Abstract
Background
Coronavirus disease 19 (COVID-19) and Mycobacterium tuberculosis (MTB) are among the top ongoing health crises globally. Both cause respiratory diseases, and the clinical presentations are similar. There is no summarized information about cases of COVID-19 patients with concomitant TB infection from different settings. Therefore this review aimed to summerize the clinical features and treatment outcomes of coronavirus and tuberculosis co-infected patients.
Methods
An electronic search of case reports published between 2020 and 2021 was conducted using Google Scholar, PubMed, Scopus, and ScienceDirect. From eligible reports, data were collected for the selected variables. We analyzed the collected information using SPSS version 27 software. Descriptive statistics were computed for the selected variables.
Results
A total of 83 patient histories were collected from 47 case reports. The majority (80%) of the cases were reported for male patients. The mean age was 42.6 years (3 months to 84 years, SD=17.3). Fever was reported in 80% of cases, followed by cough (73.3%) and hypotension (37.1%). Blood cell parameters revealed lymphopenia (52%), lower hemoglobin (30%), elevated CRP (70%), elevated ferritin (28%), and increased D-dimer (23.4%). Treatment outcome is significantly associated with blood cell count results (p-0.044) and a rise in blood inflammatory cytokines(p-0.041). The mean days for viral clearance or negative PCR was 23 days (Range 5–82 days) and the overall mean duration of hospitalization was 27 days. The total death rate was 22.4%. Recovery was reported for 76.6% of cases. Survival status (p-0.613) and disease severity (p-0.68) are not significantly associated with the gender of the participants.
Conclusion
An alteration in blood cell parameters is associated with an unfavorable treatment outcome. There is a higher death rate in COVID-19/TB co-infection. The death is associated with older age, smoking or smoking history, drug abuse, and co-morbidity of non-communicable diseases. Conversely, there is a lower death rate in HIV patients.
Keywords: coronavirus, SARSCOV-2, COVID-19, Mycobacterium tuberculosis, tuberculosis, TB, co-infection
Background
Viral and bacterial diseases continued, causing epidemics and pandemics throughout human history. A pandemic of coronavirus disease 19 (COVID-19) and the epidemic of disease caused by Mycobacterium tuberculosis (MTB) are the top ongoing health crises worldwide. Coronavirus is a virus in the kingdom of Riboviria and the family of corona-viridae.1 The viruses in this family are positive-sense RNA viruses known to cause acute respiratory syndrome (SARS).2 COVID-19 is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-Cov-2). The disease is an ongoing pandemic, claiming the lives of millions throughout the world. Until early June 2022, an estimated 529 million confirmed cases and more than 6 million deaths had been reported globally.3 Tuberculosis (TB), on the other hand, is a chronic infectious bacterial disease affecting the lungs and other organs. Annually, there are an estimated 10 million infections and 1.4 million deaths due to TB.4
COVID-19 and TB patients share plenty of common clinical and radiological features.5 Both are primarily respiratory pathogens and are highly contagious. Although the risk of COVID-19 in activating latent TB(LTBI) infection has not been studied, different case reports indicate the occurrence of TB cases either before the incidence of COVID-19,6,7 simultaneous incidence,8,9 or after infection with COVID-19.10,11 The co-occurrence of these deadly pathogens causes difficulty in identifying each other. Clinical management is also challenging.12
Clinical features such as fever, fatigue, chest pain, cough, weight loss, night sweats, and loss of appetite have been known as identifying signs and symptoms for TB screening.13 These signs were also common among COVID-19 patients.14 Thus, there is an overlapping set of clinical characteristics that are common among TB/COVID-19 co-infected patients. In a study analyzing the interface of COVID-19 and TB, patients were identified to have all these signs, including fatigue without change in test or smell.15
It has been anticipated that the COVID-19 pandemic might affect the clinical course of TB or vice versa. A study has reported TB as an independent risk factor for severe COVID-19.17 However, there is no consensus idea on the impact of the two diseases on each other’s clinical course and patient treatment outcomes. For instance, in a study describing the clinical characteristics of COVID-19 in TB patients and factors for the disease severity, a lower risk of COVID-19 and a lower mortality risk have been noted in TB patients co-infected with COVID-19.16
The various published studies have been conducted at the facility level. Reviewing experiences from various settings for individual case management provides unbiased information about what happened to patients co-infected with COVID-19 and TB. In this study, we have examined case reports of COVID-19 and TB co-infection to investigate the distinguishing clinical characteristics and treatment outcomes for individual case management.
Methods
Search Strategy
We conducted a systematic review of published case reports and summarized the common reported clinical characteristics and treatment outcomes of COVID-19 and TB co-infected patients from different settings. We systematically searched electronic databases: Google Scholar, Scopus, ScienceDirect, and PubMed for case reports published in English. We followed a sensitive searching method to capture all available articles in the databases. We have restricted our search to papers published between 2020 and 2021. The electronic database search was conducted from June 1, 2021, to July 2, 2021. We used a search strategy by combining key-terms: “Coronavirus”, “SARSCOV-2”, “COVID-19”, “Mycobacterium tuberculosis”, “tuberculosis”, “TB”, “co-infection.”
Inclusion and Exclusion Criteria
We included case reports and case series reports that described the clinical characteristics and treatment outcomes of patients with TB and COVID-19 co-infection. Eligible reports include reports containing all diagnostic and treatment parameters, from case presentation to treatment outcome, in different countries across the world. We excluded reports published in other languages than English. Studies with different study designs were also excluded. Our justification for choosing only case reports is that first, we are interested in individual clinical data from different settings. Second, we wanted to see what experiences were there from different settings while diagnosing and treating the new pandemic.
Case Report Selection Process
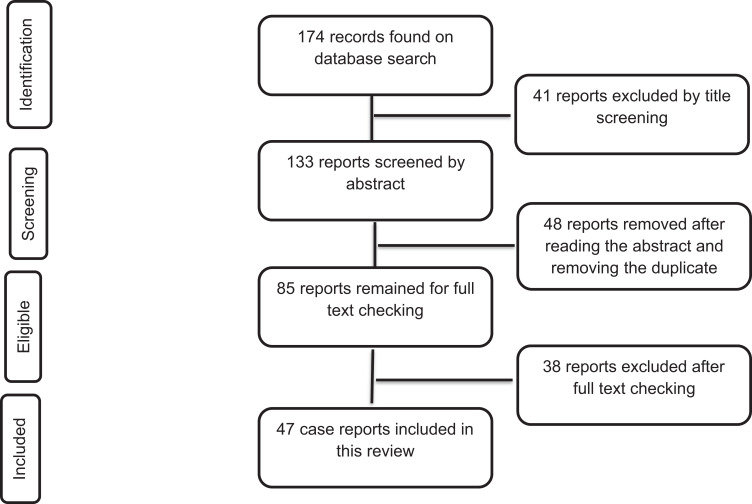
From database searches, we identified a total of 174 records. Due to a lack of either of the variables of interest for this review, a total of 41 records were excluded upon title screening. After abstract screening and removing the duplicates, a total of 48 reports were excluded based on the exclusion criteria. Full-text reading excluded 38 additional reports, and 47 reports remained to be included in the final review. Thus, a total of 47 case reports were included in this review (Figure 1). Eligibility assessment was performed independently by the three authors and disagreements were resolved by discussion.
Figure 1.
Flow chart diagram describing the selection of case reports included in the systematic review of clinical features and treatment outcomes of coronavirus and tuberculosis co-infected patients.
Data Extraction and Quality Check
Data collection was done on an excel sheet. The data is double-checked for missing information and data entry errors. One of the review authors (HM) extracted the data from included studies, and the second author (DC) checked the retrieved information. Any disagreements were resolved by discussion and confirmation by the third author (DB). We extracted information on the clinical characteristics of participants and the final treatment outcome. Furthermore, treatment status, days of hospitalization, travel, and previous history of TB were collected. After quality assurance measures, the data is exported into SPSS version 27 software for statistical analysis.
Statistical Analysis
Coding and recoding of qualitative variables were performed. Stratification of cases according to their outcome was performed to create an association with the predictor of the outcome. Descriptive statistics were computed for categorical variables. An odds ratio was calculated for the analysis of risk factors.
Results and Discussion
Clinical and Demographic Characteristics of Participants
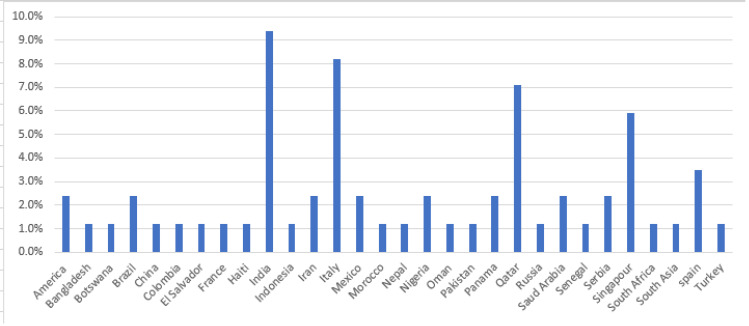
A total of 83 histories of cases (patient information) from 47 reports were collected. All the cases have reported diagnosis and treatment of COVID-19 and TB co-infections. The reports consisted of as many as eight case series or a single case per report. The proportion of male patients was 80%. The mean age was 42.6 years (3 months–84 years, SD=17.3). The highest case reported was from India (9.8%), followed by Italy (8.2%) and Qatar (7.1%). The country in which the case report was published is indicated in Figure 2. Only 10.5% of patients have reported contact with active COVID-19 cases or travel history, and 4.7% had TB contact history. Among the cases, 2.45% had been treated for COVID-19 before, 8.2% had a history of TB treatment, and 2.45% had chronic hepatitis. Upon admission, 10.6% had a negative initial COVID-19 PCR test and 2.4% had an indeterminate test result.
Figure 2.
Number of case report distribution by country.
Common Clinical Features of Coronavirus-TB Co-Infection
Only 43% of cases reported a severe illness, while others had a mild or non-apparent clinical illness. Cough was reported in 80% of cases, fever in 73%, but only 37.1% had hypotension. Half of the participants (50%) had an oxygen saturation level (SpO2) report. Most cases (30.3%) in the reviewed report had a SpO2 level of 90–97% in room air. The minimum measurement was 68% and the highest was 99%. A lower SpO2 level is an early indicator that the patient needs medical attention.18
The complete blood cell analysis revealed an altered count of blood cells and lower hemoglobin in 45.3% of cases. Most of these abnormal blood cell counts are lymphopenia (52%). Lymphopenia is consistently reported as a common clinical feature of COVID-19 cases.19–21 Therefore, given the short turnaround time for profiling blood cell parameters, such markers in addition to the clinical sign and symptom assessment might help to suspect and isolate cases for better management.19 Lower hemoglobin levels were found in 30% of the cases. The lower hemoglobin level is associated with COVID-19 pneumonia and the worsening of the disease.22 Blood chemistry also revealed half of the participants (50%) had a significant rise in liver enzymes. Among them, 70% had elevated C-reactive protein (CRP), 28% had elevated ferritin, and 23.4% had an increased D-dimer. Adenosine deaminase (ADA) and lactate dehydrogenase (LDH) were also common. Such an increase in liver enzyme activity is because of activation of both innate and adaptive immune responses and is an indication of systemic inflammation.23
There were typical COVID-19 signs and symptoms in 40.7% of cases; 22% had TB signs, and 31% had atypical or non-specific symptoms. Moreover, 7% had concurrent COVID-19 symptoms with abdominal pain. Of these, 1.2% of them had both TB and COVID-19 symptoms. Non-specific symptoms reported include muscle pain, voice changes, anxiety, and dehydration. Such nonspecific clinical manifestations may appear before the occurrence of common symptoms like fever and cough, which should be investigated closely as an initial measure for suspecting.24
The mean time for viral clearance or negative PCR was 23 days (range 5–82 days). However, the overall mean duration of hospitalization is higher (27 days). All the treatment of TB was done under a directly observed treatment short-course (DOTS). The common form of TB (86%) during COVID-19 coinfection appears in pulmonary TB (PTB), and the proportion of extrapulmonary TB (EPTB) is 14%. More TB (36%) has occurred after the onset of COVID-19. In 33.7% of cases, TB and COVID-19 occurred concurrently, and 30.2% were admitted for TB before developing COVID-19. A modeling study by WHO has reported that the pandemic might fuel the future TB epidemic mainly because of utilizing manpower and diagnostic utilities in response to the pandemic.4 In addition, studies are also reporting the effect of the pandemic in promoting the activation of latent TB cases.25
The Risk Factors for Favorable and Unfavorable Treatment Outcomes
The total death rate was 22.4%. Among the cases with unfavorable treatment outcomes, 86.7% were primarily diagnosed with TB, and COVID-19 infection was recorded as a secondary superinfection (Table 1). Recovery was reported for 76.6% of cases. Among these, 43.9% were simultaneously diagnosed with the two diseases (Table 2). The common medications recorded in the history of survivors are ceftriaxone, azithromycin, hydroxychloroquine, clarithromycin, lopinavir/ritonavir, oseltamivir, Sovodak, remdesivir, heparin, enoxaparin, oxygen supplementation, and Rifampicin (RIF), Isoniazid (INH), Pyrazinamide (PZA), and Ethambutol (EMB) anti-TB drugs.
Table 1.
Clinical Characteristics of TB Patients with COVID-19 Secondary Superinfection
| First Author and Year of Publication | Country of the Study/Citizenship | Addiction | TB Site of Infection | TB History | Comorbidity | Treatment Outcomes |
|---|---|---|---|---|---|---|
| Ata F et al 202029 | India | None | PTB | Unknown | Kidney disease | Recovered |
| Gadelha et al 20206 | Brazil | Alcohol consumption | PTB | Relapse | Cardiac disease | Recovered |
| Motta I et al 20207 | Italy/Spain | Drug/Alcohol/Smoking | PTB | Contact/Unknown | Cardiac disease/Hypertensio, kidney disease, HIV | Died |
| Sarinoglu RC et al 202037 | Turk | None | PTB | Unknown | DM, Kidney disease/ Hypertension | Recovered |
| Vilbrun SC et al 202038 | Haiti | None | PTB | Unknown | Hypertension | Recovered |
| Baskara MA, et al 202141 | Indonesia | None | PTB | Unknown | Diabetic | Recovered |
| Fard NG et al 202143 | Indonesia | None | EPTB | Unknown | Kidney disease | Recovered |
| Farias L et al 202044 | Italy | Drug abuse | PTB | Unknown | Hypertension | Recovered |
| Gbenga TA et al 202045 | Nigeria | None | PTB | Unknown | None | Died |
| Gerstein S et al 202146 | El Salvador | Alcohol consumption | EPTB | Unknown | None | Recovered |
| Sarma U et al 20205 | India | None | PTB | Unknown | Diabetic | Recovered |
Table 2.
Treatment Outcome of Patients Simultaneously Diagnosed for COVID-19 and TB
| First Author and Year of Publication | Country of the Study | Covid-19 Treatment | TB Treatment | Treatment Outcomes |
|---|---|---|---|---|
| Chen ZY et al 202030 | China | Lopinavir/ritonavir, Umifenovir hydrochloride, Interferon alpha, Corticosteroids | Standard first line (RHZE) | 1 patient died, 2 recovered |
| Essajee F et al 202031 | South Africa | dexamethasone | Standard first line (RHZE) | recovered |
| Freij BJ et al 202032 | America | Dexamethasone, hydroxychloroquine, azithromycin | Not treated | Died |
| Martínez Orozco JA et al 202033 | Mexico | Not reported | Standard first line (RHZE) | Recovered |
| Motta I et al 20207 | Spain | Hydroxy-chloroquine, ritonavir/lopinavir, azithromycin, piperacillin, tazobactam | Standard first line (RHZE) | Died |
| Mulale UK et al 202134 | Botswana | Not reported | Standard first line (RHZE) | Died |
| Rivas N et al 202035 | Panama | Levofloxacin, azithromycin | Standard first line (RHZE) | Recovered |
| Shabrawishi M et al 202136 | Saudi Arabia | ceftriaxone and azithromycin. | Standard first line (RHZE) | Recovered |
| Yadav S et al 202010 | India | Not reported | Standard first line (RHZE) | Recovered |
| Yousaf Z et al 202011 | Qatar | Ceftriaxone, Azithromycin, and Hydroxychloroquine | Standard first line (RHZE) | Recovered |
| Al Lawati R et al 202139 | Oman | ceftriaxone, clarithromycin and oseltamivir, Hydroxychloroquine and lopinavir/ritonavir | Standard first line (RHZE) | Recovered |
| AlKhateeb MH et al 202040 | South Asia | Not reported | Standard first line (RHZE) | Recovered |
| Chowdhury D et al 202042 | Bangladesh | Favipiravir, ceftriaxone, clarithromycin | first line (RHZE) | Recovered |
| Luciani M et al 202047 | Italy | lopinavir/ritonavir, hydroxychloroquine | first line (RHZE) | Recovered |
| Maaroufi A et al 202148 | Morocco | (lopinavir/ritonavir), azithromycin, dexamethasone | first line (RHZE) | Recovered |
| Ortiz-Martínez Y et al 202149 | Columbia | ampicillin/sulbactam, doxycycline, dexamethasone | first line (RHZE) | Died |
| Patil S et al 202150 | India | remdesivir, methyl prednisolone, heparin | first line (RHZE) | Recovered |
| Pujari S et al 202051 | India | methylprednisolone | first line (RHZE) | Recovered |
| Rajput D et al 202152 | India | Not reported | first line (RHZE) | Recovered |
| Rodriguez JA et al 202153 | America | convalescent plasma, remdesivir, hydroxychloroquine, azithromycin | Not treated | Died |
| Sanyaolu A et al 202054 | Nigeria | Clarithromycin, Hydroxychloroquine, and lopinavir/ritonavir | first line (RHZE) | Recovered |
| Stjepanović M et al 202155 | Serbia | azithromycin, chloroquine, fluoroquinolone, cephalosporine | first line (RHZE) | Recovered |
| Valdivieso-Jiménez JA et al 202056 | Mexico | cephalosporins, amikacin, macrolide, carbapenems | first line (RHZE) | Recovered |
| Wong SW et al 202157 | Singapore | Remdesivir | first line (RHZE) | Recovered |
Survival or death is not associated with the gender of the participants (p-0.613, Cramer’s V-0.01). The odds ratio of the unfavorable treatment outcome (death) in both sexes is 0.9 (95% CI, 0.23–3.75). Gender was reported as a risk factor for death in COVID-19 patients, in which the male gender is indicated as a risk.20 The lower association of gender as a predictor of unfavorable treatment outcomes in this review might be due to the lower female-to-male ratio. On the other hand, being older is significantly associated with death (p 0.001). Age, together with other variables, was also reported as an independent risk factor for death among such patients.26
Treatment outcome is significantly associated with blood cell count results (p 0.044). The odds ratio of an unfavorable treatment outcome is 0.236 (95% CI: 0.61–0.9) in individuals with altered blood cell counts. A study conducted in the USA, using blood samples of confirmed COVID-19 cases, also showed a significant association between lower mean lymphocyte count and death.19 Similarly, a rise in blood inflammatory cytokines such as CRP, D-dimer, ferritin, and IL-6 has a significant association with the treatment outcome (p-0.041). The odds of unfavorable treatment outcomes are 0.289 times higher in individuals having raised inflammatory cytokines (95% CI:0.084–0.99). Such a cytokine storm potentially predicts mortality and can be used as a preliminary indicator for intensive care and treatment.27
After adjusting for patients with no addiction, 66% of smokers and 37.5% of drug abusers have died. Smoking or smoking history is a risk for death (AOR=7, 95% CI;0.86–56.9). Similarly, drug abusers are more likely to die than non-drug users (AOR=1.8;95% CI;0.2–15).
A higher percentage of deaths was recorded among patients with co-morbidities such as anemia (40%), hypertensive patients (41.7%), patients with chronic renal failure (42.9%), and patients with hepatitis B virus and/or liver disease (60%). This finding was consistent with a meta-analysis that identified comorbidity as a facilitator of increased case fatality in any infectious disease.28
The death rate is comparatively lower among HIV patients (28.6%). Another case series report also showed no extensive morbidity or mortality associated with COVID-19 cases in people living with HIV (PLWH).20 Antiretroviral therapy might play a protective role in patients with HIV. However, validation of this hypothesis in a larger longitudinal cohort is important.
Only a single case (1.17%) of multi-drug-resistant TB (MDR TB) was reported, which might be an indicator of the reactivation of latent tuberculosis during the COVID-19 era. Moreover, non-tuberculosis mycobacteria (NTM) were also isolated from two cases in which untreated cases had died. The role of NTM in this regard needs further study.
The Implication of Patient Characteristics for Disease Severity
About 60% of severe cases end in death. Disease severity is higher at older ages (> 30 years), which accounts for 73.3%. Other reports were also in line with this finding.20 Disease severity is mainly dependent on the immune status of the individual, and the deterioration of the immune system at advanced age might play a crucial role in disease severity. In addition, 20% of severe cases were also reported among children 5 years of age. Many (86.7%) severe cases have reported fever. Fever is also reported as the major clinical sign of the disease.24 However, only 46.7% of severe cases had a coughing symptom. Disease severity has no significant association with gender (p-0.68, OR =0.9, 95% CI; 0.12–4.9). However, more severe cases were noted among females (27.7%) as compared to males (22.4%) in this review. Disease severity was also high among patients with another comorbidity (65%).
Conclusion
Fever, cough, hypotension, altered blood cell count and liver enzymes, and lower hemoglobin are common in COVID-19/TB co-infection. An alteration in blood cell parameters is associated with an unfavorable treatment outcome. There is a higher death rate in COVID-19/TB co-infection, and it is associated with older age, smoking or smoking history, drug abuse, and co-morbidity of non-communicable diseases. Conversely, there is a lower death rate in HIV patients. This might be the role of antiviral drugs. Further studies associating immune cell function in HIV patients need to be investigated.
Recommendation
A large-scale longitudinal study is needed to see the more precise co-appearance of the two respiratory pathogens, mainly in TB diagnostic and treatment initiating centers.
Limitation of the Review
This review is not without limitations. First, the articles collected in this review are only cases in which we are unable to calculate the prevalence of the co-occurrence of the two diseases. Next, the quality assurance measures in all reported parameters of cases are not indicated and thus are not measurable.
Acknowledgment
We acknowledge clinicians for reporting and publishing the cases used for reviewing this article.
Funding Statement
There is no funding to report.
Abbreviations
ADA, Adenosine Di Aminase; AOR, Adjusted Odds Ratio; BCG, Bacillus Calmette Guerin Vaccine; COVID 19, Corona Virus Disease −2019; CRP, Inflammatory Reactive C- Protein; DOT, Directly Observed Therapy; HIV, Human Immuno-Deficiency Virus; LDH, Lactate De Hydrogenase; MTB, Mycobacterium Tuberculosis; OR, Odds Ratio; PCR, Polymerase Chain Reaction; PLWH, Peoples Living with Human Immuno-Deficiency Virus; RHZE, Rifampicin, Isoniazid, Pyrazinamide, Ethambutol; SARS COV 2, Severe Acute Respiratory Syndrome Corona Virus; TB, Tuberculosis; WHO, World Health Organization.
Data Sharing Statement
The data related to this document can be obtained by contacting the principal investigator.
Author Information
Hilina Mollalign is an associate researcher at the National Tuberculosis Reference Laboratory, Ethiopian Public Health Institute.
Dawit Chala is the technical manager and COVID-19 testing coordinator at the National Immunohematology Reference Laboratory, Ethiopian Public Health Institute.
Dereje Beyene is an assistant professor in molecular genetics and functional genomics at Addis Ababa University College of Natural and Computational Science, Department of Microbial, Cellular, and Molecular Biology.
Author Contributions
All authors have made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
- 1.Mavrodiev EV, Tursky ML, Mavrodiev NE, Ebach MC, Williams DM. On classification and taxonomy of coronaviruses (Riboviria, Nidovirales, Coronaviridae) with a special focus on severe acute respiratory syndrome-related coronavirus 2 (SARS-Cov-2). bioRxiv. 2020. doi: 10.1101/2020.10.17.343749 [DOI] [Google Scholar]
- 2.Keni R, Alexander A, Nayak PG, Mudgal J, Nandakumar K. COVID-19: emergence, spread, possible treatments, and global burden. Frontiers in Public Health. 2020;8:216. doi: 10.3389/fpubh.2020.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Epidemiological Update on COVID-19. World Health Organization; 2022. [Google Scholar]
- 4.World Health Organization. Global Tuberculosis Report. World Health Organization; 2020. [Google Scholar]
- 5.Sarma U, Mishra V, Goel J, Yadav S, Sharma S, Sherawat RK. Covid-19 pneumonia with delayed viral clearance in a patient with active drug-resistant pulmonary tuberculosis. Ind J Crit Care Med. 2020;24(11):1132. doi: 10.5005/jp-journals-10071-23662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gadelha Farias LAB, Moreira ALG, Corrêa EA, et al. Case report: coronavirus disease and pulmonary tuberculosis in patients with human immunodeficiency virus: report of two cases. Am J Trop Med Hygiene. 2020;103:1593–1596. doi: 10.4269/ajtmh.20-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motta I, Centis R, D’Ambrosio L, et al. Tuberculosis, COVID-19, and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26(4):233–240. doi: 10.1016/j.pulmoe.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riou C, du Bruyn E, Stek C, et al. The relationship between SARS-CoV-2–specific CD4 response and COVID-19 severity, as well as the impact of HIV-1 and tuberculosis coinfection. J Clin Invest. 2021;131(12). doi: 10.1172/JCI149125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shabrawishi M, AlQarni A, Ghazali M, et al. A new disease and old threats: a case series of COVID-19 and tuberculosis coinfections in Saudi Arabia. Clin Case Rep. 2021;9. doi: 10.1002/ccr3.4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadav S, Rawal G. The case of pulmonary tuberculosis with COVID-19 in an Indian male—the first of its type ever reported from South Asia. Pan African Med J. 2020;36. doi: 10.11604/pamj.2020.36.374.24260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousaf Z, Khan AA, Chaudhary HA. Cavitary pulmonary tuberculosis with COVID-19 coinfection. IDCases. 2020;22:e00973. doi: 10.1016/j.idcr.2020.e00973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mousquer GT, Peres A, Fiegenbaum M. Pathology of TB/COVID-19 co-infection: the phantom menacer. Tuberculosis. 2021;126:102020. doi: 10.1016/j.tube.2020.102020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Lu S. COVID-19 and tuberculosis. J Transl Intern Med. 2020;8(2):59–65. doi: 10.2478/jtim-2020-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen JR, Martin MR, Martin JD, Kuhn P, Hicks JB. Modeling the onset of symptoms of COVID-19. Front Public Health. 2020;8. doi: 10.3389/fpubh.2020.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parolina L, Pshenichnaya N, Vasilyeva I, et al. Clinical characteristics of COVID-19 in TB patients and factors associated with the disease severity. Int J Infect Dis. 2022. doi: 10.1016/j.ijid.2022.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.TB/COVID-19 Global Study Group. Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur Respir J. 2022;59(3):2102538. doi: 10.1183/13993003.02538-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J. A rapid systematic review and meta-analysis of the relationship between tuberculosis and COVID-19 severity and mortality. J Med Virol. 2021;93(1):194–196. doi: 10.1002/jmv.26311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illg Z, Muller G, Mueller M, Nippert J, Allen B. Analysis of the absolute lymphocyte count in COVID-19 patients. Am J Emerg Med. 2021;46:16–19. doi: 10.1016/j.ajem.2021.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Härter G, Spinner CD, Roider J, et al. A case series of 33 patients with COVID-19 in people living with human immunodeficiency virus. Infection. 2020;48(5):681–686. doi: 10.1007/s15010-020-01438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): a systematic review and meta-analysis. J Intensive Care. 2020;8(1):36. doi: 10.1186/s40560-020-00453-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anai M, Akaike K, Iwagoe H, et al. A decrease in hemoglobin levels predicts an increased risk for severe respiratory failure. DOI COVID-19 patients with pneumonia. Respir Res. 2021;59(2):187–193. doi: 10.1016/j.resinv.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buczko M, Nita-Lazar A, Park J-H, et al. new insights on the role of cytokines in COVID-19. Nat Immunol. 2021;22(4):404–411. doi: 10.1038/s41590-021-00901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baj J, Karakua-Juchnowicz H, Teresiski G, et al. Specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med. 2020;9(6):1753. doi: 10.3390/jcm9061753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khayat M, Fan H, Vali Y. COVID-19 promoting the development of active tuberculosis in a patient with latent tuberculosis infection: a case report. Respir Med Case Rep. 2021;32:101344. doi: 10.1016/j.rmcr.2021.101344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albitar O, Ballouze R, Ooi JP, Sheikh Ghadzi SM. Risk factors for mortality among COVID-19 patients. Diabetes Res Clin Pract. 2020;166:108293. doi: 10.1016/j.diabres.2020.108293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadeem R, Elhoufi AM, Iqbal NE, et al. Prediction of cytokine storm and mortality in patients with COVID-19 admitted to the ICU: do markers tell the story? Dubai Med J. 2021;4(2):142–150. doi: 10.1159/000514406 [DOI] [Google Scholar]
- 27.Noor FM, Islam MM. Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis. J Commun Health. 2020;45(6):1270–1282. doi: 10.1007/s10900-020-00920-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar DR, Bhattacharya DB, Meena DV, Soneja DM, Wig DN. COVID-19 and TB co-infection - ‘Finishing touch” in perfect recipe to ‘severity’ or ‘death’. J Infect. 2020;81(3):e39–e40. doi: 10.1016/j.jinf.2020.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ata F, Yousaf Q, Parambil JV, Parengal J, Mohamedali MG, Yousaf Z. A 28-year-old man from India with SARS-CoV-2 and pulmonary tuberculosis co-infection with central nervous system involvement. Am J Case Rep. 2020;21:1–5. doi: 10.12659/AJCR.926034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen ZY, Wang Q, Liu W, et al. Three patients with COVID-19 and pulmonary tuberculosis, Wuhan, China, January–February 2020. Emerg Infect Dis. 2020;26(11). doi: 10.3201/EID2611.201536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Essajee F, Solomons R, Goussard P, Van Toorn R. Child with tuberculous meningitis and COVID-19 coinfection complicated by extensive cerebral sinus venous thrombosis. BMJ Case Rep. 2020;13(9):e238597. doi: 10.1136/bcr-2020-238597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freij BJ, Gebara BM, Tariq R, et al. Fatal central nervous system co-infection with SARS-CoV-2 and tuberculosis in a healthy child. BMC Pediatr. 2020;20(1). doi: 10.1186/s12887-020-02308-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez Orozco JA, Sánchez Tinajero Á, Becerril Vargas E, et al. COVID-19 and tuberculosis coinfection in a 51-year-old taxi driver in Mexico City. Am J Case Rep. 2020;21:e927628. doi: 10.12659/AJCR.927628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulale UK, Kashamba T, Strysko J, Kyokunda LT. Fatal SARS-CoV-2 and Mycobacterium tuberculosis coinfection in an infant: insights from Botswana. BMJ Case Rep. 2021;14(4):e239701. doi: 10.1136/bcr-2020-239701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivas N, Espinoza M, Loban A, et al. Case report: COVID-19 recovery from triple infection with mycobacterium tuberculosis, HIV, and SARS-CoV-2. Am J Trop Med Hygiene. 2020;103(4):1597–1599. doi: 10.4269/ajtmh.20-0756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shabrawishi M, AlQarni A, Ghazawi M, et al. New disease and old threats: a case series of COVID-19 and tuberculosis coinfection in Saudi Arabia. Clin Case Rep. 2021;9(5). doi: 10.1002/ccr3.4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarinoglu RC, Sili U, Eryuksel E, Yildizeli SO, Cimsit C, Yagci AK. Tuberculosis and COVID-19: an overlapping situation during pandemic. J Infect Dev Ctries. 2020;14(7):721–725. doi: 10.3855/jidc.13152 [DOI] [PubMed] [Google Scholar]
- 38.Vilbrun SC, Mathurin L, Pape JW, Fitzgerald D, Walsh KF. Case report: multidrug-resistant tuberculosis and COVID-19 coinfection in Port-au-Prince, Haiti. Am J Trop Med Hygiene. 2020;103(5):1986–1988. doi: 10.4269/ajtmh.20-0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al Lawati R, Al Busaidi N, Al Umairi R, et al. COVID-19 and pulmonary mycobacterium tuberculosis coinfection. Oman Med J. 2021;36(5):e298. doi: 10.5001/omj.2022.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AlKhateeb MH, Aziz A, Eltahir M, Elzouki A. Bilateral foot-drop secondary to axonal neuropathy in a tuberculosis patient with co-infection of COVID-19: a case report. Cureus. 2020;12(11). doi: 10.7759/cureus.11734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baskara MA, Makrufardi F, Dinisari A. COVID-19 and active primary tuberculosis in a low-resource setting: a case report. Annals Med Surg. 2021;62:80–83. doi: 10.1016/j.amsu.2020.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chowdhury D, Uddin MR, Bhuiyan S, Das BC, Rahman M, Khan FA. Is COVID-19 masking or delayed the diagnosis of active pulmonary tuberculosis? A case report from Bangladesh. Microbes Infect Dis. 2021;2(2):189–196. [Google Scholar]
- 43.Fard NG, Khaledi M, Afkhami H, et al. COVID-19 coinfection in patients with active tuberculosis: first case-report in Iran. 2021.
- 44.Farias LABG, Moreira ALG, Corrêa EA, et al. Case report: coronavirus disease and pulmonary tuberculosis in patients with human immunodeficiency virus: report of two cases. Am J Trop Med Hyg. 2020;103(4):1593. doi: 10.4269/ajtmh.20-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gbenga TA, Oloyede T, Ibrahim OR, Sanda A, Suleiman BM. Pulmonary tuberculosis in coronavirus disease-19 patients: a report of two cases from Nigeria. Open Access Macedonian J Med Sci. 2020;8(T1):272–275. doi: 10.3889/oamjms.2020.5298 [DOI] [Google Scholar]
- 46.Gerstein S, Khatri A, Roth N, Wallach F. Coronavirus disease 2019 and extra-pulmonary tuberculosis co-infection–A case report and review of literature. J Clin Tuberculos Other Mycobacter Dis. 2021;22:100213. doi: 10.1016/j.jctube.2021.100213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luciani M, Bentivegna E, Spuntarelli V, et al. Coinfection of tuberculosis pneumonia and COVID-19 in a patient vaccinated with Bacille Calmette-Guérin (BCG): case report. SN Comprehensive Clin Med. 2020;1–4. doi: 10.1007/s42399-020-00601-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maaroufi A, Diai A, Jebbar N, et al. Tuberculous meningitis and COVID-19 co-infection: about a case. Open J Clin Diagn. 2021;11(2):52–58. doi: 10.4236/ojcd.2021.112004 [DOI] [Google Scholar]
- 49.Ortiz-Martínez Y, Mogollón-Vargas JM, López-Rodríguez M, Rodriguez-Morales AJ. A fatal case of triple coinfection: COVID-19, HIV and tuberculosis. Travel Med Infect Dis. 2021;43:102129. doi: 10.1016/j.tmaid.2021.102129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patil S, Gondhali G. COVID-19 pneumonia with pulmonary tuberculosis: double trouble. Int J Mycobacteriol. 2021;10(2):206. doi: 10.4103/ijmy.ijmy_51_21 [DOI] [PubMed] [Google Scholar]
- 51.Pujari S, Gugale P, Shah D, et al. Pulmonary tuberculosis masquerading as coronavirus disease 2019 in an HIV-infected individual with advanced immune suppression. Aids. 2020;34(15):2165–2168. doi: 10.1097/QAD.0000000000002676 [DOI] [PubMed] [Google Scholar]
- 52.Rajput D, Kumar S, Rai A, Chezhian S. Diagnostic and surgical challenges in disseminated tuberculosis presenting as acute abdomen during COVID-19 pandemic. JRSM Open. 2021;12(2):2054270420985736. doi: 10.1177/2054270420985736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez JA, Bonnano C, Khatiwada P, Roa AA, Mayer D, Eckardt PA. COVID-19 coinfection with mycobacterium abscessus in a patient with multiple myeloma. Case Rep Infect Dis. 2021;2021:1–5. doi: 10.1155/2021/8840536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanyaolu A, Prakash S, Marinkovic A, Okorie C. Pulmonary tuberculosis in coronavirus disease-19 patients: report of cases. Asclepius Med Case Rep. 2020;3(2):1–3. [Google Scholar]
- 55.Stjepanović M, Belić S, Buha I, Marić N, Baralić M, Mihailović-Vučinić V. Unrecognized tuberculosis in a patient with COVID-19. Srp Arh Celok Lek. 2021;6:70–73. [Google Scholar]
- 56.Valdivieso-Jiménez JA, Gaxiola-Ortiz AV, Cortes-Telles A. COVID-19, HIV and pulmonary tuberculosis. A triple threat to consider. J Respir Dis. 2020;2:1–2. [Google Scholar]
- 57.Wong SW, Ng JKX, Chia YW. Tuberculous pericarditis with tamponade diagnosed concomitantly with COVID-19: a case report. Eur Heart J Case Rep. 2021;5(1):491. doi: 10.1093/ehjcr/ytaa491 [DOI] [PMC free article] [PubMed] [Google Scholar]