Abstract
Expression of the nickel-specific transport system encoded by the Escherichia coli nikABCDE operon is repressed by a high concentration of nickel. By using random transposon Tn10 insertion, we isolated mutants in which expression of the nik operon became constitutive with respect to nickel. We have identified the corresponding nikR gene which encodes a nickel-responsive regulator. Expression of nikR was partially controlled by Fnr through transcription from the nikA promoter region. In addition, a specific transcription start site for the constitutive expression of nikR was found 51 bp upstream of the nikR gene.
Nickel has been known for a long time as a heavy metal toxic to both eukaryotic and prokaryotic organisms (1, 16). Epidemiological studies have identified nickel as potentially carcinogenic and allergenic to humans (5, 9). Nickel is also an essential trace element for both eukaryotes and prokaryotes. The average daily requirement for humans is estimated to be 0.15 mg per day, and the total quantity in the body is about 10 mg (19). In microorganisms, nickel forms the active center of at least five classes of metalloenzymes: urease, NiFe-hydrogenase, methyl coenzyme M reductase, and CO dehydrogenase (7) and superoxide dismutase (26).
We demonstrated previously that nickel has an antagonistic effect on the fermentative growth of Escherichia coli (23). Nickel is essential for activities of three NiFe-hydrogenase isoenzymes and for bacterial fermentative growth. The successful production of these nickel-containing enzymes relies on the efficient uptake of nickel via the high-affinity, nickel-specific ABC transport system encoded by the nikABCDE operon (11, 21). However, when present at high concentrations (from 0.3 mM in rich medium), nickel inhibits growth and thus exhibits a toxic effect (11, 23). E. coli uses two strategies in response to the toxic concentration of nickel. First, it activates Tar- and NikA-dependent negative chemotaxis and swarms away from this repellent agent (4). Second, it blocks the entrance of nickel through the high-affinity nickel transport system. This is achieved by repression of the expression of the nik operon (21, 22). We report here the identification of the nikR gene which encodes a novel type of metallo-regulatory protein responding specifically to nickel.
Screening for Tn10 insertions in nickel-responsive regulator element.
To isolate mutants defective in the repression of nik at a high nickel concentration, we took advantage of the relatively simple phenotype plate screens for constitutive expression of β-galactosidase from the nikA-lacZ fusion. A random collection of 1,100 independent Tn10 insertions in strain HYD723 [as MC4100, but nikA::MudI(lacZ Ampr) (21)], which was performed by using phage lambda 1098 as described by Way et al. (20), was plated on MacConkey-lactose-tetracycline plates supplemented with 0.5 mM nickel at a density of about 200 cells per plate. Two colonies, KS01 and KS02, showing red color under anaerobic conditions were picked, purified, and characterized further. They exhibited constitutive expression of β-galactosidase activity in the presence of 0.5 mM nickel (Fig. 1). The constitutive expression of the nik operon in the mutants might be the result of insertion of Tn10 into a regulatory element or a consequence of transposition of phage MudI from the nik operon to other places. In order to assess these possibilities, we analyzed the phenotype of these double mutants with respect to the restoration of hydrogenase activity by nickel. Two observations confirmed that they retained the correct nikA::MudI(lacZ Ampr) genotype. First, these mutants showed a hydrogenase-negative phenotype, which was restored by 0.5 mM NiCl2. Second, β-galactosidase activity and hydrogenase activity were detected only during anaerobic growth in these double mutants, which is the same phenotype as that of the parental nik-lacZ single mutant HYD723 (21).
FIG. 1.
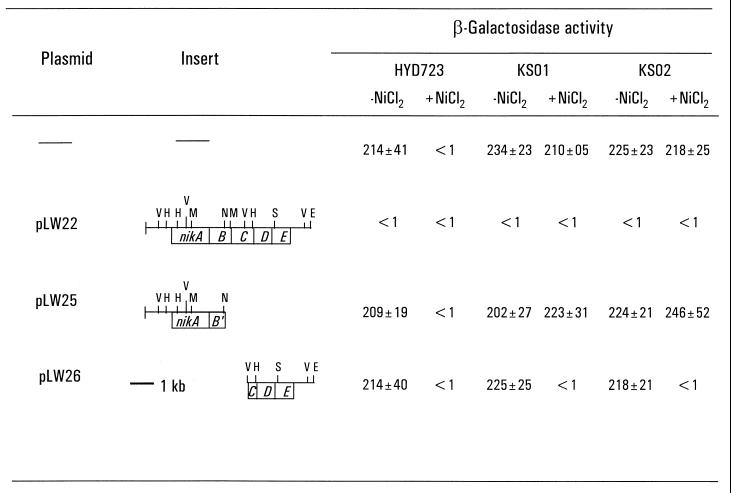
Complementation analysis of Tn10 insertion mutants with plasmids carrying the nik region. KS01 and KS02 are Tn10 insertion derivatives of HYD723 (nikA-lacZ). Cells which carried the indicated plasmid were grown microaerobically at 37°C in LB medium supplemented with 2 μM ammonium molybdate, 2 μM sodium selenite, and kanamycin (25 μg/ml) when required (21). Plasmids pLW22, pLW25, and pLW26 were described previously (24). β-Galactosidase activity was measured for cells treated by addition of 0.0025% sodium dodecyl sulfate–5% chloroform, and the specific activity is expressed as nanomoles of o-nitrophenol produced per minute per milligram bacterial dry weight. Values quoted are the averages of three separate experiments. Symbols: E, EcoRI; H, HincII; M, MluI; N, NsiI; S, SspI; V, EcoRV.
Mapping, cloning, and sequencing of the nikR gene.
In an attempt to quickly locate the mutations in mutants KS01 and KS02, the Tn10 insertions were introduced into the wild-type strain MC4100 by transduction with P1cml (10). Transductants were selected for tetracycline resistance and then screened for ampicillin resistance. This experiment also allowed us to genetically purify the Tn10 insertion mutants. In the two cases, a 98% linkage was found between ampicillin resistance and tetracycline resistance. This percentage of cotransduction indicated that the Tn10 integration is approximately 0.02 min away from the nikA-lacZ fusion on the E. coli chromosome (25). Therefore, constitutive expression of the nikA-lacZ fusion in the mutants might be the consequence of Tn10 insertion in the promoter-operator region of the nik operon or in a gene coding for a nickel-responsive regulator, which is located in the immediate vicinity.
To assess these possibilities, in trans complementation experiments were performed by using various plasmids carrying the nik operon and adjacent regions, which were described previously (22, 24). Plasmid pLW22 contains a 7-kb chromosome fragment which covers the entire nik operon as well as 0.9-kb upstream and 0.9-kb downstream adjacent regions (Fig. 1). Introduction of plasmid pLW22 into mutants KS01 and KS02 completely abolished expression of the nikA-lacZ fusion (Fig. 1). Successful in trans complementation by plasmid pLW22 indicated that transposon Tn10 had integrated into a repressor gene instead of the promoter-operator region of the nik operon in mutants KS01 and KS02. We designated the transposon-affected gene as nikR (for nickel-responsive regulator).
The nikR gene is thus located either on the 0.9-kb fragment upstream or on the 0.9-kb fragment downstream of the nik operon. Further complementation analysis showed that plasmid pLW26 containing the 0.9-kb downstream fragment was capable of complementing the Tn10 insertion mutations in mutants KS01 and KS02, whereas plasmid pLW25 carrying the 0.9-kb upstream fragment was not able to do so (Fig. 1). Therefore, the nikR gene is located downstream of the nik operon.
It should be noted that plasmid pLW22, in contrast to plasmid pLW26, repressed nikA expression in the absence and presence of nickel. Since both plasmids carry the nikR gene, it seems unlikely that the constitutive repression generated by pLW22 results from multiple copies of nikR. Interestingly, plasmid pLW22 harbors in addition the entire nik operon encoding a functional nickel-specific transport system, which should increase the intracellular nickel availability required for repression. To minimize the effects resulting from multiple copies of the nikABCDE operon, we lowered plasmid copy number by introducing a pcnB allele into strain KS01/pLW22. Expression from the nikA-lacZ fusion was relieved to half the level of that of the KS01 strain without plasmid in the absence of nickel, demonstrating that overproduction of the high-affinity nickel uptake system was responsible for the constitutive repression.
The DNA sequence of the nik locus which covers the nikABCDE genes and a 423-bp downstream region was previously reported (11). No open reading frame (ORF) was revealed by computer analysis of this 423-bp fragment. We rechecked the sequence of the 423-bp area and sequenced its 200-bp downstream region by using the T7 sequencing kit of Pharmacia with Deaza 35Sequencing Mixes to overcome G-C compression. The new sequence corrected seven errors in the old one and was in full agreement with the E. coli genome sequence in this region (15). An ORF that predicted a polypeptide of 133 amino acids was revealed. This ORF, designated nikR, was transcribed in the same direction as the nikE gene and was separated from nikE by 5 bp. This ORF corresponded to the hypothetical 15.1-kDa YhhG protein described in the E. coli genome database.
Identification of the predicted polypeptide NikR.
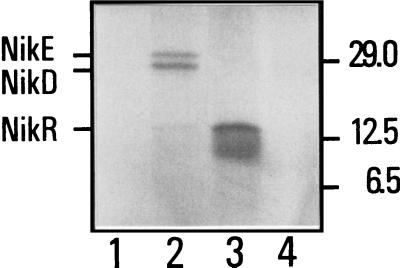
The sequence of the nikR gene predicts a polypeptide of 133 amino acids with a molecular weight of 15,093 and a calculated pI of 6.22. The hydropathy plot of the putative polypeptide revealed a highly hydrophilic protein. The product of the nikR gene was first identified by expression of nikDER genes from plasmid pHD4 by an in vivo T7 expression system (17). Autoradiography revealed two prominent bands with apparent molecular masses of 28 and 30 kDa and a much fainter band of 15 kDa (Fig. 2, lane 2). These bands were not observed in strain K38/pGP1-2 carrying vector pT7-6 alone without an insert (lane 1), and their apparent molecular masses correspond to those calculated for the nikD (26.5 kDa), nikE (29.6 kDa), and nikR (15.1 kDa) gene products, respectively. This result suggests that nikR may be expressed from the same transcriptional unit as the nikD and nikE genes from the T7 φ10 promoter under this condition, although its expression was much weaker than that of the other two genes.
FIG. 2.
Specific expression of the nikR, nikD, and nikE gene products, under control of the T7 φ10 promoter in E. coli K38/pGP1-2. Cells containing vector pT7-6 (lane 1), its derivative pHD4, harboring nikDER (lane 2), vector pKSM710 (lane 4), and its derivative p8611, harboring nikR (lane 3), were labeled with [35S]methionine and [35S]cysteine and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 17% denaturing polyacrylamide gel. Molecular mass standards (in kilodaltons) and NikE, NikD, and NikR proteins are indicated on the right and on the left, respectively.
In order to increase the specific expression of the nikR gene, we then introduced a NcoI site at the position of the ATG initiation codon of nikR and cloned nikR into plasmid pKSM710 (8). In the resulting plasmid, p8611, the nikR gene can be expressed optimally from the T7 φ10 promoter. Introduction of the NcoI site substituted the second codon, GAA (Glu), for CAA (Gln). Insertion of plasmid p8611 into strain K38/pGP1-2 led to the specific synthesis of a polypeptide with an apparent molecular mass of 15 kDa (Fig. 2; compare lanes 3 and 4), similar to that predicted from the nucleotide sequence of nikR. We concluded that the nikR gene indeed encodes a polypeptide chain. The authenticity of this polypeptide as the product of the nikR gene was proven by determination of the N-terminal sequence. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel (15%) electrophoresis and then electroblotted onto a polyvinylidene difluoride membrane. After being stained with PONCEAU S (Sigma), the NikR band was excised. The N-terminal amino acid sequence of NikR was determined by automated Edman degradation of NikR using an Applied Biosystems gas-phase sequencer. Over a stretch of seven amino acids (Met-Glu-Arg-Val-Thr-Ile-Thr), the sequence was identical to the first seven amino acids predicted from the nikR nucleotide sequence, except for the second amino acid, which was changed from Gln to Glu after introduction of the NcoI site.
Regulation of nikR gene expression.
The dependence on nickel for the NikR-mediated repression of expression of the nik operon suggests that nickel functions as either a corepressor of NikR or an inducer for the expression of nikR. In order to study nikR expression, a nikR-uidA operon fusion was constructed. The promoterless uidA-Kanr cassette encoding β-glucuronidase was obtained by SmaI digestion of plasmid pUIDK3 (2) and was inserted into the unique ApaLI site in the nikR gene of plasmid pKS1014, which is a derivative of pACYC184 containing the 7-kb BamHI-HindIII fragment of pLW21 (24). The resulting plasmid, pKS1015, was checked by restriction endonuclease digestion and was found to contain the correct fusion of the uidA-Kanr cassette within the nikR gene. Insertion of the uidA-Kanr cassette into nikR was also confirmed by the defect in nikR function. Compared with pKS1014, pKS1015 was no longer able to repress the expression of the nikA-lacZ fusion in the double mutant KS01 [nikA::MudI(lacZ Ampr) nikR::Tn10] (data not shown).
Expression of the nikR gene was first analyzed by monitoring activity of the β-glucuronidase produced as a result of plasmid pKS1015. β-Glucuronidase was not detectable in the wild-type strain, NM522 [F′ lacIq Δ(lacZ)M15 proA+B+/supE thi Δ(lac-proAB) Δ(hsdMS-mcrB)5], harboring the parental plasmid pKS1014, suggesting that the chromosomal copy of the uidA gene was not expressed under these growth conditions in the absence of inducer. Introduction of plasmid pKS1015 (nikR-uidA) conferred on strain NM522 a specific β-glucuronidase activity of about 400 units, which was increased more than sixfold under anaerobic conditions (Table 1). Neither aerobic nor anaerobic expression of nikR was affected by the addition of 0.5 mM nickel in the growth medium. Therefore, expression of the nikR gene is independent of nickel, which may function as a corepressor of NikR in the regulation of expression of the nik operon.
TABLE 1.
Expression of the nikR-uidA fusion under different growth and genetic conditions
| Strain/plasmida | Relevant genotype | β-Glucuronidase activityb
|
|||
|---|---|---|---|---|---|
| Aerobic growth
|
Anaerobic growth
|
||||
| − NiCl2 | + NiCl2 | − NiCl2 | + NiCl2 | ||
| NM522/pKS1014 | Wild type/(nikA-R)+ | < 1 | < 1 | < 1 | < 1 |
| NM522/pKS1015 | Wild type/(nikA-E)+nikR-uidA | 440 ± 37 | 400 ± 35 | 2,490 ± 83 | 2,534 ± 61 |
| MC4100 | Wild type | < 1 | < 1 | < 1 | < 1 |
| KS04 | nikR-uidA | 38 ± 01 | 33 ± 02 | 369 ± 32 | 379 ± 05 |
| KS04Fnr | nikR-uidA fnr | 34 ± 02 | ND | 105 ± 06 | ND |
| KS04/pACYC184 | nikR-uidA/vector | 37 ± 02 | 34 ± 02 | 349 ± 20 | ND |
| KS04/pKS1014 | nikR-uidA/(nikA-R)+ | 35 ± 02 | 33 ± 01 | 188 ± 08 | 191 ± 02 |
| HYD723 | nikA::MudI | < 1 | < 1 | < 1 | < 1 |
| KS06 | nikA::MudI nikR-uidA | 34 ± 04 | 39 ± 05 | 127 ± 07 | 111 ± 04 |
| KS06Fnr | nikA::MudI nikR-uidA fnr | 32 ± 02 | ND | 96 ± 04 | ND |
| KS06/pACYC184 | nikA::MudI nikR-uidA/vector | 38 ± 05 | 32 ± 02 | 92 ± 02 | 100 ± 06 |
| KS06/pKS1014 | nikA::MudI nikR-uidA/(nikA-R)+ | 37 ± 01 | 37 ± 03 | 92 ± 02 | 100 ± 06 |
All strains, except NM522, are derivatives of MC4100. Plasmid pKS1014 contains a 7-kb chromosome fragment which covers the entire nik operon. Plasmid pKS1015, a derivative of pKS1014, was constructed as described in the text. Cells were grown either aerobically or microaerobically at 37°C in LB medium supplemented with 2 μM ammonium molybdate, 2 μM sodium selenite and, when required, 20 μg of chloramphenicol per ml. When noted, 0.5 mM NiCl2 was added in the growth medium.
β-Glucuronidase activity was measured and expressed as described in Fig. 1. Values quoted (with standard deviations) are the averages of three separate experiments.
To avoid multiple-copy effect, the nikR-uidA fusion was recombined back into the chromosome of the recD strain, D355 [F− lac-3350 galK2 galT22 λ− recD1014 rpsL179 IN(rrnD-rrnE)1] (13), after linearization of plasmid pKS1015 with EcoRI and KpnI. The resulting nikR mutation was then moved into strain MC4100 (araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 ptsF25 deoC1 rbsR) via P1cml transduction (10), selecting for kanamycin resistance and giving rise to strain KS04. To ascertain a successful recombination, biosynthesis of NikA was analyzed by immunoblotting, as described previously (4). As expected, the NikA protein was not detected by anti-NikA antiserum in a crude extract of the wild-type parental strain, MC4100, grown in the presence of 0.5 mM nickel. In contrast, KS04 synthesized NikA constitutively in the absence or presence of nickel in the growth medium, indicating the defect of the chromosomal nikR gene (data not shown). Compared with expression from plasmid pKS1015, the β-glucuronidase activity was reduced by 10-fold and 6-fold in strain KS04 grown under aerobic and anaerobic conditions, respectively (Table 1). However, nikR expression remained inducible by anaerobic conditions and independent of nickel concentration.
The fnr gene product is required for the anaerobic expression of several respiratory enzymes (6). Expression of the nik operon has been reported to be under the positive control of Fnr (21). To test whether or not the anaerobic induction of the nikR-uidA expression depends on Fnr, nikR expression was examined in an fnr background. The fnr derivative of KS04 was constructed by transduction with a P1cml lysate grown on strain MC4100nir (as MC4100, but fnr-22 zcj-261::Tn10) (21), selecting for tetracycline resistance and then scoring for the absence of nitrate reductase. Since the fnr mutation led to a 3.5-fold reduction in anaerobic nikR-uidA expression in the resulting double mutant KS04Fnr compared with expression in the parental single mutant KS04, nikR expression seems to be regulated partially by Fnr (Table 1).
To assess whether nikR is autoregulated, the wild-type nikR allele was provided in trans to mutant KS04 by plasmid pKS1014 carrying the entire nik operon in addition to nikR. The anaerobic β-glucuronidase activity was reduced by about twofold in the resulting strain, KS04/pKS1014, compared with that in KS04 carrying vector pACYC184 without an insert. Therefore, nikR appears to be partially autoregulated.
The partial autoregulation and the Fnr-mediated activation of nikR expression could be the consequence of transcriptional regulation of nikR at the level of the promoter of the nik operon. This hypothesis is supported by the finding, in the promoter-operator region of the nik operon (11), of an inverted repeated sequence composed of a 14-base dyad, AATCAGTATGACGA-N10-TCGTCATACTTATT, which may serve as a NikR binding site and of a partially conserved FNR box located just upstream. Indeed, introduction, by P1cml-mediated transduction, of the nikA::MudI insertion upstream of the nikR gene in mutant KS06 had a strong polar effect on nikR-uidA expression (Table 1), which was reduced to a level similar to that of KS04Fnr. In addition, neither the fnr mutation nor the presence of multiple copies of nikR+ in trans had further effect on the nikR-uidA expression in mutant KS06 (Table 1). These results suggest that the invariable aerobic β-glucuronidase activity and the remaining anaerobic β-glucuronidase activity in the double mutant KS06 could be more a consequence of nikR-uidA expression from another promoter than that of the nikABCDE operon.
Mapping of the transcriptional start site for nikR.
To identify the promoter responsible for constitutive nikR transcription, total RNA isolated from wild-type strain NM522 harboring plasmid pLW22 was subjected to primer extension analysis by using the synthetic oligonucleotide NikR (5′-GCTCAGGCGATCCAGCG-3′). This oligonucleotide is complementary to the DNA sequence from bp 59 to bp 44 downstream of the translation start codon of nikR. The same extension product of 111 bp was detected from cultures grown anaerobically without or with 0.5 mM NiCl2 (Fig. 3), identifying the A residue located 51 bp upstream of the ATG of nikR as the transcription start site. A putative −10 box (TACAAA) was found, but a sequence homologous to the −35 box with the proper spacing was not identified. This result confirms that expression of nikR is independent of nickel concentration and indicates that this gene is constitutively expressed from its own promoter.
FIG. 3.
Determination of the constitutive nikR transcription start site. Total RNAs (50 μg) isolated from E. coli NM522/pLW22 grown anaerobically in Luria broth in the absence (lane 1) or presence (lane 2) of 0.5 mM NiCl2 were analyzed by primer extension with primer NikR (see text). The DNA sequence ladder (lanes TGCA) was obtained with the same primer and plasmid pLW22 as a template. Part of the sequence and the nikR transcription start site are indicated on the right.
Functional similarity between NikR and Fur proteins.
Bacteria require numerous metal ions, such as iron, nickel, and cobalt, for growth and have consequently evolved several distinct, high-affinity uptake systems (14). However, at high concentrations, metal ions are also potentially toxic elements because they can catalyze the formation of dangerously reactive hydroxyl radicals, which can damage virtually all cellular constituents. Therefore, expression of these systems is generally tightly regulated. A common feature of many of these uptake systems is that their expression is induced by metal ion starvation and repressed by high concentrations of metal. The best known example is the iron-responsive regulation of the expression of the high-affinity iron uptake pathway and bacterial virulence factors (12). It is mediated by the ferric uptake regulation (Fur) repressor protein or diphtheria toxin repressor (DtxR) (14, 18).
The results described above indicate that NikR resembles a Fur or DtxR counterpart and functions as a nickel-responsive regulator in nickel metabolism. Interestingly, when NikR was used as a query sequence to scan the nr database (all nonredundant GenBank CDS translations+PDB+SwissProt+PIR+PRF by using the Blastp program (http://www.expasy.ch/cgi-bin/blastncbi.p1), the 12 best-scoring sequences were as follows, in decreasing order: six conserved hypothetical proteins from Methanococcus jannaschii (Y549_METJA), Archaeoglobus fulgidus (AE001054), Helicobacter pylori (AE000635), Methanobacterium thermoautotrophicum (AE000835 and AE000842), and Methanococcus jannaschii (Y767_METJA); Fur protein from Vibrio cholerae (FUR_VIBCH); Rho1 GDP-GTP exchange protein 2 from Saccharomyces cerevisiae (ROM2_YEAST); SCP-1 from Homo sapiens (D67035); and three Fur proteins, from Vibrio anguillarum (FUR_VIBAN), Vibrio vulnificus (FUR_VIBVU), and Vibrio parahaemolyticus (AB003752). Therefore, the only apparent functionally related proteins are the four ferric uptake regulator proteins. The Smallest Sum Probability P(N) score obtained by sequence comparison between NikR and these Fur proteins ranges from 0.60 to 0.97, which is too low to establish sequence relatedness between them. However, NikR contains two motifs which are perfectly conserved in the four Fur proteins described above. The first motif consists of STQHHHXXL, corresponding to residues 73 to 81 of NikR. This motif is located in a region that shows the highest solvent accessibility score in NikR and that is composed of a histidine-rich stretch of 5 amino acids. The sequence of the stretch is His-His-His-His-Asp (HHHHD) in NikR and (His)-His-His-Asp-His [(H)HHDH] in 16 Fur-like proteins from gram-negative bacteria. The corresponding histidine-rich region of Fur-like proteins from gram-positive bacteria and Synechococcus does not contain aspartate. Some of these residues may provide a ligand(s) for metal coordination, and the position of the Asp might be of importance for metal specificity. The second motif, DXGXVXXFXDDXIXXR, corresponds to residues 104 to 119 of NikR and it is not as well conserved as the first one in the 16 Fur-like proteins.
To assess a possible functional substitution of NikR with Fur, we tested the effect of a fur deletion on nikA-lacZ expression by introducing fur::Tn903 from strain QC1732 into HYD723 or by complementation of the nikR deficiency in KS01 by the fur gene. In neither case was the regulation pattern of the nik operon expression altered (data not shown). The nonexchangeability may rely on the low level of similarity between the NikR and FurR proteins and on the difference in the structures of the repressor-binding sites. The potential NikR-binding site contains two half sites of 14 bp separated by 10 bp (see above), whereas the perfect dyad of the Fur box is separated by one nucleotide (3). In vivo and in vitro experiments will help to determine the precise DNA motif involved in the contact with NikR.
Acknowledgments
This work was supported by grants from the Centre National de la Recherche Scientifique to UMR CNRS 5577 and UPR CNRS 9043, the “Programme de Recherche Fondamentale en Microbiologie, Maladies Infectieuses et Parasitaires” (to M.A.M. and L.F.W.), and the Ligue Nationale contre le Cancer, Comité des Bouches-du-Rhône no. 471.96 (to L.F.W. and G.G.).
We thank J. Robert-Baudouy for her constant support for this work. We acknowledge C. C. Richardson, K. Ippen-Ihler, and D. Touati for plasmids pT7-6 and pKSM710 and fur strains, respectively.
REFERENCES
- 1.Babich H, Stotzky G. Toxicity of nickel to microbes: environmental aspects. Adv Appl Microbiol. 1983;29:195–265. doi: 10.1016/s0065-2164(08)70358-7. [DOI] [PubMed] [Google Scholar]
- 2.Bardonnet N, Blanco C. ′uidA-antibiotic-resistance cassettes for insertion mutagenesis, gene fusions and genetic constructions. FEMS Microbiol Lett. 1992;72:243–247. doi: 10.1016/0378-1097(92)90469-5. [DOI] [PubMed] [Google Scholar]
- 3.de Lorenzo V, Wee S, Herrero M, Neilands J B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987;169:2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Pina K, Navarro C, McWalter L, Boxer D H, Price N C, Kelly S M, Mandrand-Berthelot M A, Wu L F. Purification and characterization of the periplasmic nickel-binding protein NikA of Escherichia coli K12. Eur J Biochem. 1995;227:857–865. doi: 10.1111/j.1432-1033.1995.tb20211.x. [DOI] [PubMed] [Google Scholar]
- 5.Doll R, Mathews J D, Morgan L G. Cancers of the lung and nasal sinuses in nickel workers: a reassessment of the period of risk. Br J Ind Med. 1977;34:102–105. doi: 10.1136/oem.34.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunsalus R P, Park S J. Aerobic-anaerobic gene regulation in Escherichia coli: control by the ArcAB and Fnr regulons. Res Microbiol. 1994;145:437–450. doi: 10.1016/0923-2508(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 7.Hausinger R P. Nickel utilization by microorganisms. Microbiol Rev. 1987;51:22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maneewannakul S, Maneewannakul K, Ippen-Ihler K. The pKSM710 vector cassette provides tightly regulated lac and T7lac promoters and strategies for manipulating N-terminal protein sequences. Plasmid. 1994;31:300–307. doi: 10.1006/plas.1994.1032. [DOI] [PubMed] [Google Scholar]
- 9.Menné T, Christophersen J, Green A. Epidemiology of nickel dermatitis. In: Maibach H I, Menné T, editors. Nickel and the skin: immunology and toxicology. Boca Raton, Fla: CRC Press; 1989. p. 109. [Google Scholar]
- 10.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 11.Navarro C, Wu L-F, Mandrand-Berthelot M A. The nik operon of Escherichia coli encodes a periplasmic binding-protein-dependent transport system for nickel. Mol Microbiol. 1993;9:1181–1191. doi: 10.1111/j.1365-2958.1993.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 12.Payne S M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1993;1:66–69. doi: 10.1016/0966-842x(93)90036-q. [DOI] [PubMed] [Google Scholar]
- 13.Russell C B, Thaler D S, Dahlquist F W. Chromosomal transformation of Escherichia coli recD strains with linearized plasmids. J Bacteriol. 1989;171:2609–2613. doi: 10.1128/jb.171.5.2609-2613.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silver S, Walderhaug M. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol Rev. 1992;56:195–228. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sofia H J, Burland V, Daniels D L, Plunkett III G, Blattner F R. Analysis of the Escherichia coli genome. V. DNA sequence of the region from 76.0 to 81.5 minutes. Nucleic Acids Res. 1994;22:2576–2586. doi: 10.1093/nar/22.13.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunderman F W., Jr Search for molecular mechanisms in the genotoxicity of nickel. Scand J Work Environ Health. 1993;19:75–80. [PubMed] [Google Scholar]
- 17.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao X, Schiering N, Zeng H-Y, Ringe D, Murphy J R. Iron, DtxR, and the regulation of diphtheria toxin expression. Mol Microbiol. 1994;14:191–197. doi: 10.1111/j.1365-2958.1994.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 19.Wapnir R A. Protein nutrition and mineral absorption. Boston, Mass: CRC Press; 1990. Nutritional environmental and physiologic factors affecting selenium, chromium, and nickel sufficiency; pp. 211–242. [Google Scholar]
- 20.Way J C, Davis M A, Morisato D, Roberts D E, Kleckner N. New derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984;32:369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- 21.Wu L-F, Mandrand-Berthelot M A. Genetic and physiological characterization of new Escherichia coli mutants impaired in hydrogenase activity. Biochimie. 1986;68:167–179. doi: 10.1016/s0300-9084(86)81081-1. [DOI] [PubMed] [Google Scholar]
- 22.Wu L-F, Mandrand-Berthelot M A, Waugh R, Edmonds C J, Holt S E, Boxer D H. Nickel deficiency gives rise to the defective hydrogenase phenotype of hydC and fnr mutants in Escherichia coli. Mol Microbiol. 1989;3:1709–1718. doi: 10.1111/j.1365-2958.1989.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu L-F, Navarro C, de Pina K, Quenard M, Mandrand M A. Antagonistic effect of nickel on the fermentative growth of Escherichia coli K-12 and comparison of nickel and cobalt toxicity on the aerobic and anaerobic growth. Environ Health Perspect. 1994;102:297–300. doi: 10.1289/ehp.94102s3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu L-F, Navarro C, Mandrand-Berthelot M A. The hydC region contains a multi-cistronic operon (nik) involved in nickel transport in Escherichia coli. Gene. 1991;107:37–42. doi: 10.1016/0378-1119(91)90294-l. [DOI] [PubMed] [Google Scholar]
- 25.Wu T A. A model for three-point analysis of random general transduction. Genetics. 1966;54:405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youn H D, Kim E J, Roe J H, Hah Y C, Kang S O. A novel nickel-containing superoxide dismutase from Streptomyces spp. Biochem J. 1996;318:889–896. doi: 10.1042/bj3180889. [DOI] [PMC free article] [PubMed] [Google Scholar]