] [PubMed] [Google Scholar]
- 9. Einarson TR, Acs A, Ludwig C, Panton UH.. Economic burden of cardiovascular disease in type 2 diabetes: a systematic review. Value Health 2018;21:881–90. [DOI] [PubMed] [Google Scholar]
- 10. Adler N, Snibbe A.. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Curr Dir Psychol Sci 2003;12:119–23. [Google Scholar]
- 11. Espelt A, Borrell C, Rodriguez-Sanza M, et al. Socio-economic inequalities in diabetes mellitus across Europe at the turn of the century—tackling health inequalities in Europe: an integrated approach. Eurothine project. Rotterdam, The Netherlands, 2007.
- 12. Baldissera S, Campostrini S, Binkin N, et al. ; PASSI Coordinating Group. Features and initial assessment of the Italian Behavioral Risk Factor Surveillance System (PASSI), 2007-2008. Prev Chronic Dis 2011;8:A24. [PMC free article] [PubMed] [Google Scholar]
- 13. Mackenbach J, Stirbu I, Roskam A-J, et al. Socio-economic inequalities in mortality and morbidity: a cross-European perspective—tackling health inequalities in Europe: an integrated approach. Eurothine project. Rotterdam, The Netherlands, 2007.
- 14. Barros AJ, Hirakata VN.. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The American Association for Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 2011. Available at: http://www.aapor.org/AM/Template.cfm?Section=Standard_Definitions2&Template=/CM/ContentDisplay.cfm&ContentID=3156 (14 april 2022, date last accessed).
- 16. Egede LE, Zheng D.. Modifiable cardiovascular risk factors in adults with diabetes: prevalence and missed opportunities for physician counseling. Arch Intern Med 2002;162:427–33. [DOI] [PubMed] [Google Scholar]
- 17. Branca F, Lartey A, Oenema S, et al. Transforming the food system to fight non-communicable diseases. BMJ 2019;364:l296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du H, Li L, Bennett D, et al. Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med 2016;374:1332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McQueen DV, Wismar M, Lin V, et al. Intersectoral Governance for Health in All Policies. Structures, Actions and Experiences. Copenhagen: World Health Organization, 2012. [Google Scholar]
- 20.WHO Regional Office for Europe. Prevention and Control of Noncommunicable Diseases in the European Region. A Progress Report. 2014.
- 21. Kanjilal S, Gregg EW, Cheng YJ, et al. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among US adults, 1971-2002. Arch Intern Med 2006;166:2348–55. [DOI] [PubMed] [Google Scholar]
- 22. Nelson DE, Holtzman D, Bolen J, et al. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS). Soz Praventivmed 2001;46 (Suppl. 1):S3–42. [PubMed] [Google Scholar]
- 23. Pierannunzi C, Hu SS, Balluz L.. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004-2011. BMC Med Res Methodol 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buse JB, Ginsberg HN, Bakris GL, et al. ; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007;115:114–26. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes Care 2019;42:S103–23. [DOI] [PubMed] [Google Scholar]
- 26. Weintraub WS, Daniels SR, Burke LE, et al. ; Council on Clinical Cardiology, and Stroke Council. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation 2011;124:967–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.