Abstract
Background
Since it was first identified in early November 2021, the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread quickly and replaced the B.1.617.2 (delta) variant as the dominant variant in many countries. Data on the real-world effectiveness of vaccines against the omicron variant in children are lacking.
Methods
In a study conducted from January 21, 2022, through April 8, 2022, when the omicron variant was spreading rapidly, we analyzed data on children in Singapore who were 5 to 11 years of age. We assessed the incidences of all reported SARS-CoV-2 infections (confirmed on polymerase-chain-reaction [PCR] assay, rapid antigen testing, or both), SARS-CoV-2 infections confirmed on PCR assay, and coronavirus disease 2019 (Covid-19)–related hospitalizations among unvaccinated, partially vaccinated (≥1 day after the first dose of vaccine and up to 6 days after the second dose), and fully vaccinated children (≥7 days after the second dose). Poisson regression was used to estimate vaccine effectiveness from the incidence rate ratio of outcomes.
Results
A total of 255,936 children were included in the analysis. Among unvaccinated children, the crude incidence rates of all reported SARS-CoV-2 infections, PCR-confirmed SARS-CoV-2 infections, and Covid-19–related hospitalizations were 3303.5, 473.8, and 30.0 per 1 million person-days, respectively. Among partially vaccinated children, vaccine effectiveness was 13.6% (95% confidence interval [CI], 11.7 to 15.5) against all SARS-CoV-2 infections, 24.3% (95% CI, 19.5 to 28.9) against PCR-confirmed SARS-CoV-2 infection, and 42.3% (95% CI, 24.9 to 55.7) against Covid-19–related hospitalization; in fully vaccinated children, vaccine effectiveness was 36.8% (95% CI, 35.3 to 38.2), 65.3% (95% CI, 62.0 to 68.3), and 82.7% (95% CI, 74.8 to 88.2), respectively.
Conclusions
During a period when the omicron variant was predominant, BNT162b2 vaccination reduced the risks of SARS-CoV-2 infection and Covid-19–related hospitalization among children 5 to 11 years of age.
After the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was detected in South Africa in November 2021, it spread quickly across many countries, with a disproportionately higher incidence of hospitalization among children than that with other variants.1,2 However, the real-world effectiveness of vaccines in reducing the incidences of omicron infection and coronavirus disease 2019 (Covid-19)–related hospitalization among children is uncertain. We now report the effectiveness of the BNT162b2 messenger RNA (mRNA) vaccine (Pfizer–BioNTech) against Covid-19 in children 5 to 11 years of age during a surge of the omicron variant in Singapore.
Local transmission increased sharply after mid-January 2022, and the number of peak daily cases exceeded those reported during the B.1.617.2 (delta) wave. Schools were open with the use of masks mandated, but children younger than 12 years of age were not subject to measures that restricted adults who were not fully vaccinated from entering selected venues. In Singapore, the vaccination of children 5 to 11 years of age with two 10-μg doses of BNT162b2 administered at least 21 days apart commenced on December 27, 2021, starting first with older children (9 to 11 years of age) and then with children 5 to 8 years of age 2 weeks later. Vaccination is voluntary in Singapore, and parental consent is required.
In this national cohort study, we analyzed the incidences of SARS-CoV-2 infection and hospitalization among 255,936 children 5 to 11 years of age, according to vaccination status, and we estimated the effectiveness of partial and full vaccination with the BNT162b2 vaccine against the omicron variant. An understanding of vaccine effectiveness against SARS-CoV-2 infections and severe illness among children is important, given the effect of the Covid-19 pandemic on their education and their social and mental well-being3,4 and the high incidence of infection with the omicron variant in this population group.
Methods
Study Design
Our analysis was based on official data reported to and maintained by the Ministry of Health, Singapore.5 Data collection and analysis for this study were carried out under the Infectious Diseases Act to support policy decision making and evaluation and were exempted from ethics review. The investigators designed the study. The first and last authors wrote the initial draft of the manuscript, and the data were analyzed by the third and last authors. The authors vouch for the accuracy and completeness of the data, and they collectively decided to submit the manuscript for publication.
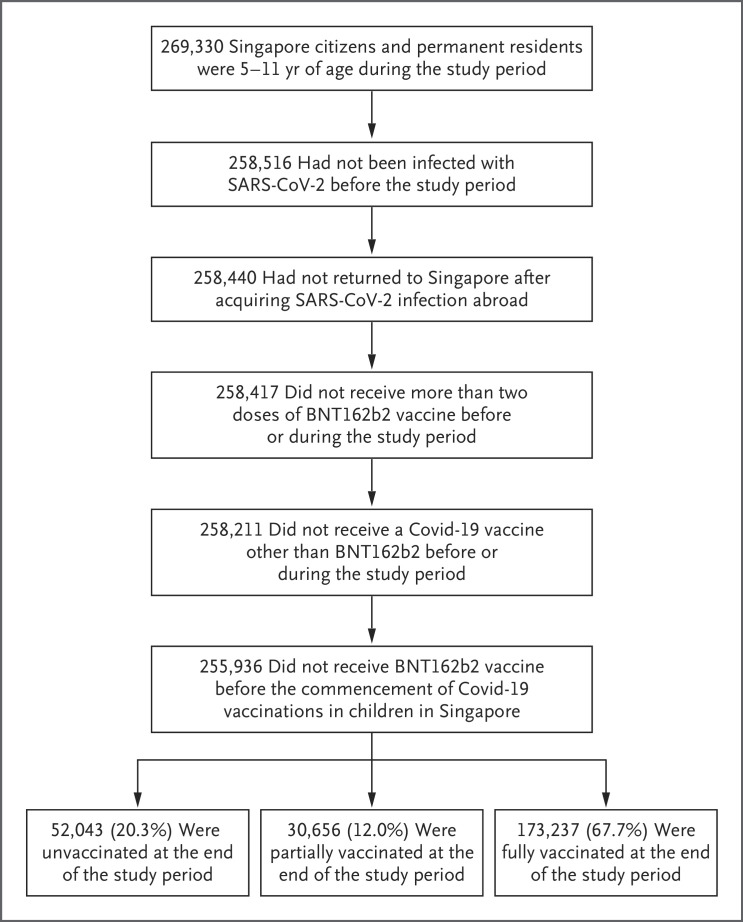
Anonymized data for our analysis were extracted on April 13, 2022, for the study period of January 21 through April 8, 2022. During this time period, the omicron variant accounted for more than 99% of sequenced cases of SARS-CoV-2 infection in Singapore.6 According to the administrative records used by the Ministry of Health to issue invitations for vaccination and to monitor vaccination uptake, 269,330 Singapore citizens and permanent residents were 5 to 11 years of age during the study period. Children who had had SARS-CoV-2 infection before the study start date and those who had returned to Singapore after acquiring SARS-CoV-2 infection abroad were excluded from the study. Children who had received vaccines other than BNT162b2, the adult dosage of BNT162b2, or more than two doses of the vaccine, as well as those who had been vaccinated before the commencement of Covid-19 vaccination in children in Singapore, were also excluded from the study. In total, 255,936 children met the inclusion criteria for the analysis (Figure 1).
Figure 1. Study Population.
Covid-19 denotes coronavirus disease 2019, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Data extracted for the study included dates of vaccination (both the first and second doses), age, sex, ethnic group (Chinese, Malay, Indian, or other), and housing type as a proxy for socioeconomic status (e.g., public housing with one to five rooms, private housing, or other housing). Outcomes included the date on which all SARS-CoV-2–positive children or their parents or guardians were notified after a positive test result, regardless of whether polymerase-chain-reaction (PCR) testing or rapid antigen testing was used, as well as the hospitalization of children with Covid-19. By law in Singapore, all clinically or laboratory diagnosed SARS-CoV-2 infections must be reported to the Ministry of Health.
Cases of Covid-19 among children in Singapore are identified through testing of symptomatic children who present with acute respiratory illness at primary or tertiary care institutions and through testing of asymptomatic children who have been identified by the Ministry of Health as being close contacts of persons with Covid-19. In accordance with the disease management protocol, symptomatic children and children who are close contacts of infected persons undergo rapid antigen testing. Children with symptoms or signs (e.g., shortness of breath, chest pain or palpitations, lethargy, and prolonged fever or respiratory symptoms for ≥5 days) and symptomatic patients with coexisting conditions undergo additional PCR testing.
We analyzed the incidences of all reported SARS-CoV-2 infections (positive on PCR, rapid antigen testing, or both) and PCR-confirmed infections, which serve as a proxy for increased severity of illness. To examine the effect of vaccines on more severe illness, we analyzed the incidence of hospitalization for Covid-19. During the omicron outbreak, admission to hospitals was based on clinical indications of symptomatic SARS-CoV-2 infection in clinically unwell children, including but not limited to severe pneumonia and multisystem inflammatory syndrome in children (MIS-C). The onset of severe illness for which hospitalization was warranted was defined as the date of confirmed infection. Because of the time lag between SARS-CoV-2 infection and hospitalization and the potential risk of excluding patients in whom infection developed during the study period but who were hospitalized after the study period, we restricted the hospitalization analysis to patients who were notified of confirmed infection up to and including April 1, 2022 (i.e., 7 days before the end of the study period).
We calculated the contribution of person-time risks to the unvaccinated, partially vaccinated, and vaccinated groups on the basis of each child’s vaccination status and vaccination dates. Children were considered to be partially vaccinated starting on the day after they received the first dose up to 6 days after they received the second dose, and they were considered to be fully vaccinated 7 days or more after they received the second dose. We chose an interval of 7 days or more to allow adequate time for antibody levels to increase after immunization and to facilitate comparisons with the results of previously reported studies of vaccine efficacy.7,8
Statistical Analysis
Using previously published methods,9 we calculated vaccine effectiveness as 1 minus the incidence rate ratio. The incidence rate ratios of outcomes between different groups were estimated with the use of Poisson regression that included sex, ethnic group, age (in years), and housing type as covariates. Calendar-date dummy variables were also included to adjust for the varying force of infection over the study period. The unvaccinated group was chosen as the reference group, and the point estimates of vaccine effectiveness as well as the 95% confidence intervals of these estimates are reported.
To assess for biases and to evaluate the robustness of our regression model, we conducted several sensitivity analyses. First, in a secondary analysis, we stratified the cohort according to the number of days after vaccination. The rates of infection and hospitalization across the partially and fully vaccinated groups according to the time since vaccination were assessed. Second, we performed analyses according to age groups and time periods. Next, we assessed vaccine effectiveness after including geographic region as an additional covariate. Finally, we analyzed the data using matching, an alternative statistical method similar to that adopted by Dagan et al.10 (see the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org). The adequacy of model fit was evaluated by comparing estimated and empirical incidence rates. The widths of the 95% confidence intervals were not adjusted for multiplicity and should not be used to infer statistical significance. The data analyses were carried out with the use of Stata software, version 17 (StataCorp).
Results
Vaccination Status of the Children
By the end of the study period, 67.7% of the children who were 5 to 11 years of age were fully vaccinated, 12.0% were partially vaccinated, and 20.3% were unvaccinated (Figure 1). Vaccination rates were similar among boys and girls, but they were higher among children in the Chinese, Malay, and Indian ethnic groups than among those in other ethnic groups. Rates of full vaccination were higher among children in older age groups than among those in younger age groups. Across housing types, the highest vaccination rates were among children living in three-to-five-room public housing units, whereas the vaccination rate was lower among those living in private housing (Table 1). The median interval between the first and second dose of vaccine was 24 days (interquartile range, 21 to 28) among the children who were fully vaccinated.
Table 1. Sociodemographic Characteristics of the Children, According to Vaccination Status.*.
| Characteristic | Unvaccinated Children |
Partially Vaccinated Children |
Fully Vaccinated Children |
|---|---|---|---|
| number of person-days at risk (percent) | |||
| Sex | |||
| Female | 2,474,089 (48.3) | 2,611,142 (48.9) | 3,654,907 (49.4) |
| Male | 2,644,379 (51.7) | 2,729,063 (51.1) | 3,750,159 (50.6) |
| Age at start of study period | |||
| 5 yr | 1,554,605 (30.4) | 565,630 (10.6) | 423,852 (5.7) |
| 6 yr | 975,943 (19.1) | 800,812 (15.0) | 835,763 (11.3) |
| 7 yr | 752,281 (14.7) | 886,631 (16.6) | 1,050,709 (14.2) |
| 8 yr | 608,456 (11.9) | 836,290 (15.7) | 1,043,724 (14.1) |
| 9 yr | 518,139 (10.1) | 831,587 (15.6) | 1,332,899 (18.0) |
| 10 yr | 417,013 (8.1) | 743,220 (13.9) | 1,382,027 (18.7) |
| 11 yr | 292,031 (5.7) | 676,035 (12.7) | 1,336,092 (18.0) |
| Ethnic group† | |||
| Chinese | 3,619,247 (70.7) | 3,720,381 (69.7) | 5,134,163 (69.3) |
| Malay | 743,466 (14.5) | 889,602 (16.7) | 1,240,769 (16.8) |
| Indian | 494,626 (9.7) | 557,723 (10.4) | 785,266 (10.6) |
| Other | 261,129 (5.1) | 172,499 (3.2) | 244,868 (3.3) |
| Housing | |||
| Public housing | |||
| One or two rooms | 179,652 (3.5) | 187,694 (3.5) | 229,088 (3.1) |
| Three rooms | 700,015 (13.7) | 822,460 (15.4) | 1,149,798 (15.5) |
| Four rooms | 1,805,300 (35.3) | 1,943,067 (36.4) | 2,671,568 (36.1) |
| Five rooms | 1,294,517 (25.3) | 1,438,387 (26.9) | 2,073,120 (28.0) |
| Private housing | 1,082,114 (21.1) | 893,930 (16.7) | 1,202,149 (16.2) |
| Other | 56,870 (1.1) | 54,667 (1.0) | 79,343 (1.1) |
The total number of person-days at risk was 5,118,468 in the unvaccinated group, 5,340,205 in the partially vaccinated group, and 7,405,066 in the fully vaccinated group. Percentages may not total 100 because of rounding.
Ethnic group was reported in the national birth registry.
SARS-CoV-2 Infection and Hospitalization
Over the 17.9 million person-days at risk, there were 53,429 infections, of which 5342 (10.0%) were PCR-confirmed infections (Table 2). Over the 16.4 million person-days at risk for the hospitalization outcome, there were 288 hospitalizations. Among hospitalized children, only five received supplemental oxygen, four of whom were admitted to the intensive care unit. Of these five children, one was unvaccinated, two were partially vaccinated, and two were fully vaccinated. No deaths attributable to Covid-19 (as determined by the cause of death reported to the Ministry of Health) were observed during the study period.
Table 2. Effectiveness of BNT162b2 Vaccine against SARS-CoV-2 Infection and Hospitalization.*.
| Group | Person-Days at Risk† | Cases of SARS-CoV-2 Infection | Crude Incidence Rate | Vaccine Effectiveness (95% CI)‡ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Confirmed Cases§ |
PCR- Confirmed Cases |
Hospitalizations | All Confirmed Cases§ |
PCR- Confirmed Cases |
Hospitalizations | All Confirmed Cases§ |
PCR- Confirmed Cases |
Hospitalizations | ||
| number | number of confirmed infections/1 million person-days at risk | percent | ||||||||
| Unvaccinated | 5,118,468 | 16,909 | 2425 | 146 | 3303.5 | 473.8 | 30.0 | Reference | Reference | Reference |
| Partially vaccinated | 5,340,205 | 16,006 | 2089 | 100 | 2997.3 | 391.2 | 19.1 | 13.6 (11.7–15.5) | 24.3 (19.5–28.9) | 42.3 (24.9–55.7) |
| Fully vaccinated | 7,405,066 | 20,514 | 828 | 42 | 2770.3 | 111.8 | 6.6 | 36.8 (35.3–38.2) | 65.3 (62.0–68.3) | 82.7 (74.8–88.2) |
Partial vaccination was defined as at least 1 day after the first dose of vaccine and up to 6 days after the second dose, and full vaccination at least 7 days after the second dose. PCR denotes polymerase chain reaction, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
The total number of person-days at risk for the hospitalization outcome was 4,869,127 in the unvaccinated group, 5,231,353 in the partially vaccinated group, and 6,338,164 in the fully vaccinated group.
Vaccine effectiveness was calculated as 1 minus the incidence rate ratio. The incidence rate ratio is obtained from the exponentiated coefficients of separate Poisson regressions on all confirmed infections, infections confirmed by means of PCR testing only, and severe infections resulting in hospitalization. The covariates of age (in years), ethnic group (Chinese, Malay, Indian, or other), sex (male or female), housing type (public housing with one or two rooms, three rooms, four rooms, or five rooms; private housing; or other housing), and calendar dates during the study period were included in the regression to control for potential confounding. Vaccine effectiveness in the partially vaccinated and fully vaccinated groups was reported with the unvaccinated group as the reference. Confidence intervals have not been adjusted for multiplicity and should not be used to infer statistical significance.
“All confirmed cases” refers to all reported SARS-CoV-2 infections confirmed by PCR testing, rapid antigen testing, or both.
Among the children in the unvaccinated group, the incidence rates of all reported infections, PCR-confirmed infections, and hospitalizations were 3303.5, 473.8, and 30.0 per 1 million person-days (Table 2). The corresponding rates were 2997.3, 391.2, and 19.1 per 1 million person-days in the partially vaccinated group and 2770.3, 111.8, and 6.6 per 1 million person-days in the fully vaccinated group.
Vaccine Effectiveness
After adjustment for covariates including the calendar date during the study period (given the changing community transmission rates over time), vaccine effectiveness against all reported infections, PCR-confirmed infections, and hospitalizations in partially vaccinated as compared with unvaccinated children was 13.6% (95% CI, 11.7 to 15.5), 24.3% (95% CI, 19.5 to 28.9), and 42.3% (95% CI, 24.9 to 55.7), respectively. In fully vaccinated children, we estimated vaccine effectiveness to be 36.8% (95% CI, 35.3 to 38.2) against all reported infections, 65.3% (95% CI, 62.0 to 68.3) against PCR-confirmed infections, and 82.7% (95% CI, 74.8 to 88.2) against hospitalization.
In our secondary analysis, the vaccine effectiveness against all confirmed infections in the fully vaccinated group as compared with the unvaccinated group was 48.8% (95% CI, 46.9 to 50.8) at 7 to 14 days after the second dose of vaccine, 37.6% (95% CI, 35.7 to 39.3) at 15 to 29 days after the second dose of vaccine, 28.5% (95% CI, 26.3 to 30.7) at 30 to 59 days after the second dose of vaccine, and 25.6% (95% CI, 19.3 to 31.5) at 60 days or more after the second dose of vaccine (Table S1 in the Supplementary Appendix). Vaccine effectiveness was similar across age groups (Table S2) and time periods (Table S3). In addition, the results obtained after accounting for geographic region (Table S4) and through matching (Table S5) were similar to those in the primary analysis. The estimated incidences of all confirmed infections among unvaccinated, partially vaccinated, and fully vaccinated children over the study period were similar to the empirical incidence rates (Figs. S1 through S3). Six children had MIS-C during the study period, of whom four were unvaccinated, one was partially vaccinated, and one was fully vaccinated.
Discussion
Our estimates of the effectiveness of two doses of the BNT162b2 vaccine against all SARS-CoV-2 infections of 36.8% (95% CI, 35.3 to 38.2) and against PCR-confirmed SARS-CoV-2 infections of 65.3% (95% CI, 62.0 to 68.3) among children 5 to 11 years of age during the omicron wave are lower than those in previous studies that were conducted when other variants were predominant.7,11 A vaccine efficacy of 90.7% (95% CI, 67.7 to 98.3) was reported in a phase 2–3 clinical trial involving 2268 children that was conducted in the United States, Spain, Finland, and Poland in June 2021, when the delta variant was predominant.7 A higher number of breakthrough infections may be due to greater immune escape of the omicron variant than the delta variant.12-14 In our study, vaccine effectiveness against PCR-confirmed infections was higher than that against all infections (confirmed by PCR, rapid antigen testing, or both). Given the national protocols for testing in Singapore during the study period, these findings suggest greater vaccine effectiveness against higher levels of disease severity, and the estimates of vaccine effectiveness against PCR-confirmed infections in Singapore may thus be higher than those reported in other countries.15,16
Nonetheless, vaccine effectiveness against hospitalization in the short term after vaccination among children 5 to 11 years of age was high at 82.7% (95% CI, 74.8 to 88.2) and was similar to that among adults who had received three doses of mRNA vaccines during the omicron-predominant period.17,18 The vaccine effectiveness of two doses of BNT162b2 against hospitalization for Covid-19 with the omicron variant was estimated to be 68% (95% CI, 42 to 82) in a case–control study involving children 5 to 11 years of age in the United States.19
Our findings indicate the protective effect of vaccination against infection and severe illness. We found that the vaccine effectiveness against hospitalization was higher with full vaccination with two doses of BNT162b2 (82.7%) than with partial vaccination with one dose (42.3%).
A strength of this study is the use of a comprehensive national data set of both vaccinations and confirmed SARS-CoV-2 infections and disease severity reported to the Ministry of Health. The results of this study may provide insights to enable decision makers to weigh the benefits against the potential risks of vaccination of children. In Singapore, 22 serious adverse events after vaccination (0.005% of all doses administered) among children 5 to 11 years of age were reported to the Health Sciences Authority as of February 28, 2022.20 Beyond the short-term and long-term health risks of SARS-CoV-2 infection in children (including MIS-C21 and “long Covid”22), high infection rates among children may lead to transmission to older adults23 and may increase the risk of overwhelming existing health care capabilities during the omicron wave. It appears that these risks can be mitigated by the vaccination of children.
Our study has certain limitations. There may have been potential confounding because of unobservable characteristics underlying the parents’ decision to vaccinate their children, the timing of vaccinations, and health care–seeking behaviors. Risk-averse persons who are less willing than others to be vaccinated24 may be more likely to reduce social interactions when infection rates are high, and parents may allow their children to interact more freely with others in social settings after vaccination; both these factors may lead to an underestimation of the true risk reduction afforded by vaccines. Although a decreased vaccine effectiveness against confirmed infections after full vaccination with two doses of BNT162b2 was observed over time, this finding may be confounded by unmeasured differences in the risk of infection between early and late adopters of the vaccine.
Although most of the fully vaccinated children in this study received the second dose of vaccine 3 weeks after the first dose (median, 24 days; interquartile range, 21 to 28), some received the second dose later (Fig. S4). Extended dosing intervals have been associated with increased neutralizing antibody levels in adults.25 However, because of the short follow-up period, we were unable to assess the effect of dosing intervals on vaccine effectiveness.
Previous studies have shown higher risks of severe Covid-19 and admission to an intensive care unit among children with coexisting conditions,26,27 but we could not assess this because data were not available in the national Covid-19 vaccination registry. Nonetheless, the prevalence of coexisting conditions predisposing children to severe Covid-19 is probably low in this age group. An observational study showed that among 130 children who were hospitalized with Covid-19 in Singapore, 13.1% presented with coexisting conditions.28 Children with disabilities in special education schools are vaccinated, and vaccinations are not contraindicated among children with stable medical conditions.
Although vaccination coverage is high among young and older adults in many countries, vaccination uptake among children has often lagged. Covid-19 vaccine hesitancy is reportedly higher for vaccination of children than for vaccination of adults.29 The omicron variant has been reported to result in less severe disease,30,31 but the absolute number32 and rate33 of hospitalizations among unvaccinated children may still be high because of increased transmissibility. Our results indicate that vaccines may play an important role in reducing infections and hospitalizations during the omicron wave.
We observed that during a period when the omicron variant was predominant, BNT162b2 vaccination reduced the risks of SARS-CoV-2 infection and Covid-19–related hospitalization among children 5 to 11 years of age.
Acknowledgments
We thank Vernon Lee, Marc Ho, Joonkiat Chua, Shannen Ho, Yongkai Lim, Yiding Lim, Darren Chan, and Jingjie Ong, all of whom are employees of the Ministry of Health, Singapore, for their input into the design of the study and their assistance with data collation and database management.
Supplementary Appendix
Disclosure Forms
This article was published on July 20, 2022, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Kozlov M. Does omicron hit kids harder? Scientists are trying to find out. Nature 2022. February 4 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 2.Shi DS, Whitaker M, Marks KJ, et al. Hospitalizations of children aged 5–11 years with laboratory-confirmed COVID-19 — COVID-NET, 14 states, March 2020–February 2022. MMWR Morb Mortal Wkly Rep 2022;71:574-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nearchou F, Flinn C, Niland R, Subramaniam SS, Hennessy E. Exploring the impact of COVID-19 on mental health outcomes in children and adolescents: a systematic review. Int J Environ Res Public Health 2020;17:8479-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meherali S, Punjani N, Louie-Poon S, et al. Mental health of children and adolescents amidst COVID-19 and past pandemics: a rapid systematic review. Int J Environ Res Public Health 2021;18:3432-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health Singapore. Vaccination statistics. February 22, 2022. (https://www.Moh.Gov.Sg/Covid-19/Vaccination/Statistics).
- 6.GISAID. Tracking of variants: VOC omicron GRA (B.1.1.529+Ba.*) first detected in Botswana/Hong Kong/South Africa. February 2022. (https://www.Gisaid.Org/Hcov19-Variants/).
- 7.Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med 2022;386:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jara A, Undurraga EA, Flores JC, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in children and adolescents: a large-scale observational study. February 15, 2022. (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4035405). preprint. [DOI] [PMC free article] [PubMed]
- 12.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature 2022;602:671-675. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann M, Krüger N, Schulz S, et al. The omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell 2022;185(3):447.e11-456.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ai J, Zhang H, Zhang Y, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect 2022;11:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5–17 years — VISION Network, 10 states, April 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowlkes AL, Yoon SK, Lutrick K, et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5–11 years and adolescents aged 12–15 years — PROTECT cohort, July 2021–February 2022. MMWR Morb Mortal Wkly Rep 2022;71:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price AM, Olson SM, Newhams MM, et al. BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med 2022;386:1899-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Health Sciences Authority of Singapore. HSA’s Covid-19 vaccine safety update #11 (30 December 2020–28 February 2022). 2022. (https://www.Hsa.Gov.Sg/Docs/Default-Source/Hprg-Vcb/Safety-Update-On-Covid19-Vaccines/Hsa-Safety-Update-No-11-On-Covid-19-Vaccines-(28-February-2022).Pdf).
- 21.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021;325:1074-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molteni E, Sudre CH, Canas LS, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health 2021;5:708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dattner I, Goldberg Y, Katriel G, et al. The role of children in the spread of COVID-19: using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLoS Comput Biol 2021;17(2):e1008559-e1008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson A, Montelpare WJ. Predictors of vaccine hesitancy: implications for COVID-19 public health messaging. Int J Environ Res Public Health 2021;18:8054-8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021;184(23):5699.e11-5714.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi JH, Choi S-H, Yun KW. Risk factors for severe COVID-19 in children: a systematic review and meta-analysis. J Korean Med Sci 2022;37(5):e35-e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward JL, Harwood R, Smith C, et al. Risk factors for PICU admission and death among children and young people hospitalized with COVID-19 and PIMS-TS in England during the first pandemic year. Nat Med 2022;28:193-200. [DOI] [PubMed] [Google Scholar]
- 28.Wong JJM, Abbas Q, Chuah SL, et al. Comparative analysis of pediatric COVID-19 infection in Southeast Asia, South Asia, Japan, and China. Am J Trop Med Hyg 2021;105:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griva K, Tan KYK, Chan FHF, et al. Evaluating rates and determinants of COVID-19 vaccine hesitancy for adults and children in the Singapore population: Strengthening Our Community’s Resilience against Threats from Emerging Infections (SOCRATEs) cohort. Vaccines (Basel) 2021;9:1415-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child 2020;106:429-439. [DOI] [PubMed] [Google Scholar]
- 31.Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis 2022;116:38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 2021;398:2126-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks KJ, Whitaker M, Agathis NT, et al. Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19 — COVID-NET, 14 states, March 2020–February 2022. MMWR Morb Mortal Wkly Rep 2022;71:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.