Abstract
AKI is a complex clinical syndrome associated with an increased risk of morbidity and mortality, particularly in critically ill and perioperative patient populations. Most AKI clinical trials have been inconclusive, failing to detect clinically important treatment effects at predetermined statistical thresholds. Heterogeneity in the pathobiology, etiology, presentation, and clinical course of AKI remains a key challenge in successfully testing new approaches for AKI prevention and treatment. This article, derived from the “AKI” session of the “Kidney Disease Clinical Trialists” virtual workshop held in October 2021, reviews barriers to and strategies for improving the design and implementation of clinical trials in patients with, or at risk of, developing AKI. The novel approaches to trial design included in this review span adaptive trial designs that increase the knowledge gained from each trial participant; pragmatic trial designs that allow for the efficient enrollment of sufficiently large numbers of patients to detect small, but clinically significant, treatment effects; and platform trial designs that use one trial infrastructure to answer multiple clinical questions simultaneously. This review also covers novel approaches to clinical trial analysis, such as Bayesian analysis and assessing heterogeneity in the response to therapies among trial participants. We also propose a road map and actionable recommendations to facilitate the adoption of the reviewed approaches. We hope that the resulting road map will help guide future clinical trial planning, maximize learning from AKI trials, and reduce the risk of missing important signals of benefit (or harm) from trial interventions.
Keywords: AKI, randomized controlled trials, pragmatic, cluster, Bayesian, heterogeneity
AKI is characterized by rapid deterioration of kidney function and is defined clinically by a rise in serum creatinine levels and/or a decrease in urine output.1,2 AKI is strongly associated with in-hospital mortality, rehospitalization, CKD, long-term dialysis, and cardiovascular events.3,4 Unfortunately, most AKI-focused randomized clinical trials have failed to identify effects on outcomes at predefined levels of statistical significance.5–7 Consequently, many aspects of AKI prevention and treatment remain unaddressed or suboptimally informed by potentially biased observational studies or underpowered trials susceptible to both falsely negative and false-positive findings. The 2021 Kidney Disease Clinical trialists (KDCT) workshop, held in October 2021, provided multiple stakeholders with a scientific forum aimed at identifying strategies to improve the state of clinical AKI research. The meeting was supported by industry (through unrestricted educational grants) and registrants. The scientific program was developed by the scientific academic committee. This report summarizes the presentations and discussions from this meeting on how the AKI community could improve outcomes through advances in trial design, innovations in implementation, and novel analytic strategies to boost the interpretability and clinical relevance of results from future trials.
Overcoming Barriers to Clinical Trials in AKI
Several factors complicate the design, conduct, analysis, and interpretation of AKI trials. AKI is in the final common pathway for many common medical conditions (e.g., sepsis, postoperative AKI, heart or liver failure). Thus, the proportion of clinical outcomes directly attributable to AKI may be small, meaning that trials focused on AKI treatment or prevention must be very large to detect these small, but clinically important, treatment effects. Further, AKI is heterogeneous in its etiology, presentation, and clinical course, and our understanding of the mechanisms of AKI remains limited. Recent efforts have focused on understanding the various phenotypes of AKI,8,9 but such testing cannot be performed on the timescale needed for evaluation for clinical trials, and it remains unclear whether recently discovered phenotypes are effective at identifying patients who are likely to respond to a given intervention.
One potential solution to both the challenge of detecting a small attributable treatment effect and potential heterogeneity in response to a given therapy, is conducting larger trials. Most AKI trials to date have been traditional, two-arm, explanatory (“efficacy”) clinical trials enrolling dozens or hundreds of patients (Table 1). These trials, sometimes termed explanatory of “efficacy” trials, are designed to determine the effect of a studied intervention under idealized conditions while intensively monitoring for (un)anticipated side effects. Although this trial design is well suited for assessing novel therapeutics, the time (often years) and effort required to enroll and monitor patients makes it impractical to conduct trials large enough to identify small but clinically relevant effects that would likely be expected for many therapies targeting AKI. Further, restrictive inclusion and exclusion criteria often result in trial populations that do not represent the broader population of patients who experience AKI, or fail to include groups of patients who might have benefited from the proposed intervention10.
Table 1.
Characteristics of explanatory versus pragmatic clinical trials
| Trial Design Phase | Feature | Explanatory Trials | Pragmatic Trials |
|---|---|---|---|
| Design/planning | Idealized example | Placebo-controlled drug trial in a highly selected population | A trial comparing available therapies commonly used in routine clinical care among a broad population |
| Cost | Usually high (high cost per subject) | May be low (low cost per subject) | |
| Sample Size | Limited by cost (usually hundreds of patients) | Large (potentially tens of thousands of patients) | |
| Typically powered only to detect large ATE sizes | May be powered to detect small but clinically relevant treatment effects and evaluate heterogenity of treatment effect | ||
| Randomization | Individual | May be at the level of the individual patients, providers, hospitals, or health systems (i.e., clusters) | |
| Approach to consent | Written informed consent when practicable | Written informed consent when practicable | |
| When consent is impracticable (cluster-level trial), consent may be waived/modified | |||
| Implementation | Trial conditions/setting | Ideal | Real world |
| Tightly controlled | Embedded in routine care | ||
| Eligibility criteria | Narrow | Broad | |
| Many exclusion criteria | Few exclusion criteria | ||
| Blinding | Blinding is commonly used to reduce bias | Blinding may not be feasible for interventions embedded into routine clinical care | |
| EHR embedding | Rare | Commonly used for • Intervention (e.g., alerts, bundles) • Data collection |
|
| Analysis/interpretation | Outcomes measured | • May be extensive, complex, or require expert assessors • May require additional blood draws or follow-up visits |
• Usually simple and patient centered • Commonly collected in routine clinical care |
| Data analysis | • “Simple” • Usually frequentist in statistical design and analysis • May employ Bayesian methods • Often explore ATE |
• May be more complex (to account for contamination or heterogeneity) • Can be frequentist or Bayesian in statistical design and analysis; Bayesian increasingly common • Ideally explore HTE |
|
| Generalizability | Lower | Higher | |
| Focused population | Broad/diverse population(s) |
Alternative trial designs, such as pragmatic trials, adaptive trials, platform trials, and alternative methods of trial analysis, have the potential to increase trial efficiency and improve the feasibility of clinical trials for AKI. The popularity of these novel techniques and their acceptance by regulators and journals have been dramatically accelerated by widespread adoption during the 2019 Coronavirus disease 2019 (COVID-19) pandemic.
Pragmatic, Cluster, and Platform Trial Designs in AKI
Pragmatic Trials
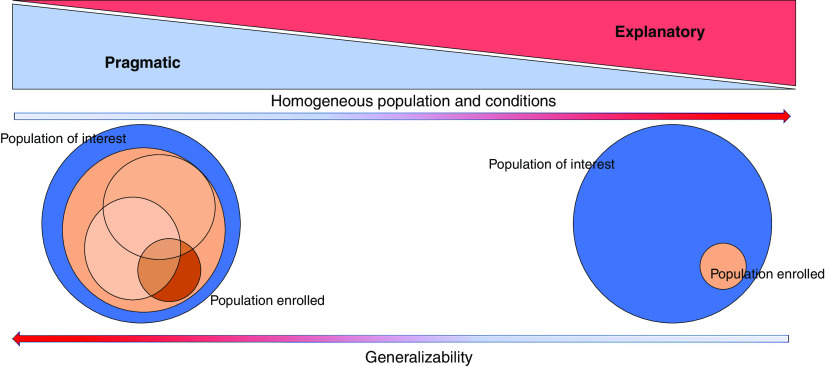
“Pragmatic” trial designs may offer a broad solution to several AKI research needs (Table 1). Designing an “explanatory” versus a “pragmatic” clinical trial is not a binary decision (Figure 1, Table 1, Supplemental Table 1). A continuum exists between the two archetypes, and AKI trials that balance a mix of pragmatic and explanatory design elements have enormous promise. Designs more to the pragmatic side of the continuum attempt to evaluate how an intervention would perform when introduced into real-world clinical practice.11 These are often called “effectiveness” trials and prioritize generalizability. Pragmatic trials typically accomplish these goals by (1) having broad and simple eligibility criteria with few exclusions, (2) embedding trial procedures and intervention delivery into routine clinical care, and (3) by using easy-to-collect, patient-centered outcomes (e.g., mortality or length of stay).12 Pragmatic design features can dramatically increase trial efficiency by expediting enrollment of sample sizes large enough to detect small but clinically meaningful treatment effects and are well suited for comparing available treatments (comparative effectiveness trials) or evaluating new processes of care (e.g., the introduction of new care bundles). Concerns have been raised that pragmatic trials with broad eligibility are predestined to fail due to overly heterogeneous patient cohorts, given the diverse pathophysiology of AKI and the likelihood that different patient groups may experience different responses to the same treatment. However, pragmatic trials may be uniquely suited to analyses exploring these differential responses to treatment (i.e., heterogeneity of treatment effect [HTE]) because these statistical methods require the large numbers of patients pragmatic trials enroll.
Figure 1.
Graphical representation of the continuum between explanatory and pragmatic trials. Less control in pragmatic trials often comes with a broader population enrolled and more generalizability of the results. The downside is more heterogeneity in response to therapies in the enrolled population.
The COVID-19 pandemic illustrated the potential for pragmatic trials to rapidly generate evidence on the effectiveness of repurposed therapeutics. The RECOVERY,13 REMAP-CAP,14–16 and SOLIDARITY17 trials enrolled tens of thousands of patients in a matter of months, providing the high-quality evidence available on several COVID-19 treatments, including corticosteroids,13 convalescent plasma,17 tocilizumab,18 hydroxychloroquine,19,20 and azithromycin.21
Clustered Trial Designs
Cluster-randomized trials are an increasingly popular pragmatic trial design that allocate interventions to groups of subjects on the basis of a location, e.g., intensive care units (ICUs), operating rooms, or hospitals, instead of individuals (Figure 2, Supplemental Table 1). All patients receiving care within a given cluster receive the same treatment, which allows intervention earlier in the course of treatment than trials that screen, consent, enroll, and randomize at the patient level. Cluster-level trials are ideal for evaluating interventions applied at the group level (e.g., approaches to contact precautions).22 There are several cluster trial design variations (Figure 2, Supplemental Table 1). The most straightforward is the parallel design, where each cluster is assigned one treatment and followed over time. Cluster crossover trials involve assigning a sequence of interventions, where each cluster receives each intervention in a separate period, thereby allowing each cluster to serve as a control and treated cluster. One successful example is the SMART Trial, which used a cluster crossover design to evaluate balanced solutions (Ringer lactate or PlasmaLyte) over normal saline for fluid resuscitation in adult patients who were critically ill in five ICUs, observing a lower risk of major adverse kidney events (MAKE) in the group of patients receiving balanced solutions.23
Figure 2.
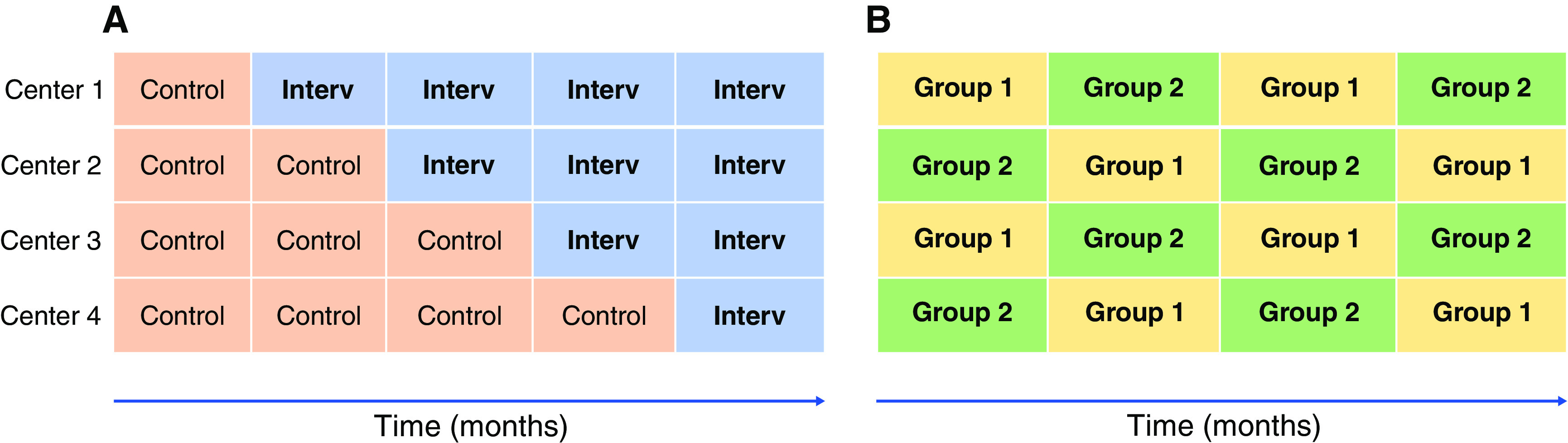
Graphical representation of two cluster-randomized trial designs. Panel (A) represents a steppedwedge cluster-randomized trial in which the intervention is implemented sequentially after a control period in each center. Panel (B) represents a crossover cluster-randomized trial where intervention and control periods alternate in each centerduring the study period.
Receipt of the nonassigned intervention (contamination) is a common problem in cluster crossover trials. Contamination is particularly problematic when evaluating treatment protocols, educational interventions, or new diagnostic tests. In these trials, clinicians are trained with the intent of delivering an intervention to a treatment group but, after training, they may unintentionally apply aspects of the intervention to patients in the control group. Stepped-wedge cluster trials attempt to address this problem. A stepped-wedge trial can also be used to evaluate treatment effects for interventions that could not be ethically deimplemented once introduced (e.g., a new and potentially life-saving therapy). In a stepped-wedge trial, all clusters start in the control group, with each cluster transitioning to the intervention group at regular intervals and remaining in the intervention group until all clusters have completed their transition. By completing the transition to the intervention only once and applying it to all future patients, these trials minimize the likelihood of contamination of the intervention and, by varying the timing of the transition, they minimize the temporal biases inherent to before-after studies.24,25 Stepped-wedge designs remain vulnerable, however, to changes in standards of care or changes in environmental factors over time, particularly during periods of rapid change as was experienced during the COVID-19 pandemic.
Another challenge of cluster trials is that patients within a cluster may be more similar to each other than patients in other clusters, if for nothing else, due to shared care structures and providers. Cluster trials, therefore, require more advanced methods of analysis than trials randomized at the patient level to account for cluster-level variation. Clustering effects also reduce the statistical power of cluster-level trials and generally require enrolling more patients than trials conducted with patient-level randomization. Further, increasing the number of patients within a cluster provides far less statistical power benefit than increasing the total number of clusters.25
Prospective, individual, patient-level consent is impossible in cluster trials because the interventions are conducted at the provider, unit, or hospital level and applied to all patients receiving care in those settings. Although the criteria for alteration or waiver of consent vary by country, they generally require that the studied intervention represent no more than minimal risk to patients. Pragmatic and clustered trial designs, therefore, lend themselves best to the evaluation of variations of the current standard of care rather than the evaluation of new drugs. Given the variability in routine clinical practice and the absence of evidence for many interventions given to treat or prevent AKI, the evaluation of these interventions may be considered to be no more than minimal risk compared with receipt of these interventions in usual care. Important questions that have been or could be evaluated using these designs include optimal fluid therapy strategies, administration of kidney replacement therapy, approaches to detect or monitor the progression of AKI, and optimization of vasoactive agents.
Platform Trials
A platform trial design permits simultaneous or consecutive comparison of multiple treatments centered around a specific disease state, such as AKI.26 A key aspect of the platform design is the ability to initiate “during-trial” adjustments such that, for example, agents can be moved in and out following prespecified rules (e.g., graduated due to efficacy or removed due to toxicity or futility), or the randomization ratio and sample size can be adjusted on the basis of interim results without stopping and initiating a new trial (Supplemental Table 1).27 Platform trials also allow the simultaneous comparison of multiple intervention groups against a single control group.28,29 Additionally, platform trials benefit from a single infrastructure with operating procedures outlined in a single master protocol. Altogether, platform trials promote greater trial efficiency and should result in a reduced number of participants needed, allowing for more interventions to be tested, at a faster pace, and with greater cost efficiency than multiple isolated trials.
However, there are various complexities that emerge from the platform design. Statistically, trialists must be cautious about how changes in population characteristics and usual care over time (such as occurred in the COVID-19 pandemic) can affect study group comparisons when using noncontemporaneous controls or adaptive designs that change allocation ratio. Real-time data entry is required for close safety monitoring and rapid adaptation.30 Funding can also be complex because the model necessary is one of sustained funding for testing multiple interventions (some of which are unknown at the onset), and thus funders must be receptive to such dynamics. Additionally, there is considerable complexity in logistical and regulatory issues.
Those caveats noted, the ICU is an ideal setting for a platform trial of AKI therapeutics because this high-risk population often presents with AKI. The I-SPY COVID Trial, inspired by a platform trial in breast cancer, is an extremely successful platform trial that has adopted both pragmatic and adaptive methods while simultaneously evaluating up to four therapeutic agents for COVID-19.28 New potential agents in a pipeline are evaluated continually for potential enrollments with funding from multiple sources. In considering a future platform trial for AKI, an assessment of already available drugs, such as sodium-glucose cotransporter-2 inhibitors, could be combined with other pharmacologic interventions, such as enhanced BP or enhanced glucose control, to create an informative platform trial.
Electronic Health Record–Embedded Clinical Trials
Data collection and monitoring represent a significant dual burden and challenge in many trials. By providing real-time granular patient data, recent advances in automated data abstraction from electronic health records (EHRs) offer the promise of improving both trial efficiency and patient safety. Several recent embedded trials, such as the SMART,23 ELAIA-1,31,32 and the ICE-AKI trials,33 have used EHR for screening, enrollment, and intervention delivery. EHR alerts may also provide the opportunity to alert clinicians to the presence of impending AKI. This approach was evaluated in a recent EHR-based, stepped-wedge trial22 that randomized centers to usual care or a “care bundle” that included volume status assessment, BP optimization, recognition and treatment of sepsis, medication review, urinalysis, and protocolized nephrology or critical care consultation for patients with AKI stage 3 or AKI complications. Mortality was not affected by the intervention, but it was associated with reductions in length of hospital stay and increased AKI recognition.
There are, of course, several challenges to using the EHR for trials.31,34 EHR structure may fundamentally differ at each collaborating facility, which compounds the inherent challenges of trial design, implementation, and data collection across various sites. In such cases, methods to enable semantic interoperability—or, the standardization of measurements, diagnoses, symptoms, medications, or EHR concepts across sites—are necessary. For example, nephrotoxins can have different formulations across sites. Likewise, common clinical variables may have different names across EHRs (e.g., pulse versus heart rate). Multicenter trials can also struggle with differential missing data across sites, including vitals or laboratory values. Data availability and patient case mix at different institutions may also affect the performance of risk scores. Data sharing may also impose obstacles to convenient data access, including the need for data use agreements that can be time consuming. However, we believe that AKI is a condition particularly well suited to EHR-based studies because AKI is one of the few acute conditions for which a reliable surrogate outcome (creatinine) is routinely obtained and routinely available in the EHR as part of routine clinical care.35 Further efficiency could be gained by embedding interventional pragmatic trials within large, preexisting, prospective, observational databases—such as the Multicenter Perioperative Outcome Group.36
The Importance of the Primary End Point
Selecting the appropriate primary end point for a trial is essential because it dictates statistical power, sample size, decisions about data collection, and, ultimately, trial interpretation.37 Table 2 outlines several advantages and considerations on common end points in AKI trials. Here, we offer a few summary comments. First, although AKI diagnosis on the basis of changes in serum creatinine may not be ideal in some settings, it may be a reasonable choice as a key safety end point or an exploratory secondary end point (giving an indication of the mechanistic aspects of an intervention), such as in the RELIEF trial.38,39 Patient-centered outcomes, like all-cause mortality, require a robust effect size and a large sample size because of the low proportion of mortality that can be attributed directly to AKI. In REVIVAL, a phase-3 study with ilofotase alfa in sepsis-associated AKI,40 mortality was selected as the primary end point on the basis of phase-2 exploratory trial results where all-cause mortality was reduced in the treatment group.41 Nonmortality outcomes must contend with issues of comparing them in the context of a competing risk from mortality and other outcomes. A popular way of managing the competing risk between outcomes of different or similarly prioritized health outcomes is to use a combined composite end point, such as MAKE (including death, need for kidney replacement therapy, and nonrenal recovery). However, MAKE may be less common than AKI and, therefore, require larger samples for a similar effect size. Furthermore, the contribution of nonrenal recovery to long-term outcomes is unclear, and the definition of nonrecovery with respect to long-term outcomes needs to be explored further. Another consideration with MAKE is that kidney replacement therapy is a clinical decision with large interclinician or institutional variability that can add noise to a trial.42 There may also be room for investigation of potential intermediate or surrogate outcomes, such as renal recovery and the risk of progression toward CKD. In this regard, longer-term changes in the eGFR might be considered as an end point in clinical trials.43 An approach for pragmatic trials with very large sample sizes to consider is to have two coprimary end points, and these may be a combination of superiority in a nonmortality outcome and noninferiority in mortality.
Table 2.
Advantages and disadvantages of several common AKI trial outcome measures
| End Point | Advantages | Disadvantages |
|---|---|---|
| AKI | • Sensitive (frequent) end point and thus may support smaller sample sizes • Associated with mortality and other organ dysfunction |
• Has been inconsistently associated with worse clinical outcomes (e.g., death) |
| Severe AKI | • Indicative of kidney damage • Associated with mortality, increased costs, hospital length of stay, and other measures of healthcare utilization |
• As a less frequently occurring event, may require larger sample size |
| Change in GFR | • Sensitive (frequent) end point and thus may support smaller sample sizes • Commonly collected value |
• Not a patient-centered outcome • Requires accurate measurement • Multiple possible ways to measure |
| Renal recovery | • Sensitive (frequent) end point and thus may support smaller sample sizes • Associated with mortality and morbidity |
• Variable definitions are used in practice, making across-trial comparisons potentially difficult |
| CKD | • Associated with mortality, morbidity, increased costs, and cardiovascular disease • Patient-centered outcome |
• Rare end point • Requires long-term follow-up to capture • Requires accurate measurement |
| RRT | • Associated with mortality, morbidity, and resource utilization | • Indications vary between institutions and providers; use of RRT is a clinical decision and not a pathophysiologic entity per se • Susceptible to survivorship bias |
| All-cause mortality | • Hard, patient-centered outcome • Easy to capture |
• Cause of death may not directly result from AKI • As a less frequently occurring event, may require larger sample size |
| MAKE at 90 days | • Occurs more frequently than individual component events | • May be driven by death and not directly related to AKI • Components may not be equally valued by different stakeholders • Low incidence in certain populations |
| Cardiovascular events | • Associated with kidney injury • Associated with mortality • Patient-centered outcome |
• May not directly result from AKI • Susceptible to survivorship bias |
This table is partially adapted from ref. 55, with permission.
A Road Map for Future AKI Trials
Here we provide several specific recommendations on how the community might more efficiently plan, run, and interpret clinical trials in patients at risk of AKI or with AKI.
Trial Planning and Design
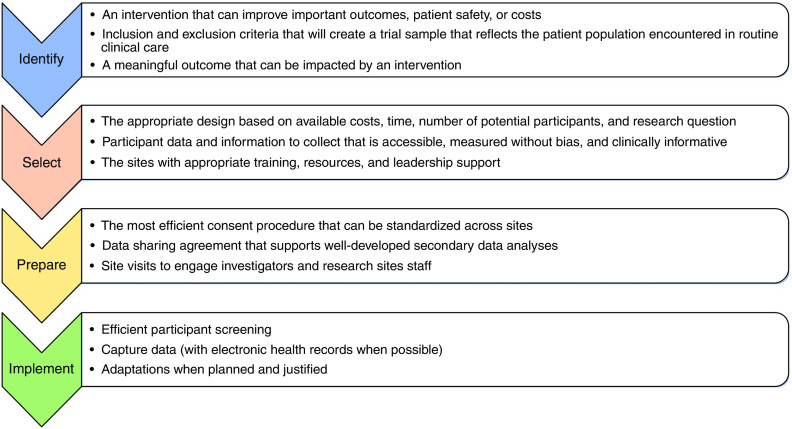
We encourage greater collaboration and planning among funders to support larger collaborative, multicenter, pragmatic trials and opportunities to incorporate multiple trial interventions into platform trials. Many treatments or interventions are applied worldwide to patients at risk for AKI (to prevent AKI) or with AKI (to prevent complications from AKI), despite a lack of evidence on their efficacy or safety. For example, interventions such as the administration of fluids, vasopressors, diuretics, albumin, or the use (or restriction) of potentially nephrotoxic drugs all lack strong experimental evidence, yet remain common in routine care. Thus, such interventions may especially benefit from testing through pragmatic trials.44 Promoting embedded trials in existing registries can be resource saving and allow faster implementation of trials with lower costs. The development of standardized definitions and measurements for a core set of outcomes would support across trial comparisons, data collection, and data synthesis. Finally, integrating diverse stakeholders and interested parties (e.g., patients, caregivers, regulators, funders, ethicists, and human subject review board members) into trial design discussions, would help inform outcome measure selection. Recommendations for optimization of clinical trial efficiency are provided in Figure 3.
Figure 3.
Road map for more efficient clinical trials in patients at risk for or with AKI (developed by the KDCT 2021 workshop and working group).
Regulatory and Data Sharing
We encourage establishing research networks and a common data language with standardized data collection and EHR interoperability. Although data collection through the EHR represents a potential method to improve the efficiency of data capture and reduce costs, most current methods use delayed data extraction and cannot transmit data in real time, which poses significant challenges for some aspects of trial conduct (e.g., safety monitoring). Thus, this is an area of great promise, but also in need of considerable innovation.
The regulations governing clinical research were largely written before the adoption of innovative trial methods, like pragmatic trials, cluster-level trials, adaptive trials, and platform trials. More widespread adoption of these methods will require a similar evaluation of the regulations governing clinical research. For example, patient-level informed consent is infeasible for cluster-level trials, EHR-embedded trials, and acute care trials that occur during medical emergencies. As noted, most countries only allow alteration or waiver of consent when the trial intervention represents no more than minimal risk to patients and informed consent is infeasible. However, the interpretation of “minimal risk” is binary and highly subjective. We believe that regulators should update regulations to specifically address minimal risk comparative effectiveness research and explicitly allow its conduct under a waiver of consent or determine other methods of respecting patient autonomy during pragmatic trials through community participation in trial design, postenrollment notification, embedding consent within clinical teams, or providing a mechanism for patients to “opt out.”45,46
Analysis
We recommend planning statistical analysis to provide both a frequentist and Bayesian trial interpretation and to assess HTE. Presently, across most of medicine, including AKI, the frequentist statistical approach is predominately used to design trials and to present and interpret their results (Figure 4). Trials also tend to be designed with a focus on the average treatment effect (ATE), or the overall effect between the trial arms. In this section, we outline how greater use of Bayesian statistical thinking and recent improvements in HTE assessment could potentially augment the knowledge derived from AKI trials.
Figure 4.
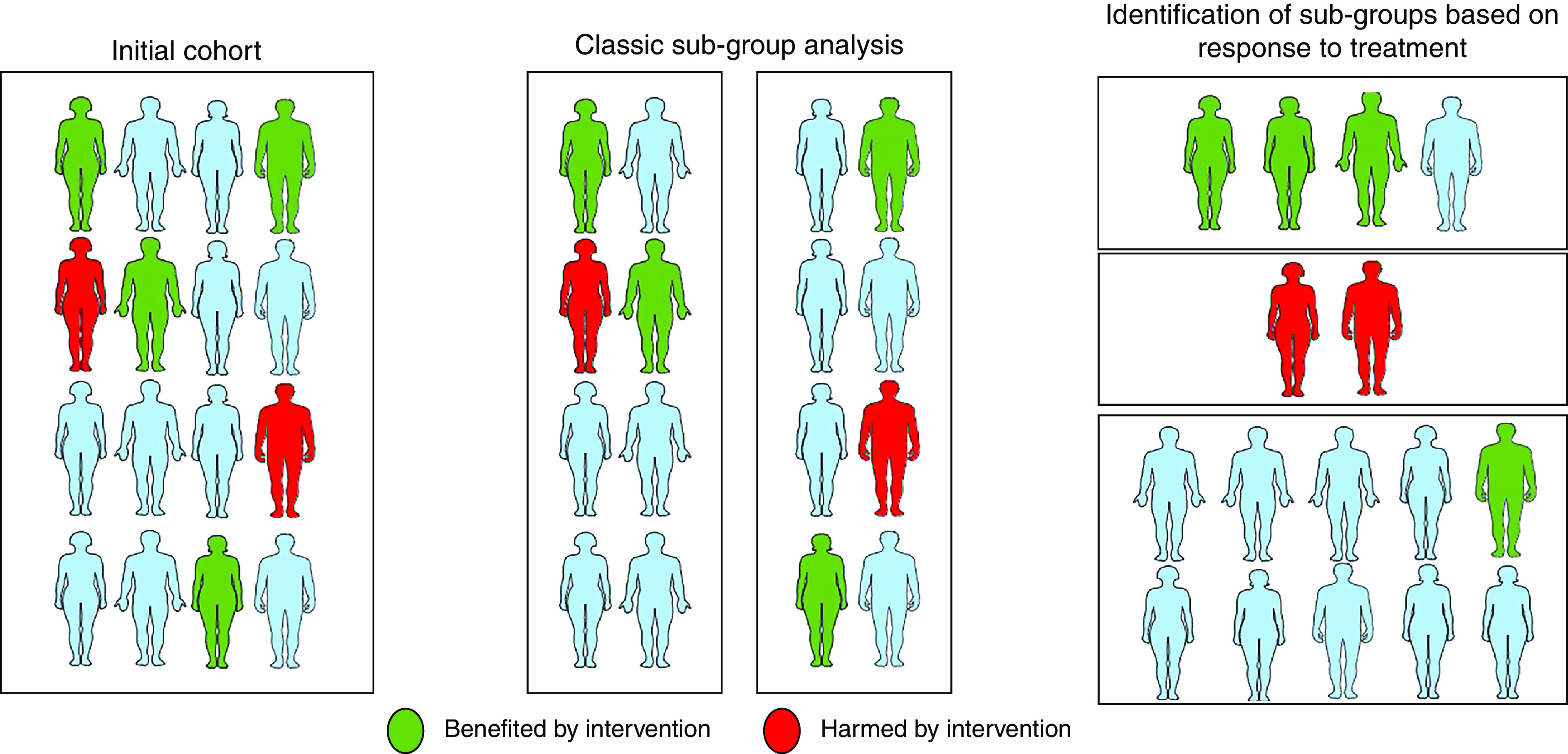
Graphical representation of heterogeneity of treatment effect in a trial population. The left panel shows how overall treatment responses may vary in the full trial population. The middle panel shows how subgroup analyses in randomized trials can fail to capture heterogeneity in treatment effects in a trial using classic subgroup analysis. The right panel shows how machine learning and other newer statistical methods may be used to estimate the treatment effects at the individual level.
Frequentist statistical thinking is rooted in the concepts of null hypotheses testing and P values. The challenge is that looking at clinical trial data through this lens does not tell you about clinical utility of the intervention. It reflects only whether the data are compatible with the null hypothesis of the trial. Acceptance of the alternative hypothesis is then conditional on meeting some prespecified difference in magnitude. This leads to two problems: (1) a temptation for investigators to plan trials targeting large treatment effects to justify a sample size that can be feasibly enrolled on the basis of trial length/funding (increasing the risk of false negative or false-positive conclusion), and (2) a binary interpretation of trial results in which trials that fail to reject the null hypothesis are incorrectly classified as proving equivalency or as being completely uninformative. Null trials, however, may be informative and affect clinical practice, as shown by recent findings that suggest that systematic early initiation of higher doses of kidney replacement therapy does not improve outcomes in AKI but leads to more resource use and invasive treatments have changed practice in many settings.47–49
Different approaches to examining trial data may also provide additional clinical insight. For example, even in a selected population (via inclusion/exclusion criteria), the ATE may be substantially different from the effect observed at the individual level (both for efficacy and potential harm).50 For example, due to extreme heterogeneity in the clinical presentation and care of patients with AKI, trial participants may respond differently to a specific treatment, and thus the data may provide information pertinent to key subpopulations. Therefore, acknowledging that such examinations are exploratory, we argue that it is critical to look for the presence of HTE, either by looking at the difference in treatment effect across predefined subgroups (or subphenotypes) or by directly estimating the variability of treatment effect explained by patient characteristics.51 For instance, in the STARRT-AKI trial investigating the effect of timing of kidney replacement therapy, no evidence of HTE was identified across subgroups on the basis of severity of illness at inclusion.49 In contrast, although no difference in mortality was noted in patients with severe acidemia and organ failure or hyperlactatemia receiving sodium bicarbonate in the BICAR-ICU study, patients with severe AKI had a lower risk of death in the intravenous sodium bicarbonate infusion group.52 These kinds of results are hypothesis generating and can help understand mechanisms of action, which is important in a field with such complexity.
Although understanding HTE enough to define individual treatment rules is the ultimate goal for trials, the ATE is still informative and could also be examined in potentially more insightful ways. One increasingly normalized approach is the use of Bayesian statistics to assess the likelihood of certain effect sizes. For example, some trials where the P value was not statistically significant, but the results were compelling, have been reanalyzed using Bayesian methods. For example, the EOLIA51 and ANDROMEDA-SHOCK53 trials had large absolute mortality reductions deemed clinically relevant and were supported by an understood biologic mechanism, and the Bayesian lens helped develop insight from these trials. The Bayesian approach provides a probabilistic interpretation of trial data, which aligns with the way many clinicians approach therapeutic interventions in terms of risk of events and response to interventions.54 In addition to interpreting trial results in a Bayesian framework, several pragmatic and platform trials have already successfully used an adaptive Bayesian model into their design, such as the REMAP-CAP trials and the I-SPY COVID Trial. In the design stage, a Bayesian approach allows adjustment in the sample size or dropping arms as trial results become available. Growing embracement of Bayesian tools can help streamline trials, especially when results are needed rapidly (such as during a pandemic), and maximize the value and knowledge translation of trials. Pragmatic, cluster, adaptive, and platform trials offer the opportunity to run more efficient trials that can potentially overcome existing barriers the AKI community faces in developing robust experimental evidence for patients suffering from AKI. However, each design brings its own complications, which will be magnified by trial-specific nuances. Although the designs and approaches to data analysis discussed in this manuscript are relatively new to AKI, they bring great promise as the community begins to experiment and innovate with their implementation.
Disclosures
S.M. Bagshaw reports receiving research funding and honoraria from, and serving on a speakers bureau for, Baxter Healthcare Corp.; having consultancy agreements with Baxter Healthcare Corp., BioPorto Inc., and CNA Diagnostics Inc.; serving in an advisory or leadership role for Baxter Healthcare Corp. (advisory), BioPorto (adjudication committee), CNA Diagnostics Inc. (advisory), and Critical Care (editorial board); being supported as a Canada Research Chair in Critical Care Outcomes and Systems Evaluation; and having ownership interest in CNA Diagnostics Inc. J. Bernholz reports being an employee of, having ownership interest in, and receiving honoraria from, AM-Pharma. M.P. Bokoch reports receiving research funding from bioMérieux, La Jolla Pharmaceuticals, and SphingoTec. S. Coca reports having consultancy agreements with 3ive, Axon, Bayer, CHF Solutions, Renalytix, Reprieve Cardiovascular, Takeda, and Vifor; serving on the editorial boards of CJASN, JASN, and Kidney International, and as associate editor for Kidney360; being employed by Icahn School of Medicine at Mount Sinai, and Mount Sinai owns part of Renalytix; receiving research funding from ProKidney, Renalytix, RRI, and XORTX; having ownership interest in pulseData and Renalytix; having patents or royalties with Renalytix; and serving in an advisory or leadership role for Renalytix and Reprieve Cardiovascular. F. Erlandsson reports being an employee of Vifor Pharma AG. M. Gallagher reports receiving honoraria from AstraZeneca; receiving research funding from Bayer Pharmaceuticals; serving in an advisory or leadership role for Ellen Medical Devices Pty Ltd.; and having other interests in, or relationships with, The George Institute for Global Health. S. Gaudry reports receiving research funding from the French ministry of health for the AKIKI and AKIKI 2 trials, and having consultancy agreements with Zambon. M.O. Harhay reports receiving honoraria, all for work unrelated to the topics in this manuscript, from the American Thoracic Society, Berkley Research Group, Elsevier, Guidepoint Advisers, Pura Vida Investments LLC, and Trinity Life Science; having consultancy agreements with Berkley Research Group, Guidepoint Advisers, Pura Vida Investments LLC, and Trinity Life Science; and receiving research funding from the National Institutes of Health (NIH) and the Patient-Centered Outcomes Research Institute. J.L. Koyner reports receiving honoraria from Acute Disease Quality Initiative, American Society of Nephrology (ASN), and Society of Critical Care Medicine; serving in an advisory or leadership role for the American Journal of Nephrology, Guard Therapeutics, Kidney360, and National Kidney Foundation (NKF) and NKF of Illinois (on the scientific advisory board); having patents or royalties with Argutus Medical for a patent for Pi GST to detect severe AKI after cardiac surgery; having consultancy agreements with Astute Medical/bioMérieux Baxter, Mallinckrodt, Novartis, and SeaStar; receiving research funding from Astute Medical, Fresenius Medical, NIH, and NxStage Medical; and serving on a speakers bureau for NxStage Medical. K.D. Liu reports serving on the editorial boards of the American Journal of Kidney Diseases, American Journal of Respiratory and Critical Care Medicine, and CJASN, and on the scientific advisory board of the NKF; having ownership interest in Amgen (hold stock only); and having consultancy agreements with AM-Pharma, bioMérieux, BOA Medical, Durect, and Seastar Medical. M.R. Mathis reports receiving research funding from NIH, National Heart, Lung, and Blood Institute (grant K01-HL141701). R.L. Mehta reports having consultancy agreements with Abiomed, Akebia, AM-Pharma, Baxter, bioMérieux, CHF Solutions, GE Healthcare, Intercept, Mallinckrodt, Nova Biomedical, Novartis, Sanofi, SphingoTec, Renasym, and Unicycive; receiving honoraria from Baxter, CHF Solutions, Medtronic, and Nova Biomedical; having ownership interest in DAECOS, CRRT Inc.; and receiving investigator-initiated grants and research funding from Fresenius and Fresenius-Kabi. R. Pirracchio reports receiving research funding from the Gate Foundation and the US Food and Drug Administration. P. Rossignol reports receiving honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer Ingelheim, CinCor, Corvidia, CVRx, Fresenius, Grunenthal, Idorsia, KBP, Novartis, Novo Nordisk, Relypsa Inc. (a Vifor Pharma Group Company), Roche, Sanofi, Sequana Medical, Servier, Stealth Peptides, and Vifor Fresenius Medical Care Renal Pharma; serving as a board member of the ASN Kidney Health Initiative work group for “Understanding and Overcoming the Challenges to Involving Patients with Kidney Disease in Cardiovascular Trials,” European Renal Association–European Dialysis and Transplant Association work group on European Renal and Cardiovascular Medicine (2021–2023), European Society of Hypertension Working Group “Hypertension and the Kidney” (since 2016), and Heart Failure Association (cardiorenal and translational group, 2016–2020; work group on biomarkers, 2020–2022); receiving personal fees from Bayer, Boehringer Ingelheim, CinCor, Idorsia, KBP, Novo Nordisk, Sanofi, Sequana Medical, Servier, and Vifor; having consultancy agreements with Bayer, CinCor, G3P, Idorsia, and KBP; being the cofounder of CardioRenal; and receiving research funding from Relypsa Inc. (a Vifor Pharma Group Company) and Vifor Fresenius Medical Care Renal Pharma. I.H. Schulman reports being employed by the National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK), NIH. D. Steubl reports being employed by Boehringer Ingelheim International, and serving as the section editor for nephrology for the journal Medicine. A. Turan reports receiving research funding from Heron and Pacira, and having consultancy agreements with Pfizer. F.P. Wilson reports serving on the editorial boards of American Journal of Kidney Disease and CJASN; receiving research funding from Amgen, AstraZeneca, Boehringer Ingelheim, NIDDK (R01DK11391, P30DK079310, and R01HS027626), Vifor Pharma, and Whoop; and serving on the board of directors for Gaylord Health Care, and as medical commentator for Medscape. A. Zarbock reports serving in an advisory or leadership role for A&A, AM-Pharma, Journal of Immunology, Guard Therapeutics, Novartis, and Piaon; having consultancy agreements with Alexion, AM-Pharma, Anomed, Astellas, Astute Medical, Baxter, bioMérieux, Guard Therapeutics, La Jolla Pharmaceuticals, Novartis, and Piaon; receiving honoraria from Amomed, Astute Medical, Baxter, bioMérieux, Braun, Fresenius, and Ratiopharm (lecture fees); receiving research funding from Astellas, Astute Medical, Baxter, Deutsche Forschungsgemeinschaft, Else-Kröner Fresenius Stiftung, and Fresenius; receiving investigator-initiated grants from Baxter, bioMérieux, and Fresenius; and having ownership interest in Vifor Pharma. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors thank Clinical Research Coordinators Emily Bi and Jillene Sturgess-DaPrato for their assistance in drafting the manuscript. The authors also thank Overcome, the organizers of the 2021 KDCT meeting.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIDDK, NIH, or the Department of Health and Human Services.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
M. Legrand, M. Harhay and J. Casey wrote the original draft. M. Legrand provided supervision; P. Rossignol was responsible for funding acquisition and resources; and all authors conceptualized the study, reviewed and edited the manuscript, and were responsible for validation.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021121605/-/DCSupplemental.
Supplemental Table 1. Advantages and caveats of the different clinical trials designs.
References
- 1.Ostermann M, Bellomo R, Burdmann EA, Doi K, Endre ZH, Goldstein SL, et al. ; Conference Participants: Controversies in acute kidney injury: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int 98: 294–309, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, et al. : Recommendations on acute kidney injury biomarkers from the Acute Disease Quality Initiative Consensus Conference: A consensus statement. JAMA Netw Open 3: e2019209, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Legrand M, Rossignol P: Cardiovascular consequences of acute kidney injury. N Engl J Med 382: 2238–2247, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. ; Acute Disease Quality Initiative Workgroup 16 : Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13: 241–257, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Makris K, Spanou L: Acute kidney injury: Definition, pathophysiology and clinical phenotypes. Clin Biochem Rev 37: 85–98, 2016 [PMC free article] [PubMed] [Google Scholar]
- 6.Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, et al. : Design of clinical trials in acute kidney injury: Report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol 7: 844–850, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Coca SG, Zabetian A, Ferket BS, Zhou J, Testani JM, Garg AX, et al. : Evaluation of short-term changes in serum creatinine level as a meaningful end point in randomized clinical trials. J Am Soc Nephrol 27: 2529–2542, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiersema R, Jukarainen S, Vaara ST, Poukkanen M, Lakkisto P, Wong H, et al. : Two subphenotypes of septic acute kidney injury are associated with different 90-day mortality and renal recovery. Crit Care 24: 150, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thau MR, Bhatraju PK: Sub-phenotypes of acute kidney injury: Do we have progress for personalizing care? Nephron 144: 677–679, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lascarrou JB, Muller G, Quenot J-P, Massart N, Landais M, Asfar P, et al. ; AfterROSC Network : Insights from patients screened but not randomised in the HYPERION trial. Ann Intensive Care 11: 156, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford I, Norrie J: Pragmatic trials. N Engl J Med 375: 454–463, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Loudon K, Zwarenstein M, Sullivan FM, Donnan PT, Gágyor I, Hobbelen HJSM, et al. : The PRECIS-2 tool has good interrater reliability and modest discriminant validity. J Clin Epidemiol 88: 113–121, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. ; RECOVERY Collaborative Group : Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384: 693–704, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arabi YM, Gordon AC, Derde LPG, Nichol AD, Murthy S, Beidh FA, et al. ; REMAP-CAP Investigators : Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial. Intensive Care Med 47: 867–886, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al. ; REMAP-CAP Investigators : Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 384: 1491–1502, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, et al. ; REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators : Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med 385: 777–789, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, et al. ; WHO Solidarity Trial Consortium : Repurposed antiviral drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med 384: 497–511, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. ; BACC Bay Tocilizumab Trial Investigators : Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 383: 2333–2344, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. ; RECOVERY Collaborative Group : Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 383: 2030–2040, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey JD, Johnson NJ, Semler MW, Collins SP, Aggarwal NR, Brower RG, et al. : Rationale and design of ORCHID: A randomized placebo-controlled clinical trial of hydroxychloroquine for adults hospitalized with COVID-19. Ann Am Thorac Soc 17: 1144–1153, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. ; Coalition Covid-19 Brazil I Investigators : Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19 [published correction appears in N Engl J Med 383: e119, 2020]. N Engl J Med 383: 2041–2052, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd M, Karahalios A, Janus E, Skinner EH, Haines T, Silva AD, et al. ; Improving Evidence-Based Treatment Gaps and Outcomes in Community-Acquired Pneumonia (IMPROVE-GAP) Implementation Team at Western Health : Effectiveness of a bundled intervention including adjunctive corticosteroids on outcomes of hospitalized patients with community-acquired pneumonia: A stepped-wedge randomized clinical trial. JAMA Intern Med 179: 1052–1060, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. ; SMART Investigators and the Pragmatic Critical Care Research Group : Balanced crystalloids versus saline in critically ill adults. N Engl J Med 378: 829–839, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selby NM, Casula A, Lamming L, Stoves J, Samarasinghe Y, Lewington AJ, et al. : An organizational-level program of intervention for AKI: A pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol 30: 505–515, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnup SJ, McKenzie JE, Hemming K, Pilcher D, Forbes AB: Understanding the cluster randomised crossover design: A graphical illustration of the components of variation and a sample size tutorial. Trials 18: 381, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JJH, Detry MA, Murthy S, Guyatt G, Mills EJ: How to use and interpret the results of a platform trial: Users’ guide to the medical literature. JAMA 327: 67–74, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ: I-SPY 2: An adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther 86: 97–100, 2009 [DOI] [PubMed] [Google Scholar]
- 28.I-SPY COVID Consortium : Clinical trial design during and beyond the pandemic: The I-SPY COVID trial. Nat Med 28: 9–11, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorlund K, Haggstrom J, Park JJ, Mills EJ: Key design considerations for adaptive clinical trials: A primer for clinicians. BMJ 360: k698, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.QuantumLeap Healthcare Collaborative : I-SPY COVID TRIAL: An adaptive platform trial to reduce mortality and ventilator requirements for critically ill patients. Available at: https://clinicaltrials.gov/ct2/show/NCT04488081. Accessed December 7, 2021
- 31.Mutter M, Martin M, Yamamoto Y, Biswas A, Etropolski B, Feldman H, et al. : Electronic alerts for acute kidney injury amelioration (ELAIA-1): A completely electronic, multicentre, randomised controlled trial: Design and rationale. BMJ Open 9: e025117, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson FP, Martin M, Yamamoto Y, Partridge C, Moreira E, Arora T, et al. : Electronic health record alerts for acute kidney injury: Multicenter, randomized clinical trial. BMJ 372: m4786, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodgson LE, Roderick PJ, Venn RM, Yao GL, Dimitrov BD, Forni LG: The ICE-AKI study: Impact analysis of a clinical prediction rule and electronic AKI alert in general medical patients. PLoS One 13: e0200584, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moledina DG, Belliveau O, Yamamoto Y, Arora T, Carey KA, Churpek M, et al. : Variation in best practice measures in patients with severe hospital-acquired acute kidney injury: A multicenter study. Am J Kidney Dis 77: 547–549, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koyner JL, Adhikari R, Edelson DP, Churpek MM: Development of a multicenter ward-based AKI prediction model. Clin J Am Soc Nephrol 11: 1935–1943, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colquhoun DA, Shanks AM, Kapeles SR, Shah N, Saager L, Vaughn MT, et al. : Considerations for integration of perioperative electronic health records across institutions for research and quality improvement: The approach taken by the Multicenter Perioperative Outcomes Group. Anesth Analg 130: 1133–1146, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisbord SD, Palevsky PM: Design of clinical trials in acute kidney injury: Lessons from the past and future directions. Semin Nephrol 36: 42–52, 2016 [DOI] [PubMed] [Google Scholar]
- 38.McCoy IE, Chertow GM: AKI-A relevant safety end point? Am J Kidney Dis 75: 508–512, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. ; Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group : Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med 378: 2263–2274, 2018 [DOI] [PubMed] [Google Scholar]
- 40.AM Pharma : (Revival) Study to Investigate the Efficacy and Safety of Alkaline Phosphatase in Patients With Sepsis-Associated AKI. Available at: https://clinicaltrials.gov/ct2/show/NCT04411472. Accessed August 16, 2021
- 41.Pickkers P, Mehta RL, Murray PT, Joannidis M, Molitoris BA, Kellum JA, et al. ; STOP-AKI Investigators : Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: A randomized clinical trial. JAMA 320: 1998–2009, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Legrand M, Darmon M, Joannidis M, Payen D: Management of renal replacement therapy in ICU patients: An international survey. Intensive Care Med 39: 101–108, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Vincenti F, Kim J, Gouveia D, Pelle G, Mayne TJ, Neylan JF: Phase 3 trial design of the hepatocyte growth factor mimetic ANG-3777 in renal transplant recipients with delayed graft function. Kidney Int Rep 6: 296–303, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.University of California San Francisco : The choice of vasopressor for treating hypotension during general anesthesia: A pilot pragmatic cluster cross-over randomized trial (the VEGA-1 Trial). Available at: https://clinicaltrials.gov/ct2/show/NCT04789330. Accessed August 15, 2021
- 45.Young PJ, Bagshaw SM, Forbes AB, Nichol AD, Wright SE, Bailey M, et al. ; PEPTIC Investigators for the Australian and New Zealand Intensive Care Society Clinical Trials Group; Alberta Health Services Critical Care Strategic Clinical Network; Irish Critical Care Trials Group : Effect of stress ulcer prophylaxis with proton pump inhibitors vs histamine-2 receptor blockers on in-hospital mortality among ICU patients receiving invasive mechanical ventilation: The PEPTIC Randomized Clinical Trial. JAMA 323: 616–626, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. ; PROPPR Study Group : Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR Randomized Clinical Trial. JAMA 313: 471–482, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. ; AKIKI Study Group : Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 375: 122–133, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, et al. ; RENAL Replacement Therapy Study Investigators : Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361: 1627–1638, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Bagshaw SM, Wald R, Adhikari NKJ, Bellomo R, da Costa BR, Dreyfuss D, et al. ; STARRT-AKI Investigators; Canadian Critical Care Trials Group; Australian and New Zealand Intensive Care Society Clinical Trials Group; United Kingdom Critical Care Research Group; Canadian Nephrology Trials Network; Irish Critical Care Trials Group : Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med 383: 240–251, 2020 [DOI] [PubMed] [Google Scholar]
- 50.Pirracchio R, Hubbard A, Sprung CL, Chevret S, Annane D; Rapid Recognition of Corticosteroid Resistant or Sensitive Sepsis (RECORDS) Collaborators : Assessment of machine learning to estimate the individual treatment effect of corticosteroids in septic shock. JAMA Netw Open 3: e2029050, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goligher EC, Tomlinson G, Hajage D, Wijeysundera DN, Fan E, Jüni P, et al. : Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome and posterior probability of mortality benefit in a post hoc Bayesian analysis of a randomized clinical trial. JAMA 320: 2251–2259, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Jaber S, Paugam C, Futier E, Lefrant J-Y, Lasocki S, Lescot T, et al. ; BICAR-ICU Study Group : Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet 392: 31–40, 2018 [DOI] [PubMed] [Google Scholar]
- 53.Zampieri FG, Damiani LP, Bakker J, Ospina-Tascón GA, Castro R, Cavalcanti AB, et al. : Effects of a resuscitation strategy targeting peripheral perfusion status versus serum lactate levels among patients with septic shock. A Bayesian reanalysis of the ANDROMEDA-SHOCK Trial. Am J Respir Crit Care Med 201: 423–429, 2020 [DOI] [PubMed] [Google Scholar]
- 54.Zampieri FG, Casey JD, Shankar-Hari M, Harrell FE Jr., Harhay MO: Using Bayesian methods to augment the interpretation of critical care trials. An overview of theory and example reanalysis of the alveolar recruitment for acute respiratory distress syndrome trial. Am J Respir Crit Care Med 203: 543–552, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lazzareschi D, Mehta RL, Dember LM, Bernholz J, Turan A, Sharma A, et al. : Overcoming barriers in the design and implementation of clinical trials for Acute Kidney Injury: A report from the 2020 Kidney Disease Clinical Trialists meeting [published online ahead of print January 12, 2022]. Nephrol Dial Transplant 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.