Abstract
The lactose-H+ symport protein (LacS) of Streptococcus thermophilus has a carboxyl-terminal regulatory domain (IIALacS) that is homologous to a family of proteins and protein domains of the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS) in various organisms, of which IIAGlc of Escherichia coli is the best-characterized member. On the basis of these similarities, it was anticipated that IIALacS would be able to perform one or more functions associated with IIAGlc, i.e., carry out phosphoryl transfer and/or affect other catabolic functions. The gene fragment encoding IIALacS was overexpressed in Escherichia coli, and the protein was purified in two steps by metal affinity and anion-exchange chromatography. IIALacS was unable to restore glucose uptake in a IIAGlc-deficient strain, which is consistent with a very low rate of phosphorylation of IIALacS by phosphorylated HPr (HPr∼P) from E. coli. With HPr∼P from S. thermophilus, the rate was more than 10-fold higher, but the rate constants for the phosphorylation of IIALacS (k1 = 4.3 × 102 M−1 s−1) and dephosphorylation of IIALacS∼P by HPr (k−1 = 1.1 × 103 M−1 s−1) are still at least 4 orders of magnitude lower than for the phosphoryltransfer between IIAGlc and HPr from E. coli. This finding suggests that IIALacS has evolved into a protein domain whose main function is not to transfer phosphoryl groups rapidly. On the basis of sequence alignment of IIA proteins with and without putative phosphoryl transfer functions and the known structure of IIAGlc, we constructed a double mutant [IIALacS(I548E/G556D)] that was predicted to have increased phosphoryl transfer activity. Indeed, the phosphorylation rate of IIALacS(I548E/G556D) by HPr∼P increased (k1 = 4.0 × 103 M−1 s−1) and became nearly independent of the source of HPr∼P (S. thermophilus, Bacillus subtilis, or E. coli). The increased phosphoryl transfer rate of IIALacS(I548E/G556D) was insufficient to complement IIAGlc in PTS-mediated glucose transport in E. coli. Both IIALacS and IIALacS(I548E/G556D) could replace IIAGlc, but in another function: they inhibited glycerol kinase (inducer exclusion) when present in the unphosphorylated form.
The phosphoenolpyruvate (PEP):carbohydrate phosphotransferase system (PTS) catalyzes the uptake of carbohydrate concomitant with its phosphorylation. The phosphoryl group is transferred from PEP via the general energy-coupling proteins enzyme I and HPr to the sugar-specific phosphoryl transfer protein/domain IIA; phosphorylated IIA (IIA∼P) transfers the phosphoryl group to the sugar-specific IIB protein domain which phosphorylates the sugar that is translocated via the sugar-specific IIC protein domain (23). Apart from its function in the uptake and phosphorylation of sugars, the PTS regulates transport and subsequent metabolism of non-PTS carbohydrates. In gram-negative enteric bacteria, this regulation is mediated by the phosphorylation state of the Glc-specific IIA (IIAGlc), which is determined by the relative rates of phosphorylation by HPr∼P and dephosphorylation by IICBGlc. For instance, IIAGlc∼P is involved in the stimulation of adenylate cyclase, whereby the expression of many catabolic enzymes is regulated through changes in cyclic AMP (cAMP) levels (1).
Unphosphorylated IIAGlc, on the other hand, binds directly to several transporters and enzymes of carbohydrate metabolism, and thereby inhibits their activities, via a phenomenon called inducer exclusion (23). The interaction of IIAGlc with one of its targets, glycerol kinase (GlpK), has been elucidated by analyzing the crystal structure of Escherichia coli glycerol kinase in complex with E. coli IIAGlc (7). This study revealed that IIAGlc binds to glycerol kinase at a region that is distant from the catalytic site of glycerol kinase, which suggests that long-range conformational changes mediate the inhibition of glycerol kinase by IIAGlc.
In gram-positive bacteria, not only can HPr be phosphorylated by PEP/enzyme I on a histidine residue (His-15), but also a metabolite-activated ATP-dependent protein kinase can phosphorylate a serine residue at position 46 (2). The serine-phosphorylated form of HPr [HPr(Ser-P)] seems to control carbohydrate metabolism both at the protein level and at the gene level, i.e., transport activities (inducer exclusion of both PTS and non-PTS sugars and/or inducer expulsion) and transcription (28).
There is no evidence for the involvement of IIAGlc or IIA-like proteins in PTS-mediated regulation in gram-positive bacteria. However, several non-PTS sugar transporters have a carboxyl-terminal domain that is homologous to IIAGlc of E. coli (18, 20). The best-characterized system of this family of transporters with a two-domain structure is the lactose transport protein (LacS) of Streptococcus thermophilus. This protein is, among others, also homologous to the melibiose transport proteins of Salmonella typhimurium and E. coli, which lack a IIA-like domain but are regulated by IIAGlc (18, 41). The carboxyl-terminal IIA domain of the LacS protein of S. thermophilus (IIALacS) is located in the cytoplasm and has 34% residue identity with IIAGlc of E. coli (20). IIALacS is phosphorylated, most likely at His-552, by HPr∼P, which inhibits the transport activity of LacS (17, 19). This histidine residue corresponds with His-90 of IIAGlc in E. coli, which has been shown to be the phosphoryl-accepting site (4, 24) (Fig. 1). The phosphorylation of LacS by HPr∼P has been assessed only qualitatively, and it is not known whether the IIALacS domain has phosphoryl transfer activity equivalent to that of IIAGlc.
FIG. 1.
Alignment of the active site regions of PTS IIA and non-PTS IIA protein domains. ∗, conserved residue; :, similar residue; s, salt bridge with residue in glycerol kinase; h, hydrophobic interaction with glycerol kinase; z, coordination of Zn(II) (7); @, charged group near phosphorylation site. Only a portion of each PTS IIA protein domain in the SwissProt database is shown. Two-letter suffixes denote S. thermophilus (St), Pseudomonas aeruginosa (Pa), Lactobacillus bulgaricus (Lb), Leuconastoc lactis, (Ll), S. typhimurium (Sty), E. coli (Ec), B. subtilis (Bs), Klebsiella pneumoniae (Kp), Brevibacterium lactofermentum (Bl), and Corynebacterium glutamicum (Cg).
In this study, we investigated (i) the kinetics of (de)phosphorylation of the IIALacS domain of S. thermophilus and (ii) the ability of the protein to carry out phosphoryl transfer to IICBGlc of E. coli (PTS-mediated glucose transport) and to inhibit glycerol kinase. Information about the (de)phosphorylation kinetics is relevant because the only known function of the IIA domain in LacS, and homologous transporters, involves the regulation of lactose-H+ symport activity, for which a very rapid phosphoryl transfer may not be critical. For this study, we expressed IIALacS in E. coli and S. typhimurium and constructed two mutants in which either the proposed phosphoryl-accepting histidine was replaced by Arg [IIALacS(H552R)] or two residues near the putative phosphorylation site were replaced by the equivalent residues conserved in all PTS members of the IIAGlc family [IIALacS(I548E/G556D)] (Fig. 1). The crystal structures of IIAGlc from E. coli and B. subtilis show that the Glu residue is exposed to the surface of the molecule and may be critical for the interaction of IIA with its partner molecules; the Asp residue is close to the active site in the tertiary structure (12, 40). For the in vitro phosphorylation assays, we purified each of the mutant proteins and assessed phosphorylation by HPr∼P from E. coli, B. subtilis, and S. thermophilus.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used were E. coli DH5α [deoR endA1 gyrA961 hsdR17 (rK− mK+) recA rel-1 supE44 thi-1 Δ(lacZYA-arg-169) Ø80ΔlacZΔλ] (8), BL21 [hsdS gal(λ cIts857) ind-1 sam-7 nin-5 lacUV5-T7 gene1] (32), LM1 [crr-1 thi-1 his-1 argG6 metB galT rpsL ptsM manI nagE] (11), and MC1061 [Δ(lacIPOZYA) F− araD139 Δ(ara-leu)7697 galU galK rK+ mK− strA] (8) and S. typhimurium PP2178 [crr-307::Tn10 nagE142 trpB223] (36). To isolate plasmid DNA, the cells were grown in Luria broth under vigorous aeration at 37°C (29). For the transport assays, the cells were grown in minimal salts medium A (31) with glycerol (0.2% [wt/vol]) as carbon and energy source as described by van der Vlag et al. (37). For large-scale protein purification, the cells were grown in Luria broth in a 10-liter fermentor (Bio Bench ADI 1065; Applicon, Inc.) with oxygen supply (50% saturation) and pH control (pH 7.0). Growth on succinate was performed on agar plates containing minimal salts medium A supplemented with succinate (0.5%) and the essential nutrients as indicated by the autotrophic markers. When necessary, carbenicillin (50 μg/ml), chloramphenicol (10 μg/ml), tetracycline (12.5 μg/ml), or isopropyl-β-d-thiogalactopyranoside (1 mM) was added to the medium.
S. thermophilus ST11 (ΔlacS)/pGKHis was grown semianaerobically at 42°C in Belliker broth supplemented with 0.5% beef extract, 20 mM lactose, and 5 mg of erythromycin per ml (9).
DNA manipulations.
DNA modifications were performed as described by Sambrook et al. (29) unless indicated otherwise. Subcloning of plasmids into E. coli strains was performed via E. coli DH5α. Plasmids used for the expression of proteins in E. coli and S. typhimurium are listed in Table 1.
TABLE 1.
Plasmids used
| Plasmid | Propertiesa | Source or reference |
|---|---|---|
| pSKII− | Ampr, high-copy-number expression vector, T7 promoter | Stratagene |
| pSKE8 | pSKII+, carrying lacS of S. thermophilus A147 as 4,073-bp EcoRI fragment (galM+ lacS+) | 19 |
| pSKE8E | pSKE8 with additional EcoRI site 21 bp upstream of the initiation codon of lacS | 9 |
| pSKE8his | pSKE8 with His-tagged lacS | 9 |
| pSKE8N | pSKE8 with NcoI site at the initiation codon of lacS | 9 |
| pSKE8(lacS-H552R) | pSKE8 with His-552 of LacS replaced by Arg | 19 |
| pSKE8(lacS-I548E/G556D) | pSKE8 with Ile-548 and Gly-556 of LacS replaced by Glu and Asp, respectively | 19 |
| pSKoppAChis | pSKE8his with lacS (1,936-bp NcoI-MluI fragment) replaced by oppA | 16a |
| pKK223-3 | Ampr, medium-copy-number expression vector, taq promoter | Pharmacia |
| pSK181 | pSKII−, carrying a 543 bp fragmentb that specifies the C-terminal 181 amino acids of LacS | This work |
| pSK173 | pSKII−, carrying a 519-bp fragmentb that specifies the C-terminal 173 amino acids of LacS | This work |
| pSK154 | pSKII−, carrying a 462-bp fragmentb that specifies the C-terminal 154 amino acids of LacS | This work |
| pKKELE | pKK223-3, carrying a 631-bp fragmentb that specifies IIALacS | This work |
| pSKIIAhis | pSKE8N with the 2,243-bp NcoI-BamHI fragment replaced by a 644-bp NcoI-BamHI fragment that specifies IIALacS-6H | This work |
| pSKIIAm1his | pSKIIAhis with H552R | This work |
| pSKIIAm2his | pSKIIAhis with I548E/G556D | This work |
| pGKHis | pGK13, carrying His-Tagged lacS of S. thermophilus as a 3,824-bp EcoRI-DraI fragment from pSKE8 ligated into the EcoRI-EcoRV sites | 9 |
Amp and Cm indicate resistance to ampicillin and chloramphenicol, respectively.
PCR fragment blunt-end ligated in the SmaI site of the vector.
Since alignments of IIALacS with other IIA proteins do not clearly reveal an optimal translation initiation site, several gene fragments specifying IIALacS were cloned. ATG initiation codons were engineered at positions 1359, 1383, and 1440 of the lacS gene, using the forward primers ARH (5′AGGAGGTGTCAACATGGCACGTCACGCTAAAATTGT), ELE (5′AGGAGGTGTCAACATGGAATTGGAACATCGCTTTAG), and VSL (5′AGGAGGTGTCAACATGGTATCTCTTGTAACCCCTAC), respectively; the corresponding protein domains are 181, 173, and 154 amino acids long. For the PCRs, use of the reverse primer BR (5′CAAAATACTTAGGATCCGAGTGAGCATC) generated a new BamHI site 82 bp downstream of the stop codon of the lacS gene. For the PCRs with pSKE8e as the template DNA, the oligonucleotide primers were treated with T4 polynucleotide kinase before use. PCR fragments were isolated with QIA quick spin columns (Qiagen, Inc.) and then blunt-end ligated into pSKII− that had been digested with SmaI and dephosphorylated by Klenow enzyme. In the resulting plasmids pSK181, pSK173, and pSK154, the gene fragments encoding IIALacS are under the control of the T7 promoter. For the expression of IIALacS from the tac promoter, plasmid pKKELE was constructed by ligating the PCR ELE/BR fragment into pKK223-3 that had been linearized with SmaI and treated with Klenow enzyme.
For the expression of IIALacS from the lacS promoter and its native ribosome binding site and to generate a six-histidine tag at the C terminus of IIALacS, the NcoI/BamHI fragment of pSKE8N was replaced by the NE/BR PCR fragment that had been treated with NcoI and BamHI. The NE/BR PCR fragment was synthesized by using the oligonucleotide primer NE (5′GTCACCATGGAATTGGAACATCGC) as the forward primer, which introduced a new NcoI restriction site at the ATG initiation codon of IIALacS, BR as the reverse primer, and pSKE8his as the template DNA. IIALacS with mutations H552R and I548E/G556D was constructed by using pSKE8(lacS-H552R) and pSKE8(lacS-I548E/G556D), respectively, as template DNAs for the PCRs. NE and LBR (5′ CGCGGATCCTTTTTTGAAGGTAAT) were used as forward and reverse primers, respectively; LBR created a BamHI restriction site 1 bp upstream of the stop codon of the lacS gene. After isolation of the PCR fragments and digestion with NcoI and BamHI, the fragments were ligated into vector pSKoppAChis that had been treated with the corresponding enzymes. In this way, the gene fragments specifying mutant IIALacS were put under control of the lacS promoter, and the corresponding proteins had a six-histidine tag at the carboxyl terminus. All plasmids constructed were checked by restriction analysis and nucleotide sequencing using the Vistra automated laser fluorescent DNA sequencer system with the labeled primer cycle sequencing kit (Amersham, Inc.).
Nomenclature.
IIALacS refers to the IIA domain of LacS in general, whereas constructs representing protein fragments of specific lengths are indicated by numbers between brackets (e.g., IIALacS[181] denotes a domain of 181 residues). The His-tagged IIALacS[173] protein, which was used in most of the experiments, is referred to as IIALacS-6H; when appropriate, mutations are indicated between parentheses [e.g., IIALacS-6H(H552R) and IIALacS-6H(I548E/G556D)]. Ile-548, His-552, and Gly-556 denote residue positions in LacS; the same numbering is used to indicate these positions in IIALacS.
Protein purification.
All purification procedures were carried out at 4°C unless indicated otherwise. Enzyme I and HPr of B. subtilis and E. coli were purified as described previously (26, 27, 38)). For the isolation and purification of HPr from S. thermophilus, the cells were lysed after lysozyme treatment (6). For the removal of lysozyme, the supernatant of the cell lysate fraction was diluted 1× in Milli Q water and incubated with S-Sepharose (10 ml/g of lysozyme) for 1 h at 4°C. Fresh S-Sepharose was added twice, after removal of old resin by decanting the supernatant, after which the cell lysate fraction was incubated for another hour at 4°C. The proteins were then precipitated by addition of ammonium sulfate to 80% (wt/vol), and incubation overnight on ice water. After centrifugation (45 min at 70,000 × g), the pellet was dissolved in 20 mM Tris-HCl (pH 8.5) and loaded on a DEAE-Sepharose fast flow column (1.6 by 40 cm; Pharmacia Biotech Inc.) that had been equilibrated with 20 mM Tris-HCl (pH 8.5). The column was washed with 10 column volumes of 20 mM Tris-HCl (pH 7.0). Proteins were eluted with 10 column volumes of 20 mM Tris-HCl (pH 7.0)–40 mM NaCl and precipitated by 80% ammonium sulfate as described above. The pellet was dissolved in 20 mM sodium acetate (pH 4.0) and desalted on a PD-10 column (Pharmacia Biotech). The resulting fraction was loaded onto an S-Sepharose fast flow column (HR 5/5; Pharmacia Biotech) that had been equilibrated with 20 mM sodium acetate (pH 4.0). The proteins were eluted with a 250-ml gradient of 0 to 250 mM NaCl in 20 mM sodium acetate (pH 4.0). The fractions containing HPr were pooled and concentrated by 80% ammonium sulfate as described above. The pellet was dissolved in 50 mM potassium phosphate (KPi; pH 7.0) to a concentration of 2 mg/ml.
For the isolation and purification of IIALacS, E. coli cells expressing IIALacS-6H were grown to late exponential phase and harvested by centrifugation. The cells were washed twice with 50 mM KPi (pH 8.0) and resuspended in buffer A (50 mM KPi [pH 8.0], 10% [wt/vol] glycerol) to a final total protein concentration of 25 mg/ml. After breaking the cells with a French pressure cell (20,000 lb/in2), DNA was removed by addition of 0.083% polyethyleneimine that had been equilibrated with buffer A and incubated for 15 min at 4°C. After centrifugation for 15 min at 70,000 × g, NaCl and imidazole were added to the supernatant to final concentrations of 400 and 10 mM, respectively. The sample was mixed and incubated with Ni-nitrilotriacetic acid (NTA) resin (∼25 mg of protein/ml of resin) for 1 h at 4°C; the resin had been equilibrated with buffer A10 (50 mM KPi [pH 8], 10% [wt/vol] glycerol, 400 mM NaCl, 10 mM imidazole). Next, the column material was poured into a Bio-Spin column (Bio-Rad Laboratories, Inc.) and washed with 10 column volumes of buffer A10 plus 10 column volumes of buffer A30 (buffer A10 containing 30 mM imidazole at pH 6.0). The protein was eluted with buffer A containing 500 mM imidazole. The fractions eluting from the column were desalted by using PD-10 columns that had been equilibrated with 50 mM Tris-HCl (pH 8.0). Eluted fractions were loaded onto a MonoQ column (HR 5/5; Pharmacia Biotech) that had been equilibrated with 50 mM Tris-HCl (pH 8.0). The proteins were eluted by running a 100-ml gradient of 0 to 500 mM NaCl in 50 mM Tris-HCl (pH 8.0).
Phosphorylation and dephosphorylation assays.
For the phosphorylation of IIALacS-6H, 5.8 μM purified IIALacS-6H was incubated in 50 mM Tris acetate (pH 7.5) containing 1 mM dithiothreitol (DTT), 2 mM MgCl2, 0.8 mM purified enzyme I, 10 mM PEP, and HPr at concentrations ranging from 1 to 90 μM. The phosphorylation reactions were carried out at 10°C in a total volume of 10 μl. The reactions were stopped by addition of 10 μl of 2× sodium dodecyl sulfate (SDS) sample buffer (29), and the samples were stored on ice. For the dephosphorylation of IIALacS-6H∼P, IIALacS-6H was first phosphorylated by PEP, enzyme I, and HPr, after which these components were removed by binding IIALacS-6H∼P to the Ni-NTA resin. Briefly, 70 μl of reaction mixture was mixed with 40 μl of Ni-NTA that had been equilibrated with 50 mM KPi (pH 7.0). Following a wash with 2 ml of 50 mM KPi, IIALacS-6H∼P was eluted with 120 μl of 50 mM KPi (pH 7.0)–500 mM imidazole. For the dephosphorylation reactions, IIALacS-6H∼P (10 mM or as indicated otherwise) was incubated in 50 mM Tris acetate (pH 7.5)–1 mM DTT–2 mM MgCl2 and HPr in the range of 1 to 100 μM in a total volume of 10 ml at 10°C. The reaction was monitored as described above. The amount of (de)phosphorylated protein was determined by SDS-polyacrylamide gel electrophoresis (PAGE) analysis (15% polyacrylamide [10]) and Coomassie brilliant blue staining (29), and the amounts of IIALacS-6H and IIALacS-6H∼P were determined by densitometry using a Dextra DF2400T scanner (Dextra Technology, Inc.).
Immunological methods.
Immunodetection of wild-type and mutant IIALacS was performed with antibodies raised against a peptide corresponding to the carboxyl-terminal 17 residues of LacS (17) or antibodies raised against purified IIALacS-6H (this work). Immunodetection of HPr from S. thermophilus was performed with antibodies raised against HPr of S. salivarius. The proteins were separated by SDS-PAGE (15% polyacrylamide gel) and transferred to polyvinylidene difluoride membranes by semidry electrophoretic blotting. A Western-light chemiluminescence detection kit (Tropix Inc.) was used to visualize the proteins.
Miscellaneous.
Uptake of labeled carbohydrates in intact cells was carried out as described by Postma (21). Protein quantification was performed by the Dc protein assay (Bio-Rad), using bovine serum albumin as the standard. N-terminal sequencing of proteins was performed by Eurosequence, Inc., Groningen, The Netherlands.
Materials.
d-[U-14C]glucose (293 mCi/mmol) and [U-14C]glycerol (150 mCi/mmol) were obtained from the Radiochemical Centre, Amersham, United Kingdom. QIA quick spin columns and Ni-NTA resin were purchased from Qiagen, the Bio-Spin columns were from Bio-Rad Laboratories, PD-10 and MonoQ columns (HR 5/5) were from Pharmacia Biotech, 2-deoxy-d-glucose grade II and polyethyleneimine were from Sigma, and the enzymes needed for DNA manipulations were obtained from Boehringer Mannheim. All other materials were reagent grade and obtained from commercial sources.
RESULTS
Expression of the IIALacS.
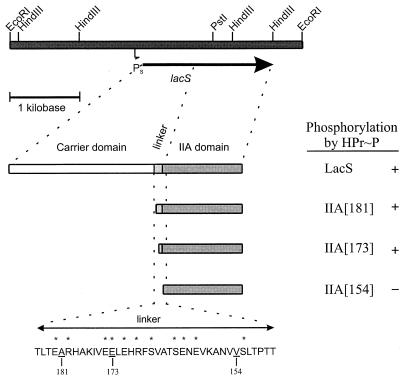
To study the functional properties of the IIALacS, this portion of the protein was expressed separately from the carrier domain. Although IIALacS is homologous to E. coli IIAGlc and various other IIA proteins, the similarity at the amino-terminal end is not significant and the start of the linker region, connecting the carrier and IIA domain of LacS, is not well defined (20, 33). Therefore, we selected three translation initiation sites near the linker region such that IIALacS proteins 181, 173, and 154 amino acids long were obtained (Fig. 2). The individual proteins were tested for the ability to be phosphorylated by HPr∼P, which was determined by monitoring the migration of the proteins on an SDS-polyacrylamide gel (4). The IIALacS proteins present in the cell extracts were detected by immunoblotting using an antibody directed against the carboxyl terminus of LacS. On an SDS-polyacrylamide gel, the IIALacS proteins migrated at a somewhat higher apparent molecular mass than predicted from the deduced amino acid sequence, which has also been observed for other IIA proteins (27). For IIALacS[173] and IIALacS[181], but not for IIALacS[154], a shift in the migration of the protein upon SDS-PAGE was observed after phosphorylation, indicating that IIALacS[173] and IIALacS[181] are phosphorylated by HPr∼P. Further experiments were performed with IIALacS[173], i.e., the smallest fragment that could be phosphorylated and having an amino terminus of the same length as that of IIAGlc.
FIG. 2.
Construction of gene fragments specifying IIALacS. The 4.1-kb EcoRI chromosomal DNA fragment of pSKE8, containing the lacS gene of S. thermophilus, is shown. Only relevant restriction sites are indicated. Shown are the lacS promoter (Ps), the lacS gene (arrow), wild-type protein and portions of the IIA domain (bars). The putative linker and flanking regions are indicated by their amino acid sequences. Amino acids that frequently occur in a Q linker are marked by asterisks, and the residues following the Met of IIALacS[181], IIALacS[173], and IIALacS[154] are indicated by the corresponding numbers. Phosphorylation of the protein was performed in the presence of enzyme I, HPr, and PEP (for details, see Materials and Methods). The ability of LacS to become phosphorylated by HPr∼P was taken from reference 19.
To amplify the expression of IIALacS[173], we made several plasmid constructs (Table 1) in which the copy number was either high or medium and the promoter was inducible (tac and T7) or constitutive (lacS). After transformation of the E. coli hosts (DH5α, MC1061, and BL21) with the appropriate plasmids, the highest expression (2 to 5% of total cell protein) was obtained from the lacS promoter present in plasmid pSKIIA with E. coli DH5α as the host (data not shown). Immunoblot detection using an antibody directed against the carboxyl terminus of LacS demonstrated that the expressed proteins corresponded to IIALacS (data not shown). To facilitate the purification of IIALacS, we constructed pSKIIAhis, in which sequences specifying a factor Xa cleavage site and a six-histidine tag are present at the 3′ end of the IIALacS gene fragment. The introduction of a carboxyl-terminal His tag had no effect on the ability of HPr∼P to phosphorylate IIALacS (data not shown). On SDS-PAGE, IIALacS-6H migrated somewhat more slowly than IIALacS.
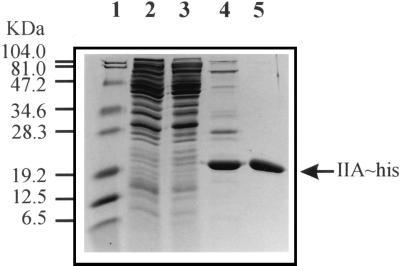
Purification of IIALacS-6H.
For the purification of IIALacS-6H, E. coli DH5α/PSKIIAhis cells were grown to late exponential growth phase (Fig. 3, lane 2). After the cells were broken in a French pressure cell and cell debris/membranes and DNA were removed (Fig. 3, lane 3), the His-tagged protein could be isolated with a purity of 90% by nickel chelate affinity chromatography (lane 4). Further purification was achieved by anion-exchange chromatography using a MonoQ column (lane 5). The protein was eluted at approximately 150 mM NaCl. From 1 liter of cells grown to an A660 of 4.5 in a computer-controlled fermentor and under vigorous aeration, 1.6 mg of IIALacS-6H was obtained. The amino-terminal sequence of the purified protein was Met-Glu-Leu-Glu-His-Arg, which is identical to the anticipated amino acid sequence (20); Glu-462 in LacS was replaced by Met to obtain the initiation codon of the IIA domain. Purification of the His-tagged IIALacS(H552R) and IIALacS(I548E/G556D) mutant proteins was performed exactly as for IIALacS.
FIG. 3.
Purification of IIALacS-6H. Shown is a Coomassie brilliant blue-stained SDS–15% polyacrylamide gel. Lane 1, protein marker; lane 2, cell extract of E. coli DH5α/pSKIIAhis (50 μg of protein); lane 3, cytosolic fraction of E. coli DH5α/pSKIIAhis (50 μg of protein); lane 4, IIALacS-6H after nickel chelate affinity chromatography (∼10 μg of protein); lane 5, IIALacS-6H after nickel chelate and anion-exchange chromatography (∼7 μg of protein).
Purification of HPr.
HPr from S. thermophilus was analyzed in column fractions by native PAGE (15% polyacrylamide gel) and immunodetection using antibodies raised against HPr of S. salivarius. HPr was isolated from cells of S. thermophilus ST11 (ΔlacS)/pGKhis that were grown to late exponential phase. After the cells were broken, most of the lysozyme present in the cell lysate was removed by adsorption to S-Sepharose. Subsequently, HPr was purified to near homogeneity in two steps, involving anion-exchange and cation-exchange chromatography. On an SDS-polyacrylamide gel, HPr migrated at ∼13 kDa. This apparent molecular mass of HPr from S. thermophilus is in the same range as found for HPr purified from other streptococci, 6.7 to 17 kDa, while the molecular mass from the nucleotide sequence is 8.9 kDa (35).
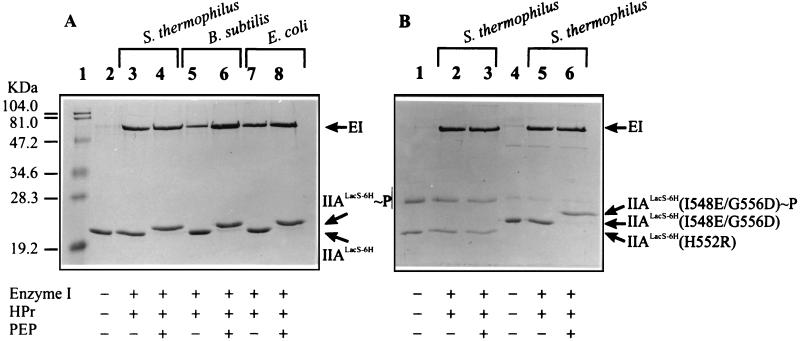
Phosphorylation of IIALacS-6H by PTS-mediated enzymes.
Phosphorylation of IIALacS-6H was analyzed by SDS-PAGE. Figure 4A shows the relative electrophoretic mobilities of purified IIALacS-6H in the absence (lane 2) and presence of PEP, enzyme I, and HPr of S. thermophilus (lane 4), B. subtilis (lane 6), and E. coli (lane 8). The phosphorylated form of IIALacS-6H exhibited a somewhat lower electrophoretic mobility than the nonphosphorylated protein (compare lanes 2 with lanes 4, 6, and 8). These experiments clearly indicate that the IIA domain of LacS can be phosphorylated via PEP and the general PTS energy-coupling proteins enzyme I and HPr of S. thermophilus as well as HPr of both E. coli and B. subtilis. Figure 4B shows the electrophoretic mobilities of the purified mutants IIALacS-6H(H552R) and IIALacS-6H(I548E/G556D). The mobility of IIALacS-6H(H552R) is similar to that of the wild type, whereas IIALacS-6H(I548E/G556D) exhibited a significantly lower mobility (lane 5). Upon addition of PEP, enzyme I of B. subtilis, and HPr of S. thermophilus, the migration of IIALacS-6H(H552R) was not affected (compare lanes 1, 2, and 3), whereas IIALacS-6H(I548E/G556D) migrated more slowly (compare lanes 5 and 7). Similar results were obtained with PEP, enzyme I, and HPr of E. coli and B. subtilis (data not shown). These results indicate that IIALacS-6H(I548E/G556D) but not IIALacS-6H(H552R) is capable of accepting the phosphoryl group from HPr∼P.
FIG. 4.
Phosphorylation of IIALacS-6H, IIALacS-6H(H552R), and IIALacS-6H(I548E/G556D). The Coomassie brilliant blue-stained SDS–15% polyacrylamide gel represents samples containing 0.8 μM enzyme I (EI) from B. subtilis, 12.5 μM HPr, 5.8 μM IIALacS-6H, and/or 10 μM PEP, as indicated at the bottom. The phosphorylation reactions (at 37°C for 15 min) were carried out in 50 mM Tris-acetate (pH 7.5)–1 mM DTT–2 mM MgCl2. (A) IIALacS. (B) Lanes 1 to 3, IIALacS(H552R); lanes 4 to 6, IIALacS-6H(I548E/G556D). The sources of HPr (S. thermophilus, B. subtilis, and E. coli) are indicated above the lanes.
Kinetics of phosphorylation and dephosphorylation of IIALacS-6H and IIALacS-6H(I548E/G556D).
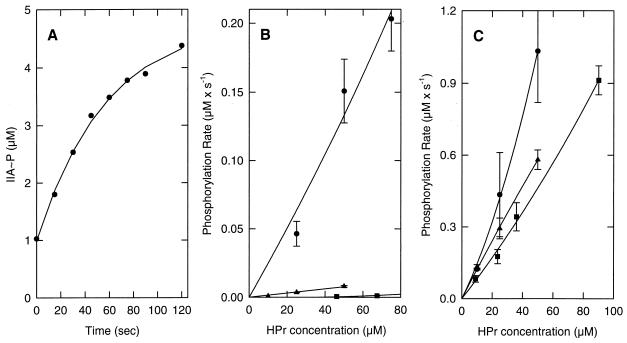
The phosphorylation kinetics of IIALacS-6H by HPr∼P from S. thermophilus is shown in Fig. 5. To determine the phosphorylation rates more precisely, the experiments were performed at 10°C, which decreased the phosphorylation rate approximately 1 order of magnitude compared to the level at 37°C. In these experiments, the concentration of HPr∼P was 5- to 50-fold greater than that of IIALacS-6H, and the kinetics of IIALacS-6H phosphorylation could be approximated as a first-order process (Fig. 5A). The initial rates of phosphorylation of IIALacS-6H at different HPr∼P concentrations and for HPr of S. thermophilus, E. coli, and B. subtilis are presented in Fig. 5B. Clearly, IIALacS-6H was phosphorylated approximately 1 and 2 orders of magnitude faster by HPr∼P from S. thermophilus than by HPr∼P from E. coli and B. subtilis, respectively. The derived rate constants obtained from these data are summarized in Table 2 (first column).
FIG. 5.
Phosphoryl transfer from HPr∼P of S. thermophilus, B. subtilis, and E. coli to IIALacS-6H and IIALacS-6H(I548E/G556D). (A) Time course of the phosphoryl transfer reaction between 25 μM HPr∼P from S. thermophilus and IIALacS-6H. (B) Phosphorylation rate of IIALacS-6H by HPr∼P from S. thermophilus (•), B. subtilis, (■), and E. coli (▴). (C) Phosphorylation rate of IIALacS-6H(I548E/G556D) by HPr∼P from S. thermophilus (•), B. subtilis (■), and E. coli (▴). The phosphorylation reaction was started by adding 5.8 μM purified IIALacS-6H or 4.1 μM IIALacS-6H(I548E/G556D) to 50 mM Tris acetate (pH 7.5) containing 1 mM DTT, 2 mM MgCl2, 0.8 μM purified enzyme I, 10 mM PEP, and HPr at concentrations ranging from 0 to 90 μM, in a total volume of 10 μl at 10°C. The reactions were stopped by addition of 10 μl of 2× SDS sample buffer.
TABLE 2.
Rate and equilibrium constants for phosphoryl transfer between HPr of S. thermophilus, B. subtilis, or E. coli and IIALacS or IIALacS(I548E/G556D)a
| Source of HPr | IIALacS
|
IIALacS (I548E/G556D), k1 (M−1 s−1) | ||
|---|---|---|---|---|
| k1 (M−1 s−1) | k−1 (M−1 s−1) | Keq(k1/k−1) | ||
| S. thermophilus | 430 ± 70 | 1,100 ± 600 | 0.5 ± 0.17 | 4,000 ± 700 |
| B. subtilis | 2.4 ± 0.45 | 12 ± 6 | 0.2 | 3,500 ± 150 |
| E. coli | 26 ± 1.6 | 130 ± 15 | 0.2 | 2,900 ± 40 |
Results are means ± standard errors.
The IIALacS-6H(I548E/G556D) mutant was constructed because the Glu and Asp residues are conserved in all PTS members of the IIA family, whereas they are replaced by neutral residues in the non-PTS IIA domains. These residues are predicted to affect the interaction of IIA with its partner molecules, e.g., HPr∼P and IIB. The phosphorylation kinetics of IIALacS-6H(I548E/G556D) is shown in Fig. 5C and Table 2 (fourth column). Indeed, the rate of phosphorylation of IIALacS-6H(I548E/G556D) by HPr∼P from S. thermophilus increased approximately 1 order of magnitude. Remarkably, however, the rate of phosphorylation of IIALacS-6H(I548E/G556D) by HPr∼P from B. subtilis and E. coli increased 2 and 3 orders of magnitude relative to that of IIALacS-6H, and the phosphorylation became nearly independent of the source of HPr∼P.
IIAGlc∼P can be dephosphorylated by transferring the phosphoryl group to IICBGlc or by redirecting the phosphoryl group to HPr. The dephosphorylation properties of IIALacS-6H∼P and IIALacS-6H(I548E/G556D)∼P were studied with HPr from S. thermophilus, B. subtilis, and E. coli as phosphoryl acceptors. Figure 6 shows the time course for the phosphoryl transfer from IIALacS-6H∼P to HPr from S. thermophilus at different concentrations of HPr. Under these conditions, IIALacS-6H∼P was quite stable and the first-order rate constant for the autodephosphorylation was 6.7 × 10−5 s−1 (Fig. 6, inset). The dephosphorylation rates of IIALacS-6H∼P were determined with HPr from S. thermophilus, B. subtilis, and E. coli as phosphoacceptors. As anticipated from the phosphorylation assays, HPr from S. thermophilus was a much better acceptor than HPr from B. subtilis or E. coli (Table 2). The phosphoryl transfer from IIALacS-6H(I548E/G556D)∼P to HPr from S. thermophilus, B. subtilis, and E. coli was too fast to be measured accurately at 10°C. The data with these three proteins as acceptors were qualitatively very similar, showing that residues Glu-548 and Asp-556 allow a better interaction between IIALacS-6H and HPr of both homologous and heterologous origin.
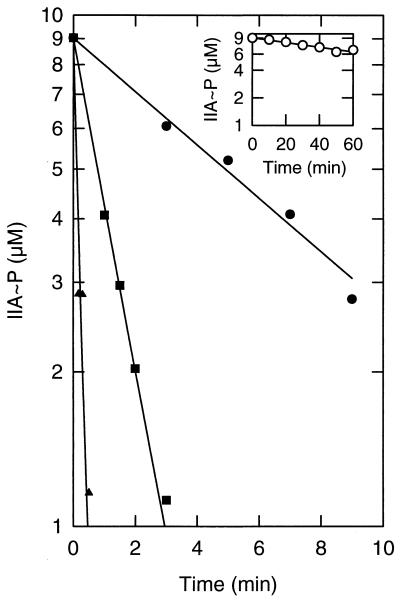
FIG. 6.
Phosphoryl transfer from IIALacS-6H∼P to HPr from S. thermophilus. The dephosphorylation reaction was started by adding 11 μM purified IIALacS-6H∼P to 50 mM Tris acetate (pH 7.5) containing 1 mM DTT, 2 mM MgCl2, and HPr at concentrations of 10 μM (•), 25 μM (■), and 50 μM (▴). The reaction volume was 10 μl, and the temperature was 10°C. The inset shows the dephosphorylation of the IIALacS-6H in the absence of HPr.
Complementation of crr strains in trans.
Since E. coli HPr∼P could phosphorylate IIALacS, we next addressed the question of whether IIALacS could substitute for IIAGlc in the phosphoryl transfer catalyzed by the glucose PTS. The phosphoryl transfer activity of IIALacS was studied in E. coli LM1 (crr manA), a strain defective in glucose transport due to a lack of IIAGlc as well as a functional mannose PTS. Since the N-terminal residues of IIALacS could be important for the interaction with the membrane IICBGlc protein, we tested IIALacS[181] and IIALacS[173] as well as IIALacS-6H(I548E/G556D) for the ability to restore glucose transport in E. coli LM1 (data not shown). The results were all negative, suggesting that these IIALacS proteins are unable to transfer the phosphoryl group rapidly enough to IICBGlc even though they can be phosphorylated by HPr∼P.
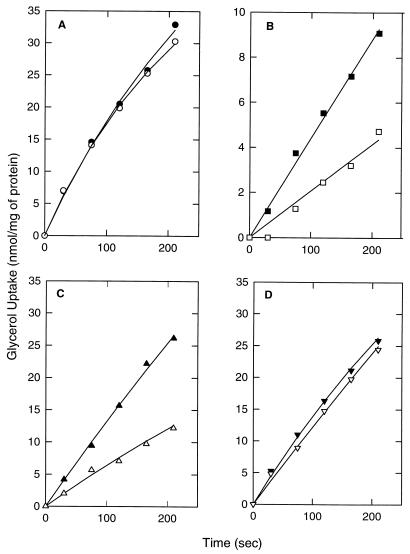
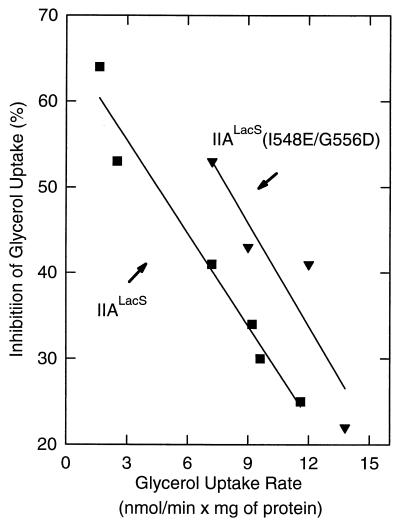
To study the ability of IIALacS to affect glycerol utilization by inhibiting glycerol kinase, IIALacS[173], IIALacS-6H(H552R), and IIALacS-6H(I548E/G556D) were expressed in S. typhimurium PP2178 (crr::Tn10 nagE). This strain lacks IIAGlc as well as IICBANag, and consequently glycerol uptake is not inhibited by the presence of a PTS sugar (Fig. 7A), which was observed in the wild-type strain. Upon transformation of this strain with a plasmid bearing a wild-type crr+ or nagE+ gene and expressing the corresponding protein, glycerol uptake is inhibited by glucose (or analogs) (36). Under these conditions, the equilibrium of IIAGlc (or IINag) is shifted from the phosphorylated to the dephosphorylated state, which is known to interact with (and inhibit) glycerol kinase (22). Similarly, upon addition of 2-deoxyglucose to S. typhimurium PP2178 expressing IIALacS or IIALacS-6H(I548E/G556D), glycerol uptake was partially inhibited (Fig. 7B and C). The glycerol uptake was not inhibited by IIALacS-6H(H552R), indicating that the residue at position 552 in IIALacS is important for the interaction with glycerol kinase (Fig. 7D). The inhibition of glycerol uptake was most clearly observed when the actual uptake rate of glycerol (amount of glycerol kinase) was low (Fig. 8), indicating that the extent of inhibition is determined by the level of glycerol kinase, as has been observed previously (37). The maximal inhibition of the glycerol uptake rate in S. typhimurium PP2178 was 65%. IIALacS-6H(I548E/G556D) inhibited glycerol kinase to a greater extent than IIALacS at equal glycerol uptake rates (Fig. 8). This difference is most likely due to the higher expression of IIALacS-6H(I548E/G556D) than of IIALacS[173], using the expression plasmids pSKIIAm2his and pKKELE, respectively. IIALacS could not be expressed to similar high levels in S. typhimurium PP2178, as pSKIIAhis was lethal to these cells.
FIG. 7.
Inhibition of glycerol kinase activity by IIALacS. S. typhimurium PP2178 (crr::Tn10 nagE) was transformed with pKK223-3 (control) (A), pKKELE (IIALacS) (B), pSKIIAm2his [IIALacS-6H(I548E/G556D)] (C), and pSKIIAm1his [IIALacS-6H(H552R)] (D). Cells were grown overnight on a minimal salts medium supplemented with 0.4% dl-lactate. After being washed with minimal salts medium, the cells were diluted to an A660 of 0.35 in minimal salts medium containing 54 mM glycerol and incubated for 30 min at 37°C; glucose was added to a final concentration of 10 mM; the cells were incubated for 1 h at 37°C and then washed with minimal salts medium. Uptake assays with 0.5 mM [14C]glycerol were performed in the presence (open symbols) and absence (filled symbols) of 10 mM 2-deoxy-d-glucose; the cells were equilibrated in the presence or absence of 2-deoxy-d-glucose for 5 min prior to the initiation of uptake.
FIG. 8.
Relationship between the activity of glycerol kinase and the extent of inhibition of glycerol uptake by IIALacS (■) and IIALacS-6H(I548E/G556D) (▾). Cells were grown as described in the legend to Fig. 7. The data are from several independent experiments that reflect partial induction by glycerol for 15, 20, or 30 min. The glycerol kinase activity was measured as [14C]glycerol uptake rate in the absence of 2-deoxyglucose, and the inhibition of [14C]glycerol uptake was measured from the ratio of the rates in the presence and absence of 10 mM 2-deoxyglucose.
To investigate a possible role of IIALacS in regulating adenylate cyclase activity, IIALacS, IIALacS-6H(H552R), and IIALacS-6H(I548E/G556D) were expressed in S. typhimurium PP2178 and grown on minimal salts A plates containing succinate or citrate. No growth was observed unless 5 mM cAMP was included in the medium. These results indicate that even though these IIALacS proteins can be phosphorylated by HPr∼P, they cannot stimulate adenylate cyclase to restore growth of crr mutants on citrate or succinate.
DISCUSSION
In this paper, we describe the functional expression and purification to near homogeneity of the IIA domain of the lactose transport protein of S. thermophilus. On the basis of the similarities in the primary sequences of IIALacS, IIAGlc, and other PTS IIA protein domains (12, 20), we anticipated that IIALacS would be able to carry out one or more functions associated with IIAGlc, i.e., complement crr strains in PTS-mediated glucose uptake, inhibit glycerol kinase, and/or activate adenylate cyclase.
The in vitro phosphorylation assays indicate that IIALacS-6H can be phosphorylated by HPr∼P from S. thermophilus, B. subtilis, and E. coli, and the results suggest that the phosphorylation site in IIALacS is His-552. This residue corresponds to His-90 of IIAGlc in E. coli, which has been shown to be the phosphoryl-accepting site (4). Although IIALacS can be phosphorylated by HPr∼P, the phosphorylation rates are lower than for phosphorylation of IIA proteins involved in PTS-mediated transport (14). The rate constant for the phosphoryl transfer between S. thermophilus HPr∼P and IIALacS at a temperature of 10°C was k1 = 4.3 × 102M−1 s−1; the rate constant for the reverse reaction (IIALacS∼P to HPr) was k−1 = 1.1 × 103 M−1 s−1. Although these phosphoryl transfer rates increased 10-fold at 37°C, it is evident that phosphorylation of IIALacS by HPr∼P from S. thermophilus is 4 orders of magnitude slower than phosphorylation of IIAGlc by HPr∼P from E. coli (14) (k1 = 6.1 × 107M−1 s−1). With HPr∼P from B. subtilis and E. coli, the differences are even much larger, as one might expect, when interacting proteins from different sources are compared (Fig. 5). The phosphorylation rate of IIALacS by HPr∼P from S. thermophilus increased 1 order of magnitude when Ile-548 and Gly-556 were substituted by Glu and Asp, respectively, and increased 3 and 4 orders of magnitude when IIALacS-6H(I548E/G556D) was phosphorylated by HPr∼P from E. coli and B. subtilis, respectively (Fig. 6). The corresponding acidic residues (Glu-86 and Asp-94 of IIAGlc in E. coli) are conserved in all PTS IIA protein domains but are not present in the non-PTS IIA protein domains (Fig. 1). The crystal structure of IIAGlc from E. coli shows that Asp-94 participates in phosphoryl transfer, as the backbone amide nitrogen of this residue is positioned to form an H bond to the phosphoryl group (16). It has been suggested that this H bond stabilizes the transition state (trigonal bipyramid) form of the P atom. The conserved Glu-86 is more exposed to the surface of the molecule and is proposed to serve as the recognition site for one of the interacting PTS proteins (12). Substitution in the β-glucoside IICBA of E. coli of Asp-551, the equivalent of Asp-94 in IIAGlc, by an alanine residue decreased the phosphorylation rate relative to the wild-type protein (30). The higher rate of phosphorylation of IIALacS-6H(I548E/G556D) than of IIALacS-6H is consistent with the predictions one could make from the three-dimensional structure of IIAGlc of E. coli and substantiates the critical role of these residues in the phosphorylation of IIA. Importantly, the rates of (de)phosphorylation of IIALacS-6H(I548E/G556D) by HPr∼P from E. coli and B. subtilis are similar to those of S. thermophilus, which allowed us to assess some of the functional properties of IIALacS in a heterologous system (see below).
The inability of IIALacS to substitute for IIAGlc in phosphorylation of E. coli IIB, as determined by assays of PTS-mediated glucose uptake, is likely due to the low phosphorylation rate of IIALacS by E. coli HPr∼P. Additionally, the inability of IIALacS to complement the lack of IIAGlc in PTS-mediated glucose uptake could also be due to an incompatible amino terminus. In this respect, it is worth noting that cell extracts of E. coli contain two electrophoretically distinguishable forms of IIAGlc, a slow form and fast form. The fast form [IIAGlc(fast)] is the product of an endopeptidase that cleaves the N-terminal heptapeptide from the mature form. IIAGlc(fast) is fully active in accepting the phosphoryl group from HPr∼P, but it has only 3% of the phosphodonor activity of the intact protein (13) and has a smaller effect on inhibition of methyl-β-d-thiogalactopyranoside uptake (inducer exclusion) than IIAGlc(slow) (15). This finding suggests that the N-terminal region of IIAGlc could participate in the interaction with its partner molecules, such as IIBGlc and the lactose permease. The IIALacS protein that was used in the majority of the experiments is 173 amino acids long and corresponds in length to IIAGlc(slow). Addition of another eight amino acids (part of putative Q-linker region in LacS) did not affect the (de)phosphorylation activity. Shortening of IIALacS[173] by 19 residues abolished the ability of the protein to accept the phosphoryl group from HPr. Future experiments are required to clarify which amino acid residues are involved in the interaction between IIAGlc and IIBGlc to facilitate phosphoryl transfer to glucose.
Apart from its function in the uptake and phosphorylation of sugars, IIAGlc interacts with several non-PTS enzymes such as glycerol kinase, the MalK component of the maltose transport system, and the melibiose and lactose transporters of E. coli, resulting in inactive complexes (23). In a previous study (36), it was shown that wild-type LacS protein, in which the IIA domain is fused to the membrane-bound carrier domain, does not inhibit glycerol kinase. This could point to the inability of membrane-bound IIALacS to interact functionally with glycerol kinase, but more likely the expression of LacS in S. typhimurium was too low for significant inhibition to be observed. It is worth noting that membrane-bound IIANag, as part of the IICBANag complex, is able to inhibit glycerol kinase (37), indicating that inducer exclusion is not exclusively mediated by cytosolic IIAGlc. Since we could express IIALacS separate from the carrier domain of LacS, we also studied the ability of the various IIALacS proteins to inhibit glycerol uptake. Although IIALacS∼P and IIALacS-6H (I548E/G556D)∼P were not able to rapidly transfer the phosphoryl group to IICBGlc, the uptake of glycerol in S. typhimurium PP2178 was inhibited by the addition of 2-deoxyglucose, most likely because IIALacS∼P or IIALacS-6H (I548E/G556D)∼P was dephosphorylated through the redirection of phosphoryl groups to HPr and subsequently to the mannose PTS. 2-Deoxyglucose is taken up by the mannose PTS and thus indirectly influences the phosphorylation state of IIALacS. The extent of inhibition of glycerol uptake was dependent on the actual uptake rate of glycerol (amount of glycerol kinase) (Fig. 8), suggesting that IIALacS inhibits glycerol kinase by forming a stoichiometric complex with the enzyme as observed for IIAGlc and glycerol kinase (37). Hurley et al. (7) identified the sites of interactions between glycerol kinase and E. coli IIAGlc, which mainly involve hydrophobic and electrostatic interactions and a Zn(II) binding site. The Zn(II) binding site is made up of the two active-site histidines of IIAGlc (His-75 and His-90), Glu-478 of glycerol kinase, and an H2O molecule. In the absence of Zn(II), IIAGlc binds to glycerol kinase primarily via the hydrophobic patch and without participation of the His residues. Except for the histidines that coordinate the Zn atom, the positions are poorly conserved in the non-PTS IIA protein domain(s). Nevertheless, IIALacS was able to inhibit glycerol kinase. Our results also indicate that substitution of His-552 for Arg (His-552 in LacS corresponds to His-90 in E. coli IIAGlc) abolished the putative interaction of IIALacS with glycerol kinase. Possibly, the binding constant of the mutant to glycerol kinase is decreased as suggested for IIAGlc(H90Q) (16). Whether the Zn atom plays an important role in the interaction of IIA proteins to glycerol kinase is unclear, as IIAGlc(H75Q) seems as effective an inhibitor as the wild-type protein (cited in reference 16).
In gram-negative enteric bacteria the phosphorylated form of IIAGlc stimulates adenylate cyclase, whereby the expression of many catabolic enzymes is regulated through changes in cAMP levels, e.g., the expression of genes for succinate and citrate catabolism (1, 23). Reddy et al. (25) suggested that the acquisition of a negative charge at His-90∼P of E. coli IIAGlc is responsible for the interaction with adenylate cyclase. Although IIALacS and IIALacS(I548E/G556D) could be phosphorylated by HPr from E. coli both in vitro and in vivo, these IIALacS proteins were not able to stimulate adenylate cyclase sufficiently, if at all, to restore growth of crr mutants on succinate and citrate. The in vivo phosphorylation is suggested by the identification of two species of IIALacS (IIALacS and IIALacS∼P) in cell extracts of E. coli cells (unpublished data); it also follows from the glycerol kinase inhibition experiments.
The equilibrium constant (Keq = k1/K−1) for the phosphoryl transfer reactions between IIALacS and HPr from S. thermophilus is 0.5, which is approximately threefold lower than the corresponding value for HPr and IIAGlc from E. coli (Keq = 1.4 ± 0.5 [14]). This implies that at a given phosphorylation potential, the phosphorylation state of IIALacS (and we assume LacS as well) in S. thermophilus will be lower than that of IIAGlc in E. coli. Since phosphorylation of LacS inhibited the lactose-H+ symport reaction (17), this condition will be met in vivo only when the [HPr(His∼P)]/[HPr] ratio is relatively high. Vadeboncoeur and coworkers (34) reported that in stationary-phase cells of S. salivarius, the [HPr(His∼P)]/[HPr] ratio is approximately 2.5 for S. salivarius. On the other hand, actively growing cells of S. mutans and S. salivarius contained mainly HPr(Ser-P) and HPr(His∼P)(Ser-P), with very little HPr(His∼P) and free HPr. Thus, the degree of phosphorylation of LacS will be low in exponentially growing cells of S. thermophilus, which implies that the system will have full activity. In resting cells of S. thermophilus, a partial inhibition of LacS-mediated methyl-β-d-thiogalactopyranoside transport was observed (17).
ACKNOWLEDGMENTS
Enzyme I and HPr from E. coli were kindly provided by G. T. Robillard (University of Groningen, Groningen, The Netherlands), enzyme I and HPr from B. subtilis were a gift from J. Reizer (University of California at San Diego), and the antibodies raised against HPr of S. salivarius were provided by C. Vadeboncoeur (Laval University, Quebec, Quebec, Canada). We thank Rechien Bader-van’t Hof for technical assistance.
This work was supported by a grant from the Dutch Organization for Scientific Research under the auspices of the Dutch Foundation for Life Science.
REFERENCES
- 1.Botsford J L, Harman J G. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deutscher J, Saier M H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci USA. 1983;80:6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deutscher J, Sauerwald H. Stimulation of dihydroxyacetone and glycerol kinase activity in Streptococcus faecalis by phosphoenolpyruvate-dependent phosphorylation catalyzed by enzyme I and HPr of the phosphotransferase system. J Bacteriol. 1986;166:829–836. doi: 10.1128/jb.166.3.829-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dörschug M, Frank R, Kalbitzer H R, Hengstenberg W, Deutscher J. Phosphoenolpyruvate-dependent phosphorylation site in enzyme IIIGlc of the Escherichia coli phosphotransferase system. Eur J Biochem. 1984;14:113–119. doi: 10.1111/j.1432-1033.1984.tb08438.x. [DOI] [PubMed] [Google Scholar]
- 5.Feese M, Pettigrew D W, Meadow N D, Roseman S, Remington S J. Cation-promoted association of a regulatory and target protein is controlled by protein phosphorylation. Proc Natl Acad Sci USA. 1994;91:3544–3548. doi: 10.1073/pnas.91.9.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foucaud C, Poolman B. Lactose transport system of Streptococcus thermophilus. Functional reconstitution of the protein and characterization of the kinetic mechanism of transport. J Biol Chem. 1992;267:22087–22094. [PubMed] [Google Scholar]
- 7.Hurley J H, Faber H R, Worthylake D, Meadow N D, Roseman S, Pettigrew D W, Remington S J. Structure of the regulatory complex of Escherichia coli IIIGlc with glycerol kinase. Science. 1993;259:673–677. [PubMed] [Google Scholar]
- 8.Huynh T V, Young R A, Daves R W. In: DNA cloning. Glover D M, editor. Oxford, England: IRL Press Limited; 1985. pp. 56–110. [Google Scholar]
- 9.Knol J, Veenhoff L, Liang W J, Henderson P J F, Leblanc G, Poolman B. Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J Biol Chem. 1996;271:15358–15366. doi: 10.1074/jbc.271.26.15358. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Lengeler J, Auburger A-M, Mayer R, Pecher A. Characterization of mutants of Escherichia coli K12, selected by resistance to streptozotocin. Mol Gen Genet. 1980;179:49–54. doi: 10.1007/BF00268445. [DOI] [PubMed] [Google Scholar]
- 12.Liao D I, Kapadia G, Reddy P, Saier M H, Jr, Reizer J, Herzberg O. Structure of the IIA domain of the glucose permease of Bacillus subtilis at 2.2-Å resolution. Biochemistry. 1991;30:9583–9594. doi: 10.1021/bi00104a004. [DOI] [PubMed] [Google Scholar]
- 13.Meadow N D, Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J Biol Chem. 1982;257:14526–14537. [PubMed] [Google Scholar]
- 14.Meadow N D, Roseman S. Rate equilibrium constants for phosphoryl transfer between active site histidines of Escherichia coli HPr and the signal transducing protein III-Glc. J Biol Chem. 1996;271:33440–33445. doi: 10.1074/jbc.271.52.33440. [DOI] [PubMed] [Google Scholar]
- 15.Misko T P, Mitshell W J, Meadow N D, Roseman S. Sugar transport by bacterial phosphotransferase system. Reconstitution of inducer exclusion in Salmonella typhimurium membrane vesicles. J Biol Chem. 1987;262:16261–16266. [PubMed] [Google Scholar]
- 16.Pelton J G, Torchia D A, Remington S J, Murphy K P, Meadow N D, Roseman S. Structures of active-site histidine mutants of IIIGlc, a major signal-transducing protein in Escherichia coli. Effects on the mechanisms of regulation and phosphoryl transfer. J Biol Chem. 1996;271:33446–33456. doi: 10.1074/jbc.271.52.33446. [DOI] [PubMed] [Google Scholar]
- 16a.Picon, A., and B. Poolman. Unpublished data.
- 17.Poolman B, Knol J, Mollet B, Nieuwenhuis B, Sulter G. Regulation of bacterial sugar-H+ symport by phosphoenolpyruvate-dependent enzyme I/HPr-mediated phosphorylation. Proc Natl Acad Sci USA. 1995;92:778–782. doi: 10.1073/pnas.92.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poolman B, Knol J, van der Does C, Henderson P J, Liang W J, Leblanc G, Pourcher T, Mus-Veteau I. Cation and sugar selectivity determinants in a novel family of transport proteins. Mol Microbiol. 1996;19:911–922. doi: 10.1046/j.1365-2958.1996.397949.x. [DOI] [PubMed] [Google Scholar]
- 19.Poolman B, Modderman R, Reizer J. Lactose transport system of Streptococcus thermophilus. The role of histidine residues. J Biol Chem. 1992;267:9150–9157. [PubMed] [Google Scholar]
- 20.Poolman B, Royer T J, Mainzer S E, Schmidt B F. Lactose transport system of Streptococcus thermophilus: a hybrid protein with homology to the melibiose carrier and enzyme III of phosphoenolpyruvate-dependent phosphotransferase systems. J Bacteriol. 1989;171:244–253. doi: 10.1128/jb.171.1.244-253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postma P W. Galactose transport in Salmonella typhimurium. J Bacteriol. 1977;129:630–639. doi: 10.1128/jb.129.2.630-639.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postma P W, Epstein W, Schuitema A R, Nelson S O. Interaction between IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system and glycerol kinase of Salmonella typhimurium. J Bacteriol. 1984;158:351–353. doi: 10.1128/jb.158.1.351-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Presper K A, Wong C Y, Liu L, Meadow N D, Roseman S. Site-directed mutagenesis of the phosphocarrier protein. IIIGlc, a major signal-transducing protein in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:4052–4055. doi: 10.1073/pnas.86.11.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy P, Kamireddi M. Modulation of Escherichia coli adenylate cyclase activity by catalytic-site mutants of protein IIAGlc of the phosphoenolpyruvate:sugar phosphotransferase system. J Bacteriol. 1998;180:732–736. doi: 10.1128/jb.180.3.732-736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reizer J, Sutrina S L, Saier M H, Stewart G C, Peterkofsky A, Reddy P. Mechanistic and physiological consequences of HPr(ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J. 1989;8:2111–2120. doi: 10.1002/j.1460-2075.1989.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reizer J, Sutrina S L, Wu L F, Deutscher J, Reddy P, Saier M H. Functional interactions between proteins of the phosphoenolpyruvate:sugar phosphotransferase systems of Bacillus subtilis and Escherichia coli. J Biol Chem. 1992;267:9158–9169. [PubMed] [Google Scholar]
- 28.Saier M H, Jr, Chauvaux S, Cook G M, Deutscher J, Paulsen I T, Reizer J, Ye J-J. Catabolite repression and inducer control in gram-positive bacteria. Microbiology. 1996;142:217–230. doi: 10.1099/13500872-142-2-217. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Schnetz K, Sutrina S L, Saier M H, Jr, Rak B. Identification of residues in the catalytic permease of beta-glucoside of Escherichia coli by site specific mutagenesis and demonstration of interdomain cross-reactivity between the beta-glucoside and glucose system. J Biol Chem. 1990;265:13464–13471. [PubMed] [Google Scholar]
- 31.Scholte B J, Postma P W. Competition between two pathways for sugar uptake by the phosphoenolpyruvate-dependent sugar phosphotransferase system in Salmonella typhimurium. Eur J Biochem. 1981;114:51–58. doi: 10.1111/j.1432-1033.1981.tb06171.x. [DOI] [PubMed] [Google Scholar]
- 32.Studier F W, Moffat B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 33.Sutrina S L, Reddy P, Saier M H, Jr, Reizer J. The glucose permease of Bacillus subtilis is a single polypeptide chain that functions to energize the sucrose permease. J Biol Chem. 1990;265:18581–18589. [PubMed] [Google Scholar]
- 34.Vadeboncoeur C, Brochu D, Reizer J. Quantitative determination of the intracellular concentration of the various forms of HPr, a phosphocarrier protein of the phosphoenolpyruvate:sugar phosphotransferase system in growing cells of oral streptococci. Anal Biochem. 1991;196:24–30. doi: 10.1016/0003-2697(91)90112-7. [DOI] [PubMed] [Google Scholar]
- 35.Vadeboncoeur C, Pelletier M. The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol Rev. 1997;19:187–207. doi: 10.1111/j.1574-6976.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 36.van der Vlag J, Postma P W. Regulation of glycerol and maltose uptake by the IIAGlc-like domain of IINag of the phosphotransferase system in Salmonella typhimurium LT2. Mol Gen Genet. 1995;248:236–241. doi: 10.1007/BF02190806. [DOI] [PubMed] [Google Scholar]
- 37.van der Vlag J, van Dam K, Postma P W. Quantification of the regulation of glycerol and maltose metabolism by IIAGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1994;176:3518–3526. doi: 10.1128/jb.176.12.3518-3526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Dijk A A, de Lange L C M, Bachovchin W W, Robillard G T. Effect of phosphorylation on hydrogen-bonding interactions of the active site histidine of the phosphocarrier protein HPr of the phosphoenolpyruvate-dependent phosphotransferase system determined by 15N NMR spectroscopy. Biochemistry. 1990;29:8164–8171. doi: 10.1021/bi00487a026. [DOI] [PubMed] [Google Scholar]
- 39.Vogler A P, Broekhuizen C P, Schuitema A, Lengeler J W, Postma P W. Suppression of IIAGlc-defects by enzymes IINag and IIBgl of the PEP:carbohydrate phosphotransferase system. Mol Microbiol. 1988;2:719–726. doi: 10.1111/j.1365-2958.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 40.Worthylake D, Meadow N D, Roseman S, Liao D-I, Herzberg O, Remington S J. Three-dimensional structure of the Escherichia coli phosphocarrier protein IIIGlc. Proc Natl Acad Sci USA. 1991;88:10382–10386. doi: 10.1073/pnas.88.23.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yazyu H, Niiya S S, Shimamoto T, Kanazawa H, Futai M, Tsuchiya T. Nucleotide sequence of the melB gene and characterization of deduced amino acid sequence of the melibiose carrier in Escherichia coli. J Biol Chem. 1984;259:4320–4326. [PubMed] [Google Scholar]