Abstract
Selenium-accumulating Astragalus spp. contain an enzyme which specifically transfers a methyl group from S-methylmethionine to the selenol of selenocysteine, thus converting it to a nontoxic, since nonproteinogenic, amino acid. Analysis of the amino acid sequence of this enzyme revealed that Escherichia coli possesses a protein (YagD) which shares high sequence similarity with the enzyme. The properties and physiological role of YagD were investigated. YagD is an S-methylmethionine: homocysteine methyltransferase which also accepts selenohomocysteine as a substrate. Mutants in yagD which also possess defects in metE and metH are unable to utilize S-methylmethionine for growth, whereas a metE metH double mutant still grows on S-methylmethionine. Upstream of yagD and overlapping with its reading frame is a gene (ykfD) which, when inactivated, also blocks growth on methylmethionine in a metE metH genetic background. Since it displays sequence similarities with amino acid permeases it appears to be the transporter for S-methylmethionine. Methionine but not S-methylmethionine in the medium reduces the amount of yagD protein. This and the existence of four MET box motifs upstream of yfkD indicate that the two genes are members of the methionine regulon. The physiological roles of the ykfD and yagD products appear to reside in the acquisition of S-methylmethionine, which is an abundant plant product, and its utilization for methionine biosynthesis.
Escherichia coli is known to possess two enzymes (methionine synthases) that catalyze the last step in methionine biosynthesis. The metE gene product, a zinc-containing monomer (9) with a molecular mass of 85 kDa (37), transfers the methyl group of N5-methyl-tetrahydrofolate to the thiolate of homocysteine. The second enzyme, the metH gene product, is also a monomer but with a size of 136 kDa (8); it catalyzes methyl transfer from N5-methyl-tetrahydrofolate to the cob(I)alamine coenzyme and from there to homocysteine. MetH exhibits a distinct structure of four domains, which can be correlated with the partial reactions catalyzed: an N-terminal homocysteine binding domain carrying a zinc ion, an N5-methyl-tetrahydrofolate binding domain, a cobalamine binding domain, and a C-terminal domain for S-adenosylmethionine binding involved in reactivation of the oxidized cob(II)alamine form (6, 10, 11). Since E. coli cannot synthesize corrinoids, MetH is only active when cobalamine is present in the medium. MetH is about 100-fold more active than MetE, which is, however, compensated by the very strong expression of the metE gene (37).
Cloning and sequencing of the gene for a selenocysteine methyltransferase (smtA) from Astragalus bisulcatus involved in selenium tolerance and comparison of the sequence with entries in the databases revealed that E. coli possesses a gene (yagD) whose derived product shares 40% amino acid sequence identity with SmtA (25). In addition, YagD shows low sequence similarities to the amino-terminal domain of MetH and to human betaine:homocysteine methyltransferase (data not shown). The regions that are conserved among these proteins comprise the GGCC motif containing Cys310 and Cys311 and the GLNCA motif around Cys247 (numbering refers to MetH from E. coli). These cysteine residues were identified as putative ligands of the zinc cofactor of MetH (10, 26). In accordance with this, human betaine:homocysteine methyltransferase was shown to also contain zinc, which presumably is ligated by cysteine residues (24). These findings indicate that YagD may be a zinc-dependent methyltransferase which uses a catalytical mechanism similar to that of MetH (10, 26). The YagD protein was purified and shown to synthesize methionine from S-methylmethionine or S-adenosylmethionine and homocysteine (25), an activity previously described for crude extracts from this organism (2) and for enriched protein preparations of Saccharomyces cerevisiae (31, 32) and Canavalia ensiformis (1). Formally, therefore, YagD constitutes a third methionine synthase in E. coli. In this communication we report on its physiological role in the sulfur metabolism of this organism.
Physiological role of the homocysteine methyltransferase.
To elucidate the role of the YagD protein in methionine metabolism, a mutant was constructed with an in-frame deletion in both the metE and metH genes. For this purpose, chromosomal copies of the two genes were amplified by PCR. After deletion of residues 493 to 1665 of metE and residues 1198 to 1554 of metH, the mutant genes were re-introduced into wild-type strain KL19 (20) by homologous recombination according to the method of Hamilton et al. (13). Stationary cells (see Fig. 1) of the resulting strain, MTD23, were then used to inoculate minimal medium containing 15 μM (each) different supplements to an optical density at 600 nm (OD600) of 0.05. Throughout this work M9 minimal medium (30) containing 0.8% glucose and the supplement indicated was used. L-S-methylmethionine was from Acros Organics (Geel, Belgium). All other compounds were purchased from Sigma (Deisenhofen, Germany). After 18 h of aerobic incubation at 37°C, the cell densities of the cultures were measured. The OD600 values (± standard deviations) obtained were 0.056 (± 0.003) without any supplement, 0.280 (± 0.002) with dl-methionine sulfoxide, 0.380 (± 0.006) with l-methionine, 0.074 (± 0.002) with S-adenosyl-l-methionine, 0.771 (± 0.001) with dl-S-methylmethionine, and 0.679 (± 0.005) with l-S-methylmethionine. These results demonstrate that l-methionine, dl-methionine sulfoxide, and S-methylmethionine complement auxotrophy of the strain. YagD does not contribute to the utilization of methionine sulfoxide, because no transfer of the methyl group of this compound to homocysteine takes place (data not shown). Presumably, methionine sulfoxide is converted to methionine via reduction (7). The lower OD value obtained for this substrate may be due to its slower utilization in comparison to methionine (7, 12). With S-adenosyl-l-methionine (AdoMet), only marginal growth could be observed, which probably resulted from utilization of impurities contained in the AdoMet preparation. This finding is in accordance with previous observations, namely that E. coli K-12 and its derivatives are largely impermeable to AdoMet (14). Growth yield with the l form of S-methylmethionine and the racemate was about twice that obtained with l-methionine.
FIG. 1.
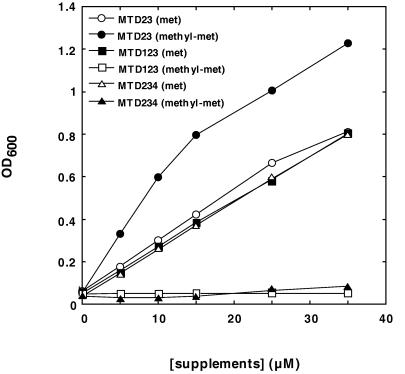
Effects of ΔyagD and ΔykfD mutations on the utilization of S-methylmethionine. Cells of strains MTD23 (ΔmetE ΔmetH), MTD123 (ΔyagD ΔmetE ΔmetH), and MTD234 (ΔmetE ΔmetH ΔykfD) were grown in minimal medium containing 100 μM l-methionine. After 18 h of aerobic incubation at 37°C, they were washed, resuspended in 0.9% NaCl to an OD600 of 10, and transferred into minimal medium containing different concentrations of l-methionine (met) or dl-S-methylmethionine (methyl-met), respectively. The initial OD600 was 0.05. After 18 h of aerobic incubation at 37°C, the resulting cell densities were determined. The data shown represent the mean values of at least two independent growth experiments.
The YagD homocysteine methyltransferase does not utilize the d stereoisomer of S-methylmethionine as a substrate (25). It was, therefore, surprising that the dl racemate yielded the same cell mass as the l form. This result was supported in an experiment in which a series of different concentrations of dl-S-methylmethionine and l-methionine were supplemented (Fig. 1). The data indicate that d-methylmethionine is converted to the l form prior to its utilization, possibly via the same pathway as the conversion of d-methionine to l-methionine (5).
To assess the involvement of the yagD gene product in S-methylmethionine metabolism, an in-frame deletion (residues 270 to 548) was introduced into the corresponding gene of strain MTD23 according to the method of Hamilton et al. (13), yielding the triple mutant MTD123 (ΔyagD ΔmetE ΔmetH). An immunoblotting analysis using antiserum directed against homocysteine methyltransferase confirmed that there was no cross-reacting material (data not shown). The results of the growth experiment shown in Fig. 1 prove that metabolism of S-methylmethionine requires a functional YagD protein, since inactivation of the yagD gene abolishes the capacity to grow on S-methylmethionine.
ykfD, a gene coding for a putative S-methylmethionine permease.
The results described above point to a role for the YagD homocysteine methyltransferase in the utilization of external S-methylmethionine as a source for methionine. Since there is no evidence in the literature that E. coli is able to synthesize S-methylmethionine, there might be a transport system for its uptake from the medium. The results presented previously do not exclude that S-methylmethionine uses the methionine uptake system (16, 17).
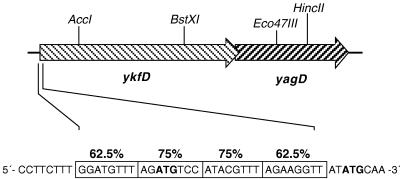
An inspection of the genomic sequence of E. coli in the vicinity of yagD revealed that upstream of yagD there is an open reading frame of 1,533 bp (ykfD) coding for a putative protein and overlapping with the yagD reading frame by 14 bp (4) (Fig. 2). Its amino acid sequence shares significant sequence similarity with that of amino acid permeases (data not shown). This similarity and the overlapping reading frames might be an indication that ykfD codes for a S-methylmethionine transporter which is cotranscribed with the yagD gene.
FIG. 2.
Chromosomal organization of the ykfD and yagD open reading frames. The restriction sites used in this study are shown. Putative MET boxes in the 5′ region of ykfD are depicted as boxed nucleotide sequences. The degrees of identity with the MET box consensus sequence 5′-AGACGTCT-3′ are indicated. Possible translational start codons are marked by boldface letters.
To test this assumption we introduced an in-frame deletion into ykfD (residues 304 to 1089) and combined this mutation with the metE metH lesions of strain MTD23 according to the method of Hamilton et al. (13). The resulting triple mutant, MTD234 (ΔmetE ΔmetH ΔykfD), was tested for growth on different supplements. Figure 1 demonstrates that the ykfD lesion prevents growth on S-methylmethionine but not on methionine. The control strain MTD23, in contrast, is able to utilize S-methylmethionine. Immunoblotting analysis of cell lysates from strain MTD234 grown in minimal medium supplemented with 20 μM l-methionine confirmed that the yagD gene was expressed (data not shown). Thus, the mutation in ykfD did not abolish YagD formation by any polarity effect. At S-methylmethionine concentrations of higher than 50 μM slow but significant growth of strain MTD234 could be observed, which may indicate nonspecific transport of the compound via other systems (data not shown).
Regulation.
The phenotypes of the ykfD and yagD mutants described above indicate a physiological role for the gene products in the acquisition of external S-methylmethionine and in its conversion into methionine. This could imply that the two genes are subject to regulation by the MetJ-S-adenosyl-methionine system. MetJ is a homodimer of 12-kDa promoters (35, 36). It binds to the MET box (5′-AGACGTCT-3′) in the upstream region of genes (metA, metBL, metC, metF, metJ, metR, and metE) whose products are involved in methionine biosynthesis and acts as a transcriptional repressor (3, 18, 28, 29). Between two and five of these MET boxes are organized in tandem repeats. Because of cooperative interactions between the repressor molecules bound, their number and match with the consensus sequence determines the level of repressibility. S-adenosylmethionine, which is synthesized from methionine and adenosine triphosphate by the metK gene product (21), acts as a corepressor (27, 33, 34) and thereby mediates methionine regulation of the met regulon.
Upstream of yagD, no sequence similar to that of the MET box motif can be found. In contrast, there are four of these motifs upstream of ykfD (Fig. 2). Depending on which of the two ATG codons of the ykfD gene is functioning for the start of the translation, the four MET boxes overlap or immediately precede the start of the reading frame.
To analyze whether the ykfD yagD putative transcriptional unit is indeed under control of methionine, cultures of E. coli KL19 (wild type) were grown in minimal medium supplemented with different concentrations of l-methionine or dl-S-methylmethionine. After 230 min of growth, cells were harvested and analyzed for the formation of YagD protein by immunoblotting (Fig. 3). It is evident that the amount of YagD protein was strongly reduced when 40 μM l-methionine or higher concentrations were present in the medium. Supplementation of S-methylmethionine only caused a slight reduction in the amount of YagD, which might be due to its intracellular conversion into methionine. Similar results were obtained when synthesis of YagD was followed by measuring enzyme activity (results not shown).
FIG. 3.
Influence of methionine and S-methylmethionine supplementation on the expression of yagD. Minimal medium containing different concentrations of l-methionine (A) or dl-S-methylmethionine (B) was inoculated to an OD600 of 0.05 with stationary cells (see legend to Fig. 1; grown without l-methionine) of wild-type strain E. coli KL19. After 230 min of aerobic incubation at 37°C, the cells were harvested and lysed. The proteins were separated on a 15% polyacrylamide gel in the presence of sodium dodecyl sulfate (19). The detection of YagD was performed by immunoblotting analysis using anti-YagD antiserum (obtained from Eurogentech, Belgium, by custom immunization of a rabbit with purified YagD) in a 1:4,000 dilution, protein A-horseradish peroxidase conjugate (Bio-Rad, Munich, Germany), and chemiluminscence blotting substrate from Boehringer (Mannheim, Germany).
The results described here identify the function of two unassigned open reading frames from E. coli. Their products are involved in the uptake of S-methylmethionine and in the methyl transfer to homocysteine, rendering two molecules of methionine. Such an activity has been described for crude extracts from E. coli cells by Balish and Shapiro (2). S-methylmethionine is a compound synthesized and stored by many plant species (15, 23), and expression of ykfD and yagD enables E. coli to utilize this compound. The abilities of other methionine auxotrophic bacterial species to use S-methylmethionine as a source of methionine (22) might be based on similar systems. In agreement with this physiological role, ykfD and yagD are subject to control of expression by methionine, thus constituting two more genes belonging to the methionine regulon. More detailed studies, however, are required to confirm that MetJ is involved as a regulatory protein. On the basis of the results obtained in this study we propose the new gene designation mmu (S-methylmethionine utilization). Accordingly, the gene coding for the putative S-methylmethionine permease (ykfD) is renamed mmuP, and the S-methylmethionine:homocysteine methyltransferase gene (yagD) is renamed mmuM.
Acknowledgments
We thank Valerie Maples and Sidney R. Kushner for providing the sequence of pMAK700.
This work was supported by grants from the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Abrahamson L, Shapiro S K. The biosynthesis of methionine: partial purification and properties of homocysteine methyltransferase of jack bean meal. Arch Biochem Biophys. 1965;109:376–382. [Google Scholar]
- 2.Balish E, Shapiro S K. Methionine biosynthesis in Escherichia coli: induction and repression of methylmethionine (or adenosylmethionine): homocysteine methyltransferase. Arch Biochem Biophys. 1966;119:62–68. doi: 10.1016/0003-9861(67)90429-8. [DOI] [PubMed] [Google Scholar]
- 3.Belfaiza J, Parsot C, Martel A, Bouthier de la Tour C, Margarita D, Cohen G N, Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci USA. 1986;83:867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 5.Cooper S. Utilization of D-methionine by Escherichia coli. J Bacteriol. 1966;92:328–332. doi: 10.1128/jb.92.2.328-332.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drummond J T, Huang S, Blumenthal R M, Matthews R G. Assignment of enzymatic function to specific protein regions of cobalamine-dependent methionine synthase from Escherichia coli. Biochemistry. 1993;32:9290–9295. doi: 10.1021/bi00087a005. [DOI] [PubMed] [Google Scholar]
- 7.Ejiri S-I, Weissbach H, Brot N. Reduction of methionine sulfoxide to methionine by Escherichia coli. J Bacteriol. 1979;139:161–164. doi: 10.1128/jb.139.1.161-164.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasca V, Banerjee R V, Dunham W R, Sands R H, Matthews R G. Cobalamine-dependent methionine synthase from Escherichia coli B: electron paramagnetic resonance spectra of the inactive form and the active methylated form of the enzyme. Biochemistry. 1988;27:8458–8465. doi: 10.1021/bi00422a025. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez J, Peariso K, Penner-Hahn J E, Matthews R G. Cobalamine-independent methionine synthase from E. coli: a zinc metalloenzyme. Biochemistry. 1996;35:12228–12234. doi: 10.1021/bi9615452. [DOI] [PubMed] [Google Scholar]
- 10.Goulding C W, Matthews R G. Cobalamine-dependent methionine synthase from Escherichia coli: involvement of zinc in homocysteine activation. Biochemistry. 1997;36:15749–15757. doi: 10.1021/bi971988l. [DOI] [PubMed] [Google Scholar]
- 11.Goulding C W, Postigo D, Matthews R G. Cobalamine-dependent methionine synthase is a modular protein with distinct regions for binding homocysteine, methyltetrahydrofolat, cobalamine, and adenosylmethionine. Biochemistry. 1997;36:8082–8091. doi: 10.1021/bi9705164. [DOI] [PubMed] [Google Scholar]
- 12.Greene R C. Methionine limitation in Escherichia coli K-12 by growth on the sulfoxides of D-methionine. J Bacteriol. 1973;116:230–234. doi: 10.1128/jb.116.1.230-234.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloway C T, Greene R C, Su C-H. Regulation of S-adenosylmethionine synthetase in Escherichia coli. J Bacteriol. 1970;104:734–747. doi: 10.1128/jb.104.2.734-747.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James F, Nolte K D, Hanson A D. Purification and properties of S-adenosyl-L-methionine:L-methionine S-methyltransferase from Wollastonia biflora leaves. J Biol Chem. 1995;270:22344–22350. doi: 10.1074/jbc.270.38.22344. [DOI] [PubMed] [Google Scholar]
- 16.Kadner R J. Transport systems for L-methionine in Escherichia coli. J Bacteriol. 1974;117:232–241. doi: 10.1128/jb.117.1.232-241.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadner R J, Watson W J. Methionine transport in Escherichia coli: physiological and genetic evidence for two uptake systems. J Bacteriol. 1974;119:401–409. doi: 10.1128/jb.119.2.401-409.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirby T W, Hindenach B R, Greene R C. Regulation of in vivo transcription of the Escherichia coli K-12 metJBLF gene cluster. J Bacteriol. 1986;165:671–677. doi: 10.1128/jb.165.3.671-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Low B. Formation of merodiploids in matings with a class of rec− recipient strains of Escherichia coli K12. Proc Natl Acad Sci USA. 1968;60:160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markham G D, Hafner E W, White Tabor C, Tabor H. S-adenosylmethionine synthetase from Escherichia coli. J Biol Chem. 1980;255:9082–9092. [PubMed] [Google Scholar]
- 22.McRorie R, Margaret A, Glazener R, Skinner C G, Shive W. Microbiological activity of the methylsulfonium derivative of methionine. J Biol Chem. 1954;211:489–497. [PubMed] [Google Scholar]
- 23.McRorie R A, Sutherland G L, Margaret S, Barton A D, Glazener Margaret R, Shive W. Isolation and identification of a naturally occurring analog of methionine. J Am Chem Soc. 1954;76:115–118. [Google Scholar]
- 24.Millian N S, Garrow T A. Human betaine-homocysteine methyltransferase is a zinc metalloenzyme. Arch Biochem Biophys. 1998;356:93–98. doi: 10.1006/abbi.1998.0757. [DOI] [PubMed] [Google Scholar]
- 25.Neuhierl B, Thanbichler M, Böck A. A family of S-methyl-methionine-dependent thiol/selenol methyltransferases: role in selenium tolerance and evolutionary relation. 1998. (submitted) [DOI] [PubMed] [Google Scholar]
- 26.Peariso K, Goulding C W, Huang S, Matthews R G, Penner-Hahn J E. Characterization of the zinc binding site in methionine synthase enzymes of Escherichia coli: The role of zinc in the methylation of homocysteine. J Am Chem Soc. 1998;120:8410–8416. [Google Scholar]
- 27.Saint-Girons I, Belfaiza J, Guillou Y, Perrin D, Guiso N, Barzu O, Cohen G N. Interactions of the Escherichia coli methionine repressor with the metF operator and with its corepressor, S-adenosylmethionine. J Biol Chem. 1986;261:10936–10940. [PubMed] [Google Scholar]
- 28.Saint-Girons I, Duchange N, Cohen G N, Zakin M M. Structure and autoregulation of the metJ regulatory gene in Escherichia coli. J Biol Chem. 1984;259:14282–14285. [PubMed] [Google Scholar]
- 29.Saint-Girons I, Duchange N, Zakin M M, Park I, Margarita D, Ferrara P, Cohen G N. Nucleotide sequence of metF, the E. coli structural gene for 5-10 methylene tetrahydrofolate reductase and its control region. Nucl Acids Res. 1983;11:6723–6732. doi: 10.1093/nar/11.19.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 31.Shapiro S K, Almenas A, Thomson J F. Biosynthesis of methionine in Saccharomyces cerevisiae. J Biol Chem. 1965;240:2512–2518. [PubMed] [Google Scholar]
- 32.Shapiro S K, Yphantis D A, Almenas A. Biosynthesis of methionine in Saccharomyces cerevisiae. J Biol Chem. 1964;239:1551–1556. [PubMed] [Google Scholar]
- 33.Shoeman R, Redfield B, Coleman T, Greene R, Smith A, Brot N, Weissbach H. Regulation of methionine synthesis in E. coli: effect of the metJ gene product and S-adenosylmethionine on the expression of the metF gene. Proc Natl Acad Sci USA. 1985;82:3601–3605. doi: 10.1073/pnas.82.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoeman R, Redfield B, Coleman T, Greene R, Smith A, Saint-Girons I, Brot N, Weissbach H. Regulation of methionine synthesis in Escherichia coli: effect of the metJ gene product and S-adenosylmethionine on the in vitro expression of the metB, metL and metJ genes. Biochem Biophys Res Commun. 1985;133:731–739. doi: 10.1016/0006-291x(85)90965-9. [DOI] [PubMed] [Google Scholar]
- 35.Smith A A, Greene R C. Cloning of the methionine regulatory gene, metJ, of Escherichia coli K12 and identification of its product. J Biol Chem. 1984;259:14279–14281. [PubMed] [Google Scholar]
- 36.Smith A A, Greene R C, Kirby T W, Hindenach B R. Isolation and characterization of the product of the methionine regulatory gene, metJ, of Escherichia coli K12. Proc Natl Acad Sci USA. 1985;82:6104–6108. doi: 10.1073/pnas.82.18.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitfield C D, Steers E J J, Weissbach H. Purification and properties of 5-methyltetrahydropteroyltriglutamate-homocysteine transmethylase. J Biol Chem. 1970;245:390–401. [PubMed] [Google Scholar]