Abstract
Full-length parathyroid hormone (PTH 1–84) is crucial for the regulation of calcium and phosphate homeostasis and bone remodeling. PTH 1–84 is metabolized into various PTH fragments, which are measured with varying levels of efficiency by PTH immunoassays. These PTH fragments, which increase in serum as CKD progresses, could potentially modulate the effects of PTH 1–84 and contribute to CKD-associated bone disorders. To obtain a true biologic representation of total PTH bioactivity, it is necessary to measure not only PTH 1–84 but also PTH fragments that are present in circulation. Traditional second-generation PTH immunoassays collectively measure PTH 1–84, PTH fragments, and post-translationally modified PTH 1–84, making it difficult to accurately predict the character of underlying renal osteodystrophy. This review highlights current advances in methods available for PTH measurement and the clinical relevance of PTH fragments in CKD. We emphasize the usefulness of mass spectrometry as a potential reference method for PTH measurement.
Keywords: parathyroid hormone, mineral metabolism, mass spectrometry
Full-length parathyroid hormone (PTH 1–84) plays an important role in the regulation of calcium and phosphate homeostasis and bone remodeling. Clinical practice guidelines recommend the measurement of PTH to monitor development and severity of mineral and bone disorder in patients with CKD. PTH is currently measured by second-generation PTH immunoassays, which use antibodies directed against different regions of PTH 1–84 and detect not only full-length PTH but also various PTH fragments with varying degrees of efficiency. The evidence suggests that these PTH fragments might modulate the PTH bioactivity and could play roles in CKD–mineral bone disorder. This review will discuss the value of measuring PTH 1–84 and its fragments by liquid chromatography–high-resolution mass spectrometry (LC-HRMS) in patients with CKD. In the review we will demonstrate the following:
To obtain a true biologic representation of total PTH bioactivity in serum, it might be beneficial to measure not only full-length PTH 1–84 but also PTH fragments that circulate in human plasma as a heterogeneous mixture of smaller PTH fragments truncated at the carboxyl terminus and/or the amino terminus. These PTH fragments could potentially demonstrate unique PTH-modulating bioactivity that may alter the bioactivity of PTH 1–84.1–10
Concentrations of circulating PTH fragments increase as CKD progresses, and increased concentrations of PTH fragments could have significant effects on bone, either by themselves or by altering the ability of PTH 1–84 to function.1,6
It is important to note that antibody-based PTH immunoassays often detect circulating PTH fragments of uncertain chemical composition, or PTH 1–84 with poorly characterized post-translational modifications, thereby leading to erroneous conclusions regarding the physiologic role of PTH fragments or post-translationally modified PTH 1–84.
Although not practical for use as a high-throughput method usable in a clinical laboratory, LC-HRMS could be used to validate serum concentrations of PTH peptides and the presence and concentrations of post-translationally modified PTH 1–84.
Biosynthesis and Bioactivity of Full-Length PTH and PTH Fragments
Human PTH is synthesized in parathyroid glands as 115-amino acid pre-pro-PTH. Pre-pro-PTH is cleaved in the endoplasmic reticulum of parathyroid chief cells to yield a 90-amino acid pro-PTH, which is ultimately cleaved in the Golgi complex to yield bioactive full-length PTH 1–84, with a molecular mass of approximately 9400 Da.11 PTH is stored in secretory granules and is secreted in response to hypocalcemia.12,13 Circulating PTH 1–84 has a plasma t1/2 of 2–4 minutes and is primarily cleared by hepatic Kupffer cells. Additionally, PTH 1–84 is cleared from the circulation by the kidney via renal peritubular uptake and, to a lesser extent, glomerular filtration.14,15 Hepatic proteolysis of PTH 1–84 results in the formation of amino-terminal and carboxy-terminal PTH fragments.16,17 The former peptides are degraded in situ, whereas carboxy-terminal PTH fragments, which have a longer t1/2 than PTH 1–84, are released into the circulation to be cleared by the kidneys, mainly via glomerular filtration.14,18–20 Carboxy-terminal PTH fragments are also generated in parathyroid glands by cathepsin-mediated intracellular proteolysis of PTH 1–84, and are subsequently secreted into circulation in a calcium-dependent manner.21–24
PTH exerts its biologic effects on bone and kidney through the PTH receptor 1 (PTHR1) by activation of protein kinase A and C pathways.25–27 In the distal tubule, PTH 1–84 stimulates the renal tubular reabsorption of calcium,28 whereas, in the proximal tubule, it reduces the renal tubular reabsorption of phosphate29,30 and stimulates the activity of the 25-hydroxyvitamin D 1α-hydroxylase, which converts 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D (calcitriol), the active metabolite of vitamin D.31,32 In bone, PTH 1–84 acts directly on osteoblasts, modifying growth and differentiation.33,34 It also modifies the activity of osteoclasts through differential changes in the expression of osteoblast RANK ligand and osteoprotegerin.35
Altered Metabolism of PTH in CKD
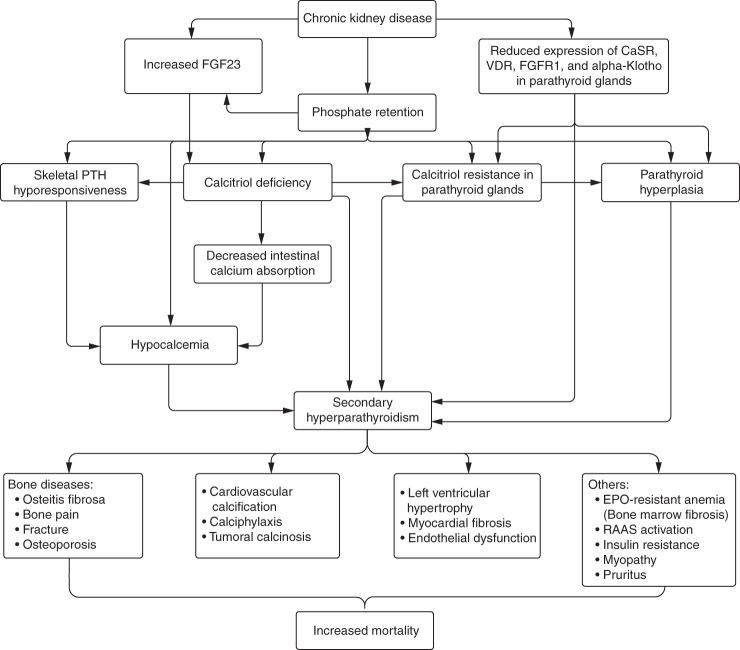
Secondary hyperparathyroidism is a central component of CKD–mineral bone disorder and leads to a worsening of laboratory parameters, renal osteodystrophy, and cardiovascular calcification. Epidemiologic studies show that serum PTH concentrations start to rise once the eGFR falls below 60 ml/min per 1.73 m2.36,37 The main factors leading to secondary hyperparathyroidism include phosphate retention, calcitriol deficiency, hypocalcemia, and fibroblast growth factor 23 hypersecretion (Figure 1). In addition, normal inhibitory feedback mechanisms become impaired in CKD due to downregulation of calcium-sensing receptor, vitamin D receptor, fibroblast growth factor 23 receptor, and Klotho in the parathyroid glands. Moreover, skeletal hyporesponsiveness to the action of PTH might aggravate the exacerbation of secondary hyperparathyroidism because higher PTH is needed to maintain bone turnover. Lastly, impaired renal clearance of PTH also contributes to secondary hyperparathyroidism in CKD.15
Figure 1.
Long-standing CKD and ESKD are associated with metabolic disturbances that lead to hypersecretion of PTH and FGF23, hyperphosphatemia, calcitriol deficiency and resistance, and hypocalcemia. Common complications of secondary hyperparathyroidism include bone diseases and cardiovascular calcifications that may contribute to cardiovascular mortality. CaSR, calcium-sensing receptor; EPO, erythropoietin; FGF23, fibroblast growth factor 23; FGFR1, fibroblast growth factor receptor 1; RAAS, renin-angiotensin-aldosterone system; VDR, vitamin D receptor.
The Chemical Identity and Concentrations of Circulating PTH Fragments Was Unknown until Recently
A reduced eGFR not only affects the biosynthesis of PTH 1–84 but also the clearance of PTH fragments, which generates the need to identify and accurately measure circulating PTH fragments and to understand their bioactivity. Until recently, the definitive chemical identification of circulating PTH fragments derived from full-length PTH 1–84 had not been undertaken.1 We identified eight PTH fragments (PTH 28–84, PTH 34–77, PTH 34–84, PTH 37–77, PTH 37–84, PTH 38–77, PTH 38–84, and PTH 45–84) of unknown bioactivity in the serum obtained from normal subjects and from individuals with CKD/ESKD using LC-HRMS.1 In line with previous research, we found that serum concentrations of PTH fragments rise progressively in patients with CKD.1,38–40 In an earlier study, using matrix-assisted laser desorption ionization mass spectrometry, Zhang et al.2 described the presence of four PTH fragments (PTH 34–84, PTH 37–84, PTH 38–84, and PTH 45–84) in the plasma of four patients with CKD, and in six normal subjects after parenteral PTH 1–84 administration. In a subsequent study using selected reaction monitoring–mass spectrometric assays, Lopez et al.3 found the same PTH fragments together with an additional five PTH fragments (PTH 28–84, PTH 34–77, PTH 37–77, PTH 38–77, and PTH 48–84) in plasma obtained from 12 healthy subjects and from 12 patients with ESKD. However, these authors did not test the bioactivity of the aforementioned PTH fragments. Table 1 summarizes the findings of these studies, indicating that, with advances in mass spectrometry, more information about PTH fragments is obtained.
Table 1.
Summary of mass spectrometry-based studies for detection of circulating PTH fragments in humans
| Features | Zhang et al.2 | Lopez et al.3 | Kritmetapak et al.1 |
|---|---|---|---|
| Method | MALDI-TOF MS or nanoLC- ESI-TOF-MS | Trypsin digestion followed by MALDI-TOF MS or SRM-MSIA | LC-HRMS |
| Immunocapture of PTH before MS analysis | C-terminal immunocapture (39–84) | C-terminal immunocapture (39–84) | C-terminal (44–84) and N-terminal immunocapture (1–37) |
| Use of stable isotope-labeled IS specific to PTH fragments | No | No | Yes |
| Number of patients | 10 (six healthy individuals receiving PTH 1–84 injection, four patients with CKD) |
24 (12 healthy individuals, 12 patients with CKD stage 5) |
221 patients (CKD stages 1–5) |
| PTH molecular forms detected | Four PTH fragments (PTH 34–84, 37–84, 38–84, and 45–84) |
PTH 1–84 and nine PTH fragments (PTH 28–84, 34–77, 34–84, 37–77, 37–84, 38–77, 38–84, 45–84, and 48–84) |
PTH 1–84 and eight PTH fragments (PTH 28–84, 34–77, 34–84, 37–77, 37–84, 38–77, 38–84, and 45–84) |
| PTH 7–84 | Not reported | Not detected (by monitoring tryptic peptide 7–13) |
Not detected (<30 pg/ml) |
| oxPTH 1–84 | Not reported | Not reported | Not detected (<50 pg/ml) |
| Serum concentration of PTH fragments in relation to eGFR | Not reported | Not reported | Reporteda |
MALDI-TOF; matrix-assisted laser desorption/ionization–time of flight; MS, mass spectrometry; nanoLC-ESI-TOF, nanoscale liquid chromatography–electrospray ionization–time of flight; SRM-MSIA, selected reaction monitoring–mass spectrometric immunoassay; C-terminal, carboxy-terminal; N-terminal, amino-terminal; IS, internal standard.
Serum concentrations of PTH 1–84 and PTH fragments increased significantly when eGFR decreased to ≤17–23 ml/min per 1.73 m2, depending on PTH species.
Identification of Novel PTH Fragments in the Serum of Subjects with and without CKD/ESKD
We identified and quantitated serum full-length PTH 1–84 and PTH fragments (PTH 28–84, PTH 34–77, PTH 34–84, PTH 37–77, PTH 37–84, PTH 38–77, PTH 38–84, and PTH 45–84) using LC-HRMS in patients with varying eGFR values (Table 2).1 Serum PTH 7–84 and oxidized forms of PTH 1–84 were not detected or were below the lower limit of quantitation. We showed that serum concentrations of PTH 1–84 and PTH fragments rose significantly when an eGFR decreased to ≤17–23 ml/min per 1.73 m2 (Figure 2). In patients with an eGFR of <30 ml/min per 1.73 m2, serum PTH concentrations measured using LC-HRMS were significantly lower than those measured using second-generation PTH immunoassays. We do not have a ready explanation for why serum PTH 1–84 measured by LC-HRMS increased only when the eGFR had reached the range of 15–30 ml/min per 1.73 m2, whereas previous work had shown an increase in PTH 1–84 measured by PTH immunoassays at higher eGFR values.36,37 It is possible that subtle increases in serum PTH 1–84 were present at higher eGFRs but were not readily detected by the LC-HRMS method. Additionally, our cohort had a low prevalence of hyperphosphatemia, which might account for the development of hyperparathyroidism only at lower eGFRs.1 Interestingly, a recent cohort study of patients with CKD found that serum PTH levels, measured by a third-generation PTH immunoassay, increased only when the eGFR declined to <20–30 ml/min per 1.73 m2.41 Similar concentrations of PTH 1–84 were noted to be present whether we used carboxy-terminal antibody or amino-terminal antibody capture methods before LC-HRMS analysis. By including analytic standards and stable isotope-labeled internal standards, we quantified serum PTH 1–84 and PTH fragments. Intact protein analysis using LC-HRMS, without tryptic digestion, allows specific detection of all captured PTH peptides in addition to PTH 1–84. An additional advantage of operating mass spectrometry systems in full scan mode, as compared with traditional immunoassays, is the ability to detect new circulating PTH fragments for which analytic standards are unavailable. We confirmed the absence of circulating amino-terminal PTH fragments (e.g., PTH 1–34) and documented the presence of carboxy-terminal and midregion PTH fragments. We show that, in patients with CKD/ESKD and diabetes mellitus, there is a trend toward an increase in serum concentrations of PTH 28–84, as compared with individuals with CKD/ESKD due to nondiabetic disease (Table 3). As noted below, PTH 28–84 blocks PTH (1–84)–mediated increases in alkaline phosphatase activity and does not alter the generation of 3′,5′-cAMP in cell culture.
Table 2.
Serum concentration of LC-HRMS PTH 1–84 and PTH fragments according to stages of kidney function
| PTH Peptides | All | eGFR (ml/min per 1.73 m2) | P for Trend | ||||
|---|---|---|---|---|---|---|---|
| ≥60 | 45–59 | 30–44 | 15–29 | <15 | |||
| PTH 1–84 | 82 (66–120) | 70 (67–95) | 72 (58–93) | 76 (61–95) | 77 (58–93) | 115 (81–189)b | 0.008 |
| PTH 28–84 | 75 (56–118) | 55 (49–70) | 58 (58–141) | 68 (61–111) | 71 (49–96) | 112 (80–180)c | 0.009 |
| PTH 34–77 | 113 (68–307) | 59 (45–78) | 56 (47–66) | 109 (97–125) | 114 (88–134)b | 285 (194–393)c | 0.004 |
| PTH 34–84 | 219 (97–554) | 99 (67–213) | 165 (81–413) | 206 (93–298)a | 231 (149–362)a | 1026 (480–1498)c | 0.006 |
| PTH 37–77 | 206 (99–349) | 105 (96–181) | 103 (82–179) | 116 (50–227) | 140 (95–206)a | 345 (206–483)c | 0.008 |
| PTH 37–84 | 126 (64–327) | 69 (54–122) | 77 (52–184) | 84 (65–175) | 121 (76–224)a | 456 (236–771)c | 0.006 |
| PTH 38–77 | 177 (91–605) | 72 (53–123) | 124 (77–221) | 133 (77–235) | 140 (87–289)a | 901 (337–2147)c | 0.008 |
| PTH 38–84 | 80 (39–206) | 35 (25–61) | 45 (29–81) | 48 (32–83) | 53 (35–109) | 265 (104–429)b | 0.009 |
| PTH 45–84 | 153 (110–277) | 132 (95–158) | 125 (74–165) | 135 (108–159) | 140 (98–156) | 381 (215–587)c | 0.005 |
Values (pg/ml) are shown as median (interquartile range).
P<0.05, compared with patients with eGFR >60 ml/min per 1.73 m2.
P<0.01, compared with patients with eGFR >60 ml/min per 1.73 m2.
P<0.001, compared with patients with eGFR >60 ml/min per 1.73 m2.
Figure 2.
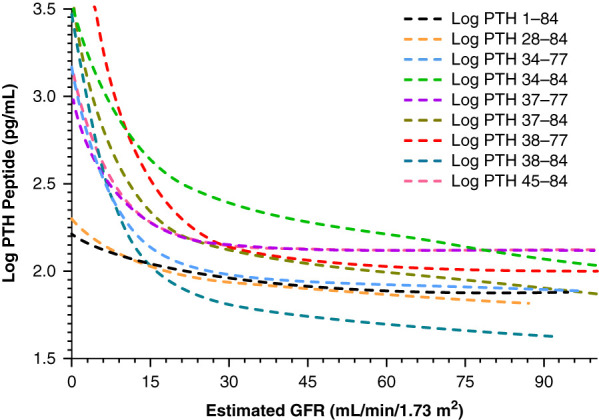
Serum concentrations of PTH 1–84 and PTH fragments increased as kidney function declined, and increased significantly when the eGFR decreased to ≤17–23 ml/min per 1.73 m2. Reprinted from ref. 1, with permission.
Table 3.
Serum concentrations of PTH peptides in subjects with CKD with versus without diabetes
| PTH Peptides | DM | Non-DM | P Values |
|---|---|---|---|
| PTH 1–84 | 94.1 (65.9–175.4) | 81.1(67.2–120.2) | 0.41 |
| PTH 28–84 | 122.5 (71.4–185.6) | 77.1 (62.6–110.8) | 0.07 |
| PTH 34–77 | 145.7 (70.5–335.4) | 136.3 (86.7–359.8) | 0.37 |
| PTH 34–84 | 280.6 (128.1–890.3) | 280.3 (119.6–700.6) | 0.62 |
| PTH 37–77 | 242.2 (95.5–399.2) | 216.1 (131.7–471.4) | 0.37 |
| PTH 37–84 | 227.5 (101.3–534.1) | 148.3 (76.0–363.5) | 0.10 |
| PTH 38–77 | 277.1 (104.1–795.9) | 181.9 (110.5–716.5) | 0.42 |
| PTH 38–84 | 342.7 (164.5–910.5) | 279.7 (161.7–859.9) | 0.67 |
| PTH 45–84 | 186.9 (129.1–318.6) | 204.2 (137.4–349.9) | 0.55 |
Serum PTH peptides (pg/ml) are presented as the median (interquartile range). Data from Kritmetapak et al.1 DM, diabetes mellitus.
Bioactivity of Amino-Terminally Truncated PTH Fragments Reveals Unique Properties in Cultured Cells
Previous work has suggested the presence of circulating truncated PTH fragments lacking the amino-terminal portion of PTH 1–84.42,43 One such peptide, the commonly cited PTH 7–84, is believed to be generated by parathyroid glands44 and blocks many of the activities of PTH 1–84, possibly through a unique receptor different from the PTHR1.6,8,10,43,45 Synthetic PTH 7–84 inhibits PTH (1–84)–mediated resorption of bone in vitro and reduces calcitriol-dependent formation of osteoclasts in murine bone marrow cultures.6 Additionally, PTH 7–84 blocks the hypercalcemic8,43,45 and phosphaturic effects43 of PTH 1–84 in rats. One study has shown that PTH 7–84 mitigates the PTH (1–84)–induced increase in bone turnover in uremic rats.10 Although in vitro and in vivo animal studies have described the unique bioactivity of synthetic PTH 7–84, human studies using mass spectrometry methods have failed to confirm the presence of circulating PTH 7–84 in readily detectable concentrations.1,3,46 We urge caution in attributing specific in vivo biologic and physiologic roles to various biosynthetic PTH fragments without confirmation of their presence in serum by robust analytic methods.
As noted earlier, we detected the presence of eight novel PTH fragments—PTH 28–84, PTH 34–77, PTH 34–84, PTH 37–77, PTH 37–84, PTH 38–77, PTH 38–84, and PTH 45–84—and confirmed their chemical identities and serum concentrations using LC-HRMS. To determine the bioactivity of these peptides, we synthesized PTH 28–84, PTH 38–84, and PTH 45–84 peptides by solid-phase peptide synthesis and tested their bioactivity in MC3T3-E1 mouse osteoblasts, either individually or together with full-length PTH 1–84, by assessing the accumulation of cAMP and the induction of alkaline phosphatase activity in osteoblasts.7,39 Increasing doses of PTH 1–84 (1–100 nM) increased the accumulation of cAMP and alkaline phosphatase activity in osteoblasts in a dose-dependent manner. PTH 28–84, PTH 38–84, and PTH 45–84 in concentrations of 1–100 nM were biologically inert. Surprisingly, 100 nM PTH 28–84, PTH 38–84, and PTH 45–84 blocked PTH (1–84)–mediated stimulation of alkaline phosphatase activity; whereas 100 nM PTH 38–84 and PTH 45–84 increased the accumulation of cAMP in osteoblasts treated with increasing doses of PTH 1–84. PTH 28–84 had no effects on cAMP activity alone or in combination with PTH 1–84. Alkaline phosphatase hydrolyzes extracellular pyrophosphate and supplies inorganic phosphate to facilitate bone mineralization. Thus, PTH 28–84 may uniquely block PTH (1–84)–mediated increases in alkaline phosphatase and, possibly, PTH (1–84)–mediated bone mineralization. Results obtained in cultured osteoblasts need to be confirmed in vivo. Although we have determined the bioactivity of PTH 28–84, PTH 38–84, and PTH 45–84 in cultured cells, we have not determined the bioactivity of these PTH fragments in in vivo animal studies. It is unknown how PTH 28–84, PTH 38–84, and PTH 45–84 bind to receptors and function at the cellular level. For example, it is unknown if they bind to PTHR1 receptors or have specific cell-surface receptors of their own. Indeed, previous publications have demonstrated the presence of specific receptors for carboxy-terminal PTH fragments on bone.8,47 Data show that, in CKD/ESKD, serum concentrations of the newly described PTH fragments are significantly increased.1 Changes in the bioactivity of PTH 1–84 may be altered by these peptides. We emphasize that further investigation of the bioactivity of PTH fragments in vivo and additional investigations regarding the mechanism of action of these truncated forms of PTH 1–84 are required. We cannot infer that the shorter PTH fragments have bioactivity in vivo from our cell culture–based studies. Nevertheless, PTH functions observed in vivo are often replicated in cell culture–based studies. Our studies highlight the value of LC-HRMS in identifying potentially important modifiers of PTH bioactivity in patients with CKD.
Use of LC-HRMS to Confirm the Existence of Post-Translationally Modified PTH 1–84; Quantitation of Oxidized PTH 1–84
Full-length PTH 1–84 has two methionine residues, at position 8 and 18, whose oxidation has been demonstrated to diminish its bioactivity and alter clearance.48 This may be significant in patients on dialysis, who are exposed to intense oxidative stress. The PTH immunoassays recognize both oxidized PTH (oxPTH) and nonoxidized PTH (n-oxPTH). Hocher et al.49 claimed that serum n-oxPTH can be measured by PTH immunoassays after prior removal of oxPTH with a specific affinity chromatography column of immobilized anti-oxPTH antibody. The researchers concluded that a large fraction (70%–90%) of measured PTH in patients on dialysis was oxPTH.49,50 Previous studies in patients with CKD stages 2–5, using the indirect method of measuring n-oxPTH, have shown that serum oxPTH was more strongly associated with mortality than n-oxPTH,51,52 possibly reflecting oxidative stress–related mortality. A subsequent study reported that histomorphometric parameters of bone turnover and circulating bone turnover markers in patients on dialysis showed similar correlations with serum n-oxPTH and PTH levels.53 In the aforementioned studies, it should be noted that the investigators did not provide data on directly measured oxPTH. Nonetheless, by using the mass spectrometry–based assay, we have found that all species of oxPTH (PTH 1–84 oxidized at methionine 8, methionine 18, and both positions) were below the limit of detection (50 pg/ml) in serum samples of patients with normal and impaired kidney function.1 This discrepancy might be explained by the limited specificity of PTH immunoassays and/or ex vivo oxidation of PTH. Further mass spectrometry–based studies are needed to establish whether oxPTH exists in the human circulation.
Current Methodologies for PTH Measurement
Measurement of PTH using immunoassay-based methods is routinely performed in patients with CKD for the management of mineral and bone disease. PTH immunoassays mostly use antibodies to capture PTH in serum and plasma matrices. The identification and quantification of circulating PTH fragments has proved to be a barrier to the development of reliable PTH immunoassays because it is recognized that some PTH fragments could crossreact with PTH immunoassay-based platforms, resulting in high assay variability. Three generations of PTH immunoassays have been developed over the past 60 years, each targeting a different portion of the PTH 1–84 molecule.54
First-generation PTH competitive RIA used a single polyclonal antibody directed toward the amino-terminal, midregion, or carboxy-terminal portion of PTH 1–84. Because these antibodies detect both PTH 1–84 and various PTH fragments, assays do not reflect levels of biologically active PTH 1–84. They have now been replaced by the second- and third-generation PTH immunoassays (“sandwich” immunoassays).55
Second-generation PTH immunoassays, also known as “intact PTH” (iPTH) assays, are currently the most common types of assays for measuring serum PTH concentrations. In these immunoassays, one “capture” antibody directed toward the carboxy-terminal portion (e.g., amino acid positions 39–84) captures PTH 1–84 or PTH fragments from serum, and a second (“reporter”) antibody directed toward the amino-terminal portion of PTH 1–84 (e.g., amino acid positions 12–24 or 26–32) is used to quantitate the amount of captured peptide.54 Because two antibodies are required for detection in the assay procedure, only relatively long PTH forms, containing both targeted epitopes, are recognized by PTH sandwich immunoassays. Small PTH fragments lacking one or both epitopes are not detected. Although second-generation PTH immunoassays improve the specificity of PTH 1–84 measurement, they also crossreact with amino-terminally truncated PTH fragments, which account for a larger proportion of PTH immunoreactivity in CKD than is found in the serum of normal subjects.9,56–59 Hence, the nomenclature “iPTH” is misleading because both full-length PTH and its fragments are detected. The term should be abandoned in favor of the terminology “second-generation PTH immunoassays.” It is to be noted that there are substantial inconsistencies between second-generation PTH immunoassays provided by different manufacturers, possibly due to differences in assay standardization.60,61 When the Allegro iPTH immunoassay (an assay validated by bone histomorphometric data)62 was used as the reference assay in the measurement of serum PTH in patients on dialysis, the median bias between different second-generation PTH immunoassays varied from −44.9% to +123%.57 This suggests that we may misestimate the true serum PTH concentration with currently available second-generation PTH immunoassays. Despite high interassay variability and limited specificity of PTH immunoassays, the 2017 Kidney Disease Improving Global Outcomes (KDIGO) CKD–Mineral and Bone Disorder guidelines still recommend the use of second-generation PTH immunoassays because of their accessibility and availability in most clinical laboratories.63 The suggested target range for serum PTH in patients on dialysis has changed from 150 to 300 pg/ml, on the basis of the Allegro iPTH immunoassay, in the Kidney Disease Outcomes Quality Initiative guidelines64 to two to nine times of the upper limit of normal, for the given assay, in the KDIGO guidelines.63 Nevertheless, the optimal serum PTH level for patients with CKD stages 3–5 not on dialysis is currently not known. We underscore the importance of defining assay-specific normative ranges in CKD, monitoring patients with assays using the same manufacturer, and basing decisions regarding treatment upon trends rather than single laboratory values.
Third-generation PTH immunoassays are called “whole,” “bioactive,” or “biointact” PTH (bioPTH) immunoassays. These immunoassays use an amino-terminal PTH antibody that is directed toward the first four to six amino acids of PTH 1–84 as the “reporter” antibody, and a carboxy-terminal PTH antibody similar to that used in second-generation PTH immunoassays as the “capture” antibody. BioPTH immunoassays are presumed to solely measure PTH 1–84, but not PTH fragments.65 Studies have revealed that bioPTH immunoassays crossreact with post-translationally modified forms of PTH 1–84, such as PTH 1–84 phosphorylated on serine 17 (“amino-PTH”), which are overproduced in patients with primary and secondary hyperparathyroidism.56,57,66 Amino-PTH represents 4%–8% of the immunoreactivity detected by bioPTH immunoassays in normal subjects and up to 15% in patients with CKD.66,67 The clinical significance of amino-PTH remains unknown. In patients with CKD stages 3–5, PTH concentrations measured using bioPTH immunoassays are 30%–50% lower than those measured using second-generation PTH immunoassays.65,68 We found that serum concentrations of PTH fragments increased significantly when an eGFR falls to ≤17–23 ml/min per 1.73 m2 and it is plausible that these fragments may cause crossreactivity in iPTH immunoassays.1 Current evidence fails to show an advantage of bioPTH immunoassays compared with iPTH immunoassays in the prediction of bone disease in CKD.69–71
PTH Standardization: A Major Problem in Laboratory Medicine
Currently, PTH immunoassays are not standardized, meaning that data generated with different immunoassays are not comparable. This lack of assay standardization affects the use of common reference intervals, which have not always been established by manufacturers.60,61 Furthermore, the presence of varying amounts of potentially interfering PTH fragments, especially in patients with CKD, warrants thorough assessments of all factors affecting inaccuracy of assays. The International Federation of Clinical Chemistry Committee on Bone Metabolism has proposed the use of a single, internationally recognized standard for PTH immunoassay calibration. The World Health Organization Expert Committee on Biologic Standardization prepared a recombinant human PTH 1–84 standard (NIBSC 95/646) for the calibration of PTH immunoassays. Nonetheless, it is not known whether this recombinant PTH standard shows the same behavior with immunoassays as native PTH in serum or plasma, which must be formally determined before imposing the NIBSC 95/646 standard. The second step in addressing the variability of results will be to develop reference measurement procedures for PTH. LC-HRMS or liquid chromatography–tandem mass spectrometry (LC-MS/MS)–based assays are promising as reference measurement methods because of the higher accuracy associated with the use of isotope dilution, and the higher specificity associated with the ability to distinguish between, and measure, multiple proteiforms when compared with immunoassays. Once appropriate reference materials and methods are in place and accurate and comparable measurements with routine clinical assays are achieved, multiethnic studies are needed to ascertain common reference intervals for PTH immunoassays. Finally, it is necessary to correlate serum PTH concentrations measured by a reference method with biologic outcomes obtained after performance of bone histomorphometry, high-resolution peripheral quantitative computed tomography, or the determination of fracture rates in a large cohort of patients with CKD/ESKD.
Unique Advantages and Limitations of Mass Spectrometry in Resolving PTH Measurement Issues
All three generations of PTH immunoassays are subject to crossreactivity from interfering PTH fragments. Mass spectrometry–based assays can readily address the limitations observed among immunoassay-based platforms used for the measurement of PTH. It should be noted that the incorporation of mass spectrometry in clinical diagnostic testing dates back decades, with the early adoption of the technique in newborn screening.72 Mass spectrometric advancements in sensitivity, specificity, speed, dynamic range, and cost have allowed for this technique to be used for a variety of applications and accessible to a large number of clinical laboratories. This is of great clinical relevance, because mass spectrometry–based assays incorporate either separation modalities for complex mixtures, high-resolution capabilities, and/or multiple mass analyzers (i.e., MS/MS) to ensure a high level of specificity from interferences, a major limitation of diagnostic immunoassays such as those used for PTH measurement. PTH is a low-abundance protein, and the enrichment method using immunoaffinity extraction with amino- or carboxy-terminal capture antibodies is generally performed to prepare the sample before PTH quantitation using mass spectrometry. Mass spectrometry allows for accurate identification of PTH forms based on their mass/charge ratio and fragmentation characteristics, as opposed to inferring the structure of PTH fragments by differential antibody crossreactivity.
When comparing conventional PTH immunoassays, mass spectrometry does require special expertise and has additional cost and less throughput, increasing turnaround time from minutes to hours. Technologic advances in instrumentation and automation may help with overcoming those limitations in the near future. Automation of sample pipetting and wash steps could also improve the throughput and precision of mass spectrometry assays.
Previous attempts to monitor full-length PTH 1–84 and endogenous peptides were successful but limited in that they lacked appropriate analytic calibrators and isotopically labeled internal standards. Internal standards increase the accuracy and reproducibility of measurements by correcting for the following: (1) variability introduced during sample preparation steps, (2) ion suppression–induced variability of the signal intensity, and (3) within-run instrument variability. Targeted mass spectrometry–based assays traditionally incorporate coeluting deuterium, carbon-13, and/or nitrogen-15, which are stable, isotopically labeled internal standards that are specific to the analyte of interest, allowing for absolute quantitation. These isotopically labeled internal standards are now available for PTH and its fragments.
Additionally, some PTH quantitation methods require trypsin digestion, which can reduce the ability to reconcile differences in PTH peptide sequence and requires additional optimization techniques to ensure complete digestion.3,73 Labile peptides, often required during digestion, may also suffer during warm incubation temperatures. The argument could be made that measuring intact peptides can be limited to only those peptides that are chromatographically resolved and ionize fully within established conditions. Although this is a limitation to our recently published method, the information obtained from our nondigestion techniques has proven beneficial in our studies, and the use of analytic calibrators and isotopically labeled internal standards has allowed a robust quantitation of PTH 1–84 and PTH fragments.1 Furthermore, monitoring intact peptides in full scan mode allows data to be collected and mined for additional variants, such as oxPTH and phosphorylated PTH forms for which no analytic standards are commercially available.
Clinical Relevance of Measuring PTH Fragments
Multiple studies have questioned the validity of serum PTH levels as a bone turnover marker and target of therapy in CKD. Alterations in PTH metabolism and signaling, together with PTH immunoassay variability, greatly influence the relationship between serum PTH and clinical outcomes (fracture, vascular calcification, and mortality) in patients with CKD. The bone and renal responses to the effect of PTH 1–84 are progressively impaired in CKD, a condition currently called PTH hyporesponsiveness. This condition has important clinical implications because it not only leads to PTH hypersecretion and parathyroid hyperplasia but also to the increasing prevalence of adynamic bone disease and vascular calcification in CKD. Factors contributing to PTH hyporesponsiveness include phosphate retention, calcitriol deficiency, aluminum overload, magnesium deficiency, oxidative stress, accumulation of uremic toxins and oxidized lipids,74 and PTHR1 downregulation/dysfunction or postreceptor defects. Previous research has shown that indoxyl sulfate, a prototypic protein-bound uremic toxin, induced skeletal hyporesponsiveness to PTH by suppressing PTH-stimulated intracellular cAMP generation and reducing PTHR1 expression in mouse osteoblasts.75 In addition, the accumulation of PTH fragments has been proposed to promote PTH hyporesponsiveness. PTH 7–84 has been reported to block PTH 1–84 signaling in bone by activating the putative carboxy-terminal PTH receptor.6 Moreover, PTH 7–34 has been reported to induce proteasome-dependent degradation of the PTHR1 in bone and kidney cells.76 Although previous studies suggested the contributing role of PTH fragments, especially PTH 7–84, to PTH hyporesponsiveness in CKD, the presence of those fragments in human circulation has not been confirmed using mass spectrometry–based methods.1,3,46 We have shown that a variety of circulating PTH fragments (PTH 28–84, PTH 34–77, PTH 34–84, PTH 37–77, PTH 37–84, PTH 38–77, PTH 38–84, and PTH 45–84), but not PTH 7–84, increased significantly in patients with CKD stages 4–5. Furthermore, we also tested the bioactivity of some of those PTH fragments (PTH 28–84, PTH 38–84, and PTH 45–84) and found that they differently modulate the effects of PTH 1–84 on osteoblasts. This might partly account for the limited correlation between serum PTH levels, measured by PTH immunoassays, and bone turnover in patients on dialysis. With CKD progression, the elevation of circulating levels of certain PTH fragments may enhance the PTH 1–84 effect on bone and help overcome the skeletal hyporesponsiveness to PTH, eventually causing osteitis fibrosa. To what extent circulating PTH fragment concentrations reflect bone histology and affect local signaling remains to be investigated.
Integration of PTH LC-HRMS Assays with Bone Histomorphometry
The bone phenotype is assessed in patients by bone biopsy. Bone histomorphometry, with an assessment of bone turnover, remains the gold standard in the diagnosis of renal osteodystrophy.77 However, this procedure is invasive and not readily available. High-resolution peripheral quantitative computed tomography provides a noninvasive method to assess bone structure and low bone mineral density.78
Recent findings suggest that bone biopsy information could be correlated with data obtained from noninvasive techniques, such as the LC-HRMS analysis of full-length PTH and PTH fragments in serum. However, it is important to know the absolute serum concentration of these PTH fragments for various stages of CKD to better understand the relationship between the PTH fragment profiles and clinical outcomes. By correlating the information obtained from full-length PTH, post-translationally modified PTH, PTH fragments, bone biopsies, and bone markers, future challenges in the assessment of mineral bone disorders in patients with CKD/ESKD could be resolved.
In conclusion, LC-HRMS and LC-MS/MS–based methods, although not readily available in most clinical laboratories, should be used as reference methods against which the validity of immunoassays is judged. LC-HRMS and LC-MS/MS can be used to accurately quantify circulating PTH 1–84 and PTH fragments in patients with varying degrees of renal dysfunction. LC-HRMS has failed to detect PTH moieties (oxPTH 1–84 and PTH 7–84) that were previously thought to exist is patient serum on the basis of immunoassay data. Additional studies are needed to translate the methodologic advances into clinical insights, such as the superior predictive ability of LC-HRMS and LC-MS/MS methods for renal osteodystrophy over PTH immunoassays. Although preliminary cell culture–based methods have shown that newly detected PTH fragments modulate the effect of PTH 1–84 on certain signaling molecules in osteoblasts, the bioactivity of these PTH fragments in vivo is unknown and deserves further study. The correlations between serum concentrations of PTH 1–84 and PTH fragments and clinical renal osteodystrophy, appropriately characterized by quantitative bone histomorphometry, may yield further definitive information regarding the role of PTH fragments in CKD–mineral bone disorder.
Disclosures
R. Kumar reports receiving research funding from the Andersen Foundation and the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases; having consultancy agreements with, receiving honoraria from, and serving in an advisory or leadership role for, Bridgebio Pharma and Orfan (as a consultant); and having ownership interest in Orfan (via stock). C. Ulmer reports serving in an advisory or leadership role for the American Society for Mass Spectrometry and the International Federation of Clinical Chemistry Committee on Bone Metabolism (both unpaid). All remaining authors have nothing to disclose.
Funding
This work was supported by the Fred C. and Katherine B. Andersen Foundation Award #21 and US Department of Health and Human Services NIH grant DK 125252.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, and the US Department of Health and Human Services.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
K. Kritmetapak, R. Kumar, R.J. Singh, and H.W. Vesper reviewed and edited the manuscript; K. Kritmetapak, R.J. Singh, and C.Z. Ulmer were responsible for validation; R. Kumar provided supervision and was responsible for funding acquisition; R. Kumar and R.J. Singh were responsible for project administration and resources; R. Kumar, R.J. Singh, C.Z. Ulmer, and H.W. Vesper conceptualized the study; C.Z. Ulmer wrote the original draft; and all authors were responsible for data curation, formal analysis, investigation, and methodology.
References
- 1.Kritmetapak K, Losbanos LA, Hines JM, O’Grady KL, Ulmer CZ, Vesper HW, et al. : Chemical characterization and quantification of circulating intact PTH and PTH fragments by high-resolution mass spectrometry in chronic renal failure. Clin Chem 67: 843–853, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang CX, Weber BV, Thammavong J, Grover TA, Wells DS: Identification of carboxyl-terminal peptide fragments of parathyroid hormone in human plasma at low-picomolar levels by mass spectrometry. Anal Chem 78: 1636–1643, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Lopez MF, Rezai T, Sarracino DA, Prakash A, Krastins B, Athanas M, et al. : Selected reaction monitoring-mass spectrometric immunoassay responsive to parathyroid hormone and related variants. Clin Chem 56: 281–290, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Ketha H, Singh RJ: Quantitation of parathyroid hormone in serum or plasma by liquid chromatography-tandem mass spectrometry. Methods Mol Biol 1378: 211–217, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Evenepoel P, Bover J, Ureña Torres P: Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int 90: 1184–1190, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Divieti P, John MR, Jüppner H, Bringhurst FR: Human PTH-(7-84) inhibits bone resorption in vitro via actions independent of the type 1 PTH/PTHrP receptor. Endocrinology 143: 171–176, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Kritmetapak K, Singh RJ, Craig TA, Hines JM, Kumar R: Short carboxyl terminal parathyroid hormone peptides modulate human parathyroid hormone signaling in mouse osteoblasts. Biochem Biophys Res Commun 572: 15–19, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, D’Amour P: Synthetic carboxyl-terminal fragments of parathyroid hormone (PTH) decrease ionized calcium concentration in rats by acting on a receptor different from the PTH/PTH-related peptide receptor. Endocrinology 142: 1386–1392, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Lepage R, Roy L, Brossard JH, Rousseau L, Dorais C, Lazure C, et al. : A non-(1-84) circulating parathyroid hormone (PTH) fragment interferes significantly with intact PTH commercial assay measurements in uremic samples. Clin Chem 44: 805–809, 1998 [PubMed] [Google Scholar]
- 10.Langub MC, Monier-Faugere MC, Wang G, Williams JP, Koszewski NJ, Malluche HH: Administration of PTH-(7-84) antagonizes the effects of PTH-(1-84) on bone in rats with moderate renal failure. Endocrinology 144: 1135–1138, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Habener JF, Amherdt M, Ravazzola M, Orci L: Parathyroid hormone biosynthesis. Correlation of conversion of biosynthetic precursors with intracellular protein migration as determined by electron microscope autoradiography. J Cell Biol 80: 715–731, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt CP, Schaefer F, Bruch A, Veldhuis JD, Schmidt-Gayk H, Stein G, et al. : Control of pulsatile and tonic parathyroid hormone secretion by ionized calcium. J Clin Endocrinol Metab 81: 4236–4243, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Schmitt CP, Schaefer F, Huber D, Zahn I, Veldhuis JD, Ritz E, et al. : 1,25(OH)2-vitamin D3 reduces spontaneous and hypocalcemia-stimulated pulsatile component of parathyroid hormone secretion. J Am Soc Nephrol 9: 54–62, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Martin KJ, Hruska KA, Lewis J, Anderson C, Slatopolsky E: The renal handling of parathyroid hormone. Role of peritubular uptake and glomerular filtration. J Clin Invest 60: 808–814, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Ballegooijen AJ, Rhee EP, Elmariah S, de Boer IH, Kestenbaum B: Renal clearance of mineral metabolism biomarkers. J Am Soc Nephrol 27: 392–397, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Amour P, Huet PM, Segre GV, Rosenblatt M: Characteristics of bovine parathyroid hormone extraction by dog liver in vivo. Am J Physiol 241: E208–E214, 1981 [DOI] [PubMed] [Google Scholar]
- 17.Rouleau MF, Warshawsky H, Goltzman D: Parathyroid hormone binding in vivo to renal, hepatic, and skeletal tissues of the rat using a radioautographic approach. Endocrinology 118: 919–931, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Martin K, Hruska K, Greenwalt A, Klahr S, Slatopolsky E: Selective uptake of intact parathyroid hormone by the liver: Differences between hepatic and renal uptake. J Clin Invest 58: 781–788, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bringhurst FR, Stern AM, Yotts M, Mizrahi N, Segre GV, Potts JT Jr: Peripheral metabolism of PTH: Fate of biologically active amino terminus in vivo. Am J Physiol 255: E886–E893, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Bringhurst FR, Stern AM, Yotts M, Mizrahi N, Segre GV, Potts JT Jr: Peripheral metabolism of [35S]parathyroid hormone in vivo: influence of alterations in calcium availability and parathyroid status. J Endocrinol 122: 237–245, 1989 [DOI] [PubMed] [Google Scholar]
- 21.D’Amour P, Räkel A, Brossard JH, Rousseau L, Albert C, Cantor T: Acute regulation of circulating parathyroid hormone (PTH) molecular forms by calcium: utility of PTH fragments/PTH(1-84) ratios derived from three generations of PTH assays. J Clin Endocrinol Metab 91: 283–289, 2006 [DOI] [PubMed] [Google Scholar]
- 22.MacGregor RR, Hamilton JW, Kent GN, Shofstall RE, Cohn DV: The degradation of proparathormone and parathormone by parathyroid and liver cathepsin B. J Biol Chem 254: 4428–4433, 1979 [PubMed] [Google Scholar]
- 23.Hashizume Y, Waguri S, Watanabe T, Kominami E, Uchiyama Y: Cysteine proteinases in rat parathyroid cells with special reference to their correlation with parathyroid hormone (PTH) in storage granules. J Histochem Cytochem 41: 273–282, 1993 [DOI] [PubMed] [Google Scholar]
- 24.D’Amour P, Palardy J, Bahsali G, Mallette LE, DeLéan A, Lepage R: The modulation of circulating parathyroid hormone immunoheterogeneity in man by ionized calcium concentration. J Clin Endocrinol Metab 74: 525–532, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Gardella TJ, Jüppner H, Wilson AK, Keutmann HT, Abou-Samra AB, Segre GV, et al. : Determinants of [Arg2]PTH-(1-34) binding and signaling in the transmembrane region of the parathyroid hormone receptor. Endocrinology 135: 1186–1194, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Lee C, Gardella TJ, Abou-Samra AB, Nussbaum SR, Segre GV, Potts JT Jr, et al. : Role of the extracellular regions of the parathyroid hormone (PTH)/PTH-related peptide receptor in hormone binding. Endocrinology 135: 1488–1495, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Luck MD, Carter PH, Gardella TJ: The (1-14) fragment of parathyroid hormone (PTH) activates intact and amino-terminally truncated PTH-1 receptors. Mol Endocrinol 13: 670–680, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Costanzo LS, Windhager EE: Effects of PTH, ADH, and cyclic AMP on distal tubular Ca and Na reabsorption. Am J Physiol 239: F478–F485, 1980 [DOI] [PubMed] [Google Scholar]
- 29.Agus ZS, Puschett JB, Senesky D, Goldberg M: Mode of action of parathyroid hormone and cyclic adenosine 3′,5′-monophosphate on renal tubular phosphate reabsorption in the dog. J Clin Invest 50: 617–626, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bank N, Aynedjian HS, Weinstein SW: A microperfusion study of phosphate reabsorption by the rat proximal renal tubule. Effect of parathyroid hormone. J Clin Invest 54: 1040–1048, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kremer R, Goltzman D: Parathyroid hormone stimulates mammalian renal 25-hydroxyvitamin D3-1 alpha-hydroxylase in vitro. Endocrinology 110: 294–296, 1982 [DOI] [PubMed] [Google Scholar]
- 32.Nesbitt T, Drezner MK, Lobaugh B: Abnormal parathyroid hormone stimulation of 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the hypophosphatemic mouse. Evidence for a generalized defect of vitamin D metabolism. J Clin Invest 77: 181–187, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wein MN, Kronenberg HM: Regulation of bone remodeling by parathyroid hormone. Cold Spring Harb Perspect Med 8: a031237, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amizuka N, Goltzman D, Ozawa H: The biologic action of parathyroid hormone-related peptide on bone and cartilage cells. Tissue Eng 2: 277–287, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ: Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20: 345–357, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Muntner P, Jones TM, Hyre AD, Melamed ML, Alper A, Raggi P, et al. : Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin J Am Soc Nephrol 4: 186–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, et al. : Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel S, Barron JL, Mirzazedeh M, Gallagher H, Hyer S, Cantor T, et al. : Changes in bone mineral parameters, vitamin D metabolites, and PTH measurements with varying chronic kidney disease stages. J Bone Miner Metab 29: 71–79, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Kritmetapak K, Losbanos L, Berent TE, Ashrafzadeh-Kian SL, Algeciras-Schimnich A, Hines JM, et al. : Hyperphosphatemia with elevated serum PTH and FGF23, reduced 1,25(OH)2D and normal FGF7 concentrations characterize patients with CKD. BMC Nephrol 22: 114, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen-Yamamoto L, Rousseau L, Brossard JH, Lepage R, Gao P, Cantor T, et al. : Origin of parathyroid hormone (PTH) fragments detected by intact-PTH assays. Eur J Endocrinol 147: 123–131, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Canney M, Djurdjev O, Tang M, Zierold C, Blocki F, Wolf M, et al. : GFR-specific versus GFR-agnostic cutoffs for parathyroid hormone and fibroblast growth factor-23 in advanced chronic kidney disease. Am J Nephrol 50: 105–114, 2019 [DOI] [PubMed] [Google Scholar]
- 42.Yamashita H, Gao P, Cantor T, Futata T, Murakami T, Uchino S, et al. : Large carboxy-terminal parathyroid hormone (PTH) fragment with a relatively longer half-life than 1-84 PTH is secreted directly from the parathyroid gland in humans. Eur J Endocrinol 149: 301–306, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Slatopolsky E, Finch J, Clay P, Martin D, Sicard G, Singer G, et al. : A novel mechanism for skeletal resistance in uremia. Kidney Int 58: 753–761, 2000 [DOI] [PubMed] [Google Scholar]
- 44.D’Amour P, Brossard JH, Rousseau L, Nguyen-Yamamoto L, Nassif E, Lazure C, et al. : Structure of non-(1-84) PTH fragments secreted by parathyroid glands in primary and secondary hyperparathyroidism. Kidney Int 68: 998–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Huan J, Olgaard K, Nielsen LB, Lewin E: Parathyroid hormone 7-84 induces hypocalcemia and inhibits the parathyroid hormone 1-84 secretory response to hypocalcemia in rats with intact parathyroid glands. J Am Soc Nephrol 17: 1923–1930, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Singh RJ, Hines JM, Lopez MF, Krastins B, Hoofnagle AN: Mass spectrometric immunoassay raises doubt for the existence of parathyroid hormone fragment 7-84. Clin Chem 61: 558–560, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Divieti P, Geller AI, Suliman G, Jüppner H, Bringhurst FR: Receptors specific for the carboxyl-terminal region of parathyroid hormone on bone-derived cells: Determinants of ligand binding and bioactivity. Endocrinology 146: 1863–1870, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Hruska KA, Korkor A, Martin K, Slatopolsky E: Peripheral metabolism of intact parathyroid hormone. Role of liver and kidney and the effect of chronic renal failure. J Clin Invest 67: 885–892, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hocher B, Armbruster FP, Stoeva S, Reichetzeder C, Grön HJ, Lieker I, et al. : Measuring parathyroid hormone (PTH) in patients with oxidative stress--do we need a fourth generation parathyroid hormone assay? PLoS One 7: e40242, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hocher B, Oberthür D, Slowinski T, Querfeld U, Schaefer F, Doyon A, et al. : Modeling of oxidized PTH (oxPTH) and non-oxidized PTH (n-oxPTH) receptor binding and relationship of oxidized to non-oxidized PTH in children with chronic renal failure, adult patients on hemodialysis and kidney transplant recipients. Kidney Blood Press Res 37: 240–251, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Tepel M, Armbruster FP, Grön HJ, Scholze A, Reichetzeder C, Roth HJ, et al. : Nonoxidized, biologically active parathyroid hormone determines mortality in hemodialysis patients. J Clin Endocrinol Metab 98: 4744–4751, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Seiler-Mussler S, Limbach AS, Emrich IE, Pickering JW, Roth HJ, Fliser D, et al. : Association of nonoxidized parathyroid hormone with cardiovascular and kidney disease outcomes in chronic kidney disease. Clin J Am Soc Nephrol 13: 569–576, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ursem SR, Heijboer AC, D’Haese PC, Behets GJ, Cavalier E, Vervloet MG, et al. : Non-oxidized parathyroid hormone (PTH) measured by current method is not superior to total PTH in assessing bone turnover in chronic kidney disease. Kidney Int 99: 1173–1178, 2021 [DOI] [PubMed] [Google Scholar]
- 54.Arya AK, Sachdeva N: Parathyroid Hormone (PTH) Assays and Applications to Bone Disease: Overview on Methodology. In: Biomarkers in Bone Disease: Methods, Discoveries and Applications, edited by Preedy V, The Netherlands, Springer, 2015, pp 1–29 [Google Scholar]
- 55.Torres PU: The need for reliable serum parathyroid hormone measurements. Kidney Int 70: 240–243, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Cavalier E, Daly AF, Betea D, Pruteanu-Apetrii PN, Delanaye P, Stubbs P, et al. : The ratio of parathyroid hormone as measured by third- and second-generation assays as a marker for parathyroid carcinoma. J Clin Endocrinol Metab 95: 3745–3749, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Souberbielle JC, Boutten A, Carlier MC, Chevenne D, Coumaros G, Lawson-Body E, et al. : Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int 70: 345–350, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barré M, D’Amour P: Accumulation of a non-(1-84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: Importance in the interpretation of PTH values. J Clin Endocrinol Metab 81: 3923–3929, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Brossard JH, Lepage R, Cardinal H, Roy L, Rousseau L, Dorais C, et al. : Influence of glomerular filtration rate on non-(1-84) parathyroid hormone (PTH) detected by intact PTH assays. Clin Chem 46: 697–703, 2000 [PubMed] [Google Scholar]
- 60.Cavalier E, Delanaye P, Vranken L, Bekaert AC, Carlisi A, Chapelle JP, et al. : Interpretation of serum PTH concentrations with different kits in dialysis patients according to the KDIGO guidelines: Importance of the reference (normal) values. Nephrol Dial Transplant 27: 1950–1956, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Cavalier E, Vasikaran S, Bhattoa HP, Heijboer AC, Makris K, Ulmer CZ: The path to the standardization of PTH: Is this a realistic possibility? A position paper of the IFCC C-BM. Clin Chim Acta 515: 44–51, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherrard DJ, Hercz G, Pei Y, Maloney NA, Greenwood C, Manuel A, et al. : The spectrum of bone disease in end-stage renal failure--an evolving disorder. Kidney Int 43: 436–442, 1993 [DOI] [PubMed] [Google Scholar]
- 63.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group : KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 7: 1–59, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42: S1–S201, 2003 [PubMed] [Google Scholar]
- 65.John MR, Goodman WG, Gao P, Cantor TL, Salusky IB, Jüppner H: A novel immunoradiometric assay detects full-length human PTH but not amino-terminally truncated fragments: Implications for PTH measurements in renal failure. J Clin Endocrinol Metab 84: 4287–4290, 1999 [DOI] [PubMed] [Google Scholar]
- 66.D’Amour P, Brossard JH, Rousseau L, Roy L, Gao P, Cantor T: Amino-terminal form of parathyroid hormone (PTH) with immunologic similarities to hPTH(1-84) is overproduced in primary and secondary hyperparathyroidism. Clin Chem 49: 2037–2044, 2003 [DOI] [PubMed] [Google Scholar]
- 67.D’Amour P, Brossard JH, Räkel A, Rousseau L, Albert C, Cantor T: Evidence that the amino-terminal composition of non-(1-84) parathyroid hormone fragments starts before position 19. Clin Chem 51: 169–176, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Gao P, Scheibel S, D’Amour P, John MR, Rao SD, Schmidt-Gayk H, et al. : Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1-84: Implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res 16: 605–614, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Salusky IB, Goodman WG, Kuizon BD, Lavigne JR, Zahranik RJ, Gales B, et al. : Similar predictive value of bone turnover using first- and second-generation immunometric PTH assays in pediatric patients treated with peritoneal dialysis. Kidney Int 63: 1801–1808, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Lehmann G, Stein G, Hüller M, Schemer R, Ramakrishnan K, Goodman WG: Specific measurement of PTH (1-84) in various forms of renal osteodystrophy (ROD) as assessed by bone histomorphometry. Kidney Int 68: 1206–1214, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, et al. : Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis 67: 559–566, 2016 [DOI] [PubMed] [Google Scholar]
- 72.Heaney LM: Advancements in mass spectrometry as a tool for clinical analysis: Part I. Clin Chem Lab Med 58: 639–642, 2020 [DOI] [PubMed] [Google Scholar]
- 73.Kumar V, Barnidge DR, Chen LS, Twentyman JM, Cradic KW, Grebe SK, et al. : Quantification of serum 1-84 parathyroid hormone in patients with hyperparathyroidism by immunocapture in situ digestion liquid chromatography-tandem mass spectrometry. Clin Chem 56: 306–313, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Sage AP, Lu J, Atti E, Tetradis S, Ascenzi MG, Adams DJ, et al. : Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res 26: 1197–1206, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nii-Kono T, Iwasaki Y, Uchida M, Fujieda A, Hosokawa A, Motojima M, et al. : Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int 71: 738–743, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Alonso V, Magyar CE, Wang B, Bisello A, Friedman PA: Ubiquitination-deubiquitination balance dictates ligand-stimulated PTHR sorting. J Bone Miner Res 26: 2923–2934, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drüeke TB: Is parathyroid hormone measurement useful for the diagnosis of renal bone disease? Kidney Int 73: 674–676, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Digby MG, Bishop NJ, Paggiosi MA, Offiah AC: HR-pQCT: a non-invasive ‘biopsy’ to assess bone structure and strength. Arch Dis Child Educ Pract Ed 101: 268–270, 2016 [DOI] [PubMed] [Google Scholar]