The significance of maintaining intracellular homeostasis cannot be overestimated. When challenged with hypotonicity, cells counteract the initial swelling by releasing osmolytes: K+, Cl−, and other organic anions in order to restore volume. This mechanism is widely known as the regulatory volume decrease.1 Volume-regulated anion currents (VRACs) were first described in 1988,2 and their electrophysiologic properties have been examined in a variety of cell types.3,4 However, the molecular identity of VRACs remained enigmatic for a long time until the leucine-rich repeat–containing 8 member A channel (LRRC8A; also known as SWELL1) was identified in 2014.5,6 LRRC8A can itself form functional hexamers, or it may assemble in herteromers with other members, including LRRC8B, LRRC8C, LRRC8D, and LRRC8E, to produce anion-conducting channels with unique biophysical properties. The leucine-rich repeat is largely responsible for the heterotetramer-specific differences in the physiologic functions of LRRC8/VRAC channels, including permeability and gating. LRRC8-mediated VRACs are activated not by a direct stretch of the plasma membrane but rather, by reductions in the intracellular ionic power due to osmotically driven water influx.7
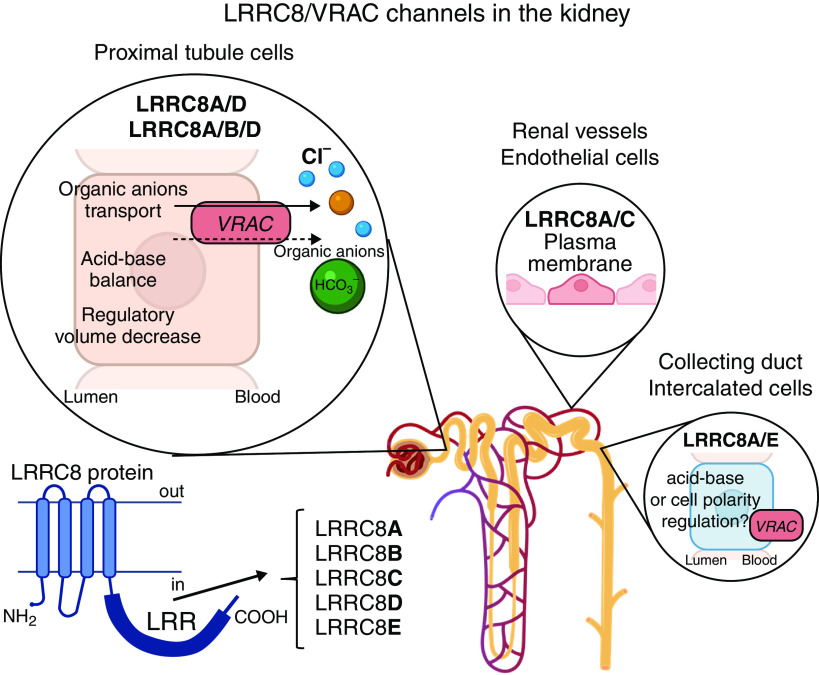
The kidneys are essentialfor tight regulation of water and electrolytes in the body. As a result, kidney cells are commonly exposed to a broad range of extracellular osmolarities. Variations in transmembrane osmotic gradients would lead to activation of the LRRC8-mediated currents; the highest magnitude would be anticipated in the hypertonic medullary regions. These were the expectations. Using newly created transgenic LRRC8 reporter models, the study by Lopez-Cayuqueo et al.8 provides the first direct evidence of site-specific expression of distinct LRRC8 members in renal tissue and more importantly, investigated the consequences of cell-specific LRRC8 deletion on renal function (Figure 1). It was found that LRRC8A was uniformly expressed in renal cells, whereas other subunits were localized in distinct cell populations. LRRC8C was restricted to vascular endothelium, LRRC8E was found exclusively in intercalated cells of the collecting duct, and LRRC8B/LRRC8D resided on the basolateral membrane of proximal tubule cells. These strikingly selective localizations of LRRC8 would be in line with region-specific functions as well as distinct osmotic gradients existing in the different regions of the kidney. However, this is where the clever experiments and results reported overturned our preconceived expectations.
Figure 1.
Site-specific expression of osmo-activated LRRC8 in the kidney. A VRAC is formed by LRRC8 subunits containing leucine-rich repeat (LRR), which determines pore properties and gating of the heterotetrameric channel. LRRC8/VRAC mediates passive efflux of Cl− ions, bicarbonate (HCO3−), and small organic osmolytes, including amino acids, sorbitol, taurine, etc. LRRC8 composition is uniquely region specific in the kidney. Proximal tubule cells are the central hub responsible for VRAC renal function, presumably mediated by LRRC8A/D and LRRC8A/B/D channels.
LRRC8A and LRRC8D deletion led to dysfunction and structural damage along the proximal tubule, whereas no adverse phenotype was evident with LRRC8A deletion in the distal segments. Furthermore, the severity of proximal tubule injury exhibited a positive correlation with the magnitude of LRRC8 deletion using different promoters. The authors reported that LRRC8A deletion correlates with intracellular accumulation of VRAC-permeable organic anions, including taurine, myo-inositol, gluconate, and lactate, which could partially account for the observed cortical damage. Interestingly, LRRC8A deficiency caused metabolic acidosis, pointing to a role in bicarbonate transport that can also occur through the VRAC channel.9 Considering that proximal tubule reabsorption occurs in an isosmotic manner, it is unlikely that LRRC8 is activated in response to decreased intracellular osmolarity. Future studies are charged with solving the mystery of LRRC8 stimulation in the proximal tubule in the absence of apparent osmotic stimuli.
The specific expression of the LRRC8E isoform localized to the basolateral membrane of intercalated cells indicates fundamentally different mechanisms of cell volume regulation in principal and intercalated cells. The latter type seems to be highly vulnerable to acute perturbations in luminal/interstitial osmolarity due to low water permeability and lack of aquaporins type 2, 3, and 4 (AQP2/AQP3/AQP4) water channels in contrast to their expression in principal cells.10 At the same time, deletion of LRRC8E does not provoke structural and functional defects in intercalated cells, arguing that this is an adaptive mechanism that is not essential for baseline homeostasis. Further studies are required to reveal the role of LRRC8E-mediated anion flux in adaptation of intercalated cells to osmotic stress.
In summary, the article by Lopez-Cayuqueo et al.8 represents a breakthrough moment of pure scientific excitement. The osmo-activated LRRC8 channels are perfectly suited to serve an essential role in maintaining cellular homeostasis/volume in the face of rapidly changing transport rates along the renal tubule. Before this study, it seemed that the only rather humdrum question was the relative contribution of different members of the LRRC8 family. Instead, we have learned that LRRC8 is critical for the function of proximal tubule, where no osmotic gradients exist. In contrast, deletion of LRRC8 members does not interfere with normal operations in the distal tubule, where osmotically driven cell volume increases and decreases are common. Solving one puzzle generated many more to decipher. Although we may have been deceived in our expectations, these unexpected findings open a new exciting area for future kidney physiology research. How and why are LRRC8s activated in the absence of osmotic gradients? Why is their function so crucial for the survivability of proximal tubule cells? Can we use stimulation of LRRC8 as a novel renoprotective strategy during pronounced injury of the proximal tubule that occurs during ischemia/hypoxic states, diabetic nephropathy, and CKD? We look forward to the next installments and answers in the future issues of JASN.
Disclosures
O. Palygin reports other interests or relationships with the American Heart Association the Council on the Kidney in Cardiovascular Disease (KCVD) (member) and the American Physiological Society (member). The remaining author has nothing to disclose
Funding
This research was supported by American Heart Association grant EIA35260097 (to O. Pochynyuk), endowed funds from the South Carolina SmartState Centers of Excellence (to O. Palygin), and National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants DK117865, DK126720 (to O. Palygin), and DK095029 (to O. Pochynyuk).
Acknowledgments
The authors thank Dr. Alicia A. McDonough from the Department of Physiology and Neuroscience, Keck School of Medicine of the University of Southern California (Los Angeles, CA) for valuable discussion and help with the editorial.
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or JASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “Renal Deletion of LRRC8/VRAC Channels Induces Proximal Tubulopathy,” on pages 1528–1545.
Author Contributions
O. Palygin and O. Pochynyuk wrote the original draft and reviewed and edited the manuscript.
References
- 1.Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S: Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol 532: 3–16, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazama A, Okada Y: Ca2+ sensitivity of volume-regulatory K+ and Cl- channels in cultured human epithelial cells. J Physiol 402: 687–702, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahalan MD, Lewis RS: Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser 43: 281–301, 1988 [PubMed] [Google Scholar]
- 4.Doroshenko P, Neher E: Volume-sensitive chloride conductance in bovine chromaffin cell membrane. J Physiol 449: 197–218, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, et al. : SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157: 447–458, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voss FK, Ullrich F, Münch J, Lazarow K, Lutter D, Mah N, et al. : Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344: 634–638, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Jentsch TJ: VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 17: 293–307, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Cayuqueo K, Planells-Cases R, Pietzke M, Oliveras A, Kempa S, Bachmann S, et al. : Renal deletion of LRRC8/VRAC channels induces proximal tubulopathy. J Am Soc Nephrol 33: 1528–1545, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilius B, Prenen J, Droogmans G: Modulation of volume-regulated anion channels by extra- and intracellular pH. Pflugers Arch 436: 742–748, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE: Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10: 135–146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]