Abstract
Background
Cellulite is the dimpled appearance of skin that commonly occurs on the buttocks and thighs of postpubertal women. Cellulite can be emotionally distressing, causing many individuals to seek medical attention. A previous first-in-human study established the safety and feasibility of a novel minimally invasive method for selectively identifying and manually releasing the specific septa responsible for causing cellulite depressions in a precise manner.
Objectives
The objective of this open-label, multicenter study was to evaluate the safety, efficacy, and feasibility of this method for reducing the appearance of moderate and severe cellulite in adult women.
Methods
Female patients (n = 20) 21 to 55 years old with a BMI ≤ 35 kg/m2 with moderate or severe cellulite on the buttocks and/or thighs were enrolled at 4 clinics. Patients were evaluated on posttreatment Days 7, 30, 120, and 180. The primary endpoint was a mean ≥1-point reduction in the Cellulite Severity Scale at 180 days.
Results
Most patients (n = 18, 95%) achieved the primary endpoint. All patients (n = 19, 100%) were rated as much improved or very much improved in Cellulite Severity Scale scores. Adverse events and investigator findings were mild and transient, and none were unexpected.
Conclusions
A novel method for selectively identifying and manually releasing the specific septa responsible for causing cellulite depressions in a precise manner is a safe and effective means for improving the appearance of moderate and severe cellulite in adult women with a wide variety of skin types.
Level of Evidence: 4

Cellulite is the dimpled skin appearance that commonly occurs on the buttocks and thighs of 80% to 90% of postpubertal women.1 Magnetic resonance imaging has demonstrated that cellulite depressions are associated with underlying thickened fibrous septa that tether the dermis to the underlying superficial fascia, resulting in the characteristic dimpled appearance of the skin.2,3 Increased tension on these septa from sitting, squeezing, or muscle contraction worsens their clinical appearance.4 Risk factors for cellulite include genetic predisposition, female gender, ethnicity, advancing age, obesity and weight change, and sedentary lifestyle.5,6
The unattractive appearance of cellulite can have a significant negative impact on affected individuals, adversely affecting self-esteem and social activity and causing many to seek medical attention.7,8 Common treatments for moderate or severe cellulite are surgical and involve severing the fibrous septa, referred to as subcision. One method of subcision is performed by inserting a hypodermic needle through the skin into the superficial layer between the dermis and superficial fascia to cut the fibrous septa below the dimples seen on the skin surface.9 Another method of subcision employs a tissue-stabilized, guided device comprised of a motorized reciprocating cutting blade inserted through the skin that is moved through the superficial layer between the dermis and superficial fascia to cut the fibrous septa.10 A third method is laser-based, where a cannula is inserted through the skin into the superficial layer between the dermis and superficial fascia. A side-firing laser is inserted through the cannula, which cuts the fibrous septa and liquefies local fat.11 These are procedures that often require numerous incisions with inaccurate targeting of the thickened fibrous septa and can result in bruising, discomfort, ecchymosis, and edema.4
An innovation for treating cellulite is a controlled focal fibrous septa release method designed for in-office use (Revelle Aesthetics, Inc.; Mountain View, CA). The location of cellulite depressions is marked while the patient is in a relaxed, standing position. With the patient lying in the prone position, the device is inserted through the skin and advanced to a marked depression location. A light indicates the position of the end of the device beneath the skin. The device is opened and retracted proximally to engage the target septa. If pulling the septa recreates the appearance of a depression in the target area, the device is used to sever the septa. If pulling the septa does not recreate the depression, the device is repositioned and another attempt is made to recreate the depression. The device can be advanced to numerous locations from a single insertion site. Multiple depressions can be treated through a single-entry point, minimizing the risk for scarring and other adverse events (AEs). The device preserves the connective tissue not responsible for cellulite depressions by allowing the physician to test and release septa.
The objective of this study is to further evaluate the safety, efficacy, and feasibility of the novel controlled focal fibrous septa release method for improving the appearance of moderate to severe cellulite in adult women at multiple centers in the United States following a single-center, first-in-woman safety study in Australia that demonstrated the safety and feasibility of the device (unpublished data, Revelle Aesthetics, Inc., Mountain View, CA).
METHODS
Study Patients
This study was initiated on December 3, 2019, and the last procedure was performed on February 28, 2020. Eligible patients who were female (n = 20), 21 to 55 years old, and seeking treatment of moderate or severe cellulite were enrolled. A BMI up to 35 kg/m2 was allowed; however, no more than 25% of patients could have a BMI between 30 and 35 kg/m2. Eighteen patients underwent treatment on the buttocks and thighs, and the remaining 2 patients underwent treatment on the thighs only. Moderate cellulite was defined as depressions that spontaneously appear on the thighs or buttocks when standing but not when lying down. Severe cellulite was defined as depressions that spontaneously appear on the thighs or buttocks when standing and remain present when lying down (Table 1). Patients of childbearing potential provided a negative urine pregnancy test prior to treatment, and all patients agreed to avoid other cellulite treatments for the duration of the trial.
Table 1.
Cellulite Severity Assessment
| None: There is no alteration of skin surface. |
| Mild: The skin of the affected area is smooth while the patient is standing or lying, but the alterations to the skin’s surface can be seen by pinching the skin or with muscle contraction. |
| Moderate: The orange skin or mattress appearance is evident when standing without the utilization of any manipulation (skin pinching or muscle contraction). |
| Severe: The alterations described in grade I or grade II are present together with raised areas and nodules. |
Criteria for exclusion from the study included any cellulite procedure performed on the planned treatment area during the previous 12 months; any prior liposuction on the planned treatment area at any time; >10% change in body weight during the previous 6 months or any weight loss >60 kg; evidence of an active infection or fever >38°C; current or recent smoker (within 6 months); history of hypertension, diabetes, hypoglycemia, coagulopathy, pneumopathy, or severe anemia; history of atrophic or hypertrophic scarring or keloids; utilization of nonsteroidal anti-inflammatory drugs, vitamin E, herbal teas, or dietary supplements during the past 14 days; pregnancy or lactation; or any other condition that might place the patient at risk or jeopardize the objectives of the study.
Ethics
The protocol employed in this study and related materials were approved by an IRB (WCG IRB; Puyallup, WA). Each patient provided written informed consent prior to participating in any study-related activities and were advised they may voluntarily withdraw from the study at any time. A non-significant risk evaluation was presented to and accepted by WCG IRB; therefore, the study was conducted in accordance with the abbreviated requirements listed in 21 CFR 812.2(b) for non-significant risk devices. The trial was carried out in accordance with the International Conference on Harmonization Good Clinical Practice and the United States Code of Federal Regulations (CFR) applicable to clinical studies (45 CFR Part 46, 21 CFR Parts 11, 50, 54, 56).12
Investigational Procedures
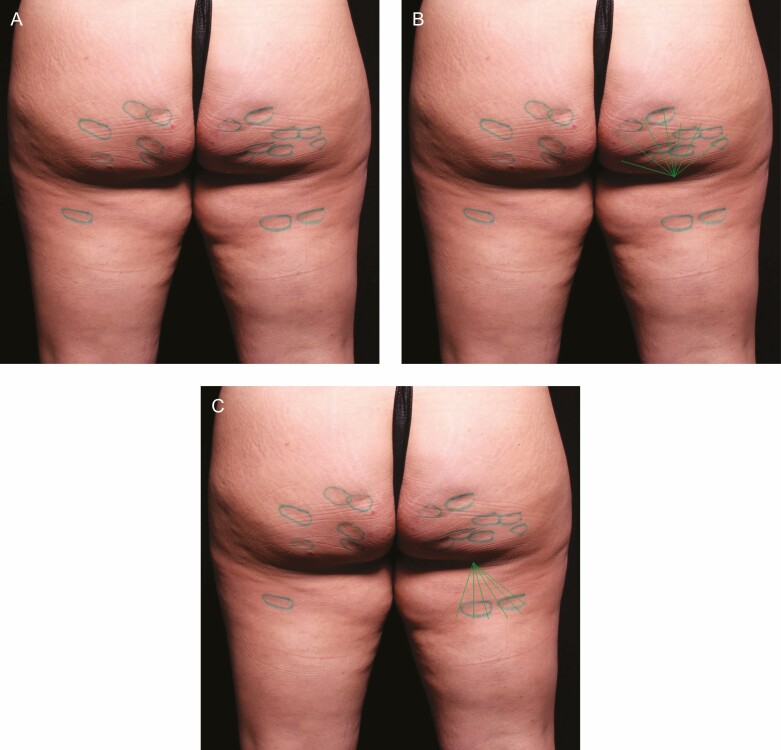
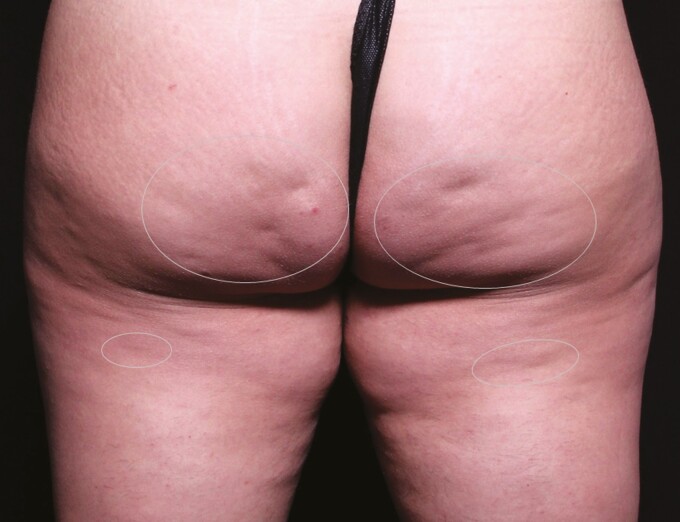
With patients in a relaxed standing position, a surgical marker was used to mark the planned treatment sites on the thighs and buttocks. For this study, patients were treated bilaterally with the baseline photo serving as a control. With treatment targets marked, procedural planning could be performed. In the case of more discrete depressions on the buttocks, a single access could be made from an entry on the gluteal crease. Longer linear depressions, typically located on the thigh, could also be accessible from the same gluteal crease entry, or it may have been desirable to approach from an additional entry on the thigh or hip. The number of depressions and the choice of access sites was left to the discretion of the investigator. The treatment areas were prepared utilizing a normal sterile technique. Examples of marked target areas for cellulite reduction and treatment planning are shown in Figure 1.
Figure 1.
With patients in a relaxed standing position, the planned treatment sites on the thighs and buttocks were marked. Photographed here is a 30-year-old female patient. (A) For discrete depressions on the buttocks, a single access could be made from an entry on the gluteal crease. (B) Longer linear depressions on the thigh could also be accessible from the same gluteal crease entry, or (C) it may have been desirable to approach from an additional entry on the thigh or hip.
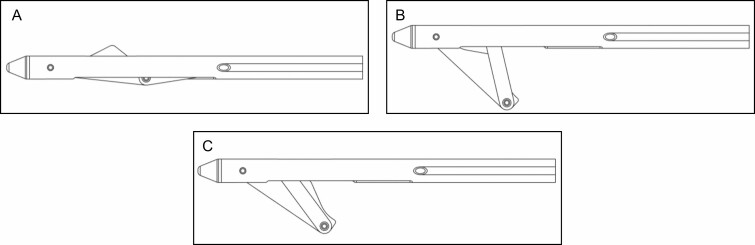
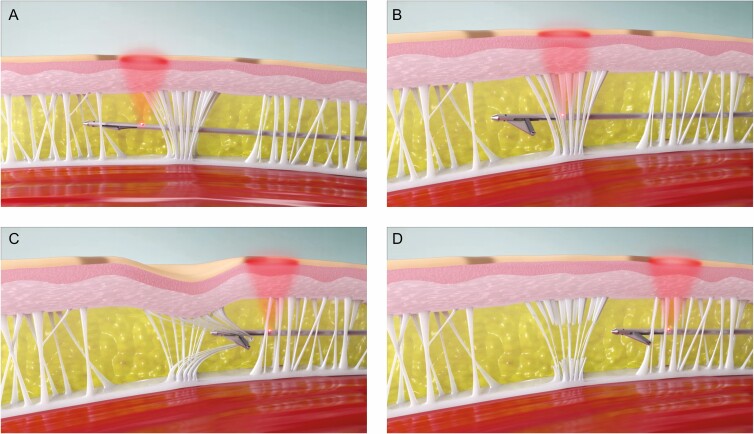
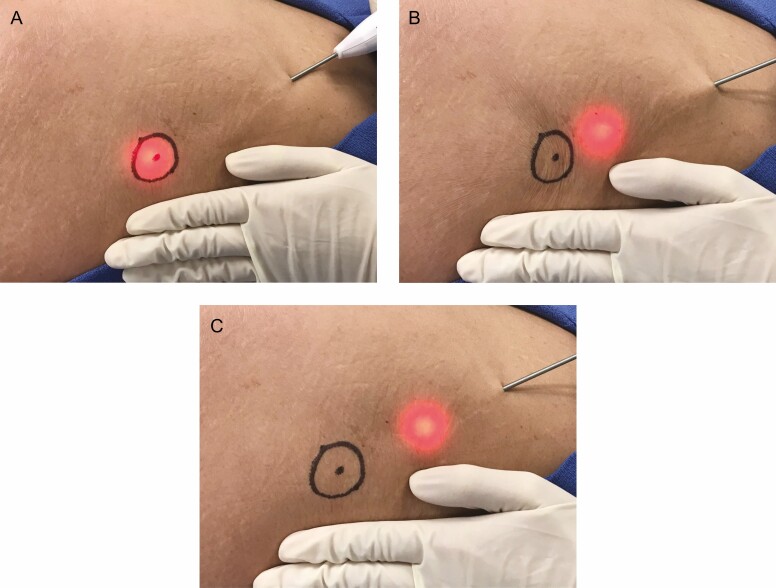
With patients lying prone on the procedure table, local anesthesia (minimum 0.06%, maximum 0.32% lidocaine) was introduced into the planned treatment areas employing a standard needle injection such as a wet technique. The design of the distal end of the device is shown in Figure 2. The device was introduced through the skin and advanced through the superficial sub-dermal plane to the site of a previously marked cellulite depression. With the light illuminated, the location of the distal end of the device could be observed through the skin. The deployable links are stored coaxially in the device shaft for advancement and withdrawal from the target treatment area. An actuator on the handle is utilized to deploy the hook under the target area. The blunt link is employed to pull on the septa to recreate a depression on the skin. When the depression is recreated, the investigator confirms the location of the septa responsible for creating the cellulite depression. The sharpened link is then exposed and utilized to cut the septa. If needed, the steps are repeated until the depression can no longer be recreated on the skin surface. If pulling the hooked septa does not recreate the previously marked depression, the hook is retracted into the shaft of the device and repositioned. The procedure is repeated until all the depression locations are treated. The stepwise procedure is shown in Figures 3 and 4. The approximately 3-mm entry site does not require suturing after the procedure. The entry site was closed with a steri-strip and covered with an adhesive dressing following the procedure, and patients were instructed to keep the area clean and dry.
Figure 2.
The device in the (A) closed position, (B) open position, and (C) cut position.
Figure 3.
(A) The light enables correct positioning of the distal end of the device. (B) After advancing to the target septa, (C) pulling with the deployed blunt hook will confirm its correct location. (D) The septa can then be cut by deploying and pulling with the sharpened link.
Figure 4.
(A) The device is advanced in the closed position to the previously marked cellulite depression location. (B) Pulling on the fibrous septa in the open position recreates the appearance of the cellulite depression. (C) The captured fibrous septa is cut by pulling with the device in the cut position.
Posttreatment Follow-Up
Patients were contacted by phone on posttreatment Day 1 for a pain assessment and were queried about possible AEs. Office assessments occurred on posttreatment Days 7, 30, 120 (originally planned for day 90 but modified due to COVID-19 clinic access restrictions), and 180. During each visit, safety assessments included a physical examination for hematomas, ecchymosis, hemosiderosis, seromas, areas of firmness or softness, erythema, wound drainage, scarring, dyspigmentation, tissue atrophy, contour irregularities, and other AEs. Standardized digital images were obtained, and patients were asked by the research staff to rate their level of overall outcome employing a 6-point scale ranging from very satisfied to very unsatisfied.
Efficacy Assessment and Study Endpoints
The efficacy assessment was the reduction in cellulite severity determined by digital images evaluated by an independent blinded physician assessor. Employing a random generator function (Excel, Microsoft Corporation, Redmond, WA), the blinded assessor was provided with baseline and Day 120 and 180 images, which were graded employing the first 2 components of the Cellulite Severity Scale: CSS-A (number of evident depressions) and CSS-B (mean depth of the evident depressions) (Tables 1, 2). When assigning the CSS, the assessor was blind to the image time point and therefore did not know whether the image was prior to or postprocedure. Once the CSS evaluation was completed, randomized image pairs (before and after for the same patient) were then presented, and the assessor was asked to identify the follow-up image. Finally, the image pairs were presented again with the baseline and follow-up known for the unblinded Global Aesthetic Improvement Scale (GAIS) assessment. The scheme for performing blinded evaluations is shown in Figure 5.
Table 2.
Cellulite Severity Scoringa
| A: Evident depressions, No. | |
| 0 | None |
| 1 | Mild (<4 depressions) |
| 2 | Moderate (>5 to < 9 depressions) |
| 3 | Severe (>10 depressions) |
| B: Mean depression depth | |
| 0 | None |
| 1 | Mild (1-2 mm) |
| 2 | Moderate (3-4 mm) |
| 3 | Severe (>5 mm) |
aThe 2 elements of morphology and laxity were excluded. Change in Cellulite Severity Scale (CSS) = (CSS-A + CSS-B) – 1. Mean baseline mCSS [(CSS-A + CSS-B) – 1] - Day 180 mCSS [(CSS-A + CSS-B) – 1] = > 1.0.
Figure 5.
This figure depicts the same 30-year-old female patient from Figure 1 and illustrates the marking scheme employed with outlines around a group of treated depressions for the blinded reviewer evaluations.
The primary efficacy endpoint was the change in the baseline CSS scores at Day 180, where CSS = (CSS-A + CSS-B) –1 (Table 2).13 The primary endpoint was considered reached with a mean ≥1-point reduction in the CSS at Day 180. Additional study analyses included responses to a patient satisfaction survey and GAIS (Table 3).14
Table 3.
Global Aesthetic Improvement Scale Scores
| Very much improved | Excellent corrective result |
|---|---|
| Much improved | Marked improvement of appearance but not completely optimal |
| Improved | Improvement of appearance, better compared with initial condition, but “touch-up” is advised |
| No change | Appearance substantially remains the same compared with original condition |
| Worse | Appearance has worsened compared with original condition |
Safety Endpoint
The primary safety endpoint for this study was the absence of device-related serious AEs at Day 30. Patients reported pain/discomfort on an 11-point scale (0-10; 0 = no pain) at each visit.
Statistical Analysis
The study was not powered to make statistically valid comparisons. Patients served as their own control with qualitative comparisons with preprocedure baseline images. Sufficient data were captured to enable accurate analysis of outcomes, including independent blinded physician assessments.
RESULTS
Demographics and baseline characteristics of enrolled patients are summarized in Table 4. Despite restrictions imposed by the COVID-19 pandemic, the retention rate was high. Follow-up visits were completed on Day 7 (n = 20, 100%), Day 30 (n = 20, 100%), Day 120 (n = 20, 100%), and Day 180 (n = 20, 100%); however, some patient images could not be obtained on Day 120 (n = 3) and Day 180 (n = 1).
Table 4.
Demographics and Baseline Characteristics
| n = 20 | |
|---|---|
| Mean age (SD), range, y | 39.5 (10.4), 22-55 |
| Mean (SD) BMI, range, kg/m2 | 25.6 (3.5), 18.6-32.4 |
| Fitzpatrick skin types, no. (%) | |
| II | 8 (40) |
| III | 9 (45) |
| IV | 2 (10) |
| V | 1 (5) |
| Baseline Cellulite Severity Assessment Scoresa, no. (%) | |
| I | 1 (5) |
| II | 16 (80) |
| III | 3 (15) |
| Mean Baseline Cellulite Severity Assessment Score (SD) | 2.1 (0.45) |
SD, standard deviation.
aReference Table 1 for Cellulite Severity Assessment Scoring categories.
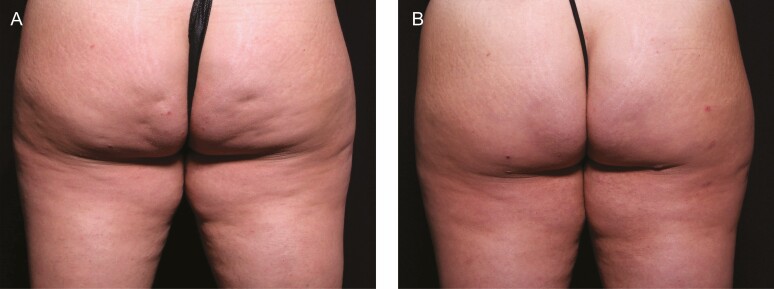
Among the 19 enrolled patients who completed a photography visit at Day 180, as shown in Table 5, 18 (95%) patients achieved a ≥1-point improvement in cellulite severity and 11 patients (58%) achieved a ≥3-point improvement. All patients (n = 19, 100%) were rated as much improved or very much improved on the GAIS scale at Day 180 (Table 6). Patients rated their posttreatment satisfaction, and overall, 17/20 (85%) patients were at least somewhat satisfied and 15/20 (75%) were satisfied or very satisfied with the posttreatment appearance of treated areas at 180 days (Table 7). Pre- and posttreatment images of 1 patient are shown in Figure 6.
Table 5.
Cellulite Severity Score Change Magnitude at Day 180
| No. (%) | |
|---|---|
| Patients with ≥1-point improvement | 18 (95) |
| Patients with ≥2-point improvement | 13 (68) |
| Patients with ≥3-point improvement | 11 (58) |
| Mean (SD) point improvement at 180 d | 2.6 (1.5) |
SD, standard deviation.
Table 6.
Change in CSS and GAIS Scores at Day 180
| CSS (primary endpoint, n = 19) | |
|---|---|
| Patients with ≥1-point improvement, no. (%) | 19 (100) |
| GAIS (n = 19) | |
| Patients with any improvement, no. (%) | 19 (100) |
CSS, Cellulite Severity Assessment; GAIS, Global Aesthetic Improvement Scale.
Table 7.
Patient Satisfaction at Day 180
| Rating (n = 20) | No. (%) |
|---|---|
| Very satisfied | 9 (45) |
| Satisfied | 6 (30) |
| Somewhat satisfied | 2 (10) |
| Somewhat unsatisfied | 2 (10) |
| Unsatisfied | 1 (5) |
| Very unsatisfied | 0 |
Figure 6.
These images of the same patient as above were obtained (A) prior to treatment and (B) after 90 days.
There were 50 AEs and investigator findings (Table 8); however, none were unexpected, and all were consistent with other surgical treatments for cellulite. These included mild-to-moderate bruising/ecchymosis (n = 20; mean duration, 17.6 days), mild to moderate temporary soreness/pain/tenderness (n = 9), mild edema (n = 9), temporary nodule (n = 2) and scar tissue/nodule (n = 1), and hematoma (Table 8). The hematoma was of minor severity and resolved spontaneously without treatment as did all other AEs. There were no serious AEs. The mean pain score was 1.9 at Day 7 and 0.7 at Day 30.
Table 8.
AEs and Investigator Findings
| AE or finding term | No. (%) | % Of all events |
|---|---|---|
| Dizziness | 1 (5) | 2 |
| Dry blisters | 1 (5) | 2 |
| Dry scab | 1 (5) | 2 |
| Ecchymosis/bruising | 20 (100) | 40 |
| Edema | 9 (45) | 18 |
| Hematoma | 1 (5) | 2 |
| Hyperpigmentation | 1 (5) | 2 |
| Induration | 1 (5) | 2 |
| Nausea | 1 (5) | 2 |
| Nodule | 2 (10) | 4 |
| Scar tissue/nodule | 1 (5) | 2 |
| Scraped skin | 1 (5) | 2 |
| Single out pouch present (investigator term for a small, raised area of skin) | 1 (5) | 2 |
| Soreness/pain/tenderness | 9 (45) | 18 |
| Total | 50 |
AE, adverse event.
DISCUSSION
The novel, minimally invasive method described enables selective identification and precise manual release of the specific septa responsible for causing cellulite depressions. The results of this study support the safety and feasibility initially demonstrated in a first-in-woman safety and feasibility study. In that study, 10 patients were treated unilaterally on the buttocks and thighs. By increasing the number of patients and treating buttocks and thighs bilaterally, the study confirmed the ability to identify target cellulite by subcutaneously advancing the device to the identified area, confirming the correct location by initially pulling on the target septa to recreate a marked depression on the skin, and then releasing the septa. To provide local anesthesia, all investigators employed diluted lidocaine delivered subcutaneously with a spinal needle.
The results represent the first real demonstration of the effectiveness at 180 days of the novel method for treating cellulite depressions with substantial improvements in several measures of efficacy.13-16 Notably, patient satisfaction was high. The study confirmed the ability to locate the position of the distal end of the device utilizing transillumination through the skin in patients with Fitzpatrick skin types II to V during the procedure. Three patients (3/20, 15%) had skin types IV and V.
The review of video-recorded procedures revealed several important and previously unknown features of cellulite that can influence treatment. In contrast with previous depictions of cellulite suggesting a single fibrous strand for each depression, the 3-dimensional septa structure is complex, with webbing, walls, and branching structures that extend beyond the center of the depression. There is more than 1 fibrous strand per cellulite depression. Evidence for septa complexity was obtained from the results of the video analysis, which revealed a mean number of 8.4 cuts performed per cellulite depression.
It appears that septa arrangement and structure vary across cellulite depressions and patients, making it important to methodically treat each depression, cutting from right to left to accommodate the location of the sharpened link. The process should be repeated until no septa remain that can recreate a depression on the skin surface. The number of required cuts will vary for each depression. It was found that some septa are very elastic or long, and the device should be pulled proximally approximately 1 inch past the marked cellulite target to ensure that all septa have been identified and cut. Although there is a potential for a protruding area of the skin, the risk can be minimized because of the precision control and by avoiding overly aggressive treatment. The illuminated distal end of the device is visible through the skin and helps the user limit its utilization to the space between the dermis and superficial fascia.
This pilot study also demonstrated the acceptable safety profile of the novel, minimally invasive method for selectively identifying and manually releasing the specific septa responsible for causing cellulite depressions in a precise manner. Most AEs were considered by the investigators to be expected events normally associated with similar procedures. All were mild or moderate in severity, transient in duration, and most resolved spontaneously. The safety profile of the method compares favorably with other marketed cellulite treatments, including the utilization of collagenase clostridium histolyticum-aesthetic formulation (CCH-aaes).10 Among patients treated in 2 large phase 3 studies, overall patient satisfaction was high, although efficacy results cannot be directly compared because different cellulite scales were utilized.17 AEs reported in >50% of CCH-aaes patients were injection-site bruising, pain, and nodule formation. Although there is no incision with CCH-aaes, there is the potential for immunogenicity with neutralizing antibody formation and potential hypersensitivity reactions, similar to other therapeutic proteins.18
There were several limitations to the present study, including the open-label study design. Due to the small number of patients, it was inadequately powered to assess for statistical significance. Other limitations were a single-blinded physician assessor and limited length of follow-up.
CONCLUSIONS
The results of this study demonstrated the safety and efficacy of a novel, minimally invasive method for selectively identifying and manually releasing the specific septa responsible for causing cellulite depressions in a precise manner for improving the appearance of moderate and severe cellulite in adult women with a wide variety of skin types. Enrolled patients achieved substantial improvements in several measures of cellulite severity, and AEs were mild and transient. Clinical research is ongoing.
Acknowledgments
The authors acknowledge the editorial assistance of Dr Carl S. Hornfeldt, Apothekon, Inc. (Woodbury, MN).
Disclosures
The authors are consultants to and/or shareholders of the study sponsor, Revelle Aesthetics, Inc. (Mountain View, CA).
Funding
This study was sponsored by Revelle Aesthetics, Inc. (Mountain View, CA).
REFERENCES
- 1. Christman M, Belkin D, Oula ML, Geronemous R, Brauer J. An anatomical approach to evaluating and treating cellulite. J Drugs Dermatol. 2017;16(1):58-61. [PubMed] [Google Scholar]
- 2. Bass LS, Kaminer MS. Insights into the pathophysiology of cellulite: a review. Dermatol Surg. 2020;46(1):S77-S85. Doi: 10.1097/DSS.0000000000002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hexsel D, Mazzuco R. Cellulite. In: Tosti A, Hexsel D, eds. Update in Cosmetic Dermatology. Berlin, Germany: Springer-Verlag; 2013:21-32. [Google Scholar]
- 4. Friedmann DP, Vick GL, Mishra V. Cellulite: a review with a focus on subcision. Clin Cosmet Investig Dermatol. 2017;10:17-23. Doi: 10.2147/CCID.S95830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hexsel DM, Abreu M, Rodrigues TC, Soirefmann M, do Prado DZ, Gamboa MM. Side-by-side comparison of areas with and without cellulite depressions using magnetic resonance imaging. Dermatol Surg. 2009;35(10):1471-1477. Doi: 10.1111/j.1524-4725.2009.01260.x. [DOI] [PubMed] [Google Scholar]
- 6. Khan MH, Victor F, Rao B, Sadick NS. Treatment of cellulite: Part I. Pathophysiology. J Am Acad Dermatol. 2010;62(3):361-370. Doi: 10.1016/j.jaad.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 7. Green J, Cohen J, Kaufman J, Metelista A, Kaminer M. Therapeutic approaches to cellulite. Semin Cutan Med Surg. 2015;34(3):140-143. Doi: 10.12788/j.sder.2015.0169. [DOI] [PubMed] [Google Scholar]
- 8. Hexsel D, Siega C, Schilling-Souza J, Stapenhorst A, Costa Rodrigues T, Brum C. Assessment of psychological, psychiatric, and behavioral aspects of patients with cellulite: a pilot study. Surg Cosmet Dermatol. 2012;4(3):131-136. [Google Scholar]
- 9. Hexsel DM, Mazzuco R. Subcision: a treatment for cellulite. Int J Dermatol. 2000;39(7):539-544. Doi: 10.1046/j.1365-4362.2000.00020.x [DOI] [PubMed] [Google Scholar]
- 10. Kaminer MS, Coleman WP 3rd, Weiss RA, Robinson DM, Coleman WP 4th, Hornfeldt C. Multicenter pivotal study of vacuum-assisted precise tissue release for the treatment of cellulite. Dermatol Surg. 2015;41(3):336-334. Doi: 10.1097/DSS.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 11. DiBernardo BE, Sasaki GH, Katz BE, Hunstad JP, Petti C, Burns AJ. A multicenter study for cellulite treatment using a 1440-nm Nd:YAG wavelength laser with side-firing fiber. Aesthet Surg J. 2016;36(3):335-343. Doi: 10.1093/asj/sjv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dixon JR, Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur. 1998;6(2):65-74. Doi: 10.1080/105294199277860. [DOI] [PubMed] [Google Scholar]
- 13. Russe-Wilflingseder K, Russe E, Vester JC, Haller G, Novak P, Krotz A. Placebo controlled, prospectively randomized, double-blinded study for the investigation of the effectiveness and safety of the acoustic wave therapy (AWT(®)) for cellulite treatment. J Cosmet Laser Ther. 2013;15(3):155-162. Doi: 10.3109/14764172.2012.759235. [DOI] [PubMed] [Google Scholar]
- 14. Cohen JL, Sadick NS, Kirby MT, et al. Development and validation clinician and patient reported photonumeric scales to assess buttocks cellulite severity. Dermatol Surg. 2020;46(12):1628-1635. Doi: 10.1097/DSS.0000000000002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geronemus RG, Kilmer SL, Wall SH Jr, et al. An observational study of the safety and efficacy of tissue stabilized-guided subcision. Dermatol Surg. 2019;45(8):1057-1062. Doi: 10.1097/DSS.0000000000001911. [DOI] [PubMed] [Google Scholar]
- 16. Hexsel D, Fabi SG, Sattler G, et al. Validated assessment scales for cellulite dimples on the buttocks and thighs in female patients. Dermatol Surg. 2019;45(8S)S2-11. Doi: 10.1097/DSS.0000000000001993. [DOI] [PubMed] [Google Scholar]
- 17. Kaufman-Janette J, Joseph JH, Kaminer MS, et al. Collagenase Clostridium histolyticum-aaes for the treatment of cellulite in women: results from two phase 3 randomized, placebo-controlled trials. Dermatol Surg. 2021;47(5):649-656. Doi: 10.1097/DSS.0000000000002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. QWO (Collagenase Clostridium Histolyticum-aaes) for Injection [prescribing information 2020]. Malvern, PA: Endo Aesthetics LLC. [Google Scholar]