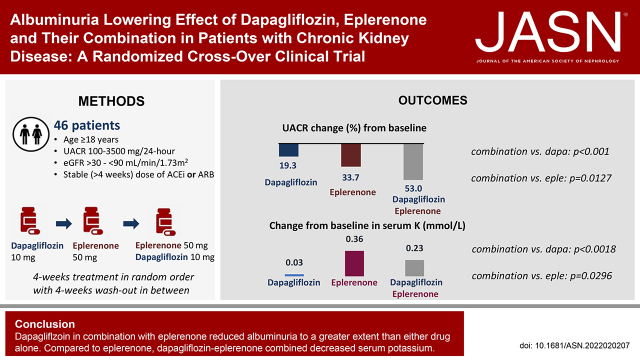
Significance Statement
In this randomized crossover clinical trial in patients with CKD with and without type 2 diabetes, we assessed the albuminuria-lowering effect of the sodium glucose co-transporter 2 (SGLT2) inhibitor dapagliflozin and mineralocorticoid receptor antagonist (MRA) eplerenone individually and in combination. We demonstrated that the albuminuria-lowering effects of dapagliflozin and eplerenone alone are additive when they are used in combination, resulting in a clinically relevant albuminuria reduction of 53% after 4 weeks of dapagliflozin-eplerenone treatment. The incidence of hyperkalemia was significantly less with combination treatment compared with eplerenone alone. These data support future clinical trials to confirm long-term efficacy and safety of combined SGLT2 inhibitor and MRA treatment.
Keywords: albuminuria, aldosterone, chronic kidney disease, randomized controlled trials, sodium glucose co transporter, mineralocorticoid receptor antagonist, dapagliflozin, eplerenone
Visual Abstract
Abstract
Background
Sodium glucose cotransporter 2 (SGLT2) inhibitors and mineralocorticoid receptor antagonists (MRAs) reduce the urinary albumin-to-creatinine ratio (UACR) and confer kidney and cardiovascular protection in patients with CKD. We assessed efficacy and safety of the SGLT2 inhibitor dapagliflozin and MRA eplerenone alone and in combination in patients with CKD.
Methods
We conducted a randomized open-label crossover trial in patients with urinary albumin excretion ≥100 mg/24 hr, eGFR 30–90 ml/min per 1.73 m2, who had been receiving maximum tolerated stable doses of an ACE inhibitor (ACEi) or angiotensin receptor blocker (ARB). Patients were assigned to 4-week treatment periods with dapagliflozin 10 mg/day, eplerenone 50 mg/day, or their combination in random order, separated by 4-week washout periods. Primary outcome was the correlation in UACR changes between treatments. Secondary outcome was the percent change in 24-hour UACR from baseline.
Results
Of 57 patients screened, 46 were randomly assigned (mean eGFR, 58.1 ml/min per 1.73 m2; median UACR, 401 mg/g) to the three groups. Mean percentage change from baseline in UACR after 4 weeks of treatment with dapagliflozin, eplerenone, and dapagliflozin-eplerenone was –19.6% (95% confidence interval [CI], –34.3 to –1.5), –33.7% (95% CI, –46.1 to –18.5), and –53% (95% CI, –61.7 to –42.4; P<0.001 versus dapagliflozin; P=0.01 versus eplerenone). UACR change during dapagliflozin or eplerenone treatment did not correlate with UACR change during dapagliflozin-eplerenone (r=–0.13; P=0.47; r=–0.08; P=0.66, respectively). Hyperkalemia was more frequently reported with eplerenone (n=8; 17.4%) compared with dapagliflozin (n=0; 0%) or dapagliflozin-eplerenone (n=2; 4.3%; Pbetween-groups=0.003).
Conclusions
Albuminuria changes in response to dapagliflozin and eplerenone did not correlate, supporting systematic rotation of these therapies to optimize treatment. Combining dapagliflozin with eplerenone resulted in a robust additive UACR-lowering effect. A larger trial in this population is required to confirm long-term efficacy and safety of combined SGLT2 inhibitor and MRA treatment.
Clinical Trial registry name and registration number:
European Union Clinical Trials Register, EU 2017–004641–25.
Introduction
Angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) have been the mainstay of treatment for prevention of kidney failure in patients with CKD for the last few decades. Despite the proven efficacy of ACE inhibitors and ARBs, the risk of kidney failure remains high. New therapeutic options to slow the progressive loss of kidney function in patients with CKD have now become available.
Sodium glucose co-transporter 2 (SGLT2) inhibitors reduce the risk of kidney failure and slow the progression of eGFR decline.1,2 These benefits were first demonstrated in the CREDENCE trial—a dedicated kidney outcome trial that recruited patients with type 2 diabetes and CKD.1 The DAPA-CKD trial confirmed and extended these findings by demonstrating that the 39% reduction in risk of the composite kidney end point achieved with dapagliflozin was similar in patients with CKD with or without type 2 diabetes.2 Mineralocorticoid receptor antagonists (MRA) is another class of agents that reduces albuminuria in patients with CKD. Finerenone, a nonsteroidal MRA, reduced the risk of a composite kidney outcome of 57% eGFR decline, ESKD, or renal death in patients with type 2 diabetes and CKD in the FIDELIO-DKD and FIGARO-DKD trials.3,4 However, despite the clinical benefits of these new therapies, a number of patients still progress toward kidney failure. The persistently high risk of kidney failure with these new therapies is associated with high albuminuria, possibly due to suboptimal treatment response.5 Additional strategies to decrease albuminuria and risk of kidney failure further are thus desired.
Post hoc analyses from clinical trials with SGLT2 inhibitors and finerenone have reported that the kidney protective effects of these drugs remained present when dapagliflozin was added to conventional MRAs (spironolactone or eplerenone) or when finerenone was added to SGLT2 inhibitors.6,7 These data suggest that the nephroprotective effects of both drug classes are possibly independent and complementary. However, the post hoc analyses do not answer the question of whether the initiation of combined SGLT2 inhibitor and MRA treatment further reduces albuminuria compared with either therapy alone. In addition, whether therapy resistance to a SGLT2 inhibitor or MRA can be overcome by rotation to another or their combination is unknown.
Because there are possible complementary effects of SGLT2 inhibitors and MRA that may synergistically affect kidney function, the ROTATE-3 study was designed to assess the albuminuria-lowering efficacy of dapagliflozin and eplerenone alone and in combination in patients with CKD.
Methods
Study Design and Patients
We conducted a prospective randomized open-label crossover clinical trial. The study was conducted at three clinical trial sites in Italy and Spain. The study was approved by the institutional ethics committees of the participating centers. The study was registered with the European Union Clinical Trials Register (EU 2017–004641–25). The study was done in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent before any specific study procedure commenced.
Patients aged ≥18 years with urinary albumin excretion ≥100 mg/24 hr and ≤3500 mg/24 hr, eGFR >30 ml/min per 1.73 m2 and <90 ml/min per 1.73 m2, serum potassium ≤5 mmol/L, and who were on stable doses of ACEis or ARBs for more than 4 weeks were eligible for participation. Key exclusion criteria were type 1 diabetes, autosomal dominant polycystic kidney disease or autosomal recessive polycystic kidney disease, lupus nephritis, or ANCA-associated vasculitis, indication for immunosuppressants as per the treating physician’s judgment, receiving immunosuppressive therapy or other immunotherapy for primary or secondary renal disease within 6 months before enrolment, active malignancy, and women who were pregnant or breast feeding or who had a positive pregnancy test at screening.
Procedures
After a screening visit to assess eligibility, all participants proceeded to a run-in period. During the run-in period, two 24-hour urine samples were collected 2 weeks apart for patients who were already on a stable maximum tolerated dose of an ACEi or ARB. Patients who were not using a stable dose of an ACEi or ARB or who did not use an ACEi or ARB at all continued the run-in period until they used these agents at a stable maximum tolerated dose for at least 4 weeks. Subsequently, at the end of the run-in, patients collected two 24-hour urine samples 2 weeks apart to determine changes in 24-hour albumin-to-creatinine ratio (UACR) during stable ACEi or ARB treatment. Eligible patients then proceeded to the randomization visit. The study used three consecutive open-label crossover treatment periods of 4 weeks each, in which patients were treated with eplerenone 50 mg once daily, dapagliflozin 10 mg once daily, or a combination of eplerenone 50 mg once daily and dapagliflozin 10 mg once daily in random order, with washout periods of 4 weeks after each active treatment period. Patients were randomly allocated to one of the three treatment orders generated by an independent pharmacist using a computer software tool before the inclusion of the first patient. Four-week treatment and washout periods were considered sufficient on the basis of prior studies demonstrating that the effects of SGLT2 inhibitors and MRAs are fully present after 4 weeks.8,9 Study medication was dispensed at the beginning of each treatment period. Patients were instructed to take the tablets in the morning. The use and dosage of all concomitant medications had to be stable during follow-up.
Measurements
Patients collected 24-hour urine samples to assess urinary albumin and creatinine concentration at the beginning and end of each treatment period to determine urinary UACR. Urinary sodium and potassium were also measured. Urinalysis was performed at the end of each treatment period to evaluate potential urinary tract infections. Systolic and diastolic BP and body weight were also recorded at these time points. BP measurements were performed with a calibrated automated sphygmomanometer in a seated position after at least 5 minutes of rest. Five BP readings were taken each time; the first two were discarded, and the mean of three readings was recorded. Blood samples were taken in a fasting condition at the beginning and end of each treatment to assess glucose, HbA1c, creatinine, hemoglobin, hematocrit, and N-terminal pro B-type natriuretic peptide (NT-proBNP). eGFR was calculated from the CKD-epi equation using serum creatinine concentration. Treatment adherence was determined by pill count.
End Points
The primary end point of the study was the correlation between the individual 24-hour UACR change from baseline during treatment with dapagliflozin, eplerenone, and their combination. Secondary end points were the percentage change in 24-hour UACR, systolic BP, eGFR, and potassium from baseline during each treatment period. The proportion of patients with a 30% or 50% reduction in 24-hour UACR from baseline was a prespecified exploratory end point. The proportion of patients with serum potassium >5 mmol/L at the 4-week treatment visit was a post hoc exploratory end point. Safety end points were investigator reported adverse events and serious adverse events.
Statistical Analyses
Power of the primary analysis was based on the concordance in 24-hour UACR response to dapagliflozin, eplerenone, and their combination. The null hypothesis assumes no correlation in UACR response between eplerenone, dapagliflozin, and their combination. A Pearson correlation of 0.4 is considered to be clinically relevant. A sample size of 46 patients provides 80% power to detect a Pearson correlation coefficient of 0.4 between the log transformed 24-hour UACR responses to eplerenone, dapagliflozin, and their combination assuming a type 1 error of 5%. A sample size of 46 patients also provided 80% power to detect a 25% difference in 24-hour UACR between combined dapagliflozin-eplerenone treatment compared with either drug alone assuming a standard deviation of 0.7 in log transformed 24-hour UACR and a type 1 error of 2.5% (the nominal α level was divided by two).
Efficacy analyses were conducted in the intention-to-treat population. Pearson correlations were calculated to assess concordance in 24-hour UACR response to dapagliflozin, eplerenone, and dapagliflozin-eplerenone. To assess the effects of dapagliflozin, eplerenone, and their combination on UACR, we used a mixed-effects repeated-measures analysis with an unstructured covariance matrix. The model included sequence, period, treatment, and baseline 24-hour UACR as a covariate and subject as a random effect. UACR was log transformed before entering the data in the repeated-measures model. The between-group geometric mean change in 24-hour UACR was derived by 100×[exp(least squares mean change)–1], and the same transformation was applied to the 95% confidence limits. We used the same repeated-measures model as was used as for the UACR analysis for the change from baseline in other clinical variables, with the exception that baseline 24-hour UACR was replaced with the baseline value of the parameter of interest. We calculated the proportion of patients achieving a 30% or 50% reduction in 24-hour UACR from baseline during dapagliflozin, eplerenone, and dapagliflozin-eplerenone treatment and used logistic regression analysis to compare treatment groups. We performed chi-squared tests to assess differences in proportion of patients with adverse events related to hyperkalemia or urinary tract infection. Statistical analyses were performed with R v4.1 (R Foundation for Statistical Computing, Vienna, Austria).10
Results
Patient Disposition and Baseline Characteristics
Between May 2019 and April 2021, 57 patients were screened for eligibility of whom 48 entered the run-in period. Of these, 46 were randomized into the study. During follow-up, two patients died (one during eplerenone treatment and the other during dapagliflozin-eplerenone treatment), and two other patients withdrew consent (Supplemental Figure 1). Forty-two patients completed all treatment periods. All randomly allocated patients with nonmissing baseline data and at least one assessment during follow-up were included in analysis (full analysis set), resulting in no losses from the intention-to-treat population. The baseline demographics and clinical and biochemical characteristics of the 46 randomized patients are shown in Table 1. The proportion of patients who adhered to study medication was 86.3% (SD=11).
Table 1.
Baseline characteristics
| Characteristic | Value |
|---|---|
| Age, yr | 69.5 (7.6) |
| Sex, n (%) | |
| Women | 11 (23.9) |
| Men | 35 (76.1) |
| Race, n (%) | |
| White | 45 (97.8) |
| Other | 1 (2.2) |
| Type 2 diabetes, n (%) | |
| No | 14 (30.4) |
| Yes | 32 (69.6) |
| HbA1c, mmol/mol | 50.3 (13) |
| Without type 2 diabetes | 39.0 (6) |
| With type 2 diabetes | 55.5 (12) |
| BP | |
| Systolic | 136.3 (9.0) |
| Diastolic | 80 (7.5) |
| BMI, kg/m2 | 30.8 (6.2) |
| eGFR, ml/min per 1.73 m2 | 58.1 (18.6) |
| UAERa, mg/24 hr | 569 (250, 919) |
| UACRa, mg/g | 401 (225, 629) |
| Medication, n (%) | |
| Antihypertensive medication use | |
| ACEi | 17 (37.0) |
| ARB | 29 (63.0) |
| β blocker | 17 (37.0) |
| Calcium channel blockers | 24 (52.2) |
| Diureticsb | 22 (47.8) |
| Thiazide diuretic | 12 (26.1) |
| Loop diuretic | 11 (23.9) |
| Metformin use | 22 (47.8) |
| Insulin use | 11 (23.9) |
| Statin use | 38 (82.6) |
Data are given as mean (SD) unless otherwise indicated. BMI, body mass index.
Geometric mean (25th to 75th percentile).
One patient was using both a thiazide and a loop diuretic.
Effects on Albuminuria
At the start of dapagliflozin, the median UACR was 430 mg/g (interquartile range [IQR] 183, 809). At week 4, the percentage change from baseline in UACR was –19.6% (95% confidence interval [CI], –34.3 to –1.5). At the start of eplerenone, the median UACR was 435 mg/g (IQR 171, 666), which was reduced by –33.7% (95% CI, –46.1 to –18.5) at week 4. At the start of dapagliflozin-eplerenone, the median UACR was 404 mg/g (IQR 176, 715). At week 4, the mean percentage change from baseline in UACR in the dapagliflozin-eplerenone period was –53% (95% CI, –61.7 to –42.4; P<0.001 versus dapagliflozin; P=0.01 versus eplerenone; Figure 1A). Four weeks after the discontinuation of dapagliflozin, eplerenone, or dapagliflozin-eplerenone, UACR had increased relative to levels during the treatment period, and the mean percentage difference between treatment groups did not significantly differ at the end of the 4-week washout period from baseline. The mean percentage change in 24-hour urinary albumin excretion during each treatment was similar compared with UACR (Supplemental Table 1).
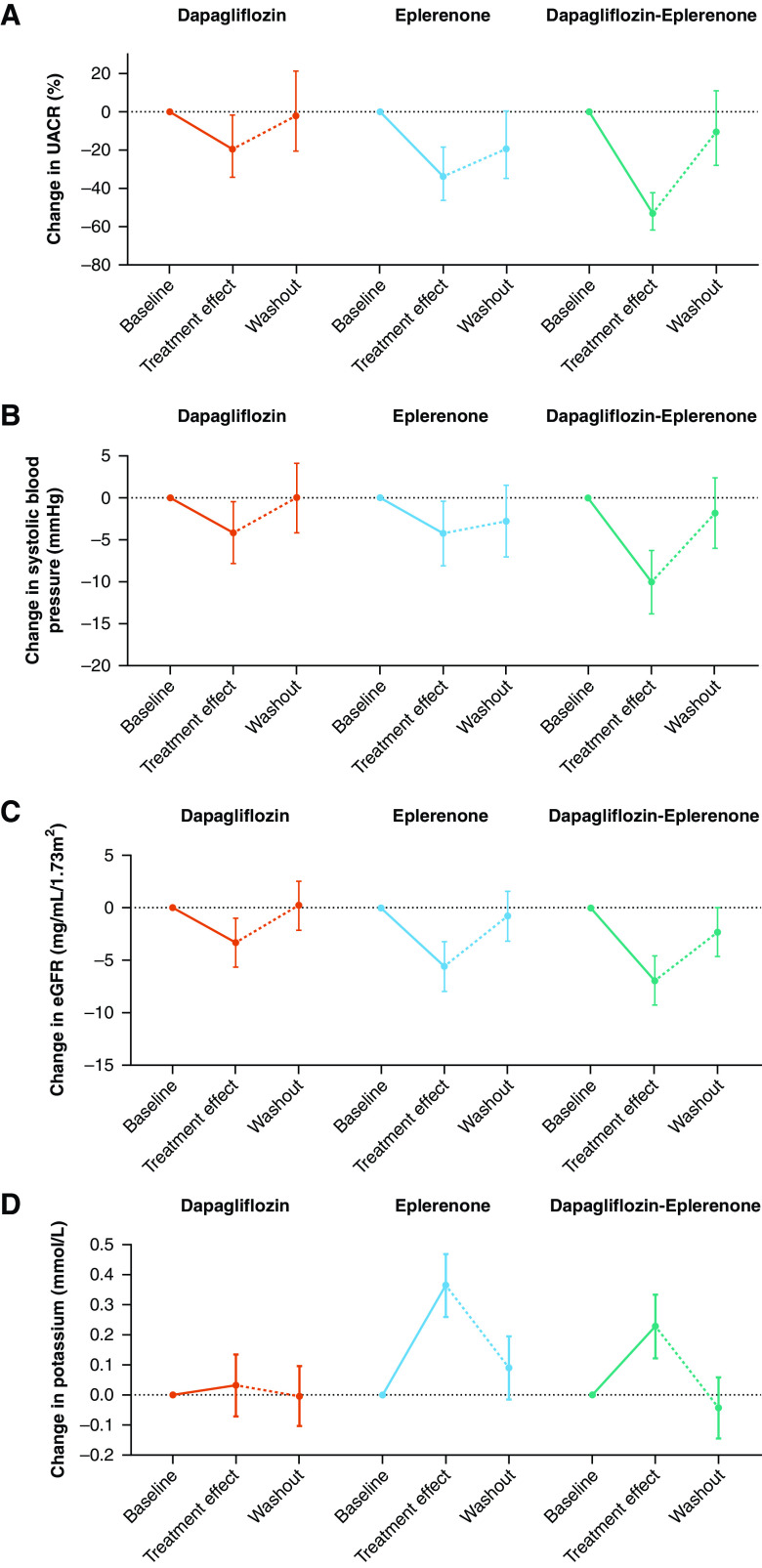
Figure 1.
Changes in UACR, systolic BP, eGFR, and potassium during treatment with dapagliflozin, eplerenone, and dapagliflozin-eplerenone. The figure shows the percent change in UACR from baseline (A) and absolute changes from baseline in systolic BP (B), eGFR (C), and potassium (D). The error bars indicate the 95% confidence interval.
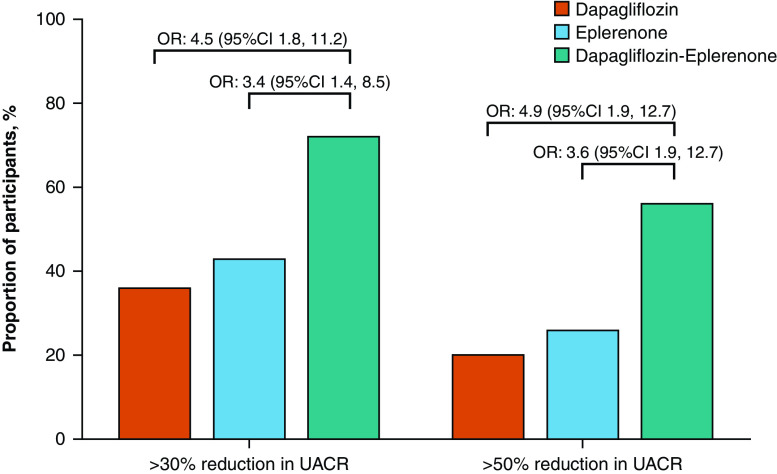
After 4 weeks of dapagliflozin, eplerenone, or dapagliflozin-eplerenone treatment, 16 (36%), 18 (43%), and 31 (72%) patients achieved a ≥30% reduction in UACR. The odds ratio of achieving a ≥30% UACR reduction during dapagliflozin-eplerenone treatment compared with dapagliflozin was 4.5 (95% CI, 1.8 to 11.2) and 3.4 (95% CI, 1.4 to 8.5) compared with eplerenone (Figure 2). There were 9 (20%), 11 (26%), and 24 (56%) patients during dapagliflozin, eplerenone, and dapagliflozin-eplerenone treatment, respectively, who achieved a ≥50% reduction in UACR at week 4. The odds ratio of achieving a ≥50% UACR reduction with dapagliflozin-eplerenone treatment compared with dapagliflozin was 4.9 (95% CI, 1.9 to 12.7) and 3.6 (95% CI, 1.4 to 8.9) compared with eplerenone.
Figure 2.
Proportion of patients and odds ratio for achieving a ≥30% and ≥50% reduction in UACR during treatment with dapagliflozin, eplerenone, and dapagliflozin-eplerenone. The bars show the proportion of patients achieving 30% or 50% UACR reduction from baseline.
The individual UACR change from baseline during dapagliflozin, eplerenone, and dapagliflozin-eplerenone varied markedly between individuals (Figure 3). There was no association between the UACR change during dapagliflozin and eplerenone (r=0.07; P=0.63; Figure 3). Changes in UACR during dapagliflozin or eplerenone were also not correlated with UACR changes during dapagliflozin-eplerenone (r=–0.13; P=0.47; r=–0.08; P=0.66, respectively; Figure 3). Of 20 patients who did not show a UACR reduction during dapagliflozin, UACR was reduced in 15 and 17 patients during eplerenone and eplerenone-dapagliflozin, respectively. Vice versa, of 11 patients who did not show a UACR reduction during eplerenone, UACR was reduced in six and eight patients during dapagliflozin and eplerenone-dapagliflozin, respectively.
Figure 3.
Correlations in percent UACR changes from baseline. (A) Correlation in UACR between dapagliflozin and eplerenone. (B) Correlation between dapagliflozin and dapagliflozin-eplerenone. (C) Correlation between eplerenone and dapagliflozin-eplerenone.
Subgroup analyses showed that the UACR changes during dapagliflozin, eplerenone, and dapagliflozin-eplerenone were generally consistent across subgroups (Figure 4). The only exceptions were subgroups defined by diabetes status and baseline eGFR. In these subgroups, the change in UACR during treatment with dapagliflozin was more pronounced in patients with type 2 diabetes compared with those without type 2 diabetes and in patients with baseline eGFR <60 ml/min per 1.73 m2 compared with those with eGFR ≥60 ml/min per 1.73 m2.
Figure 4.
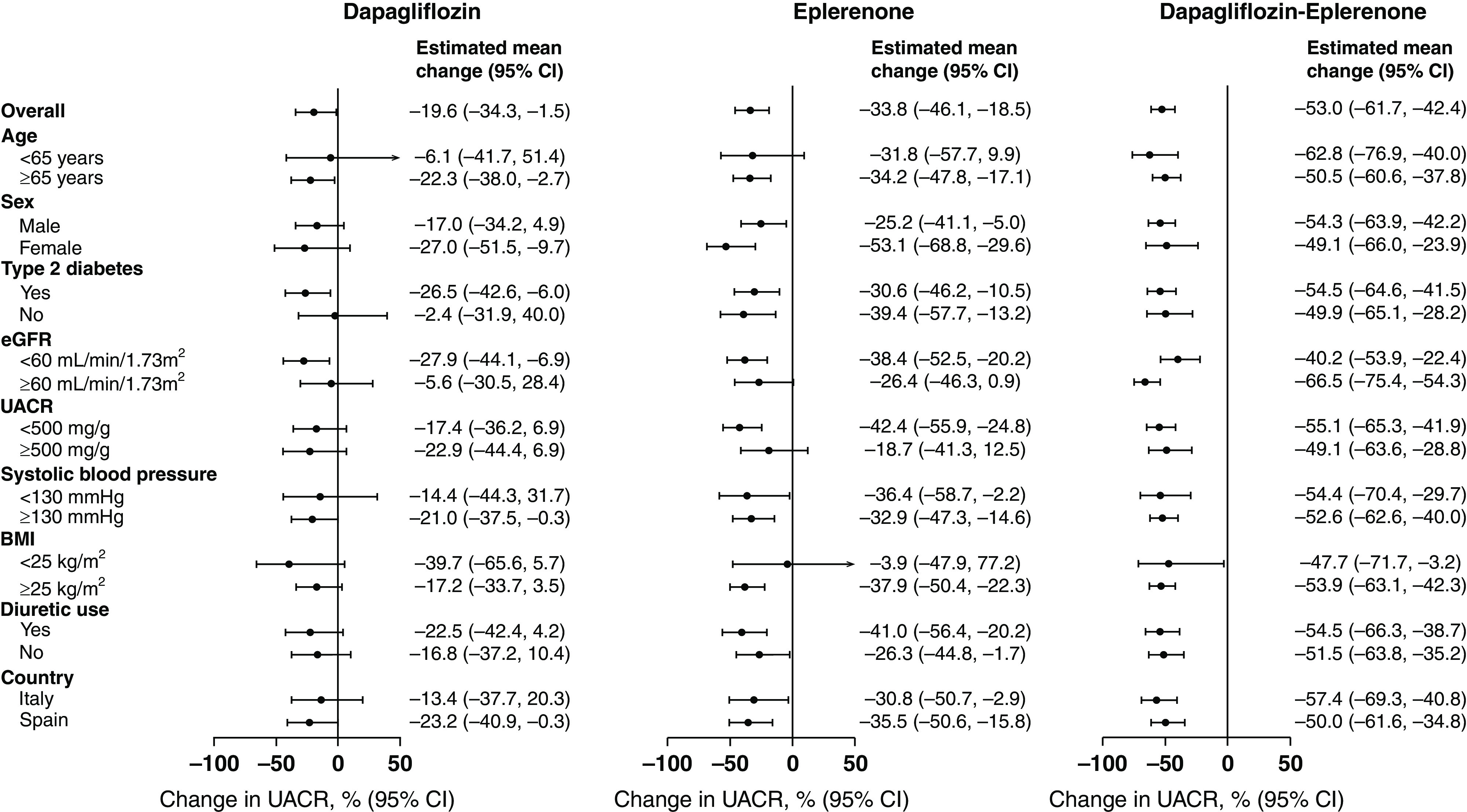
Percent change from baseline in UACR after 4 weeks of treatment with dapagliflozin, eplerenone, and dapagliflozin-eplerenone in patient subgroups defined by baseline characteristics. The effects on UACR are presented in prespecified subgroups. The solid dot represents the estimate of the treatment effect in subgroups; the horizontal line the corresponding 95% confidence interval.
Effects on BP, eGFR, and Potassium
Mean systolic BP was 135 mm Hg (SD=12) at the start of dapagliflozin, 135 mm Hg (SD=14) at the start of eplerenone, and 133 mm Hg (SD=11) at the start of dapagliflozin-eplerenone. The mean change from baseline at week 4 was –4.1 mm Hg (95% CI, –7.9 to –0.4) during dapagliflozin, –4.2 mm Hg (95% CI, –8 to –0.4) during eplerenone, and –10 mm Hg (95% CI, –13.7 to –6.2) during dapagliflozin-eplerenone (P=0.005 versus dapagliflozin; P=0.007 versus eplerenone; Figure 1B). Systolic BP increased 4 weeks after discontinuation of each treatment. At the end of the 4-week washout period, systolic BP did not differ from start of treatment for each treatment group. Post hoc subgroup analyses showed that systolic BP effects were consistent in patient subgroups defined by baseline eGFR, UACR, and systolic BP (Supplemental Figure 2). Another post hoc analysis demonstrated that the UACR-lowering effect observed during each treatment period was maintained after adjusting for concomitant changes in systolic BP or body weight (Supplemental Table 2).
Mean eGFR was 56.8 ml/min per 1.73 m2 (SD=18) at the start of dapagliflozin, 57.2 ml/min per 1.73 m2 (SD=18) at the start of eplerenone, and 58.4 ml/min per 1.73 m2 (SD=19) at the start of dapagliflozin-eplerenone. The mean change from baseline at week 4 was –3.3 ml/min per 1.73 m2 (95% CI, –5.6 to –1) during dapagliflozin, –5.6 ml/min per 1.73 m2 (95% CI, –7.9 to 3.2) during eplerenone, and –6.9 ml/min per 1.73 m2 (95% CI, –9.2 to –4.6) during dapagliflozin-eplerenone (P=0.02 versus dapagliflozin; P=0.4 versus eplerenone; Figure 1C). eGFR increased 4 weeks after dapagliflozin, eplerenone, and dapagliflozin-eplerenone discontinuation by 3.7 ml/min per 1.73 m2 (95% CI, 1.5 to 5.9), 4.9 ml/min per 1.73 m2 (95% CI, 2.6 to 7.2), and 4.5 ml/min per 1.73 m2 (95% CI, 2.3 to 6.8), indicating that the decline in eGFR was directly reversible after discontinuation. Post hoc subgroup analyses showed that eGFR effects were consistent in patient subgroups defined by baseline eGFR, UACR, and systolic BP (Supplemental Figure 3).
Mean serum potassium was 4.4 mmol/L (SD=0.4) at the start of dapagliflozin, 4.4 mmol/L (SD=0.3) at start of eplerenone, and 4.4 mmol/L (SD=0.4) at start of dapagliflozin-eplerenone. The mean change from baseline at week 4 was 0.03 mmol/L (95% CI, –0.07 to 0.13) during dapagliflozin, 0.36 mmol/L (95% CI, 0.26 to 0.47) during eplerenone, and 0.23 mmol/L (95% CI, 0.12 to 0.33) during dapagliflozin-eplerenone (P=0.002 versus dapagliflozin; P=0.03 versus eplerenone; Figure 1D). Potassium decreased 4 weeks after discontinuation of each treatment (Figure 1D). The number of patients with serum potassium concentration >5 mmol/L after 4 weeks of treatment in the dapagliflozin, eplerenone, and dapagliflozin-eplerenone groups were 1 (2.2%), 12 (26.1%), and 5 (10.9%), respectively (P=0.002 across treatment groups).
A post hoc analysis demonstrated that a reduction in eGFR during dapagliflozin treatment was associated with a reduction in potassium (r=0.42; P=0.005). An inverse correlation was observed during eplerenone or eplerenone-dapagliflozin treatment such that a reduction in eGFR correlated with an increase in potassium (r=–0.29; P=0.06; r=–0.39; P=0.01, respectively).
Effects on Metabolic and Extracellular Volume-Related Parameters
Changes in HbA1c, body weight, NT-proBNP, hemoglobin, hematocrit ratio, and fractional excretion of sodium and potassium are shown in Table 2. As expected in this cohort of patients with CKD, there was no reduction in HbA1c during each treatment period. Body weight was reduced by both dapagliflozin and eplerenone, and the combination resulted in a numerically higher body weight reduction than either treatment alone. NT-proBNP was reduced to a similar degree during each treatment period. Hematocrit ratio and hemoglobin increased during dapagliflozin and dapagliflozin-eplerenone treatment but did not change during eplerenone treatment. Fractional sodium excretion increased with dapagliflozin and eplerenone and further increased with dapagliflozin-eplerenone. Dapagliflozin also increased fractional potassium excretion, whereas it did not significantly change during treatment with eplerenone or dapagliflozin-eplerenone.
Table 2.
Mean values and changes from baseline after 4 weeks in exploratory biochemical parameters during treatment with dapagliflozin, eplerenone, or dapagliflozin-eplerenone
| Parameter | Dapagliflozin | Eplerenone | Dapagliflozin/Eplerenone |
|---|---|---|---|
| HbA1c, mmol/mol | |||
| Baseline | 48.9 (1.7) | 49.5 (1.7) | 50 (1.7) |
| Week 4 | 48.2 (1.7) | 50.3 (1.7) | 49.6 (1.7) |
| Change from baseline | −0.7 (0.5) | 0.7 (0.5) | −0.5 (0.5) |
| P value | 0.16 | 0.16 | 0.37 |
| Body weight, kg | |||
| Baseline mean | 85.8 (2.9) | 86 (2.9) | 86.4 (2.9) |
| Week 4 mean | 85 (2.9) | 85.6 (2.9) | 85.3 (2.9) |
| Change from baseline | −0.8 (0.3) | −0.4 (0.3) | −1 (0.3) |
| P value | 0.01 | 0.18 | <0.001 |
| Hemoglobin, g/dl | |||
| Baseline mean | 13.7 (0.3) | 13.9 (0.3) | 13.7 (0.3) |
| Week 4 mean | 14.1 (0.3) | 13.9 (0.3) | 14.1 (0.3) |
| Change from baseline | 0.37 (0.1) | −0.04 (0.1) | 0.36 (0.1) |
| P value | 0.002 | 0.73 | 0.003 |
| Hematocrit, % | |||
| Baseline mean | 42 (0.7) | 42.6 (0.8) | 41.9 (0.8) |
| Week 4 mean | 43.5 (0.7) | 42.3 (0.7) | 42.8 (0.7) |
| Change from baseline | 1.5 (0.3) | −0.2 (0.3) | 0.8 (0.3) |
| P value | <0.001 | 0.52 | 0.02 |
| NT-proBNP, pg/ml | |||
| Baseline mean | 93.9 (14.7) | 72.8 (12.2) | 78.3 (13.2) |
| Week 4 mean | 74.5 (12.4) | 59.1 (9.9) | 62.2 (10.4) |
| Change from baseline | −19.4 (6.6) | −13.7 (5.1) | −16.1 (5.6) |
| P value | 0.004 | 0.008 | 0.004 |
| Fractional Na+ excretion, % | |||
| Baseline mean | 0.75 (0.06) | 0.78 (0.07) | 0.73 (0.07) |
| Week 4 mean | 0.87 (0.06) | 0.93 (0.07) | 0.96 (0.07) |
| Change from baseline | 0.12 (0.05) | 0.17 (0.05) | 0.21 (0.05) |
| P value | 0.03 | 0.003 | <0.001 |
| Fractional K+ excretion, % | |||
| Baseline mean | 8.9 (0.6) | 9.2 (0.6) | 9.4 (0.6) |
| Week 4 mean | 10.5 (0.6) | 9.8 (0.6) | 10 (0.6) |
| Change from baseline | 1.4 (0.5) | 0.7 (0.5) | 0.7 (0.5) |
| P value | 0.006 | 0.20 | 0.16 |
Shown are mean and standard errors except for NT-proBNP, which is reported as geometric mean.
Safety
Adverse events are described in Table 3. Overall, the pattern of adverse events in these patients with CKD was similar compared with what was previously reported in dapagliflozin or eplerenone studies. Hyperkalemia occurred in eight (17.4%) patients during eplerenone treatment compared with 0 (0%) patients during dapagliflozin treatment and 2 (4.3%) during dapagliflozin-eplerenone treatment. Urinalysis findings at week 4 showed urinary tract infection in 10 (21.7%) patients during dapagliflozin compared with 3 (6.5%) patients during eplerenone and 4 (8.7%) during dapagliflozin-eplerenone treatment (Table 3). All but two urinary tract infections were asymptomatic and required treatment in only one participant.
Table 3.
Number of patients with adverse events by treatment periods
| Adverse Event Type | Dapagliflozin | Eplerenone | Dapagliflozin-Eplerenone | Wash-out |
|---|---|---|---|---|
| Any adverse event | 12 | 17 | 13 | 1 |
| Serious adverse event | 0 | 1 (2.2) | 1 (2.2) | 0 |
| Death | 0 | 1 (2.2) | 1 (2.2) | 0 |
| Adverse events | ||||
| Hyperkalemia | 0 | 8 (17.4) | 2 (4.3)* | 0 |
| Hypotension | 0 | 1 (2.2) | 3 (6.5) | 0 |
| Acute renal insufficiency | 0 | 0 | 1 (2.2) | 0 |
| Urinary tract infection | 1 (2.2) | 1 (2.2) | 0 | 0 |
| Asymptomatic bacteriuria | 9 (19.6) | 2 (4.3) | 4 (8.7) | 0 |
| Hypoglycemia | 0 | 0 | 0 | 0 |
| Dyspnea | 1 (2.2) | 0 | 0 | 0 |
| Mild abdominal pain | 1 (2.2) | 0 | 0 | 0 |
| Constipation | 0 | 1 (2.2) | 0 | 1 (2.2) |
| Toothache | 0 | 1 (2.2) | 0 | 0 |
| Renal colic | 0 | 1 (2.2) | 0 | 0 |
| Lipothymia | 0 | 0 | 1 (2.2) | 0 |
Among 32 patients with type 2 diabetes, 7 and 2 hyperkalemia adverse events were recorded during eplerenone and dapagliflozin-eplerenone treatment, respectively. Urinary tract infections were reported in one patient with type 2 diabetes during treatment with dapagliflozin and in one patient without diabetes during treatment with eplerenone.
P value across treatment groups, 0.00328.
Discussion
In this prospective randomized crossover trial in patients with CKD and increased albuminuria, the SGLT2 inhibitor dapagliflozin and the MRA eplerenone both reduced albuminuria. The combination of dapagliflozin-eplerenone produces a robust and clinically meaningful reduction in albuminuria, suggesting that the effects of both agents on albuminuria were independent and additive. We observed a large variation in albuminuria responses during dapagliflozin, eplerenone, and their combination. Changes in albuminuria did not correlate during each treatment period, suggesting that patients who failed to respond to one of these treatments may benefit from the other. The potassium increase and frequency of hyperkalemia adverse events were higher during treatment with eplerenone compared with dapagliflozin-eplerenone. These data support a large outcome trial to confirm long-term safety and efficacy in reducing clinical outcomes with combined SGLT2 inhibitor and MRA treatment.
That SGLT2 inhibitors and MRAs have complementary biologic mechanisms of actions that could lead to large reductions in albuminuria and clinically meaningful reductions in kidney outcomes has been suggested on the basis of experimental studies and secondary analyses from large clinical outcome trials. A preclinical rat study in a model of cardiorenal disease demonstrated that combination therapy with empagliflozin and finerenone resulted in a synergistic anti-albuminuric effect and improved survival compared with either therapy alone.11 A post hoc analysis from the FIDELIO-DKD trial demonstrated that finerenone compared with placebo in patients with diabetic kidney disease reduced UACR in those treated with SGLT2 inhibitors at baseline (25% reduction; 95% CI, 10 to 38) and those not treated (31% UACR reduction; 95% CI, 29 to 34).6 Conversely, in the DAPA-CKD trial, 229 (5.3%) patients with CKD were treated with conventional MRAs (spironolactone or eplerenone). The benefits of dapagliflozin on kidney and cardiovascular outcomes were consistent in patients treated or not treated with MRAs at baseline.7 However, in the FIDELIO and DAPA-CKD analyses patients were already treated with SGLT2 inhibitors or MRAs before enrollment into the trial. As a result, the studies could not directly determine whether initiation of SGLT2 inhibition in combination with MRA treatment results in additive effects. Our data therefore extend the previous studies and show that initiation of combined treatment with a SGLT2 inhibitor and MRA results in a robust additive albuminuria-lowering effect.
A large variability in albuminuria changes was observed after 4 weeks of treatment with dapagliflozin, eplerenone, and dapagliflozin-eplerenone. The individual albuminuria changes were reversible 4 weeks after treatment discontinuation and inversely correlated with the albuminuria reduction during treatment, suggesting that the between-patient variability in albuminuria change is a true pharmacologic response variation rather than reflecting random day-to-day variability. The albuminuria responses to dapagliflozin, eplerenone, or dapagliflozin-eplerenone did not correlate. This finding supports the notion that the mechanisms of albuminuria-lowering effects of both drug classes are likely independent and suggests that treatment resistance to dapagliflozin or eplerenone may be overcome by rotating to the other agent before considering combination treatment. Understanding which underlying biologic mechanisms regulate individual treatment response is an area of great interest. Future studies may provide more insight into which molecular pathways and biomarkers predict individual drug responses in order to tailor therapy.
There may also be safety advantages of combined SGLT2 inhibitor and MRA treatment. Agents that inhibit the renin-angiotensin-aldosterone system cause hyperkalemia, in particular in patients with type 2 diabetes and CKD. Indeed, eplerenone increased potassium by 0.36 mmol/L and hyperkalemia-related adverse events were reported during eplerenone treatment in 17.4% of patients in our study. A meta-analysis of all cardiovascular or kidney outcome trials with SGLT2 inhibitors demonstrated that these agents modestly reduce potassium levels and decrease the risk of hyperkalemia compared with placebo treatment.12 In addition, a post hoc analysis from the FIDELIO-DKD trial showed that hyperkalemia-related adverse events occurred less frequently in patients who were using finerenone in combination with SGLT2 inhibitors compared with those who did not use SGLT2 inhibitors.13 In our study, combining dapagliflozin with eplerenone resulted in less potassium increase and led to significantly fewer hyperkalemia-related adverse events. This effect makes the combination of SGLT2i with MRAs an attractive treatment option from a safety perspective, although additional long-term safety data are required.
The effects of dapagliflozin and eplerenone on kidney function were additive, leading to a relatively large acute decline in eGFR. This effect may cause safety concerns and can lead to early treatment discontinuation and clinical inertia. However, it is important to emphasize that the acute decline in eGFR was completely reversible 4 weeks after treatment discontinuation. In addition, the acute decline in eGFR correlated with the degree of eGFR increase during washout, highlighting that patients with a large acute decline experienced a more pronounced increase after treatment discontinuation. This response pattern suggests these agents reduce glomerular hyperfiltration, which has been associated with long-term kidney function stabilization during treatment with SGLT2 inhibitors or MRAs alone.14,15 Thus, the reversible decline in eGFR soon after dapagliflozin-eplerenone treatment likely reflects the protective mechanism of action and should not necessarily lead to increased safety concerns.
The directional inverse correlations between eGFR and potassium changes during dapagliflozin and eplerenone treatment are of interest and underpin the different mechanism of action of the two drug classes. SGLT2 inhibition increases sodium chloride delivery to the distal tubule, which restores tubuloglomerular feedback and causes an acute decline in eGFR. Increased distal sodium delivery increases the electronegative charge in the tubular lumen that drives potassium secretion via principal cells in the cortical collecting duct.16,17 Increased distal sodium delivery during SGLT2 inhibition may thus result in a more pronounced restoration of TGF and decline in eGFR and potassium secretion. On the other hand, a larger eGFR decline during eplerenone likely reflects more pronounced renin-angiotensin-aldosterone system inhibition, resulting in an increase in potassium.
In addition to additive effects on kidney parameters, we also observed additive effects of both agents on BP. BP lowering per se can reduce albuminuria, and it could be possible that the reduction in albuminuria during dapagliflozin-eplerenone treatment is a result of the potent BP–lowering effect of the combination. However, adjusting the treatment effect on albuminuria for concomitant changes in BP or body weight did not alter our main findings, suggesting that the albuminuria-lowering effect of dapagliflozin-eplerenone is independent of its effect on BP.
Dapagliflozin and eplerenone both exert natriuretic/diuretic effects as indicated by the increase in fractional sodium excretion and reduction in body weight. These natriuretic/diuretic effects were more pronounced when the two drugs were combined and, together with the observed reduction in NT-proBNP, support the combination of SGLT2 inhibitors with MRAs for the prevention and treatment of heart failure. Hematocrit increased with dapagliflozin and dapagliflozin-eplerenone but not with eplerenone. An increase in hematocrit may reflect contraction of plasma volume but may also reflect direct effects of SGLT2 inhibition on hematopoiesis as shown in other studies.18
This study has limitations. First, the study used a surrogate outcome measure as primary outcome, and additional studies are required to confirm the long-term safety and impact of dapagliflozin-eplerenone on clinical outcomes more definitely. Second, the study follow-up period was only 4 weeks. Whether reductions in albuminuria persist over longer time periods could not be investigated but is likely because monotherapy with dapagliflozin or eplerenone causes a durable reduction in UACR.19,20 Third, we note that the assumptions of the crossover design include no carryover effects and correlations among treatment responses. Fourth, we did not include a placebo treatment period. We were therefore only able to compare albuminuria responses during active treatment. However, we believe the reductions in albuminuria are real because there was no change in UACR (geometric mean change 0%; IQR –18.1%, 22.1%) between the two visits at the end of the run-in period when all patients were using stable ACEi or ARB treatment. In addition, the observed albuminuria reductions with dapagliflozin or eplerenone alone are consistent with results from placebo controlled clinical trials.8,21 Finally, the study had an open-label design, which may have resulted in bias in selectively reporting adverse events in particular hyperkalemia and urinary tract infections. However, the lower frequency of hyperkalemia-reported adverse events with dapagliflozin-eplerenone compared with eplerenone was in agreement with the less pronounced potassium increase.
In conclusion, the SGLT2 inhibitor dapagliflozin and MRA eplerenone reduce albuminuria, with variability among individual patients supporting a schedule to rotate these therapies to find the optimal treatment for each individual. Combining dapagliflozin with eplerenone as adjunct to ACEis or ARBs resulted in a robust UACR-lowering effect, whereas potassium levels and incidence of hyperkalemia with dapagliflozin-eplerenone were significantly lower compared with eplerenone alone. These data suggest that dapagliflozin in combination with eplerenone is an attractive option to slow the progression of kidney disease in patients with CKD, which requires confirmation in a large prospective clinical trial.
Disclosures
L. De Nicola received fees for lectures and scientific consultations from Amgen, Astellas, AstraZeneca, MundiPharma, NovoNordisk, and Vifor Fresenius; honoraria from Amgen, Astellas, AstraZeneca, and Vifor; and has an advisory or leadership role for Astellas, AstraZeneca, the Italian Journal of Nephrology, and Vifor Fresenius. J.L. Gorriz received fees for lectures and scientific consultations from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Mundipharma, Novartis, NovoNordisk, and Traveere Pharmaceuticals; research funding from AstraZeneca; and has an advisory or leadership role for AstraZeneca, Bayer, Boehringer-Ingelheim, and NovoNordisk. H.J.L. Heerspink is a consultant for AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Eli Lilly, Gilead, GoldFinch, Janssen, Merck, NovoNordisk, and Traveere Pharmaceuticals, and has a policy that all honoraria are paid to his employer; received research funding from AstraZeneca, Janssen (grant funding directed to employer), NovoNordisk, is supported by a VIDI (917.15.306) grant from the Netherlands Organisation for Scientific Research; and participated in a speakers’ bureau for AstraZeneca. M. Provenzano received fees for lectures and support for attending scientific congress from AstraZeneca, Menarini, and NovoNordisk. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We thank all patients and research staff for their participation and assistance in this study. Writing support to create figures included in this publication was provided by Springer and sponsored by AstraZeneca. H.J.L. Heerspink is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: ROTATE-3 study group members, Luigi Pennino, Ilaria De Gregorio, Lucio Polese, Alfredo Caturano, Roberto Minutolo, Ferdinando Sasso, Ida Gagliardi, Mariateresa Zicarelli, Giuseppina Crugliano, Elena Gimenez Civera, Nayara Panizo, Taha Sen, and Di Xie
Author Contributions
M. Andreucci, L. De Nicola, L. D’Marco, C. Garofalo, J.L. Gorriz, H.J.L. Heerspink, and M.J. Puchades reviewed and edited the manuscript; M. Andreucci, L. De Nicola, J.L. Gorriz, and H.J.L. Heerspink were responsible for supervision; M. Andreucci, L. D’Marco, C. Garofalo, J.L. Gorriz, H.J.L. Heerspink, M. Provenzano, and M.J. Puchades were responsible for the investigation; L. De Nicola, C. Garofalo, M.J. Provenzano, and M.J. Puchades were responsible for the methodology; L. De Nicola, J.L. Gorriz, H.J.L. Heerspink, and M. Provenzano were responsible for conceptualization; H.J.L. Heerspink and N. Jongs were responsible for the formal analysis; H.J.L. Heerspink and M. Provenzano wrote the original draft of the manuscript; N. Jongs was responsible for resources; M. Provenzano was responsible for data curation; and M. Provenzano and M.J. Puchades were responsible for project administration.
Data Sharing Statement
Data cannot be shared. EU privacy regulations and informed consent do not permit sharing of individual patient data.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2022020207/-/DCSupplemental.
Supplemental Appendix. Full list of ROTATE-3 investigators.
Supplemental Table 1. Percent change from baseline in 24-hour urinary albumin excretion at week 4.
Supplemental Table 2. UACR change after adjustment for concomitant BP and body weight changes.
Supplemental Figure 1. Trial profile.
Supplemental Figure 2. Changes in systolic BP in patient subgroups during treatment with dapagliflozin, eplerenone, and dapagliflozin-eplerenone.
Supplemental Figure 3. Changes in eGFR in patient subgroups during treatment with dapagliflozin, eplerenone, and dapagliflozin-eplerenone.
References
- 1.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. ; CREDENCE Trial Investigators : Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. ; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. ; FIDELIO-DKD Investigators : Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383: 2219–2229, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. ; FIGARO-DKD Investigators : Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 385: 2252–2263, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Oshima M, Neuen BL, Li J, Perkovic V, Charytan DM, de Zeeuw D, et al. : Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: A post hoc analysis from the CREDENCE Trial. J Am Soc Nephrol 31: 2925–2936, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossing P, Filippatos G, Agarwal R, Anker SD, Pitt B, Ruilope LM, et al. ; FIDELIO-DKD Investigators : Finerenone in predominantly advanced CKD and type 2 diabetes with or without sodium-glucose cotransporter-2 inhibitor therapy. Kidney Int Rep 7: 36–45, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provenzano M, Jongs N, Vart P, Stefánsson BV, Chertow GM, Langkilde AM, et al. : The kidney protective effects of the sodium–glucose cotransporter-2 inhibitor, dapagliflozin, are present in patients with chronic kidney disease treated with mineralocorticoid receptor antagonists. Kidney Int Rep 7: 436–443, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollock C, Stefánsson B, Reyner D, Rossing P, Sjöström CD, Wheeler DC, et al. : Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 7: 429–441, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD: Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol 20: 2641–2650, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.R Core Team : R: A language and environment for statistical computing, 2017. Available at: https://www.R-project.org. Accessed March 31, 2022
- 11.Kolkhof P, Hartmann E, Freyberger A, Pavkovic M, Mathar I, Sandner P, et al. : Effects of finerenone combined with empagliflozin in a model of hypertension-induced end-organ damage. Am J Nephrol 52: 642–652, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neuen BL, Oshima M, Agarwal R, Arnott C, Cherney DZ, Edwards R, et al. : Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: A meta-analysis of individual participant data from randomized controlled trials [published online ahead of print April 8, 2022]. Circulation 10.1161/CIRCULATIONAHA.121.057736 [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Joseph A, Anker SD, Filippatos G, Rossing P, Ruilope LM, et al. ; FIDELIO-DKD Investigators : Hyperkalemia risk with finerenone: Results from the FIDELIO-DKD Trial. J Am Soc Nephrol 33: 225–237, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. : An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, et al. : Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int 99: 999–1009, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Palmer BF: Regulation of potassium homeostasis. Clin J Am Soc Nephrol 10: 1050–1060, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karet FE: Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol 20: 251–254, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J: Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15: 853–862, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jongs N, Greene T, Chertow GM, McMurray JJV, Langkilde AM, Correa-Rotter R, et al. ; DAPA-CKD Trial Committees and Investigators : Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 9: 755–766, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T; EVALUATE Study Group : Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: A double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2: 944–953, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, et al. : Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 1: 940–951, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.