Abstract
Impaired endothelial insulin signaling and consequent blunting of insulin-induced vasodilation is a feature of type 2 diabetes (T2D) that contributes to vascular disease and glycemic dysregulation. However, the molecular mechanisms underlying endothelial insulin resistance remain poorly known. Herein, we tested the hypothesis that endothelial insulin resistance in T2D is attributed to reduced expression of heat shock protein 72 (HSP72). HSP72 is a cytoprotective chaperone protein that can be upregulated with heating and is reported to promote insulin sensitivity in metabolically active tissues, in part via inhibition of JNK activity. Accordingly, we further hypothesized that, in individuals with T2D, 7 days of passive heat treatment via hot water immersion to waist level would improve leg blood flow responses to an oral glucose load (i.e., endogenous insulin stimulation) via induction of endothelial HSP72. In contrast, we found that: 1) endothelial insulin resistance in T2D mice and humans was not associated with reduced HSP72 in aortas and venous endothelial cells, respectively; 2) after passive heat treatment, improved leg blood flow responses to an oral glucose load did not parallel with increased endothelial HSP72; and 3) downregulation of HSP72 (via small-interfering RNA) or upregulation of HSP72 (via heating) in cultured endothelial cells did not impair or enhance insulin signaling, respectively, nor was JNK activity altered. Collectively, these findings do not support the hypothesis that reduced HSP72 is a key driver of endothelial insulin resistance in T2D but provide novel evidence that lower-body heating may be an effective strategy for improving leg blood flow responses to glucose ingestion-induced hyperinsulinemia.
Keywords: endothelial insulin resistance, heat shock protein 72, insulin signaling, leg blood flow, passive heating
INTRODUCTION
The prevalence and incidence of type 2 diabetes (T2D) are growing in the United States and worldwide (1, 2), with the number of US adults diagnosed with T2D expected to nearly triple by 2060 (3). Notably, individuals with T2D are at increased risk of developing and subsequently dying from cardiovascular disease (4, 5). Endothelial cell dysfunction plays an important role in the pathogenesis of cardiovascular disease in T2D (6–9). Particularly, impaired endothelial insulin signaling through the PI3K-Akt pathway and consequent blunting of insulin-induced vasodilation and blood flow, also referred to as selective endothelial insulin resistance (9, 10), contributes to vascular disease and glycemic dysregulation (6, 7, 10, 11). Indeed, genetic disruption of insulin signaling in endothelial cells promotes atherosclerosis (12, 13) and limits skeletal muscle glucose uptake (14), while conversely, selective activation of the insulin receptor-PI3K-Akt signaling pathway protects against atherosclerosis formation (15). Notwithstanding the unquestionable recognition that vascular insulin resistance is implicated in the pathogenesis of cardiovascular and metabolic diseases, the molecular underpinnings underlying endothelial insulin resistance in T2D remain poorly known. A deeper understanding of such molecular mechanisms can help identify new strategies for the prevention and treatment of T2D-associated vasculometabolic derangements.
Heat shock protein 72 (HSP72) is a heat-inducible cytoprotective chaperone protein whose constitutive expression appears to be suppressed in obesity and T2D, particularly in metabolically active tissues (16–18). Importantly, deficiency of HSP72 promotes insulin resistance (17, 19) and these effects are likely mediated through activation of c-Jun amino-terminal kinase (JNK) (17, 19–23), a classic stress-activated kinase shown to disrupt the PI3K-Akt insulin-signaling pathway (24–26). However, the role of HSP72 in modulating insulin signaling in the vasculature remains unknown. Herein, we hypothesized that endothelial insulin resistance in T2D can be attributed to reduced expression of HSP72.
Accumulating evidence indicates that chronic passive heating is therapeutically effective in treating and preventing cardiometabolic diseases (27–36). Along these lines, we recently showed that one bout of lower-limb heating subsequently increases insulin-stimulated leg blood flow in healthy individuals (37). It is possible that the multifaceted beneficial effects of passive heating, including its insulin-sensitizing actions, are at least in part driven by induction of HSP72. As such, it can be reasoned that passive heating may be an effective strategy for restoring HSP72 expression in T2D and thus correcting endothelial insulin resistance. Specifically, we hypothesized that, in individuals with T2D, passive heat treatment via hot water immersion would improve leg blood flow responses to an oral glucose load (i.e., endogenous insulin stimulation), and that this improvement would be accompanied by an induction of HSP72 in endothelial cells. Congruently, we also posited that knockdown of HSP72 in endothelial cells would impair insulin signaling and that the converse would also be true; that is, induction of HSP72 in endothelial cells would enhance insulin signaling.
METHODS
Experimental Protocol in Isolated Arteries from Mice
All animal procedures were approved by the University of Missouri Institutional Animal Care and Use Committee. The University of Missouri is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Isolated aortic rings were used from 20-wk-old control db+ (n = 12, 32.5 ± 0.73 g body wt) and diabetic db/db (n = 10, 58.1 ± 1.02 g body wt) male mice (Strain No. 000642; The Jackson Laboratory, Bar Harbor, ME). Males were used because of availability. Aortas were harvested and cleaned of perivascular adipose tissue in ice-cold physiological saline solution (pH 7.4) and cut into 2-mm segments. Abdominal aortic rings were then mounted on wire myograph organ bath chambers (620M; Danish Myo Technology, Hinnerup, Denmark) containing warmed physiological saline solution gassed with 95% O2-5% CO2 and maintained at 37°C as previously described (38, 39). Aortic rings were treated with 80 mM KCl to ensure viability. Next, aortas were preconstricted with the thromboxane A2-mimetic, U-46619 (20 nM) to test vasorelaxation responses to increasing insulin concentrations (10−9 to 10−5 M, Humulin R; Eli Lilly, Indianapolis, IN). The remaining aorta was flash-frozen and subsequently processed for analysis of HSP72 and phospho-JNK via Western blotting.
Experimental Protocol in Human Subjects
Human participants.
The study was approved by the University of Missouri Institutional Review Board (IRB, No. 2008181), registered at ClinicalTrials.gov (NCT03203694), and conducted in accordance with the Declaration of Helsinki. Subjects with a self-reported clinical diagnosis of noninsulin-dependent T2D (n = 20), along with age- and sex-matched healthy subjects (n = 20), were recruited from the Columbia, MO area. All subjects provided written informed consent and completed a medical health history questionnaire before participating in the study. All subjects were 35–65 yr old and free of overt cardiovascular, renal, hepatic, autoimmune diseases, cancers, exogenous insulin use, immunosuppressant therapies, gout, diabetic neuropathy, tobacco or nicotine use, excessive alcohol consumption (>14 drinks/wk for men, and >7 drinks/wk for women), pregnancy or nursing, and mobility limitations. Furthermore, subjects with T2D were also excluded if they had a body mass index (BMI) of ≥50 kg/m2, self-reported participating in more than 60 min of exercise per week, had uncontrolled hypertension (≥180 mmHg systolic, or ≥110 mmHg diastolic), or had any fungal infections or disorders. In addition, based on manufacturer contraindications for the sensor used for measuring core temperature during the heating sessions, subjects were excluded if they presented with any of the following: swallowing or esophageal disorders, gag-reflex impairment, gastrointestinal tract diseases or disorders, any previous gastrointestinal surgeries, any implanted electromedical device, or any scheduled nuclear magnetic resonance/magnetic resonance imaging scanning unrelated to the study. Healthy subjects were also excluded if they had a BMI outside of the 19–29 kg/m2 range, a history of prediabetes or diabetes mellitus, self-reported participating in <150 min of moderate-intensity exercise per week, had hypertension (≥130 mmHg systolic, or ≥90 mmHg diastolic) or were taking any antihypertensive medications. The use of non-obese, physically active adults as healthy control subjects allowed us to characterize the optimal vascular and metabolic phenotype to be used for reference. This idea that control subjects should be physically active has been persuasively advocated by Booth et al. (40–44). Five women (n = 2 from T2D group, n = 3 from healthy group) were premenopausal and had their experimental visits scheduled during the early follicular phase (days 1–7 of the menstrual cycle) or oral contraceptive placebo week (if applicable) to minimize any potential impact of hormonal fluctuations across the menstrual or oral contraceptive cycle on metabolic and vascular outcomes. Therefore, ∼28 days elapsed between experimental visits for these participants.
Experimental design.
Healthy subjects participated in a single experimental visit. Subjects with T2D participated in two experimental visits, one occurring before and the other after seven consecutive days of passive heating sessions. The second experimental visit occurred 16–24 h after their last passive heating session, as previously described in other passive heating studies (45). Such short-term course of treatment (i.e., 7 days) was purposely used to examine the vascular effects of heating before overt improvements in metabolic function, known to occur with longer heating interventions (36, 46, 47), which could in turn lead to secondary vascular effects. That same strategy has been used in exercise studies with individuals with T2D using similar outcomes (48).
The 7-day lead up to experimental visits.
Before the experimental visits, subjects wore an accelerometer for seven consecutive days. Subjects with T2D also wore an accelerometer during the seven consecutive days of passive heating sessions before their second experimental visit. All subjects filled out a 3-day food diary before an experimental visit. Subjects with T2D were given a copy of their food intake record and instructed to replicate the meals and timing of food intake, as much as possible, for the three days before their second experimental visit.
Experimental visit.
Participants arrived at the laboratory after an overnight fast. Subjects refrained from medications the morning of testing and also abstained from exercise for 24–48 h, caffeine for 12 h, and alcohol for 24 h before testing. Subjects underwent anthropometric measurements including height, weight, and body composition via dual-energy X-ray absorptiometry (HorizonA, Hologic Inc., Bedford, MA). After 15 min of supine rest in a darkened, temperature-controlled room (∼21°C), aortic stiffness via carotid-to-femoral pulse-wave velocity (cfPWV) and femoral artery endothelial function via flow-mediated dilation (FMD) were assessed to further characterize vascular function. An intravenous catheter was then placed in an antecubital vein for blood sampling and collection of venous endothelial cells. Thereafter, subjects underwent an oral glucose tolerance test to assess leg blood flow responses to endogenous insulin stimulation (i.e., a physiological readout of vascular insulin sensitivity).
Experimental Measurements
Physical activity.
Subjects were fitted with an accelerometer (ActiGraph GTX3; ActiGraph, Pensacola, FL) on the right hip to record physical activity. Accelerometers collected data at a rate of 30 Hz for seven consecutive days. Subjects were instructed to wear the accelerometer upon waking until just before sleeping and were given a diary to note what periods they wore the accelerometer and any reason for taking the device off (e.g., sleeping, bathing, etc.).
Recorded data were downloaded over 60-s epochs and analyzed with the manufacturer’s software (ActiLife v6.13.3, ActiGraph, Pensacola, FL). ActiLife wear-time validation was performed and, subsequently, the amount of time spent in moderate-vigorous physical activity, light physical activity, and sedentary activity per week was calculated. Moderate-vigorous physical activity, light physical activity, and sedentary activity were determined by the ActiLife software using device counts per minute and cut-off thresholds for adults published by Freedson et al. (49). Current physical activity guidelines for Americans use time spent in moderate-vigorous physical activity as the metric to guide and promote health benefits (50). Healthy subjects wore the accelerometer for 14 ± 0.4 h/day over 7 ± 0 days. Before the 7-day heating intervention, subjects with T2D wore the accelerometer for 12 ± 0.3 h/day over 6.9 ± 0.1 days, and during the intervention period wore the accelerometer for 11 ± 0.3 h/day over 7 ± 0 days.
Aortic stiffness via carotid-to-femoral pulse-wave velocity.
cfPWV was measured using the cuff-based SphygmoCor XCEL (AtCor Medical, Itasca, IL) to assess aortic stiffness, according to current recommendations and as previously described (51, 52). Briefly, the SphygmoCor XCEL device enables simultaneous acquisition of carotid (via tonometer) and femoral (via cuff) pulse waves. Transit time between carotid and femoral pressure waves was calculated using the foot-to-foot method. Wave foots were identified using intersecting tangent algorithms. cfPWV (reported in m/s) was calculated as distance traveled by the pulse wave divided by pulse transit time. For nine subjects with T2D, the carotid pulse wave signal was of insufficient quality and thus cfPWV data could only be generated for a subset of the subjects.
Femoral artery endothelial function via FMD.
FMD in the superficial femoral artery was performed via two-dimensional (2-D)/Doppler ultrasound (GE Logiq P5) according to published guidelines (53) and as previously described (54–56). Two minutes of arterial diameter and velocity were recorded using an 11-MHz linear array transducer. Signals were obtained in duplex mode at a pulsed frequency of 5 MHz and corrected with an insonation angle of 60°. Sample volume was adjusted to encompass the entire lumen of the vessel without extending beyond the walls, and the cursor was set at midvessel and parallel to the vessel wall. A cuff placed on the calf muscle was then inflated to a pressure of 250 mmHg for 5 min. Continuous diameter and blood velocity measures were recorded during this occlusion period and 3 min following cuff deflation. For subjects with T2D, the ultrasound probe placement was marked on the skin, markings were scaled against a tape measure, and a picture was taken to ensure consistency between visits.
Video recordings of the ultrasound images were obtained with real-time capture software (Elgato Video Capture, Elgato, CA), later exported and analyzed using an automated wall detection software (Cardiovascular Suites 4, Quipu Srl, Pisa, Italy). FMD percent change was calculated as [(peak diameter − base diameter)/base diameter] × 100. Shear rate, an estimate of shear stress without blood viscosity, was calculated as 4 × mean blood velocity/diameter. Postocclusion hyperemic shear rate total area under the curve (AUC) up to 60 s was also calculated and used an index of reactive hyperemia (57, 58). For five subjects with T2D, the borders of the arterial wall in the 2-D ultrasound image were not of sufficient resolution for accurate diameter measurements (confirmed by an independent investigator) and thus these videos were not processed for final FMD analysis.
Venous endothelial cell collection and processing.
After creating a sterile field, a 20-gauge intravenous catheter was placed in an antecubital vein using aseptic techniques. A 0.18 in. spring-wire J-tip guidewire (AW-16402, Arrow International, Reading, PA) was advanced ∼2–5 in. through the catheter, moved back and forth 10 times to collect endothelial cells. The wire was cut and placed into a conical tube containing a disassociation buffer. This process was repeated for a total of four guidewires. Intravenous catheter placement was not possible in two subjects with T2D.
The collected wires were rinsed with dissociation buffer for 10 min using a motorized serological pipette. Red blood cell lysis buffer (eBioscience 1× RBC Lysis Buffer No. 00-4333-57, Invitrogen, Thermo Fisher Scientific, Carlsbad, CA) was added to the collected cells for 10 min at room temperature. Cells were then centrifuged, washed, and subsequently fixed in pellet form with 4% paraformaldehyde (PFA, No. 15710, Electron Microscopy Sciences) for 10 min at room temperature. Fixed cells were washed, centrifuged, resuspended, and 10 μL of fixed cell suspension was plated per well onto a 15-well chambered microscope slide (μ-Slide Angiogenesis ibiTreat No. 81506, Ibidi GmbH, Gräfelfing, Germany) pretreated with poly-l-lysine solution (P4832, Sigma Aldrich, St. Louis, MO). Slides were then incubated at 37°C for 5 h to allow cells-to-slide adherence and then rinsed with 50 mM glycine/PBS solution to quench any remaining PFA. Slides were stored at −80°C until further analysis of proteins of interest via quantitative immunofluorescence staining.
Quantitative immunofluorescence staining of venous endothelial cells.
Slides were thawed and rehydrated with PBS for 10 min. Slides were then incubated with permeabilization buffer (0.05% Triton X-100 + 2% BSA + PBS) and then blocking buffer (goat serum + 0.05% Triton X-100 + 1% BSA + PBS) for 1 h each at room temperature. Slides were incubated overnight at 4°C with primary antibodies for the following targets: von Willebrand factor (vWF) (1:1,000; Invitrogen, No. PA1-43057), phospho-JNK [Thr183/Tyr185 (1:100; Cell signaling No. 9255)], JNK (1:100; Cell Signaling, No. 9252), and HSP72 (1:50; Enzo Life Sciences No. ADI-SPA-810). Slides were incubated the following day with corresponding fluorophore-conjugated secondary antibodies and 4′,6-diamidino-2-phenylindole (DAPI) (1:500 or 1:1,000; Sigma Aldrich, No. D9542) for 1 h at room temperature. The fluorophore-conjugated secondary antibodies used were Alexa Fluor 488 (1:2,000; Abcam No.150177), Alexa Fluor Plus 555 (1:250; Invitrogen No. A32727; or 1:500; A32732), and Alexa Fluor 633 (1:500; Invitrogen No. A21071).
Each multiwell-chambered slide accommodated the simultaneous probing of phospho-JNK, JNK, and HSP72. Phospho-JNK and JNK were probed for within the same wells. All wells were probed for DAPI and vWF. DAPI identified intact cell nuclei, whereas vWF was used to identify endothelial cells with intact cell membranes. Staining of slides was performed in several batches. Each batch contained four slides: one slide from a healthy subject, two slides from a subjects T2D (before and after their heating intervention), and one slide containing human umbilical vein endothelial cells (HUVECs, No. CC-2519, Lonza, Walkersville, MD) at passage four used for normalization (59, 60). Slides were imaged on an ×63 oil objective (1.4 numerical aperture) using a Leica Thunder Imager (Leica Microsystems, Wetzlar, Germany). Images were captured at the same exposure time and corrected for background fluorescence using Leica Thunder software. The mean fluorescence intensity per unit area of our proteins of interest was calculated from 27 ± 2 cells/subject and normalized to the respective mean fluorescence intensity from HUVECs (simultaneously stained) to account for any batch effect. The fluorescence intensity was analyzed using a custom MATLAB script that automated the identification of positively stained endothelial cells (i.e., only signal from endothelial cells was used for analysis). Cells with a compromised membrane and considered not spherical by the script’s algorithm were excluded from the analysis. The imaging and fluorescence intensity analysis was completed by an investigator blinded to the characteristics of subjects and treatments.
Leg blood flow responses to glucose ingestion.
After a 40-min period of supine rest, a 75-g oral glucose load (No. 100075, Azer Scientific, Morgantown, PA) was ingested within 5 min. Before and during the postprandial state (15, 30, 45, 60, 90, and 120 min after ingestion), blood samples were collected from the intravenous catheter, and superficial femoral artery blood flow was assessed via 2-D/Doppler ultrasound. Each blood flow recording period was at least 4 min long. At these time points, brachial artery blood pressure readings were also collected in duplicate (i.e., immediately before and after the blood flow recording) using an automated blood pressure monitor (SunTech Tango M2, SunTech Medical, Morrisville, NC). Blood flow (reported in mL/min) was calculated as: 3.14 × [diameter (cm)/2]2 × mean blood velocity (cm/s) × 60. Mean arterial pressure (MAP) (reported in mmHg) was calculated as: (2 × diastolic pressure + systolic pressure)/3. Leg vascular conductance was calculated as leg blood flow/MAP. Throughout, blood samples were placed in EDTA vacutainers and immediately analyzed for glucose using the YSI 2300 STAT PLUS glucose analyzer (YSI Inc., Yellow Springs, OH) or frozen for later determination of glycosylated hemoglobin (HbA1c) at the University of Missouri Diabetes Diagnostic Lab. The remaining blood samples were centrifuged (4°C, 3,500 rpm, for 15 min), and the plasma was aliquoted and stored at −80°C for later analysis of insulin levels using a commercially available enzyme-linked immunosorbent assay (No. 80-INSHU-E10.1, ALPCO, Salem, NH). Postprandial glucose and insulin incremental AUC were calculated with the trapezoidal method over the 2-h period. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as: [fasting glucose (mg/dL) × fasting insulin (μU/mL)]/405.
Passive heating sessions.
Subjects were instructed to maintain their usual physical activity, dietary habits, and medication intake during the 7 days of the heating intervention. At least 7–9 h before arriving for their first and last session, the subject ingested a telemetry sensor (CorTemp Temperature Sensor HT150002, HQ, Inc., Palmetto, FL) to measure core temperature (reported in °C). This sensor emits radio frequencies picked up by the receiver (HQinc, Palmetto, FL), placed near the lumbosacral region. Before each heating session, subjects provided a urine sample to assess hydration status using urine specific gravity (USG) readings from a digital refractometer (PAL-10S, Atago Co., Ltd., Japan). If the USG reading was >1.02, indicative of dehydration, the subject drank 500 mL of water. After the subject rested for 5 min in a semirecumbent chair, tympanic membrane temperature (TMT) was assessed with an infrared digital thermometer (Braun ThermoScan 5 IRT6500, Kaz USA, Inc.) and brachial artery blood pressure was assessed with an automated blood pressure monitor (SunTech Tango M2). After nude body weight was recorded using a digital scale (Patient Aid Scale PA-550XL, Patient Aid LLC), subjects were also fitted with a heart rate monitor (Polar H10, Polar Electro Oy, Finland). Nude body weight and TMT were recorded for safety monitoring purposes only, hence these data are not reported.
Subjects were immersed at waist level in 40.5°C water for 60 min in an inflatable hot tub (GoRelax inflatable hot tub PH050013, Shanghai Sunshine Development Co., Ltd., Shanghai, China), every day for 7 days. This modality of passive heating has been shown to increase core temperature (61), a stimulus known to upregulate HSP72 expression (62). Every 5 min during immersion, core temperature (on first and last session), TMT, rating of perceived exertion (RPE) using the Borg’s 6–20-point scale (63), thermal sensation using the 13-point McGinnis categorical scale (64), and signs/symptoms were recorded. Heart rate and blood pressure were recorded every 10 min. For comfort, subjects were allowed to rest their arms on the sides of the hot tub and out of the water. Also for comfort, a box fan continuously blew over them. During immersion, subjects were given a 500-mL bottle of water to drink ad libitum.
After the 60-min water immersion, subjects sat in a semirecumbent chair for a 10-min cool-down period, during which the fan continued to blow over them. Signs and symptoms, vitals, and TMT remained monitored during the cool-down. Next, subjects recorded a postimmersion nude body weight. If there was a 1% loss in nude body weight between pre- and postimmersion, subjects consumed 500 mL of water before leaving the facility. To document any heat acclimation, mean whole body sweat rate (reported as L/h) for each session was calculated by taking the difference in dry nude body weight between pre- and postimmersion and correcting for water intake, as previously described (32, 46).
Experimental Protocol in Human Skeletal Muscle Microvascular Endothelial Cells in Culture
Human skeletal muscle microvascular endothelial cells (hSMMECs, No. H-6220; Cell Biologics; Chicago, IL) were cultured in complete VascuLife EnGS medium with 10% FBS. Cells were maintained in a humidified incubator at 37°C and 5% CO2 unless otherwise stated. Experiments occurred when cells were at passage four on 60-mm dishes and ∼90% confluency unless otherwise stated.
Small interfering-RNA knockdown of HSP72 and insulin stimulation.
hSMMECs were passaged onto 6-well plates and maintained in VascuLife EnGS medium with 10% FBS containing growth factors and no antibiotics. Twenty-four hours after plating, cells were switched to VascuLife EnGS medium with 1% FBS containing growth factors and no antibiotics. At ∼70%–80% confluency, cells were transfected for 6 h with small interfering-RNA (siRNA) targeting HSP72 (20 nM; No. sc-29352, Santa-Cruz Biotechnology, Santa-Cruz, CA) or siRNA-scramble (20 nM; siRNA-control A No. sc-37007, Santa-Cruz Biotechnology) with Lipofectamine RNAiMAX transfection reagent (No. 13778150, Invitrogen, Life Technologies, Corp, Carlsbad, CA) complexed in Opti-MEM reduced serum medium (No. 11058021, Gibco, Grand Island, NY). After the transfection period, media was replaced with VascuLife EnGS medium with 1% FBS containing growth factors and no antibiotics. Cells recovered for 48 h, and this transfection protocol and recovery were repeated. After the second recovery period, cells were incubated with versus without insulin (100 nM; Humulin R; Eli Lilly, Indianapolis, IN) for 30 min and collected.
Heating to overexpress HSP72 and insulin stimulation.
Twenty-four hours before initiation of the experiment, hSMMECs were serum starved in complete VascuLife EnGS medium with 0.5% FBS. Cells were then placed in a water-jacketed incubator set to 40.5°C and 5% CO2, or kept at 37°C and 5% CO2 (sham-treated), for 60 min, over five consecutive days. Treatments co-occurred in two adjacent incubators. After the 60-min heat exposure, cells were placed back in the 37°C incubator (with the sham-treated cells) and allowed to recover. After a 60-min recovery period, cell culture media for all dishes was refreshed. Twenty-four hours after the last heat/sham treatment (day 5), cells were incubated with versus without 100 nM insulin for 30 min and collected.
Exposure to a glucolipotoxic milieu and insulin stimulation.
Twenty-four hours before initiation of the experiment, hSMMECs were serum-starved in complete VascuLife EnGS medium with 0.5% FBS. Cells were then treated with a glucolipotoxic milieu or osmotic vehicle solution for 24 h and subsequently incubated with versus without 100 nM insulin for 30 min and collected. The glucolipotoxic milieu was used as an approach to suppress HSP72 and overall Akt activity. The glucolipotoxic milieu contained complete VascuLife EnGS medium with 0.5% FBS, 30 mM d-glucose (24 mM added to the 6 mM present in the basal medium), and 0.1 mM palmitic acid-BSA conjugate (1 mM:0.25 mM; 4:1 molar ratio complex). The osmotic vehicle solution contained complete VascuLife EnGS medium with 0.5% FBS, 24 mM d-mannitol, and 0.1 mM BSA-vehicle (0.25 mM BSA).
Heating to restore expression of HSP72 in hSMMECs exposed to the glucolipotoxic milieu and insulin stimulation.
Twenty-four hours before initiation of the experiment, cell culture media was switched to media containing the glucolipotoxic milieu. Cells were then placed in a water-jacketed incubator set to 40.5°C and 5% CO2, or kept at 37°C and 5% CO2 (sham treated), for 60 min. Treatments co-occurred in two adjacent incubators. After the 60-min heat exposure, cells were placed back in the 37°C incubator (with the sham-treated cells) and allowed to recover. After a 60-min recovery period, the media containing the glucolipotoxic milieu was refreshed. Twenty-four hours after the heat/sham treatment, cells were incubated with versus without 100 nM insulin for 30 min and collected. Only a single bout of heating was used for this experiment, rather than the 5-day paradigm, because exposure to the glucolipotoxic milieu for 5 days compromised cell viability (data not shown).
Cell lysate collection after insulin stimulation.
Immediately after the 30-min insulin stimulation, hSMMECs were washed with cold, sterile PBS and lysed in RIPA buffer (R0278, Millipore Sigma, St. Louis, MO) supplemented with EDTA and protease and phosphatase inhibitors. Cell lysates were collected via cell scraping and were subsequently sonicated and centrifuged. The supernatant was collected after the centrifugation and stored at −80°C for subsequent Western blotting.
Western blotting.
Cell lysates were prepared in 2× Laemmli buffer (No. 1610737, Bio-Rad Laboratories, Inc.) following protein quantification using Pierce BCA protein assay. Prepared protein samples (3–5 µg/lane) were separated in Criterion Tris-Glycine-eXtended Stain-Free precast gels (No. 5678085, Bio-Rad Laboratories, Inc.). Proteins were transferred overnight onto polyvinylidene difluoride membranes and blocked with 5% BSA for 1 h at room temperature. Membranes were probed overnight at 4°C for: phospho-Akt (Ser473) (1:1,000; Cell Signaling, No. 4060), Akt (1:1,000; Cell Signaling, No. 9272), HSP72 (1:1,000; Enzo Life Sciences, No. ADI-SPA-810), phospho-JNK (T183/Y185) (1:1,000; Cell Signaling No. 9251), JNK (1:1,000; Cell Signaling, No. 9252), and GAPDH (1:1,000; Cell Signaling, No. 5174). Secondary antibodies were applied the following day for 1 h at room temperature, which included: anti-rabbit (1:2,000; Bio-Rad No. 1705046) and anti-mouse (1:2,000; Cell Signaling, No. 7076). Blots were imaged (Bio-Rad ChemiDoc XRS+ System, Bio-Rad, Hercules, CA). The intensity of individual protein bands was quantified via densitometry using Image Lab Software (v.6.0.1 build 35, Bio-Rad, Laboratories, Inc.). Proteins of interest were normalized to GAPDH expression. Values are expressed as fold differences.
Statistical Analyses
GraphPad Prism (v.9.3.0, GraphPad Software LLC, La Jolla, CA) was used for statistical analysis. Statistical comparisons were performed by two-tailed t test, or analysis of variance (ANOVA), as appropriate, followed by Bonferroni adjusted pair-wise comparisons when significant interactions were found. No sex differences were detected on outcome variables, therefore, data from male and female subjects were pooled for analysis. Individual responses and means ± SE are presented, as appropriate. For all statistical tests, significance was accepted at P < 0.05.
RESULTS
Endothelial Insulin Resistance in T2D Mice and Humans is Not Associated with Reduced HSP72 in Aortas and Venous Endothelial Cells, Respectively
As displayed in Fig. 1A, using wire myography, aortic rings from diabetic db/db mice exhibited suppressed insulin-induced relaxation relative to aortic rings from control db+ mice (interaction P = 0.002). Impaired insulin-induced relaxation in aortic rings from db/db mice was not accompanied with a reduction in HSP72 content (P = 0.81, Fig. 1B) nor with an elevation in phospho-JNK (in fact, there was a strong trend for its reduction, P = 0.05, Fig. 1C) in aortic tissues.
Figure 1.
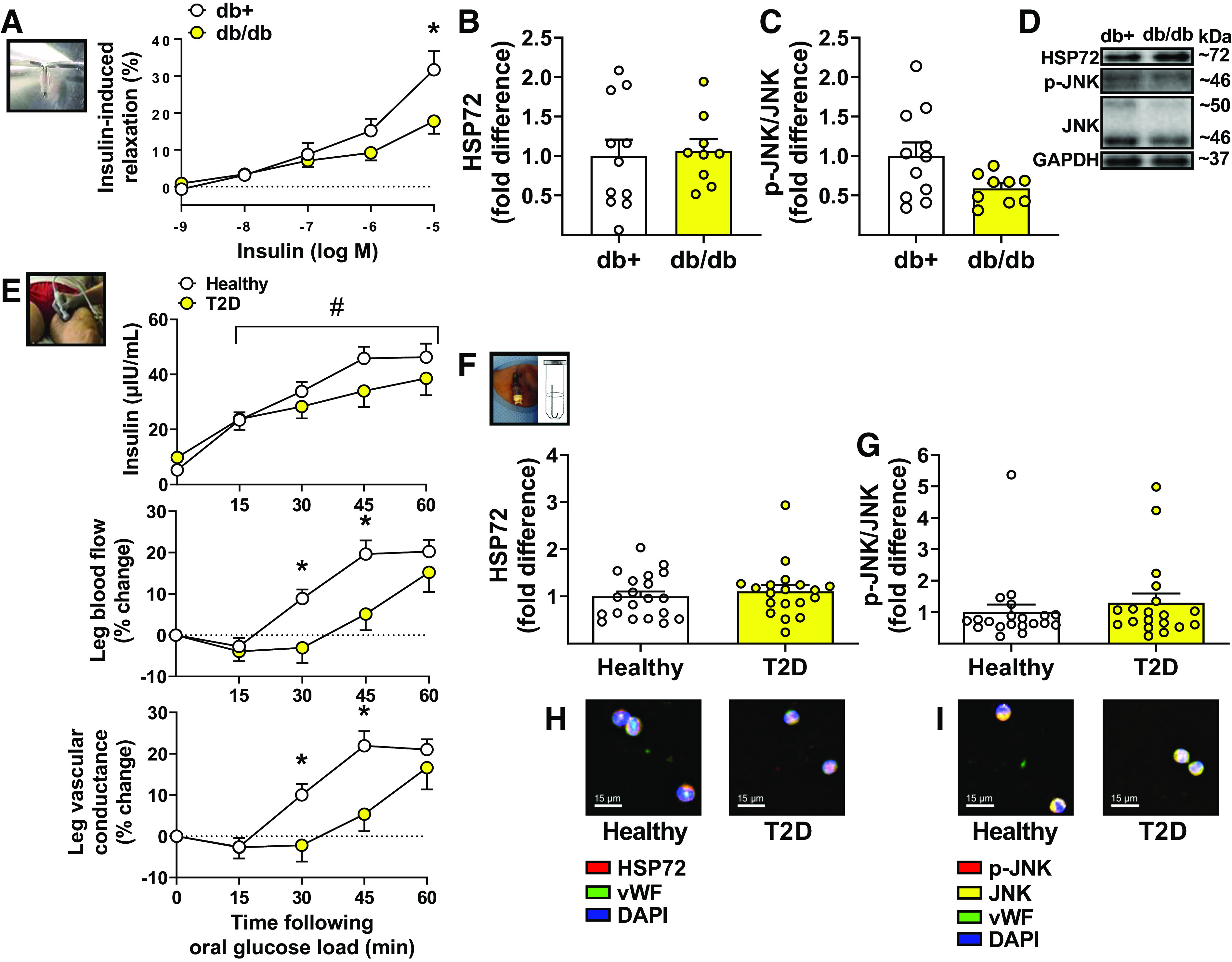
Endothelial insulin resistance in type 2 diabetes (T2D) mice and humans is not associated with reduced heat shock protein 72 (HSP72) in aortas and endothelial cells, respectively. A: insulin-induced relaxation, via wire-myography, in aortic rings collected from male db+ and db/db mice (db+ n = 11; db/db n = 7). Two-way ANOVA with repeated-measures and Bonferroni post hoc test. HSP72 (B) and phospho-c-Jun amino-terminal kinase (JNK)/JNK protein content (C) in aortas from male db+ and db/db mice, measured by Western blotting (db+ n = 11; db/db n = 9). Unpaired two-tailed Student’s t test, respectively. D: representative Western blot bands for proteins of interest in mouse aortas. E: plasma insulin concentration (top; healthy n = 20; T2D n = 18), relative leg blood flow (percent change from baseline) (middle; healthy n = 20; T2D n = 20), and relative leg vascular conductance (percent change from baseline) (bottom; healthy n = 20; T2D n = 20) in response to an oral glucose load (75 g) in healthy and T2D subjects. Two-way ANOVA with repeated-measures and Bonferroni post hoc test. HSP72 (F) and phospho-JNK/JNK protein content (G) in venous endothelial cells collected from healthy and T2D subjects, measured by quantitative immunofluorescence intensity (healthy n = 20; T2D n = 19). Unpaired two-tailed Student’s t test, respectively. H and I: representative images of quantitative immunofluorescence intensities of HSP72 and phospho-JNK/JNK, respectively. Means ± SE and individual data, when appropriate, are reported. In A and E (middle and bottom), *significance using Bonferroni-adjusted pairwise comparisons (P < 0.05). In E (top), #significant main effect of time (P < 0.05). vWF, von Willebrand factor.
Similarly, as shown in Fig. 1E, individuals with T2D exhibited a blunted leg blood flow response (interaction P = 0.005) to an oral glucose load and concomitant acute hyperinsulinemia compared with healthy subjects, notably between 30 and 45 min after glucose ingestion. Blood flow responses beyond 60 min after ingestion were not different between groups (at 90 min: healthy subjects, 23.6 ± 4.2%, subjects with T2D, 19.2 ± 6.6%, P = 0.58; at 120 min: healthy subjects, 25.2 ± 5.9%, subjects with T2D, 25.1 ± 6.9%, P > 0.99). Impaired blood flow responses to the oral glucose load in T2D, suggestive of vascular insulin resistance, were not paralleled with a reduction in HSP72 content (P = 0.52, Fig. 1F) nor with an elevation in phospho-JNK (P = 0.43, Fig. 1G) in endothelial cells. Demographic information and subject characteristics are summarized in Table 1. As expected, relative to healthy controls, individuals with T2D also exhibited increased aortic stiffness as assessed via cfPWV (P < 0.001, Table 1) and impaired endothelial function in the femoral artery as assessed via FMD (P < 0.001, Table 2).
Table 1.
Subject characteristics, anthropometrics, hemodynamic measurements, and blood profile parameters in healthy subjects and subjects with type 2 diabetes
| Healthy Subjects | Subjects With T2D | P Value | n (Healthy/T2D) | |
|---|---|---|---|---|
| Age, yr | 52 ± 2.1 | 53 ± 1.9 | 0.62 | 20/20 |
| Sex (Female/Male) | (12/8) | (12/8) | 20/20 | |
| Race, n, % | ||||
| Asian | (0, 0%) | (0, 0%) | 0/0 | |
| Black | (0, 0%) | (5, 25%) | 0/5 | |
| Caucasian/Hispanic | (4, 20%) | (0, 0%) | 4/0 | |
| Caucasian/Non-Hispanic | (16, 80%) | (15, 75%) | 16/15 | |
| Other | (0, 0%) | (0, 0%) | 0/0 | |
| Height, cm | 168 ± 2.5 | 170 ± 1.9 | 0.58 | 20/20 |
| Weight, kg | 67.8 ± 2.9 | 104.0 ± 4.1 | <0.001 | 20/20 |
| BMI, kg/m2 | 23.7 ± 0.5 | 35.9 ± 1.2 | <0.001 | 20/20 |
| Body fat, % | 30.0 ± 1.7 | 39.8 ± 1.8 | <0.001 | 20/20 |
| Lean mass, kg | 48.3 ± 2.7 | 63.0 ± 3.1 | <0.001 | 20/20 |
| VAT, kg | 0.33 ± 0.04 | 0.83 ± 0.07 | <0.001 | 20/20 |
| Android/gynoid ratio | 0.82 ± 0.03 | 1.07 ± 0.03 | <0.001 | 20/20 |
| Systolic BP, mmHg | 116 ± 2 | 129 ± 3 | <0.001 | 20/20 |
| Diastolic BP, mmHg | 72 ± 2 | 79 ± 2 | 0.005 | 20/20 |
| MAP, mmHg | 87 ± 1 | 96 ± 2 | 0.003 | 20/20 |
| cfPWV, m/s | 6.1 ± 0.2 | 8.5 ± 0.5 | <0.001 | 20/11 |
| Fasted leg blood flow, mL/min | 153 ± 9.3 | 186 ± 19.6 | 0.14 | 20/20 |
| Fasted blood glucose, mg/dL | 79.3 ± 1.2 | 106.0 ± 6.9 | <0.001 | 20/18 |
| Postprandial glucose 2 h iAUC, mg/dL·min | 6,045 ± 560 | 8,633 ± 1016 | 0.03 | 20/18 |
| Fasted insulin, μIU/mL | 5.3 ± 0.6 | 9.8 ± 1.7 | 0.01 | 20/18 |
| Postprandial insulin 2 h iAUC, μIU/mL·min | 3,802 ± 457 | 3,106 ± 522 | 0.32 | 20/18 |
| HOMA-IR | 1.0 ± 0.1 | 2.8 ± 0.6 | 0.006 | 20/18 |
| HbA1c, % | 5.2 ± 0.07 | 6.8 ± 0.2 | <0.001 | 20/19 |
| MVPA, min/wk | 285.0 ± 27.7 | 88.4 ± 19.4 | <0.001 | 20/20 |
| LPA, min/wk | 2,110 ± 140 | 1,444 ± 138 | 0.002 | 20/20 |
| Sedentary activity, min/wk | 3,326 ± 102 | 3,130 ± 149 | 0.28 | 20/20 |
| Length of T2D diagnosis, yr | 7.4 ± 1.2 | 0/20 | ||
| Medications | ||||
| α-Glucosidase inhibitor | 1 | |||
| Biguanide | 17 | |||
| Dipeptidyl peptidase-IV inhibitor | 1 | |||
| Glucagon-like peptide-1 agonist | 3 | |||
| Sodium-glucose cotransporter 2 inhibitor | 3 | |||
| Sulfonylurea | 6 | |||
| Thiazolidinedione | 1 | |||
| Aldosterone receptor antagonist | 3 | |||
| Angiotensin-converting enzyme inhibitor | 7 | |||
| Angiotensin II receptor antagonist | 3 | |||
| β Blocker | 2 | |||
| Calcium channel blocker | 3 | |||
| Thiazide | 2 | |||
| Fibric acid agent | 1 | |||
| HMG-CoA reductase inhibitor | 13 | |||
| Omega-3-acid ethyl ester | 1 | |||
| Thyroid hormone replacement | 4 | |||
| Hormone replacement therapy | 1 | |||
| Intrauterine device | 2 | |||
| Oral contraceptive | 1 |
Data are presented as means ± SE. n, sample sizes. Significant P values are in bold. BMI, body mass index; BP, blood pressure; cfPWV, carotid-femoral pulse-wave velocity; HbA1C, hemoglobin A1c; HOMA-IR, homeostatic model assessment for insulin resistance; iAUC, incremental area-under-the-curve; LPA, light physical activity; MAP, mean arterial pressure; MVPA, moderate-vigorous physical activity; T2D, type 2 diabetes; VAT, visceral adipose tissue.
Table 2.
Femoral artery hemodynamics in healthy subjects and subjects with type 2 diabetes
| Healthy Subjects | T2D Subjects | P Value | n (Healthy/T2D) | |
|---|---|---|---|---|
| Baseline diameter, cm | 0.63 ± 0.02 | 0.65 ± 0.03 | 0.60 | 20/15 |
| Peak diameter, cm | 0.66 ± 0.02 | 0.66 ± 0.03 | 0.97 | |
| Time-to-peak diameter, s | 74.6 ± 7.7 | 97.2 ± 9.5 | 0.07 | |
| Hyperemic shear rate, s−1 AUC | 10,610 ± 1,030 | 9,215 ± 856 | 0.33 | |
| FMD, % | 4.91 ± 0.56 | 2.04 ± 0.31 | <0.001 |
Data are presented as means ± SEM. n, sample sizes. Significant P values are in bold. AUC, area under the curve; FMD, flow-mediated dilation; T2D, type 2 diabetes.
Improved Leg Blood Flow Responses to an Oral Glucose Load after Passive Heat Treatment Does Not Parallel with Increased HSP72 in Venous Endothelial Cells
Passive heating via water immersion in individuals with T2D (illustrated in Fig. 2A) effectively increased core temperature over the course of the 60-min heating period (time effect P < 0.001, Fig. 2B). Passive heating also increased heart rate (time effect P < 0.001, Fig. 2C) and reduced MAP (time effect P < 0.001, Fig. 2D). It should be noted that the fall in blood pressure during passive heating was further accentuated at the seventh session of heating (interaction P = 0.004, Fig. 2D). The passive heating intervention was well-tolerated as during immersion subjects registered thermal sensation as “comfortable” and RPE as “extremely light”; perceptions that did not change over the course of the 7-day intervention. Furthermore, whole body sweat rates during immersion, while trending to increase, were not significantly different between the first and seventh heating sessions (1st session: 0.468 ± 0.04 L/h; 7th session: 0.610 ± 0.08 L/h, P = 0.09), suggesting subjects only became partially heat acclimated to the intervention.
Figure 2.
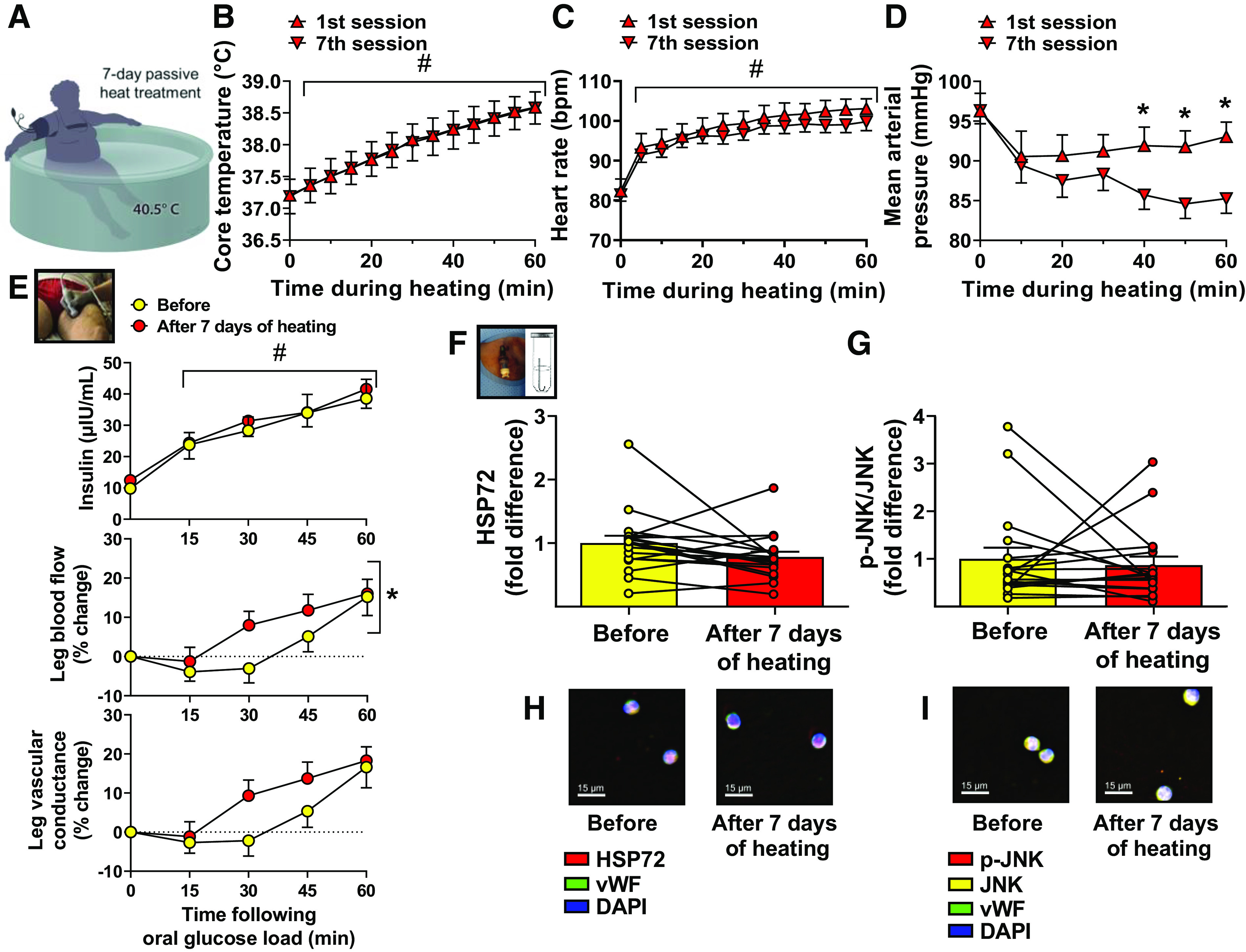
Improved leg blood flow responses to an oral glucose load after passive heat treatment does not parallel with increased heat shock protein 72 (HSP72) in endothelial cells. A: illustration of a single heating session during the 7-day intervention in which a subject with type 2 diabetes (T2D) is immersed at waist-level in 40.5°C water for 60 min. Core temperature (B), heart rate (C), and mean arterial pressure (D) measured during the 1st and 7th heating sessions. Two-way ANOVA with repeated-measures and Bonferroni post hoc test, respectively. E: plasma insulin concentration (top; n = 18), relative leg blood flow (percent change from baseline) (middle; n = 20) and relative leg vascular conductance (percent change from baseline) (bottom; n = 20) in response to an oral glucose load (75 g) in subjects with T2D, before and after the 7 days of heating. Two-way ANOVA with repeated-measures and Bonferroni post hoc test. HSP72 (F) and phospho-c-Jun amino-terminal kinase (JNK)/JNK protein content (G) in venous endothelial cells collected from subjects with T2D before and after the 7 days of heating, measured by quantitative immunofluorescence intensity (n = 18). Paired two-tailed Student’s t test, respectively. H and I: representative images of quantitative immunofluorescence intensities of HSP72 and phospho-JNK/JNK, respectively. Means ± SE and individual data, when appropriate, are reported. In B and C, #significant main effect of time during heating (P < 0.05). In D, *significant Bonferroni-adjusted pairwise comparisons (P < 0.05). In E (top), #significant main effect of time. In E (middle), *significant main effect of heat treatment (P < 0.05). In E (bottom), the main effect of heat treatment on leg vascular conductance was trending (P = 0.07). vWF, von Willebrand factor.
Importantly, as shown in Fig. 2E, the heating intervention in individuals with T2D improved leg blood flow responses (treatment effect P = 0.03) to the oral glucose load and the concomitant acute hyperinsulinemia. This improvement in leg blood flow responsiveness during the postprandial state after 7 days of heating occurred despite no evidence of increased HSP72 expression in venous endothelial cells (P = 0.10, Fig. 2F). Phospho-JNK was also not affected by the heating intervention (P = 0.66, Fig. 2G). As expected by design, this short-term heating intervention was of insufficient duration to alter metabolic outcomes. It was also insufficient to increase FMD or reduce cfPWV, although a trend was observed for the latter (P = 0.07). These data and other phenotypic parameters are summarized in Tables 3 and 4.
Table 3.
Effects of 7 days of passive heating on subject characteristics, anthropometrics, hemodynamic measurements, and blood profile parameters in subjects with type 2 diabetes
| Before | After 7 Days of Heating | P Value | n | |
|---|---|---|---|---|
| Age, yr | 53 ± 1.9 | 20 | ||
| Sex (Female/Male) | (12/8) | 20 | ||
| Height, cm | 170 ± 1.9 | 170 ± 1.9 | 0.20 | 20 |
| Weight, kg | 104 ± 4.1 | 104 ± 4.0 | 0.33 | 20 |
| BMI, kg/m2 | 35.9 ± 1.2 | 35.8 ± 1.3 | 0.32 | 20 |
| Body fat, % | 39.8 ± 1.7 | 40.0 ± 1.7 | 0.21 | 20 |
| Lean mass, kg | 63.0 ± 3.1 | 62.3 ± 2.8 | 0.17 | 20 |
| VAT, kg | 0.83 ± 0.07 | 0.79 ± 0.07 | 0.30 | 20 |
| Android/gynoid ratio | 1.07 ± 0.03 | 1.07 ± 0.03 | 0.98 | 20 |
| Systolic BP, mmHg | 129 ± 3 | 127 ± 2 | 0.27 | 20 |
| Diastolic BP, mmHg | 79 ± 2 | 78 ± 2 | 0.58 | 20 |
| MAP, mmHg | 96 ± 2 | 94 ± 2 | 0.24 | 20 |
| cfPWV, m/s | 8.5 ± 0.5 | 7.8 ± 0.3 | 0.07 | 11 |
| Fasted leg blood flow, mL/min | 186 ± 19.6 | 184 ± 19.1 | 0.71 | 20 |
| Fasted blood glucose, mg/dL | 106 ± 6.9 | 108 ± 7.2 | 0.68 | 18 |
| Postprandial glucose 2 h iAUC, mg/dL·min | 8,633 ± 1016 | 9,207 ± 859 | 0.45 | 18 |
| Fasted insulin, μIU/mL | 9.8 ± 1.7 | 12.4 ± 2 | 0.07 | 18 |
| Postprandial insulin 2 h iAUC, μIU/mL·min | 3,106 ± 522 | 3,029 ± 461 | 0.74 | 18 |
| HOMA-IR | 2.8 ± 0.6 | 3.4 ± 0.6 | 0.14 | 18 |
| HbA1c, % | 6.8 ± 0.2 | 6.8 ± 0.2 | >0.99 | 19 |
| MVPA, min/wk | 88.4 ± 19.4 | 86.4 ± 15.4 | 0.87 | 20 |
| LPA, min/wk | 1,444 ± 138 | 1,352 ± 105 | 0.24 | 20 |
| Sedentary activity, min/wk | 3,130 ± 149 | 3,235 ± 111 | 0.41 | 20 |
Data are presented as means ± SE. Sample sizes are presented as n. Significant P values are in bold. BMI, body mass index; BP, blood pressure; cfPWV, carotid-femoral pulse wave velocity; HbA1C, hemoglobin A1c; HOMA-IR, homeostatic model assessment for insulin resistance; iAUC, incremental area-under-the-curve; LPA, light physical activity; MAP, mean arterial pressure; MVPA, moderate-vigorous physical activity; T2D, type 2 diabetes; VAT, visceral adipose tissue.
Table 4.
Effects of 7 days of passive heating on femoral artery hemodynamics in subjects with type 2 diabetes
| Before | After 7 Days of Heating | P Value | n | |
|---|---|---|---|---|
| Baseline diameter, cm | 0.65 ± 0.03 | 0.65 ± 0.03 | 0.48 | 15 |
| Peak diameter, cm | 0.66 ± 0.03 | 0.67 ± 0.03 | 0.55 | |
| Time-to-peak diameter, s | 97.2 ± 9.5 | 101 ± 12.7 | 0.84 | |
| Hyperemic shear rate, s−1 AUC | 9,215 ± 856 | 9,161 ± 973 | 0.95 | |
| FMD, % | 2.04 ± 0.3 | 2.67 ± 0.4 | 0.17 |
Data are presented as means ± SE. Sample sizes are presented as n. Significant P values are in bold. AUC, area under the curve; FMD, flow-mediated dilation; T2D, type 2 diabetes.
Downregulation or Upregulation of HSP72 in Cultured hSMMECs Does Not Impair or Enhance Insulin Signaling, Respectively
As shown in Fig. 3, siRNA-mediated knockdown of HSP72 (P = 0.002, Fig. 3A) did not enhance phospho-JNK (P = 0.58, Fig. 3B) and did not blunt insulin-induced activation of Akt (interaction P = 0.14, Fig. 3C) in cultured endothelial cells. Similarly, HSP72 induction after five consecutive days of heating (P < 0.001, Fig. 3E) did not suppress phospho-JNK (P = 0.36, Fig. 3F) and did not enhance insulin-induced activation of Akt (interaction P = 0.93, Fig. 3G).
Figure 3.
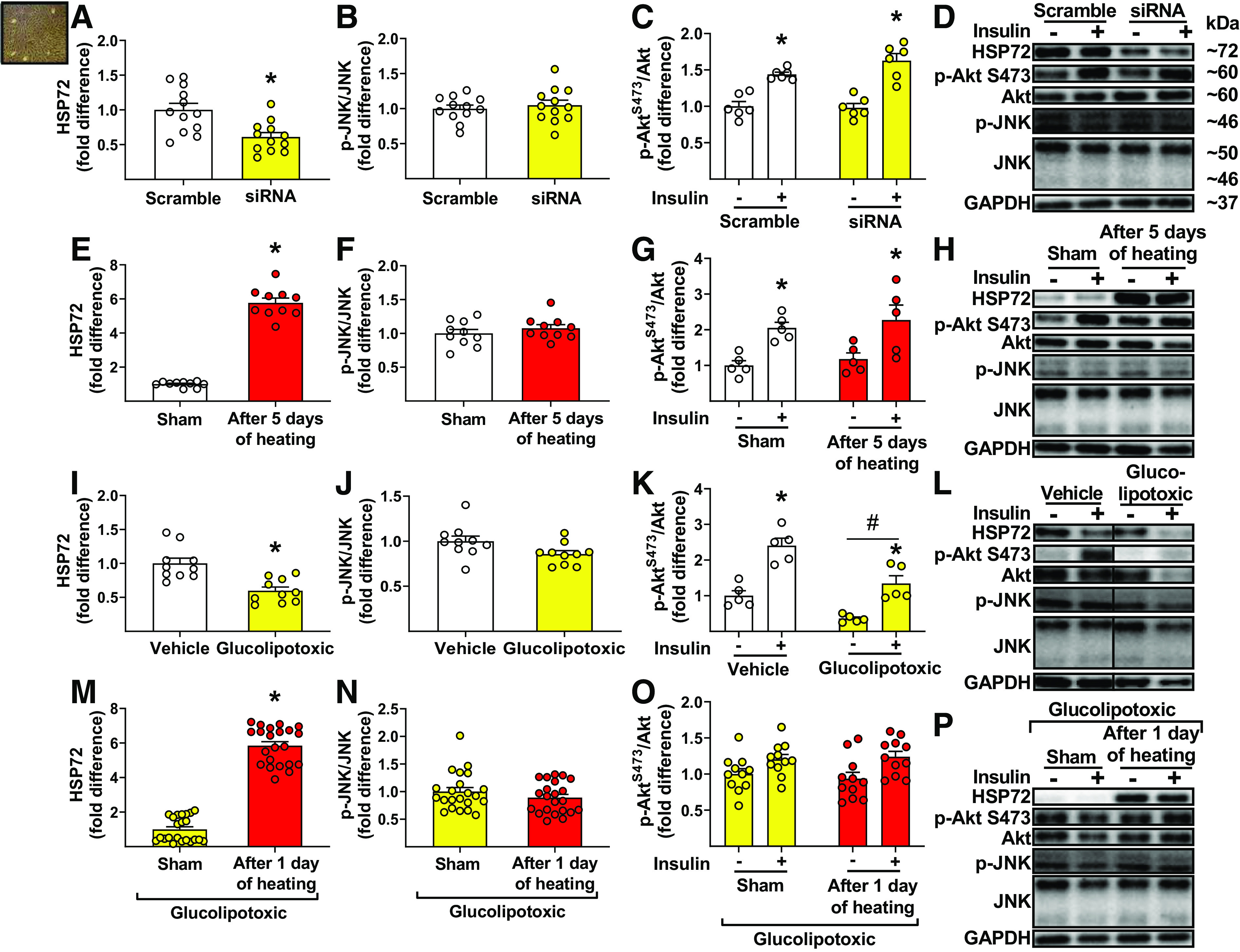
Downregulation of heat shock protein 72 (HSP72) (via small interfering RNA) or upregulation HSP72 (via heating) in cultured endothelial cells does not impair and enhance insulin signaling (i.e., activation of Akt), respectively, nor is c-Jun amino-terminal kinase (JNK) activity altered. HSP72 (A), phospho-JNK/JNK (B), and insulin-stimulated phospho-AktS473/Akt expression (C) in human skeletal muscle microvascular endothelial cells (hSMMECs), 48 h after 2nd transfection with scramble or HSP72 siRNA (20 nM for 6 h) (A, n = 12/condition; B, n = 12/condition; C, n = 6/condition). HSP72 (E), phospho-JNK/JNK (F), and insulin-stimulated phospho-AktS473/Akt expression (G) in hSMMECs, 24 h after sham (37°C) or heat treatment (40.5 °C) for 60 min/day, over 5 days (E, n = 10/condition; F, n = 10/condition; G, n = 5/condition). HSP72 (I), phospho-JNK/JNK (J), and insulin-stimulated phospho-AktS473/Akt expression (K) in hSMMECs, 24 h after incubation with a glucolipotoxic or vehicle milieu (I, n = 10/condition; J, n = 10/condition; K, n = 5/condition). HSP72 (M), phospho-JNK/JNK (N), and insulin-stimulated phospho-AktS473/Akt expression (O) in glucolipotoxic-treated hSMMECs, 24 h after sham (37°C) or heat treatment (40.5°C) for 60 min (M, n = 22/condition; N, n = 22/condition; O, n = 11/condition). D, H, L, and P: representative Western blot bands for proteins of interest (bands in L were obtained from the same gel but are noncontiguous, as denoted by separated boxes). Cells were incubated with insulin (100 nM) for 30 min before collection. Comparisons of HSP72 (A, E, I, M) and phospho-JNK/JNK (B, F, J, N) between conditions within each experiment were performed with unpaired two-tailed Student’s t test. Comparisons of phospho-AktS473/Akt (C, G, K, O) between conditions in each experiment were performed with two-way ANOVA and Bonferroni post hoc test. Means ± SE and individual data are reported. In A, E, I, M, *significant effect of condition (P < 0.05). In C, G, K, *significant main effect of insulin stimulation (P < 0.05). In K, #significant main effect of glucolipotoxicity (P < 0.05).
Furthermore, exposure of endothelial cells to a glucolipotoxic milieu reduced HSP72 (P < 0.001, Fig. 3I) but did not enhance phospho-JNK (in fact, there was a strong trend for its reduction, P = 0.05, Fig. 3J). Although downregulation of HSP72 caused by glucolipotoxicity was associated with an overall suppression of phospho-Akt (i.e., main effect of glucolipotoxic treatment, P < 0.001), it did not impair activation of Akt in response to insulin (i.e., interaction P = 0.23, Fig. 3K). Similarly, restoration of HSP72 via heating in glucolipotoxic cells (P < 0.001, Fig. 3M) did not reduce phospho-JNK (P = 0.25, Fig. 3N) and did not elicit an improvement in insulin-induced activation of Akt (interaction P = 0.52, Fig. 3O).
DISCUSSION
The primary findings of this investigation are threefold. First, endothelial insulin resistance in T2D mice and humans was not associated with reduced HSP72 in aortas and venous endothelial cells, respectively. Second, after passive heat treatment in individuals with T2D, improved leg blood flow responses to an oral glucose load did not parallel with increased venous endothelial HSP72. Finally, downregulation or upregulation of HSP72 in cultured endothelial cells did not impair or enhance insulin signaling, respectively, nor was JNK activity altered. In aggregate, these findings do not support the notion that reduced HSP72 is a key determinant of endothelial insulin resistance in T2D but do demonstrate that lower body heating may be efficacious for improving leg blood flow responses to glucose ingestion, likely due to increased vascular insulin sensitivity.
The finding that mouse diabetic arteries with impaired insulin-induced relaxation did not display evidence of suppressed HSP72 expression suggests that loss of HSP72 cannot be an obligatory mechanism causing insulin resistance in vascular tissue. This disconnect between HSP72 expression and vascular insulin sensitivity is corroborated in our cohort of individuals with T2D who showed evidence of blunted leg blood flow responses to endogenous insulin stimulation (using an oral glucose load) despite no decrease in venous endothelial expression of HSP72. In further support of this disassociation, we found that 7 days of passive heating in individuals with T2D improved leg blood flow responses to insulin stimulation but that this improvement was not accompanied with an induction of HSP72, suggesting the idea that upregulation of HSP72 with heating may not be a required mechanism to promote vascular insulin sensitivity. Additional evidence supporting the lack of a causal relationship between HSP72 and endothelial insulin sensitivity is revealed by our endothelial cell culture loss- and gain-of-function experiments. That is, we show that knockdown of HSP72 via siRNA does not impair endothelial insulin signaling (i.e., activation of Akt in response to insulin stimulation) and that the converse is also true. Induction of HSP72 via heating, in naïve cells or cells exposed to a glucolipotoxic milieu, does not enhance insulin-induced Akt activity.
This “uncoupling” between HSP72 and endothelial insulin signaling could be explained by the fact that, contrary to our hypothesis, modulation of HSP72 did not influence JNK activity, a known stress-activated kinase shown to disrupt the PI3K-Akt insulin-signaling pathway (24–26). Our finding that alteration of HSP72 in endothelial cells did not influence JNK activity suggests the link between HSP72 and JNK, supported by other studies in metabolically active cells and tissues (16–23, 65–67), may not extrapolate to the endothelium. Notably, although our data do not support a role of HSP72 in regulating endothelial insulin sensitivity, this is not to infer that endothelial HSP72 is not implicated in other important physiological processes. In this regard, Shiota et al. (68) found that siRNA-mediated knockdown of HSP72 in endothelial cells impairs VEGF-stimulated PI3K-Akt signaling and angiogenesis.
Although it was hypothesized that repeated bouts of lower-body heating, and consequent increases in core temperature, would be a sufficient stimulus for upregulation of HSP72 in endothelial cells (62), there is also some precedence in the literature for heating interventions in humans to not result in an induction of HSP72 [e.g., in biopsied skeletal muscle (47) and adipose tissue (46), or collected immune cells (36)]. This is in contrast to heating studies in animals that typically demonstrate an induction of HSP72, likely attributable to exposure to higher temperatures (17, 19, 65, 66, 69–72). Of note, previous studies have shown that exercise-induced (73) and heat-induced (74) HSP72 expression in metabolically active tissues is blunted in rat models of insulin resistance. Furthermore, increased plasma HSP72 observed in humans following either an acute session of exercise or hot water immersion has been reported to be impaired in individuals with increased body mass (75). In this context, it is plausible that T2D (76, 77) and/or excess body mass (75) could be associated with a deficiency in the heat stress response and that this may have contributed to the lack of upregulation of HSP72 with heating. However, additional studies are needed to test these hypotheses.
Importantly, despite the lack of induction of HSP72 after 7 days of passive heating in individuals with T2D, we observed an improvement in leg blood flow responses to an oral glucose load. This improvement is reminiscent of findings by Mikus et al. (48) where 7 days of aerobic exercise was shown to enhance leg blood flow responses to an oral glucose load in subjects with T2D. Because both lower-body heating and exercise increase leg blood flow and shear stress, it is reasonable to speculate that increased shear stress may be the shared mechanism whereby these two interventions promote insulin-sensitizing effects in the vasculature. In support of this proposition, we recently showed in cultured endothelial cells and in isolated arteries that application of shear stress for 1 h subsequently augmented insulin signaling and insulin-induced dilation, respectively (37). The observation that heating and exercise produce analogous insulin-sensitizing effects in the vasculature is even true after a single, 1-h bout of unilateral leg heating (37) and unilateral leg exercise (78) in young healthy subjects, again reinforcing the idea that increased shear stress may be the principal driving stimulus mediating these beneficial vascular effects.
The notion that heating can recapitulate some of the beneficial vascular adaptations to exercise in human limbs is clinically relevant (79), particularly when considering that a significant fraction of patients with T2D are incapable of performing sustained aerobic exercise (80–84) or would choose not to do so (85, 86). Notably, our findings extend those from previous studies indicating that lower body heating also prevents inactivity-induced leg vascular dysfunction in healthy subjects (87) and, when applied for 8 wk, it improves several indices of vascular function in overweight sedentary individuals (32) and in women who are obese with polycystic ovary syndrome (33). Further underscoring the clinical significance of heat therapy, data from a large prospective cohort study revealed that increased frequency of sauna bathing is associated with a reduced risk of fatal cardiovascular diseases and all-cause mortality (29). Furthermore, heat therapy has been demonstrated to improve the prognosis of patients with chronic heart failure (35), and both vascular outcomes and physical functioning in patients with coronary artery disease (88), chronic heart failure (89–95), and peripheral arterial disease (34, 96–99).
In our study, improvements in leg blood flow responsiveness during the postprandial state after the seven-day heating intervention were not accompanied by improvements in femoral artery FMD or a significant destiffening of the aorta, likely owing to the short-term regimen and/or lower sample size (and thus lesser statistical power) for these two variables. The observation that improved leg blood flow responses to glucose ingestion following the heating intervention occurred before manifestation of other overt vascular and metabolic changes stimulates the idea that vascular insulin sensitivity may be a phenotypic attribute that is largely amenable to change. In support of this concept, previous work in genetic models of obesity and diet-induced obesity (100–103) indicates that endothelial insulin resistance is an early event in the disease process that develops before manifestation of other indices of vascular endothelial dysfunction (e.g., impaired acetylcholine-induced dilation). Because endothelial insulin resistance is causally implicated in the development and progression of vascular and metabolic disease (9–12, 14), strategies that correct insulin resistance in the vasculature should be deemed of high relevance.
Another interesting finding of the present study is that although arterial pressure did not change after the heating intervention in subjects with T2D, it decreased during heating, a response that was further amplified by the last session. In relation to this finding, recent data show that blood pressure during heating is well maintained in young healthy subjects and that this is due to increased sympathetic nerve activity (104). Conversely, older adults demonstrate a fall in blood pressure with heating, similar to our observation in subjects with T2D, and this fall in blood pressure does not lead to compensatory (i.e., baroreflex-mediated) increases in sympathetic nerve activity (104). Accordingly, it is likely that the drop in blood pressure during heating in older adults and subjects with T2D is mediated by a sympathoinhibitory effect that alters the compensatory neural response to hypotension (104). The fact that this blood pressure-lowering effect of heating in T2D is augmented with more sessions of heating may be attributed to such sympathoinhibitory effects now compounded with improved peripheral vasodilation. Supporting this, 8 wk of passive heating sessions has been shown to decrease sympathetic nerve activity and arterial blood pressure in women with polycystic ovary syndrome (33). Additional studies are warranted to further elucidate the mechanisms and implications of such blood pressure effects of acute and chronic heating.
Several aspects of this investigation warrant further consideration. First, the present study used lean and physically active adults as healthy control subjects. The addition of sedentary lean and obese nondiabetic groups would have allowed us to disentangle the effects of obesity versus inactivity, conditions that commonly coexist in subjects with T2D (105–107). Second, it should be acknowledged that a more robust experimental design would have involved the randomization of participants with T2D to passive heating versus sham immersion. We expect the present findings will stimulate the conduct of a larger-scale clinical trial involving randomization of patients to passive heating versus sham immersion using a longer treatment paradigm. Also, it should be noted that only subjects with T2D underwent the passive heating therapy and thus it remains unknown if the observed effects of heating occur in healthy subjects. Third, an oral glucose load was used for endogenous insulin production with concurrent assessment of leg blood flow responsiveness (48, 108), rather than the hyperinsulinemic-euglycemic clamp, an approach also commonly used by our group (37, 58, 109–112). Although the use of an oral glucose load is more physiological, one of the main caveats is that the magnitude of insulin release by pancreatic β cells is largely variable across individuals. That is, individuals with T2D with β cell dysfunction exhibit limited insulin production. In addition, while insulin is likely the primary driver of increased leg blood flow during the postprandial state, an oral glucose load not only stimulates production of insulin but also other hormones (e.g., incretins) that can have vascular effects. Notably, these vascular effects may also be modulated by hyperglycemia itself, which can promote vasoconstriction. These are considerations that have been well presented and discussed in the literature (113, 114). Fourth, endothelial cells were collected from an antecubital vein. This was decided on the basis that our modality of passive heating effectively increases core temperature and thus presumably the entire vasculature is subjected to repeated bouts of heat, a stimulus for HSP72 induction. We acknowledge that a better approach would have been the collection of arterial endothelial cells from the lower limbs; however, this procedure carries greater risk to the subject. Fifth, while it is well established that during lower-body heating there is an increase in leg blood flow (37, 87, 115–117), these measurements were not performed in the present study and thus this should be considered as a limitation. Finally, we used conventional ex vivo and in vitro preparations to study insulin signaling and insulin-induced vasodilation; nonetheless, it should be acknowledged that the higher insulin concentrations used extend into supraphysiological levels.
Perspectives and Significance
Data from the present investigation using various experimental models including isolated mouse arteries, humans, and cultured endothelial cells suggest that endothelial insulin resistance in T2D is not causally attributed to reduced expression of HSP72. Furthermore, we show for the first time that lower-body heating may be a promising approach for restoring leg blood flow responses to an oral glucose load and consequent hyperinsulinemia in individuals with T2D.
GRANTS
This work was supported by the National Institutes of Health Grants R01 HL137769 (to J. Padilla), R01 HL151384 (to L. A. Martinez-Lemus and J. Padilla), R01 HL142770 (to C. Manrique-Acevedo), and R21 DK116081 (to C. Manrique-Acevedo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.P-M. and J.P. conceived and designed research; R.J.P-M., G.P., F.J.C-A., N.S., and Y.L. performed experiments; R.J.P-M., F.J.C-A., F.I.R-P., R.N.S., L.A.M-L., and J.P. analyzed data; R.J.P-M., R.N.S., J.A.K., L.A.M-L., and J.P. interpreted results of experiments; R.J.P-M., F.J.C-A., F.I.R-P., and J.P. prepared figures; R.J.P-M., and J.P. drafted manuscript; R.J.P-M., G.P., F.J.C-A., F.I.R-P., R.N.S., N.S., Y.L., D.D.C., J.A.K., L.A.M-L., C.M.M-A., and J.P., edited and revised manuscript; R.J.P-M., G.P., F.J.C-A., F.I.R-P., R.N.S., N.S., Y.L., D.D.C., J.A.K., L.A.M-L., C.M.M-A., and J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors appreciate the time and effort of all volunteer participants. In addition, the authors thank Becky Shafer for all technical and administrative help.
REFERENCES
- 1.CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017. [Google Scholar]
- 2.Emerging Risk Factors C, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375: 2215–2222, 2010. [Erratum in Lancet 376: 958, 2010]. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin J, Thompson TJ, Cheng YJ, Zhuo X, Zhang P, Gregg E, Rolka DB. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr 16: 9, 2018. doi: 10.1186/s12963-018-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinesh Shah A, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1.9 million people. Lancet 385, Suppl 1: S86, 2015. doi: 10.1016/S0140-6736(15)60401-9. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, Del Canizo-Gomez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes 5: 444–470, 2014. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Turco S, Gaggini M, Daniele G, Basta G, Folli F, Sicari R, Gastaldelli A. Insulin resistance and endothelial dysfunction: a mutual relationship in cardiometabolic risk. Curr Pharm Des 19: 2420–2431, 2013. doi: 10.2174/1381612811319130010. [DOI] [PubMed] [Google Scholar]
- 7.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 8.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord 14: 5–12, 2013. doi: 10.1007/s11154-012-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King GL, Park K, Li Q. Selective insulin resistance and the development of cardiovascular diseases in diabetes: the Edwin Bierman Award Lecture . Diabetes 65: 1462–1471, 2016. doi: 10.2337/db16-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 11.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 301: E252–E263, 2011. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab 11: 379–389, 2010. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gage MC, Yuldasheva NY, Viswambharan H, Sukumar P, Cubbon RM, Galloway S, Imrie H, Skromna A, Smith J, Jackson CL, Kearney MT, Wheatcroft SB. Endothelium-specific insulin resistance leads to accelerated atherosclerosis in areas with disturbed flow patterns: a role for reactive oxygen species. Atherosclerosis 230: 131–139, 2013. doi: 10.1016/j.atherosclerosis.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 13: 294–307, 2011. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Kanter JE, Kramer F, Barnhart S, Duggan JM, Shimizu-Albergine M, Kothari V, Chait A, Bouman SD, Hamerman JA, Hansen BF, Olsen GS, Bornfeldt KE. A novel strategy to prevent advanced atherosclerosis and lower blood glucose in a mouse model of metabolic syndrome. Diabetes 67: 946–959, 2018. doi: 10.2337/db17-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 52: 2338–2345, 2003. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- 17.Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA 105: 1739–1744, 2008. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 51: 1102–1109, 2002. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- 19.Kitano S, Kondo T, Matsuyama R, Ono K, Goto R, Takaki Y, Hanatani S, Sakaguchi M, Igata M, Kawashima J, Motoshima H, Matsumura T, Kai H, Araki E. Impact of hepatic HSP72 on insulin signaling. Am J Physiol Endocrinol Metab 316: E305–E318, 2019. doi: 10.1152/ajpendo.00215.2018. [DOI] [PubMed] [Google Scholar]
- 20.Meriin AB, Yaglom JA, Gabai VL, Zon L, Ganiatsas S, Mosser DD, Zon L, Sherman MY. Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol Cell Biol 19: 2547–2555, 1999. doi: 10.1128/MCB.19.4.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaglom JA, Gabai VL, Meriin AB, Mosser DD, Sherman MY. The function of HSP72 in suppression of c-Jun N-terminal kinase activation can be dissociated from its role in prevention of protein damage. J Biol Chem 274: 20223–20228, 1999. doi: 10.1074/jbc.274.29.20223. [DOI] [PubMed] [Google Scholar]
- 22.Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, Sherman MY. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem 272: 18033–18037, 1997. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- 23.Morino S, Kondo T, Sasaki K, Adachi H, Suico MA, Sekimoto E, Matsuda T, Shuto T, Araki E, Kai H. Mild electrical stimulation with heat shock ameliorates insulin resistance via enhanced insulin signaling. PLoS One 3: e4068, 2008. doi: 10.1371/journal.pone.0004068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537, 2002. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 271: 665–668, 1996. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 26.Breton-Romero R, Feng B, Holbrook M, Farb MG, Fetterman JL, Linder EA, Berk BD, Masaki N, Weisbrod RM, Inagaki E, Gokce N, Fuster JJ, Walsh K, Hamburg NM. Endothelial dysfunction in human diabetes is mediated by Wnt5a-JNK signaling. Arterioscler Thromb Vasc Biol 36: 561–569, 2016. doi: 10.1161/ATVBAHA.115.306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunutsor SK, Khan H, Zaccardi F, Laukkanen T, Willeit P, Laukkanen JA. Sauna bathing reduces the risk of stroke in Finnish men and women: a prospective cohort study. Neurology 90: e1937–e1944, 2018. doi: 10.1212/WNL.0000000000005606. [DOI] [PubMed] [Google Scholar]
- 28.Laukkanen JA, Laukkanen T. Sauna bathing and systemic inflammation. Eur J Epidemiol 33: 351–353, 2018. doi: 10.1007/s10654-017-0335-y. [DOI] [PubMed] [Google Scholar]
- 29.Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med 175: 542–548, 2015. doi: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- 30.Laukkanen T, Kunutsor SK, Khan H, Willeit P, Zaccardi F, Laukkanen JA. Sauna bathing is associated with reduced cardiovascular mortality and improves risk prediction in men and women: a prospective cohort study. BMC Med 16: 219, 2018. doi: 10.1186/s12916-018-1198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med 341: 924–925, 1999. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- 32.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ely BR, Francisco MA, Halliwill JR, Bryan SD, Comrada LN, Larson EA, Brunt VE, Minson CT. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in obese women with polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol 317: R630–R640, 2019. doi: 10.1152/ajpregu.00078.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akerman AP, Thomas KN, van Rij AM, Body ED, Alfadhel M, Cotter JD. Heat therapy vs. supervised exercise therapy for peripheral arterial disease: a 12-wk randomized, controlled trial. Am J Physiol Heart Circ Physiol 316: H1495–H1506, 2019. doi: 10.1152/ajpheart.00151.2019. [DOI] [PubMed] [Google Scholar]
- 35.Kihara T, Miyata M, Fukudome T, Ikeda Y, Shinsato T, Kubozono T, Fujita S, Kuwahata S, Hamasaki S, Torii H, Lee S, Toda H, Tei C. Waon therapy improves the prognosis of patients with chronic heart failure. J Cardiol 53: 214–218, 2009. doi: 10.1016/j.jjcc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Hoekstra SP, Bishop NC, Faulkner SH, Bailey SJ, Leicht CA. The acute and chronic effects of hot water immersion on inflammation and metabolism in sedentary, overweight adults. J Appl Physiol (Bethesda, MD: 1985) 125: 2008–2018, 2018. doi: 10.1152/japplphysiol.00407.2018. [DOI] [PubMed] [Google Scholar]
- 37.Walsh LK, Ghiarone T, Olver TD, Medina-Hernandez A, Edwards JC, Thorne PK, Emter CA, Lindner JR, Manrique-Acevedo C, Martinez-Lemus LA, Padilla J. Increased endothelial shear stress improves insulin-stimulated vasodilation in skeletal muscle. J Physiol 597: 57–69, 2019. doi: 10.1113/JP277050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunewald ZI, Jurrissen TJ, Woodford ML, Ramirez-Perez FI, Park LK, Pettit-Mee R, Ghiarone T, Brown SM, Morales-Quinones M, Ball JR, Staveley-O’Carroll KF, Aroor AR, Fadel PJ, Paradis P, Schiffrin EL, Bender SB, Martinez-Lemus LA, Padilla J. Chronic elevation of endothelin-1 alone may not be sufficient to impair endothelium-dependent relaxation. Hypertension 74: 1409–1419, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winn NC, Grunewald ZI, Gastecki ML, Woodford ML, Welly RJ, Clookey SL, Ball JR, Gaines TL, Karasseva NG, Kanaley JA, Sacks HS, Vieira-Potter VJ, Padilla J. Deletion of UCP1 enhances ex vivo aortic vasomotor function in female but not male mice despite similar susceptibility to metabolic dysfunction. Am J Physiol Endocrinol Metab 313: E402–E412, 2017. doi: 10.1152/ajpendo.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol (1985) 93: 3–30, 2002. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- 41.Booth FW, Chakravarthy MV, Spangenburg EE. Exercise and gene expression: physiological regulation of the human genome through physical activity. J Physiol 543: 399–411, 2002. doi: 10.1113/jphysiol.2002.019265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol (1985) 88: 774–787, 2000. doi: 10.1152/jappl.2000.88.2.774. [DOI] [PubMed] [Google Scholar]
- 43.Booth FW, Lees SJ. Physically active subjects should be the control group. Med Sci Sports Exerc 38: 405–406, 2006. doi: 10.1249/01.mss.0000205117.11882.65. [DOI] [PubMed] [Google Scholar]
- 44.Lees SJ, Booth FW. Sedentary death syndrome. Can J Appl Physiol 29: 447–460, 2004. discussion 444–446. doi: 10.1139/h04-029. [DOI] [PubMed] [Google Scholar]
- 45.Rivas E, Newmire DE, Crandall CG, Hooper PL, Ben-Ezra V. An acute bout of whole body passive hyperthermia increases plasma leptin, but does not alter glucose or insulin responses in obese type 2 diabetics and healthy adults. J Therm Biol 59: 26–33, 2016. doi: 10.1016/j.jtherbio.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Ely BR, Clayton ZS, McCurdy CE, Pfeiffer J, Needham KW, Comrada LN, Minson CT. Heat therapy improves glucose tolerance and adipose tissue insulin signaling in polycystic ovary syndrome. Am J Physiol Endocrinol Metab 317: E172–E182, 2019. doi: 10.1152/ajpendo.00549.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pallubinsky H, Phielix E, Dautzenberg B, Schaart G, Connell NJ, de Wit-Verheggen V, Havekes B, van Baak MA, Schrauwen P, van Marken Lichtenbelt WD. Passive exposure to heat improves glucose metabolism in overweight humans. Acta Physiol (Oxf) 229: e13488, 2020. doi: 10.1111/apha.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikus CR, Fairfax ST, Libla JL, Boyle LJ, Vianna LC, Oberlin DJ, Uptergrove GM, Deo SH, Kim A, Kanaley JA, Fadel PJ, Thyfault JP. Seven days of aerobic exercise training improves conduit artery blood flow following glucose ingestion in patients with type 2 diabetes. J Appl Physiol (Bethesda, MD: 1985) 111: 657–664, 2011. doi: 10.1152/japplphysiol.00489.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30: 777–781, 1998. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA 320: 2020–2028, 2018. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang MH, Yoo JK, Kim HK, Hwang CL, Mackay K, Hemstreet O, Nichols WW, Christou DD. Validity and reliability of aortic pulse wave velocity and augmentation index determined by the new cuff-based SphygmoCor Xcel. J Hum Hypertens 28: 475–481, 2014. doi: 10.1038/jhh.2013.144. [DOI] [PubMed] [Google Scholar]
- 52.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on H. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walsh LK, Restaino RM, Neuringer M, Manrique C, Padilla J. Administration of tauroursodeoxycholic acid prevents endothelial dysfunction caused by an oral glucose load. Clin Sci (Lond) 130: 1881–1888, 2016. doi: 10.1042/CS20160501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol Heart Circ Physiol 311: H177–H182, 2016. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Padilla J. Prior exercise and standing as strategies to circumvent sitting-induced leg endothelial dysfunction. Clin Sci (Lond) 131: 1045–1053, 2017. doi: 10.1042/CS20170031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGarity-Shipley EC, Schmitter SM, Williams JS, King TJ, McPhee IAC, Pyke KE. The impact of repeated, local heating-induced increases in blood flow on lower limb endothelial function in young, healthy females. Eur J Appl Physiol 121: 3017–3030, 2021. doi: 10.1007/s00421-021-04749-7. [DOI] [PubMed] [Google Scholar]
- 58.Park LK, Parks EJ, Pettit-Mee RJ, Woodford ML, Ghiarone T, Smith JA, Sales ARK, Martinez-Lemus LA, Manrique-Acevedo C, Padilla J. Skeletal muscle microvascular insulin resistance in type 2 diabetes is not improved by eight weeks of regular walking. Journal of applied physiology (Bethesda, Md: 1985) 129: 283–296, 2020. doi: 10.1152/japplphysiol.00174.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hwang MH, Yoo JK, Luttrell M, Kim HK, Meade TH, English M, Segal MS, Christou DD. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci (Lond) 125: 513–520, 2013. doi: 10.1042/CS20130200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang MH, Yoo JK, Luttrell M, Kim HK, Meade TH, English M, Talcott S, Jaffe IZ, Christou DD. Acute effect of mineralocorticoid receptor antagonism on vascular function in healthy older adults. Exp Gerontol 73: 86–94, 2016. doi: 10.1016/j.exger.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carter HH, Spence AL, Atkinson CL, Pugh CJ, Naylor LH, Green DJ. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol 114: 859–865, 2014. doi: 10.1007/s00421-013-2817-2. [DOI] [PubMed] [Google Scholar]
- 62.Gibson OR, Tuttle JA, Watt PW, Maxwell NS, Taylor L. Hsp72 and Hsp90α mRNA transcription is characterised by large, sustained changes in core temperature during heat acclimation. Cell Stress Chaperones 21: 1021–1035, 2016. doi: 10.1007/s12192-016-0726-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borg G. Borg's perceived exertion and pain scales. Champaign, IL: Human Kinetics, 1998, p. viii. [Google Scholar]
- 64.Lee JY, Stone EA, Wakabayashi H, Tochihara Y. Issues in combining the categorical and visual analog scale for the assessment of perceived thermal sensation: methodological and conceptual considerations. Appl Ergon 41: 282–290, 2010. doi: 10.1016/j.apergo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 58: 567–578, 2009. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol (1985) 110: 451–457, 2011. doi: 10.1152/japplphysiol.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J 20: 446–456, 2001. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiota M, Kusakabe H, Izumi Y, Hikita Y, Nakao T, Funae Y, Miura K, Iwao H. Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arterioscler Thromb Vasc Biol 30: 491–497, 2010. doi: 10.1161/ATVBAHA.109.193631. [DOI] [PubMed] [Google Scholar]
- 69.Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 63: 1881–1894, 2014. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Archer AE, Rogers RS, Von Schulze AT, Wheatley JL, Morris EM, McCoin CS, Thyfault JP, Geiger PC. Heat shock protein 72 regulates hepatic lipid accumulation. Am J Physiol Regul Integr Comp Physiol 315: R696–R707, 2018. doi: 10.1152/ajpregu.00073.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kavanagh K, Davis AT, Jenkins KA, Flynn DM. Effects of heated hydrotherapy on muscle HSP70 and glucose metabolism in old and young vervet monkeys. Cell Stress Chaperones 21: 717–725, 2016. doi: 10.1007/s12192-016-0699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silverstein MG, Ordanes D, Wylie AT, Files DC, Milligan C, Presley TD, Kavanagh K. Inducing muscle heat shock protein 70 improves insulin sensitivity and muscular performance in aged mice. J Gerontol A Biol Sci Med Sci 70: 800–808, 2015. doi: 10.1093/gerona/glu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hanninen O, Sen CK. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol (1985) 97: 605–611, 2004. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- 74.Rogers RS, Morris EM, Wheatley JL, Archer AE, McCoin CS, White KS, Wilson DR, Meers GM, Koch LG, Britton SL, Thyfault JP, Geiger PC. Deficiency in the heat stress response could underlie susceptibility to metabolic disease. Diabetes 65: 3341–3351, 2016. doi: 10.2337/db16-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faulkner SH, Jackson S, Fatania G, Leicht CA. The effect of passive heating on heat shock protein 70 and interleukin-6: a possible treatment tool for metabolic diseases? Temperature (Austin ) 4: 292–304, 2017. doi: 10.1080/23328940.2017.1288688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henstridge DC, Whitham M, Febbraio MA. Chaperoning to the metabolic party: The emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol Metab 3: 781–793, 2014. doi: 10.1016/j.molmet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hooper PL, Balogh G, Rivas E, Kavanagh K, Vigh L. The importance of the cellular stress response in the pathogenesis and treatment of type 2 diabetes. Cell Stress Chaperones 19: 447–464, 2014. doi: 10.1007/s12192-014-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sjoberg KA, Frosig C, Kjobsted R, Sylow L, Kleinert M, Betik AC, Shaw CS, Kiens B, Wojtaszewski JFP, Rattigan S, Richter EA, McConell GK. Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes 66: 1501–1510, 2017. doi: 10.2337/db16-1327. [DOI] [PubMed] [Google Scholar]
- 79.Cheng JL, MacDonald MJ. Effect of heat stress on vascular outcomes in humans. J Appl Physiol (1985) 126: 771–781, 2019. doi: 10.1152/japplphysiol.00682.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bilak JM, Gulsin GS, McCann GP. Cardiovascular and systemic determinants of exercise capacity in people with type 2 diabetes mellitus. Ther Adv Endocrinol Metab 12: 2042018820980235, 2021. doi: 10.1177/2042018820980235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fang ZY, Sharman J, Prins JB, Marwick TH. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes care 28: 1643–1648, 2005. doi: 10.2337/diacare.28.7.1643. [DOI] [PubMed] [Google Scholar]
- 82.Kuziemski K, Słomiński W, Jassem E. Impact of diabetes mellitus on functional exercise capacity and pulmonary functions in patients with diabetes and healthy persons. BMC Endocr Disord 19: 2, 2019. doi: 10.1186/s12902-018-0328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ribisl PM, Lang W, Jaramillo SA, Jakicic JM, Stewart KJ, Bahnson J, Bright R, Curtis JF, Crow RS, Soberman JE, Look ARG; on behalf of the Look AHEAD Research Group. Exercise capacity and cardiovascular/metabolic characteristics of overweight and obese individuals with type 2 diabetes: the Look AHEAD clinical trial. Diabetes care 30: 2679–2684, 2007. doi: 10.2337/dc06-2487. [DOI] [PubMed] [Google Scholar]
- 84.Roberts TJ, Barros-Murphy JF, Burns AT, MacIsaac RJ, MacIsaac AI, Prior DL, La Gerche A. Reduced exercise capacity in diabetes mellitus is not associated with impaired deformation or twist. J Am Soc Echocardiogr 33: 481–489, 2020. doi: 10.1016/j.echo.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Korkiakangas EE, Alahuhta MA, Laitinen JH. Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: a systematic review. Health Promot Int 24: 416–427, 2009. doi: 10.1093/heapro/dap031. [DOI] [PubMed] [Google Scholar]
- 86.Shultz JA, Sprague MA, Branen LJ, Lambeth S. A comparison of views of individuals with type 2 diabetes mellitus and diabetes educators about barriers to diet and exercise. J Health Commun 6: 99–115, 2001. doi: 10.1080/108107301750254457. [DOI] [PubMed] [Google Scholar]
- 87.Teixeira AL, Padilla J, Vianna LC. Impaired popliteal artery flow-mediated dilation caused by reduced daily physical activity is prevented by increased shear stress. J Appl Physiol (Bethesda, MD: 1985) 123: 49–54, 2017. doi: 10.1152/japplphysiol.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sobajima M, Nozawa T, Ihori H, Shida T, Ohori T, Suzuki T, Matsuki A, Yasumura S, Inoue H. Repeated sauna therapy improves myocardial perfusion in patients with chronically occluded coronary artery-related ischemia. Int J Cardiol 167: 237–243, 2013. doi: 10.1016/j.ijcard.2011.12.064. [DOI] [PubMed] [Google Scholar]
- 89.Gruner Svealv B, Cider A, Tang MS, Angwald E, Kardassis D, Andersson B. Benefit of warm water immersion on biventricular function in patients with chronic heart failure. Cardiovasc Ultrasound 7: 33, 2009. doi: 10.1186/1476-7120-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haseba S, Sakakima H, Kubozono T, Nakao S, Ikeda S. Combined effects of repeated sauna therapy and exercise training on cardiac function and physical activity in patients with chronic heart failure. Disabil Rehabil 38: 409–415, 2016. doi: 10.3109/09638288.2015.1044032. [DOI] [PubMed] [Google Scholar]
- 91.Ohori T, Nozawa T, Ihori H, Shida T, Sobajima M, Matsuki A, Yasumura S, Inoue H. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol 109: 100–104, 2012. doi: 10.1016/j.amjcard.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 92.Sobajima M, Nozawa T, Fukui Y, Ihori H, Ohori T, Fujii N, Inoue H. Waon therapy improves quality of life as well as cardiac function and exercise capacity in patients with chronic heart failure. Int Heart J 56: 203–208, 2015. doi: 10.1536/ihj.14-266. [DOI] [PubMed] [Google Scholar]
- 93.Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Tanaka N, Toyama Y. Effects of hot water bath or sauna on patients with congestive heart failure: acute hemodynamic improvement by thermal vasodilation. J Cardiol 24: 175–183, 1994. [PubMed] [Google Scholar]
- 94.Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91: 2582–2590, 1995. doi: 10.1161/01.CIR.91.10.2582. [DOI] [PubMed] [Google Scholar]
- 95.Tei C, Imamura T, Kinugawa K, Inoue T, Masuyama T, Inoue H, Noike H, Muramatsu T, Takeishi Y, Saku K, Harada K, Daida H, Kobayashi Y, Hagiwara N, Nagayama M, Momomura S, Yonezawa K, Ito H, Gojo S, Akaishi M, Miyata M, Ohishi M, Investigators W-CS, WAON-CHF Study Investigators. Waon therapy for managing chronic heart failure—results from a multicenter prospective randomized WAON-CHF Study. Circ J 80: 827–834, 2016. doi: 10.1253/circj.CJ-16-0051. [DOI] [PubMed] [Google Scholar]
- 96.Thomas KN, van Rij AM, Lucas SJ, Cotter JD. Lower-limb hot-water immersion acutely induces beneficial hemodynamic and cardiovascular responses in peripheral arterial disease and healthy, elderly controls. Am J Physiol Regul Integr Comp Physiol 312: R281–R291, 2017. doi: 10.1152/ajpregu.00404.2016. [DOI] [PubMed] [Google Scholar]
- 97.Neff D, Kuhlenhoelter AM, Lin C, Wong BJ, Motaganahalli RL, Roseguini BT. Thermotherapy reduces blood pressure and circulating endothelin-1 concentration and enhances leg blood flow in patients with symptomatic peripheral artery disease. Am J Physiol Regul Integr Comp Physiol 311: R392–R400, 2016. doi: 10.1152/ajpregu.00147.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pellinger TK, Neighbors CB, Simmons GH. Acute lower leg heating increases exercise capacity in patients with peripheral artery disease. J Cardiovasc Nurs 34: 130–133, 2019. doi: 10.1097/JCN.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 99.Shinsato T, Miyata M, Kubozono T, Ikeda Y, Fujita S, Kuwahata S, Akasaki Y, Hamasaki S, Fujiwara H, Tei C. Waon therapy mobilizes CD34+ cells and improves peripheral arterial disease. J Cardiol 56: 361–366, 2010. doi: 10.1016/j.jjcc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 100.Olver TD, Grunewald ZI, Jurrissen TJ, MacPherson REK, LeBlanc PJ, Schnurbusch TR, Czajkowski AM, Laughlin MH, Rector RS, Bender SB, Walters EM, Emter CA, Padilla J. Microvascular insulin resistance in skeletal muscle and brain occurs early in the development of juvenile obesity in pigs. Am J Physiol Regul Integr Comp Physiol 314: R252–R264, 2018. doi: 10.1152/ajpregu.00213.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab 293: E1134–E1139, 2007. doi: 10.1152/ajpendo.00516.2006. [DOI] [PubMed] [Google Scholar]
- 102.Katakam PV, Tulbert CD, Snipes JA, Erdos B, Miller AW, Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol 288: H854–H860, 2005. doi: 10.1152/ajpheart.00715.2004. [DOI] [PubMed] [Google Scholar]
- 103.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol 28: 1982–1988, 2008. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Engelland RE, Hemingway HW, Tomasco OG, Olivencia-Yurvati AH, Romero SA. Neural control of blood pressure is altered following isolated leg heating in aged humans. Am J Physiol Heart Circ Physiol 318: H976–H984, 2020. doi: 10.1152/ajpheart.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eaton SB, Eaton SB. Physical inactivity, obesity, and type 2 diabetes: an evolutionary perspective. Res Q Exerc Sport 88: 1–8, 2017. doi: 10.1080/02701367.2016.1268519. [DOI] [PubMed] [Google Scholar]
- 106.Mayer-Davis EJ, Costacou T. Obesity and sedentary lifestyle: modifiable risk factors for prevention of type 2 diabetes. Curr Diab Rep 1: 170–176, 2001. doi: 10.1007/s11892-001-0030-x. [DOI] [PubMed] [Google Scholar]
- 107.Venables MC, Jeukendrup AE. Physical inactivity and obesity: links with insulin resistance and type 2 diabetes mellitus. Diabetes Metab Res Rev 25, Suppl 1: S18–S23, 2009. doi: 10.1002/dmrr.983. [DOI] [PubMed] [Google Scholar]
- 108.Olver TD, Hazell TJ, Hamilton CD, Shoemaker JK, Lemon PW. Impaired superficial femoral artery vasodilation and leg blood flow in young obese women following an oral glucose tolerance test. Appl Physiol Nutr Metab 37: 176–183, 2012. doi: 10.1139/h11-148. [DOI] [PubMed] [Google Scholar]
- 109.Reynolds LJ, Credeur DP, Manrique C, Padilla J, Fadel PJ, Thyfault JP. Obesity, type 2 diabetes, and impaired insulin-stimulated blood flow: role of skeletal muscle NO synthase and endothelin-1. J Appl Physiol (Bethesda, MD: 1985) 122: 38–47, 2017. doi: 10.1152/japplphysiol.00286.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Young BE, Padilla J, Finsen SH, Fadel PJ, Mortensen SP. Role of endothelin-1 receptors in limiting leg blood flow and glucose uptake during hyperinsulinemia in type 2 diabetes. Endocrinology 163: bqac008, 2022. doi: 10.1210/endocr/bqac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Limberg JK, Soares RN, Power G, Harper JL, Smith JA, Shariffi B, Jacob DW, Manrique-Acevedo C, Padilla J. Hyperinsulinemia blunts sympathetic vasoconstriction: a possible role of β-adrenergic activation. Am J Physiol Regul Integr Comp Physiol 320: R771–R779, 2021. doi: 10.1152/ajpregu.00018.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Limberg JK, Smith JA, Soares RN, Harper JL, Houghton KN, Jacob DW, Mozer MT, Grunewald ZI, Johnson BD, Curry TB, Baynard T, Manrique-Acevedo C, Padilla J. Sympathetically mediated increases in cardiac output, not restraint of peripheral vasodilation, contribute to blood pressure maintenance during hyperinsulinemia. Am J Physiol Heart Circ Physiol 319: H162–H170, 2020. doi: 10.1152/ajpheart.00250.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roberts-Thomson KM, Parker L, Betik AC, Wadley GD, Gatta PAD, Marwick TH, Keske MA. Oral and intravenous glucose administration elicit opposing microvascular blood flow responses in skeletal muscle of healthy people: role of incretins. J Physiol 600: 1667–1681, 2022. doi: 10.1113/JP282428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberts-Thomson KM, Betik AC, Premilovac D, Rattigan S, Richards SM, Ross RM, Russell RD, Kaur G, Parker L, Keske MA. Postprandial microvascular blood flow in skeletal muscle: Similarities and disparities to the hyperinsulinaemic-euglycaemic clamp. Clin Exp Pharmacol Physiol 47: 725–737, 2020. doi: 10.1111/1440-1681.13237. [DOI] [PubMed] [Google Scholar]
- 115.Romero SA, Gagnon D, Adams AN, Cramer MN, Kouda K, Crandall CG. Acute limb heating improves macro- and microvascular dilator function in the leg of aged humans. Am J Physiol Heart Circ Physiol 312: H89–H97, 2017. doi: 10.1152/ajpheart.00519.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol 310: H648–H653, 2016. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Heinonen I, Brothers RM, Kemppainen J, Knuuti J, Kalliokoski KK, Crandall CG. Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol (Bethesda, MD: 1985) 111: 818–824, 2011. doi: 10.1152/japplphysiol.00269.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


