Abstract
High follicle-stimulating hormone (FSH) doses during ovarian stimulation protocols for assisted reproductive technologies (ART) are detrimental to ovulatory follicle function and oocyte quality. However, the mechanisms are unclear. In a small ovarian reserve heifer model, excessive FSH doses lead to phenotypic heterogeneity of ovulatory size follicles, with most follicles displaying signs of premature luteinization and a range in severity of abnormalities. By performing whole transcriptome analyses of granulosa cells, cumulus cells, and oocytes from individual follicles of animals given standard or excessive FSH doses, we identified progressive changes in the transcriptomes of the 3 cell types, with increasing severity of follicular abnormality with the excessive doses. The granulosa and cumulus cells each diverged progressively from their normal phenotypes and became highly similar to each other in the more severely affected follicles. Pathway analysis indicates a possible dysregulation of the final stages of folliculogenesis, with processes characteristic of ovulation and luteinization occurring concurrently rather than sequentially in the most severely affected follicles. These changes were associated with disruptions in key pathways in granulosa and cumulus cells, which may account for previously reported reduced estradiol production, enhanced progesterone and oxytocin production and diminished ovulation rates. Predicted deficiencies in oocyte survival, stress response, and fertilization suggest likely reductions in oocyte health, which could further compromise oocyte quality and ART outcomes.
Keywords: excessive FSH doses, ovarian stimulation, transcriptome analysis, cumulus cells, granulosa cells, oocytes, ovulatory-size follicles
High follicle-stimulating hormone (FSH) doses are incorporated into assisted reproductive technologies (ART) in women and cattle to increase the number of ovulatory-size follicles and oocytes available for in vitro or in vivo fertilization and embryo transfer. However, high FSH doses during ovarian stimulation are inversely related to oocyte recovery and live birth rates in women (1, 2), and decreased ovarian function and embryo transfer success in cattle (3-7). These observations support the hypothesis that high FSH doses during ovarian stimulation protocols are detrimental to ovulatory follicle function and oocyte quality, but the mechanisms of these effects are unclear.
During natural reproductive cycles, ovulatory follicles undergo luteinization in response to the preovulatory luteinizing hormone (LH) surge, characterized by an intrafollicular shift from primarily estradiol to progesterone production. However, high doses of recombinant human FSH (hFSH) administered to women (8, 9) and animals (10) induces premature luteinization, characterized by a reduced circulating estradiol:progesterone (E:P) ratio on the day of the ovulatory stimulus (9, 11-17). Premature luteinization occurs in 12.3 to 46.7% of ART cycles in women but its impact on ART outcomes is unclear, due in part to inconsistent assignment of a premature luteinization phenotype (18). We are not aware of any FSH dose–response studies in women or other relevant animal models that have directly examined the ovulatory-size follicles developing in response to ovarian stimulation with excessive FSH doses (i.e., doses greater than needed to achieve a maximum ovulatory response). Consequently, it is unknown if excessive FSH doses during ovarian stimulation cause differentiation (e.g., luteinization) of its target sites, the granulosa cells (GCs) and cumulus cells (CCs), resulting in premature luteinization (9, 11-17), impaired oocyte quality, and effects on ART ouitcomes.
The small ovarian reserve heifer (SORH; 11-12 months old) is a unique and ideal model for identifying excessive FSH doses and their impacts on ovulatory follicle function. Aside from being a mono-ovulatory model that, like women, is subjected to ARTs, it mimics the well-established characteristics of women with small ovarian reserves. These include a diminished number of morphologically healthy follicles (and thus oocytes) in the ovarian reserve, FSH hypersecretion, low circulating anti-Müllerian hormone, progesterone, and androgen concentrations during reproductive cycles, and reduced responsiveness to ovarian stimulation compared with age-matched individuals with a larger ovarian reserve (19-21). Our previous work demonstrated that increasing the FSH dose to elicit a greater response to ovarian stimulation during ART is detrimental to the number of oocytes retrieved and live birth rate (1, 2). Although the primary reason women seek ART is due to having a small ovarian reserve, it is unclear why these individuals respond poorly to high FSH doses during ovarian stimulation (22, 23).
Using the SORH model in a dose–response study, we found that doses 3-fold higher than industry-standard doses of Folltropin-V, a commercial FSH-enriched porcine pituitary preparation (cpFSH), during ovarian stimulation were excessive because they did not increase the number of ovulatory-size follicles but reduced the human chorionic gonadotropin (hCG)–induced ovulation rate and decreased circulating estradiol concentrations (3). In response to the industry-standard cpFSH dose, ovulatory-size follicles contained compact cumulus–oocyte complexes (cCOCs), and most (80%) had higher intrafollicular E:P (24). These follicles were considered morphologically healthy, estrogen-active (EA) dominant follicles, mimicking the well-established phenotype of dominant preovulatory follicles developing during the follicular phase of estrous cycles prior to the preovulatory gonadotropin surge in cattle (25-27). In stark contrast, most (72%) of the ovulatory-size follicles developing in response to the excessive cpFSH doses contained expanded COCs (eCOCs) and >50% of these eCOC-containing follicles had lower E:P (estrogen-inactive, EI) and/or high oxytocin concentrations (24). These EI ovulatory-size follicles with eCOCs mimicked the phenotypes of dominant ovulatory follicles developing during the follicular phase of estrous cycles following the preovulatory gonadotropin surge (but prior to ovulation), or the dominant nonovulatory follicles developing during the luteal phase of estrous cycles as they lose dominance and undergo atresia during a nonovulatory follicular wave in cattle (25, 26, 28). Thus, most ovulatory-size follicles developing in response to ovarian stimulation with excessive cpFSH doses undergo premature luteinization, characterized by CC expansion, reduced estradiol but enhanced intrafollicular progesterone and/or oxytocin production, and a diminished sensitivity to an LH-like (hCG) stimulus resulting in a reduced ovulation rate.
FSH is the predominant gonadotropin in Folltropin-V; however, LH is a minor (<1%) contaminant (29). Regardless, high Folltropin-V doses during ovarian stimulation do not enhance circulating progesterone or estradiol production, or induce early ovulation, as would be expected if the preparation contained biologically significant amounts of LH (3). Additionally, Folltropin-V injections do not enhance overall circulating LH concentrations in cattle (30). These combined observations indicate that the LH contamination even in high doses of Folltropin-V is likely insufficient to impact ovarian function. Other reports support the conclusion that premature luteinization is likely attributable to excessive FSH concentrations (10, 18). Overall, this supports the hypothesis that excessive FSH doses during ovarian stimulation disrupt pathways in GC and CC directly and indirectly regulated by FSH, resulting in premature luteinization of ovulatory-size follicles, leading to impaired oocyte quality.
To test this hypothesis, and to discover the molecular and cellular mechanisms responsible for adverse effects of excessive FSH doses, we used the SORH model and applied RNA sequencing (RNA-seq) and a bioinformatics analysis pipeline incorporating Ingenuity Pathway Analysis (IPA; QIAGEN Inc., https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa/ (31)) to identify transcriptome differences between ovulatory-size follicles developing during ovarian stimulation with industry-standard vs excessive cpFSH doses. We acquired transcriptome data for each cell type (GC, CC, and oocyte) from individual ovulatory-size follicles either without (industry-standard dose) or with (excessive dose) varying degrees (based on COC morphology and intrafollicular estradiol, progesterone, and oxytocin concentrations) of the premature luteinization phenotype. The results provide new mechanistic insight into the impact of excessive cpFSH doses on cellular phenotypes within individual ovulatory-size follicles, including remarkable changes in molecular and cellular pathways, and functions predictive of diminished oocyte quality that could not fully be explained by premature luteinization alone. These results point to potential risks of using excessive FSH doses during ART.
Materials and Methods
Supplemental data files are available at Figshare https://figshare.com/search?q=1920860410.6084%2Fm9.figshare.19208604 (RRID:SCR_004328, https://scicrunch.org/resolver/RRID:SCR_004328) doi: 10.6084/m9.figshare.19208604 (32).
SORH Selection, Ovarian Stimulation Protocol, and Excision of Ovulatory-size Follicles
Heifers with a small ovarian reserve used in this study (n = 14) were selected as described (3, 24). Briefly, ultrasonography was used to determine the antral follicle count (AFC; number of antral follicles ≥ 3 mm in diameter) of 2 groups of 50 Holstein heifers (11-12 months old; Green Meadow Farms Inc., Ovid-Elsie, MI). Using the AFC, animals were ranked and the 25 heifers with the lowest AFC in each group were administered 2 prostaglandin F2α (PG; 12.5 mg/mL, Lutylase HighCon, Zoetis) injections 10 days apart to induce ovulation and the initiation of the first follicular wave. The AFC was measured prior to each PG injection and 4 days after the final PG injection. Animals with an AFC ≤ 15 (14 heifers) on these days were selected for inclusion in this study and housed at the Michigan State University Beef Cattle Teaching and Research Centre for the duration of the study. Our previous study has shown that age-matched individuals with an AFC ≤ 15 also have an 80% smaller ovarian reserve (total number of morphologically healthy follicles/oocytes in ovaries) than heifers with a high (≥25) AFC (20). All procedures described herein were approved by the Institutional Animal Care and Use Committee at Michigan State University.
The 2 cpFSH doses investigated herein were chosen as the industry standard (70 IU) and excessive (210 IU) doses based on previous data (3, 24). For ovarian stimulation, heifers were treated with either 70 IU (40 mg, n = 7 heifers) or 210 IU (120 mg, n = 7) cpFSH (Folltropin-V, Lot# 499213, Vetoquinol USA Inc.) per injection. The cpFSH contains 700 IU (equivalent to 400 mg NIH-FSH-P1 with <1 mg NIH-LH-S19) of FSH per 20-mL vial (29). One heifer treated with the 210 IU dose was excluded from the final analysis due to an absence of response to ovarian stimulation. Heifers were synchronized by administering PG injections 10 days apart followed by a third PG injection 12 hours later. At the time of ovulation or emergence of the first follicular wave (~36 hours after the last PG injection), animals were randomly assigned to treatment with either 70 or 210 IU cpFSH twice daily for 4 days (8 injections total). Daily ultrasonography was used to monitor the AFC and diameter of these follicles. The AFC prior to superovulation did not differ between cpFSH dose (24). Heifers were euthanized 12 hours after the final cpFSH injection and the pairs of ovaries were recovered in lieu of administration of an ovulatory dose of hCG that is standard for this superovulation protocol (3). The number and size of ovulatory-sized follicles produced in response to ovarian stimulation did not differ between cpFSH doses (24).
Ovaries were washed briefly in 70% ethanol, then Dulbecco’s phosphate-buffered saline (DPBS) and then placed in fresh DPBS for the duration of tissue collection. For a subset of follicles (5-15/heifer), ovulatory-size (≥10 mm) follicles were individually dissected from the ovary. Follicular fluid was aspirated to recover the COC. The aspirated follicular fluid was immediately transferred to a separate dish and processed as described below. The follicular fluid was recovered and stored for measurement of progesterone, estradiol, and oxytocin.
Measurement of Progesterone, Estradiol, and Oxytocin and Determination of Estradiol:Progesterone Ratio in Follicular Fluid of Ovulatory-size Follicles
As described (24), estradiol, progesterone and oxytocin concentrations were measured in follicular fluid samples from all ovulatory-size follicles where a COC was recovered (n = 5-15/heifer). Estradiol and progesterone concentrations were measured using commercially available enzyme-linked immunosorbent assay kits (estradiol, RRID:AB_328054; progesterone, RRID:AB_2811273; Cayman Chemical Company, MI), enabling the calculation of the E:P ratio. The E:P ratios were used to classify follicles as EA (E:P ratio ≥ 1) or EI (E:P ratio < 1). Follicular fluid oxytocin concentrations, another established marker of premature luteinization (33-36), were also measured using the oxytocin enzyme-linked immunosorbent assay kit (RRID:AB_2815012; ENZO Life Sciences, Inc, NY, USA).
Follicle Types and Nomenclature, and Statistical Analyses of Follicle Characteristics
Type 1 follicles were those from cattle that receive the industry standard dose (70 IU) of cpFSH. Three of the high-dose (210 IU) follicular phenotypes were chosen for analysis by RNA-seq, designated as Types 2 to 4 (Table 1; Table S1 (32)). The statistical analyses performed on data in Table 1 were undertaken using the Statistical Analysis System (SAS 9.4) software program (RRID:SCR_008567, https://www.sas.com/en_us/legal/editorial-guidelines.html) (37). Data were arcsine (E:P ratio) or log (estradiol, progesterone and oxytocin) transformed before overall treatment (ie, follicle type) effects were evaluated, and Fisher Least Significant Difference was used to determine if means differed (P < .05).
Table 1.
Summary of phenotypic characteristics of ovulatory-size follicles subjected to RNA-seq analysis
| Follicle type (cpFSH dose) | COC morphology | Follicles/heifer (%)a | Estrogen-active (EA) or inactive (EI) (E:P ratio) |
Follicle diameter (mm) | Concentration (ng/mL) | ||
|---|---|---|---|---|---|---|---|
| Estradiol | Progesterone | Oxytocin | |||||
| Type 1 (70 IU) | Compact | 79 ± 6 | EA (2.99 ± 0.51) |
12.9 ± 1.2 | 155 ± 15 (n = 7, 7)b |
54 ± 9 (n = 7, 7) |
0.27 ± 0.04 (n = 7, 7) |
| Type 2 (210 IU) | Compact | 28 ± 3 | EA (1.80 ± 0.51) |
12.2 ± 0.6 | 153 ± 37 (n = 6, 6) |
85 ± 30 (n = 6, 6) |
0.36 ± 0.05 (n = 6, 6) |
| Type 3 (210 IU) | Expanded | 26 ± 5 | EA (2.60 ± 0.89) |
12.5 ± 0.9 | 217 ± 51 (n = 6, 6) |
83 ± 16 (n = 6, 6) |
0.31 ± 0.06 (n = 6, 6) |
| Type 4(210 IU) | Expanded | 20 ± 3 | EI (0.01 ± 0.01)** |
10.4 ± 0.2 | 8 ± 7** (n = 5, 5) |
747 ± 546*** (n = 5, 5) |
4.04 ± 0.86*** (n = 5, 5) |
Follicle characteristics include COC morphology, proportion of ovulatory-size follicles per heifer, estrogen-active (EA) or estrogen-inactive (EI) and estradiol:progesterone ratio (E:P), follicle diameter (mm), estradiol, progesterone and oxytocin concentrations. Data for E:P ratio, estradiol, progesterone, and oxytocin concentration were generated from Table S1.
Asterisks (**P < .01, ***P < .001) denote differences between the geometric means (±SEM). All comparisons were relative to Type 1 follicles, since these were considered representative of preovulatory follicles in cattle and were from standard (70 IU) cpFSH dose treated heifers.
a Proportion of each follicle type per heifer (number of Type 1, 2, 3, or 4 follicles, respectively/total number of ovulatory-size follicles).
b n = number of heifers, total number of ovulatory-size follicles.
Type 1 and Type 2 follicles were highly similar to each other and presumably represent the most healthy, normal follicles having cCOC and EA characteristics. Based on these parameters, it is important to note that the only difference between Type 1 and 2 follicles was the cpFSH dose used during ovarian stimulation, 70 and 210 IU, respectively. With progressively more severe abnormalities, Type 3 and 4 follicles contained eCOC, and Type 4 follicles additionally displayed an EI endocrine profile, making them phenotypically the most divergent from Type 1 follicles.
Samples from 7 heifers receiving the standard dose and 6 heifers receiving the excessive dose were obtained. One sample per relevant follicle type per heifer was included where possible and were randomly selected and excised from the ovaries, and processed to yield a GC sample, a CC sample, and an oocyte sample for RNA-seq. Only high-quality RNA-seq libraries were included in the analysis of transcriptome effects for GC (Type 1 n = 7, Type 2 n = 6, Type 3 n = 5, Type 4 n = 4), CC (Type 1 n = 6, Type 2 n = 6, Type 3 n = 6, Type 4 n = 4), and oocytes (Type 1 n = 5, Type 2 n = 3, Type 3 n = 6, Type 4 n = 4). Transcriptome profiles were compared between doses within cell type, and between cell types to assess the molecular changes that accompany and may underlie the observed morphological and endocrine features of these 4 follicle types.
Oocyte and Cumulus Cell Isolation
The COCs were identified within the follicular fluid that was aspirated from each individual follicle. Dishes were examined under a heated (38.5°C) stereomicroscope and then were moved to a 35-mm dish filled with HH medium (HEPES buffered medium with 3 mg/mL BSA) using a Drummond Micropipette with a glass tip. Morphology of the COCs, either cCOC or eCOC were recorded (24). COCs were incubated in 0.1% hyaluronidase (Sigma-Aldrich, St. Louis, MO) in HH medium at 37°C for 5 minutes to separate the CCs from the oocyte. The CC mass and oocyte were then transferred to a dish with HH medium to remove the hyaluronidase. The CC mass was divided into 2 or 3 aliquots and then washed through 3 drops of ~200 µL of HH medium. Cumulus cell aliquots were transferred to 200 µL drops of DPBS and moved to a 1.5-mL tube. Tubes were spun down until cells pelleted. The DPBS was removed and 100 µL of PicoPure Extraction Buffer™ was used to resuspend the cell pellet. Tubes were transferred to a 42°C incubation for 30 minutes and stored at –80°C. After separation from the CC, the oocyte was incubated in 0.1% pronase in HH medium and observed for removal of the zona pellucida. Denuded oocytes were transferred into 3, ~150 µL drops of HH medium to wash off the pronase solution. The oocytes were then moved into a tube with 50 µL of PicoPure Extraction buffer™, which was incubated at 42°C for 30 minutes and stored at –80°C.
Granulosa Cell Isolation
The follicle wall was bisected, and the GCs were gently scraped into DPBS. The follicle wall was removed from the dish and small sections of GC were isolated and transferred into a tube where 100 µL of PicoPure Extraction Buffer™ was added and processed as described above for the CC. All samples were stored at –80°C until RNA extraction.
RNA Extraction and RNA-seq Library Preparations
Following the manufacturer’s protocol, total RNA was isolated from oocytes, CC, and GC using the PicoPure RNA Isolation Kit™ (ThermoFischer Scientific, Waltham, MA) including a DNase digestion (RNase-Free DNase; Qiagen, Hiden, Germany) to remove contaminating DNA. A randomly selected subset of samples was assessed for RNA quality and all samples fell in the range of 9.2 to 10.0 RNA Integrity (RIN) values (mean ± SEM = 9.9 ± 0.1), indicating that the RNA isolation method was robust and yielded high-quality RNA. For RNA-seq library preparation of oocytes, the Ovation® SoLo RNA-Seq System kit (TECAN, Redwood, CA) was used, including bead purification, end repair, adaptor ligation, and first round library amplification and purification steps. Then 20 to 30 ng of each library was used for the remainder of library preparation, which included the use of AnyDeplete bovine primers for rRNA depletion, and a second round of library amplification and purification. With the SoLo kit, enzymatic shearing was applied rendering RNA-seq libraries between 300 and 350 bp in length.
For CCs and GCs, library construction was conducted using the TECAN Universal RNA-Seq with NuQuant kit which included Bovine AnyDeplete primers for rRNA depletion. Library construction steps included cDNA synthesis, fragmentation using a Covaris-2 sonicator that mechanically fragments the cDNA to an average length of 300 bp, end repair, adaptor ligation, strand selection, AnyDeplete, and final library amplification steps following the manufacturer’s protocol.
All RNA-seq libraries were assessed for quantity using the Qubit DNA high-sensitivity assay kit (ThermoFisher Scientific) and quality using the Agilent high-sensitivity DNA kit (Agilent Technologies) on a bioanalyzer following product instructions. A total of 23 oocytes (1 oocyte was excluded due to poor library quality), 24 CC, and 24 GC barcoded libraries were pooled and sequenced on an Illumina HiSeq 4000 (Illumina, San Diego, CA, USA) at the Michigan State University – Genomics Core. During the demultiplexing of the oocyte sequencing results, 2 samples were flagged for potential inversion of barcode identification and were subsequently removed from downstream analysis. An additional 2 CC, 2 GC, and 2 oocyte samples were excluded due to low-quality output relating to low generation of reads, low complexity, etc. This resulted in a total of high-quality samples numbering 22 for CCs and GCs, and 18 for oocytes. RNA sequencing data are available at the Gene Expression Omnibus (RRID:SCR_005012, https://scicrunch.org/resolver/SCR_005012) (accession number GSE197116) (38).
Sequencing Data Processing
Sample information is listed elsewhere (Table S1 (32)), including follicle type membership, dose, morphology, and exonic reads. Raw sequencing data in fastq format underwent initial sequencing quality metrics using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc). Resultant FastQC data identified aberrant nucleotide distribution in the first 5 base pairs. Trimming was conducted using TrimGalore (39) with the following parameters: minimum quality threshold of 20, minimum length of 20 base pairs, removal of Ns from ends of reads, and a hard trim of the first 5 base pairs. The bovine cDNA genome (ARS-UCD1.20, build 102) was downloaded from Ensembl and indexed with Kallisto (v0.44.0) (40) and mRNA abundance quantitation were calculated using standard settings.
Differential Expression Calculation
Resultant Kallisto outputs were processed with DESeq2 (v1.30.1) (41) within R (v4.1.0). For normalization and differential expression calculations, the somatic cells (CC and GC) were processed jointly while the oocyte samples were processed independently. Transcript abundance was collapsed to gene abundance using biomaRt (v2.45.8) (42), using Ensembl gene identifiers.
Pre-DEG (differentially expressed genes) filtering of lowly expressed genes was conducted, using a threshold of at least 1 Fragments Per Kilobase of transcript per Million mapped reads (FPKM) in at least 1 sample. For CCs and GCs, all pairwise comparisons were conducted: comparing follicle types within cell type and comparing follicle types across cell type. Within the oocyte samples, pairwise comparisons were calculated comparing follicle types. Level of significance for DEG was set at false discovery rate (FDR) < 0.01.
We considered limiting the analysis to a set of highly homologous genes identified using the MetaPhOrs database to address possible concerns related to gene annotation differences between bovine and the other species from which much of the data in the IPA database have been derived (43). However, because the application of sequence-based cross-species consistency scores can exclude some well-studied genes, even apparent gene homologs that share a gene symbol annotation, we elected not to apply this additional filter in our analysis.
IPA
Identification of canonical pathways (CPs), disease and functions (DFs), and upstream regulator (URs) was conducted by submitting the resultant DEG lists to IPA (QIAGEN Inc., https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa/) (IPA database content as of 01/2021) (31). IPA was selected due to the robustness of its manually curated Knowledge Base, which contains >7 million observations (Qiagen.com, March 2021) including molecular interactions organized into >700 pathways, >800 000 expression datasets and reported associations of molecules with diseases and biological functions, and >30 integrated third-party databases (Qiagen IPA in-program description), and its ability to compare multiple datasets. Like standard gene set enrichment methodology, submitted gene lists were compared with the genes associated with each CP/DF/UR to calculate a level of statistically significant overlap (P value; significance set at .05). With the known impact of up- or downregulating genes on a given IPA CP or biological function (BF) entry, the software can also calculate a direction of CP or BF modulation (activation or inhibition), denoted as positive and negative z-scores, respectively (significance set at z>|1.96|). For the UR analysis, the activity of an UR is predicted based on the direction of change for the downstream DEG targets. It should be noted that the magnitudes of gene expression changes do not factor into the calculations, only the direction of change. To remove potential spurious results, IPA data were limited to those entries with greater than 1 DEG present. DF entries were filtered to remove disease/cancer related entries, and the term BF applied.
To prioritize the consideration and presentation of IPA results, we employed a hierarchical approach. IPA natively identifies entities that satisfy 2 statistical measures. The P value is the level of statistically significant overlap between the number of DEGs and the number of molecules in an IPA entry; significance set at P < .05. The z-score is the level of directionality based on the known interactions; significance set at |z|>1.96. All entries are limited to statistically significant P values. We then noted those IPA entries with significant z-scores. The remaining IPA entries, lacking a significant z-score, were examined with respect to P value and other parameters, such as mRNA expression level and regulation.
The combined approach of whole-transcriptome RNA-seq plus IPA allows a robust assessment of gene expression changes coupled with the identification of potentially affected pathways and functions, which can further implicate affected upstream regulators, providing novel direction for hypothesis-driven research. While other methods such as reverse transcription quantitative polymerase chain reaction can be useful for quantifying the expression of a selected set of genes, such methods are limited in scope as they can only assay known sequences, and can be subject to artifacts related to hybridization. The combination of RNA-seq and IPA can provide an evaluation of whether a pathway or network is affected in a given situation. An important value of the whole-transcriptome approach is that it avoids bias and assays a large number of interacting or target molecules, the expression of which provides a statistically powerful assessment of whether a pathway or function is affected.
Luteinization Marker Identification
Because the IPA database did not include luteinization as a BF, we generated a manually curated list of 71 markers of luteinization, selected from previously published works evaluating changes in DEGs that were characteristic of the transition to a luteinized state (44-52). The expected changes during luteinization were determined based on the observed increase or decrease during this transition to luteinization. We acknowledge that this is not an exhaustive list of markers characteristic of luteinization (i.e., occurring in response to the preovulatory LH surge). Moreover, they are not considered exclusive markers of premature luteinization, that is, luteinization occurring before or in the absence of an ovulatory stimulus, such as LH or hCG.
DEG and IPA Figures
The R package ComplexHeatmap (v2.6.2) (53) was utilized to generate heatmaps of DEGs and IPA results arranged with cowplot (v1.1.1) (54). Correlation data were generated with the R package corrplot (v0.90) via the Pearson method and plotted using the ComplexHeatmap package.
Results
Study Design and Data Set
The overall goal of the study was to compare the transcriptomes of oocytes, CCs, and GCs isolated from ovulatory-size follicles in ovaries of SORH that received either a standard (70 IU) or excessive (210 IU) dose of cpFSH, to ascertain the effects of cpFSH dose and to assess potential cell–cell interactions at the transcriptome level. In an earlier report (24) we determined the different ovulatory-size follicle phenotypes based on morphological and endocrine features (ie, cCOC vs eCOC, and EA vs EI). Ovulatory-size follicles from ovaries of animals receiving the industry standard dose were predominantly of a single phenotype (Type 1), whereas multiple follicular phenotypes were identified in animals receiving the excessive dose. Samples from Type 1 follicles were classified as the control follicle type for subsequent major comparisons (i.e., 2 vs 1, 3 vs 1, and 4 vs 1), based on the published characteristics (e.g., EA and containing a cCOC (25-27)) of the ovulatory follicle in cattle and the fact that these samples were collected from heifers treated with the industry-standard, 70 IU, cpFSH dose.
RNA-seq library preparation yielded high quality libraries (Table S1 (32)). We calculated an average of 13 million exonic reads for CCs, 17.9 million for GCs, and 16.9 million for oocytes. This resulted in detection and quantitation of mRNAs from an average of 14 865 genes for CCs, 15 255 for GCs, and 14 658 for oocytes. Principal component analysis indicated a high degree of reproducibility within cell type and follicle type (Figure S1 (32)).
Progressively More Severe Follicular Abnormality is Accompanied by Progressively More Severe Transcriptome Alterations in all 3 Cell Types
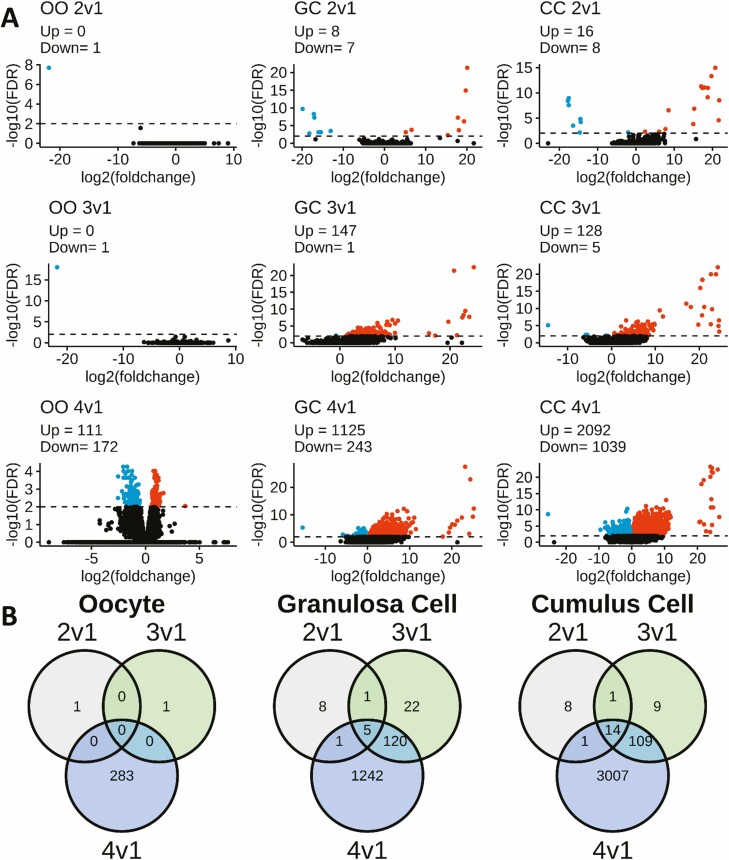
DEGs were identified between follicle Types 2 to 4 and control, Type 1, follicles (Tables S2-S4 (32)). Among the excessive dose follicles analyzed, transcriptome divergence from Type 1 follicles increased progressively from Type 2 to Type 3 and then to Type 4 (Figures S1 and 1A (32)). The principal component analysis plot (Figure S1 (32)) indicated that all 3 cell types from Type 4 follicles were most divergent from the control, Type 1 follicles. Volcano plots (Fig. 1A) reveal not only an increasing number of DEGs in the progression from Type 2 to Type 4, but an additional shift to more genes being overexpressed (indicated in red) in Type 3 and 4 relative to Type 1 follicles for CC and GC. Comparisons of DEGs between Types 2 to 4 and Type 1 revealed significant overlap in DEGs for follicle Types 2 to 4 for CC and GC, but also reveal unique DEGs for each follicle type (Fig. 1B).
Figure 1.
Volcano plots comparing Type 2 to 4 with Type 1 follicles, within cell type, and Venn diagram summarizing the number of DEGs and their overlaps. (A) Each plot displays DESeq2 results (–log10 [FDR]), DEGs were called at a significance threshold greater than 2.0 (FDR < 0.01), denoted by horizontal dashed line. Point color key: black = non-DEG, Red = upregulated DEGs, blue = downregulated DEGs. (B) Summary of DEG overlaps for each cell type comparing follicle Types 2-4 vs Type 1: oocyte, granulosa cell, and cumulus cell. All data were generated from Tables S2-S4 (32).
Oocytes displayed the fewest DEGs, with just a single DEG for Type 2 and 3 follicles, and 283 DEGs for Type 4 follicles (FDR < 0.01), with no overlaps between follicle types (Fig. 1B). DEGs with reduced expression include oogenesis factors: Folliculogenesis-specific BHLH Transcription Factor (FIGLA), Serine-threonine kinase 11-interacting protein (STK11IP, a.k.a. LIP1), and ADP ribosylation factor GTPase activating protein 3 (ARFGAP3).
CCs displayed 24, 133, and 3131 DEGs (FDR < 0.01) from follicle Types 2, 3, and 4, respectively, and thus there were many more affected DEGs for Type 4 CCs than GCs (see below). Most of the Type 2 CC DEGs overlapped with Types 3 and/or 4, and most of the Type 3 CC DEGs overlapped with Type 4. Again, CCs from each follicle type displayed a small number of unique DEGs. Of the 24 transcripts in the 2 vs 1 follicle type with FDR < 0.01, 8 were identified as downregulated in Type 2 relative to Type 1 follicles. The DEGs with the largest magnitude differences (±15 log2 (fold change), relative to Type 1) included both upregualted (e.g., FGG, MEPE, NGFR, LIF, MDFI, NCS1, RGS2, VGF, AREG, and TGFA) and downregulated (KIAA1324 and AQP11) transcripts (Table S2) (32). All of the Type 2 upregulated DEGs were also identified as the DEGs with the largest magnitude changes in the Type 3 and 4 follicles.
GCs displayed 15, 148, and 1368 DEGs for Type 2, 3, and 4 follicles, respectively. Fewer than half the Type 2 GC DEGs were shared with Type 3 and 4 follicles, whereas a majority of Type 3 GC DEGs were shared with Type 4 follicles. A small number of DEGs were unique to GCs of each follicle type. Of the 15 transcripts in the 2 vs 1 follicle types with FDR < 0.01, 8 were annotated (SERPINA14, NPS, and MASP1 were upregulated and TMEM255A, FGG, LRRC7, TNFSF4, and CRM1 were downregulated; Table S3 (32)). SERPINA14 and MASP1 were also identified as DEGs (FDR < 0.01) in the GCs of Type 3 and 4 follicles. In Type 3 follicles, only 5 of 148 DEG were downregulated. The DEGs with the largest differences were all upregulated (≥10 log2 (fold change)), and included SCG3, SERPINA11, EDN2, SERPINA14, GRO1, CSRP3, XCL1, and ADAMTS20 (Table S3 (32)). In Type 4 follicles, <20% of the 1368 DEGs (FDR < 0.01) were downregulated in GC. The genes with the largest differences were again all upregulated (≥10 log2 (fold change), relative to Type 1 GC), and included SERPINA11, SCG3, SERPINA14, GRO1, NPS, EDN2, ADAMTS20, TNN, XCL1, CSRP3, and AMKRD55 (Table S3 (32)).
More Severe Follicular Phenotypes are Associated with Enhanced Expression of a Subset Luteinization Markers
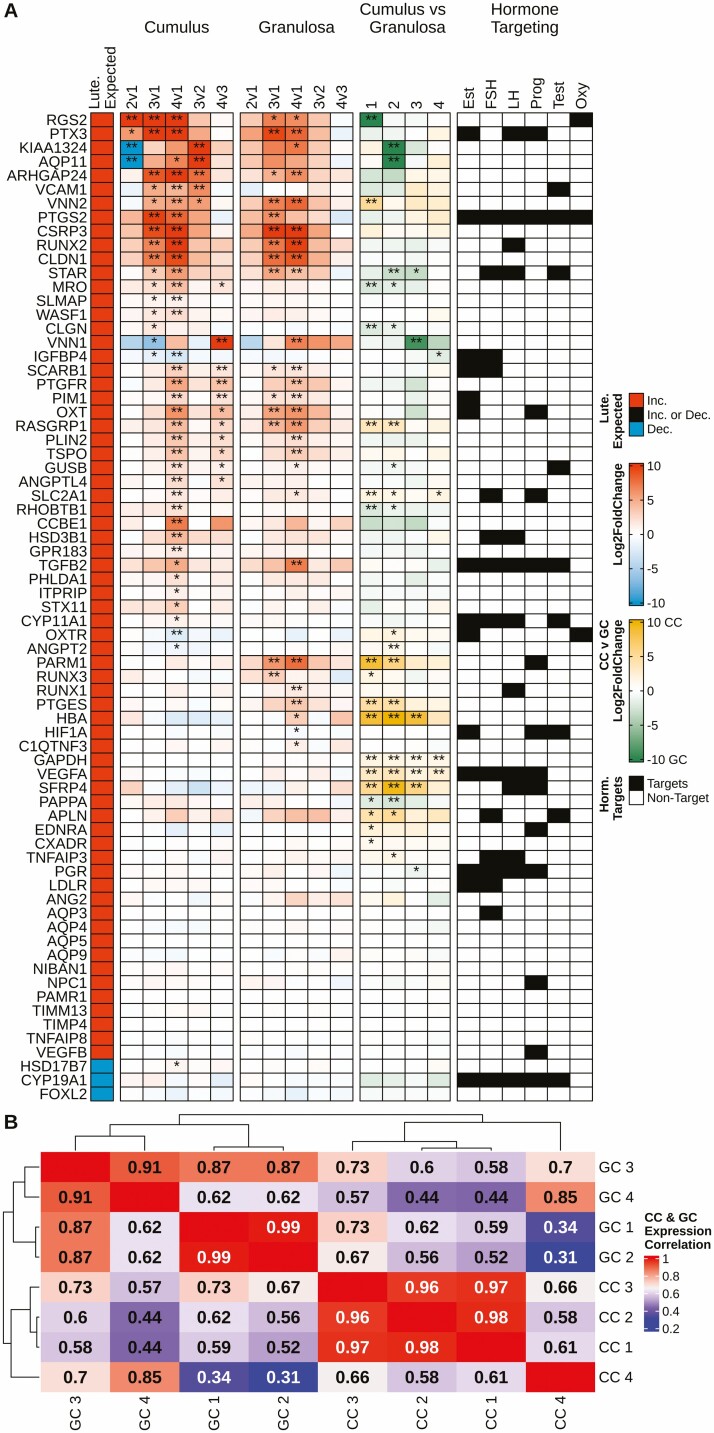
Our previous study reported that the effects of excessive cpFSH dose on COC morphology and intrafollicular endocrine profile resembled a premature luteinization effect (24). To explore this idea further, we compared Types 2 to 4 with Type 1 follicles for effects in GCs and CCs on a manually curated panel of 71 luteinization markers (Fig. 2A, Column 1) identified from the literature (44-52). In comparison with Type 1 follicles, progressively more markers were altered in a manner consistent with luteinization in Type 2, 3, and 4 ovulatory-size follicles in GCs (26/71 in Type 4) and CCs (33/71 in Type 4), even so, less than half of this list of luteinization markers were affected in any of the follicle types. Many of the affected markers were progressively upregulated in both cell types in Type 3 and 4 (with eCOC morphology) follicles. Many of the luteinization markers were more highly expressed in the CCs than GCs (Fig. 2A column 4, denoted by yellow coloring). Three luteinization markers (KIAA1324, VNN1, and AQP11) were affected opposite to the predicted directions in Type 2 or 3 CCs. An additional 4 markers (IGFBP4, OXTR, ANGPT2, HSD17B7) displayed changes opposite to the predicted directions in Type 4 CCs. Analysis of the correlation between CCs and GCs of the different follicle types with respect to the expression of these luteinization markers revealed that Type 1, 2, and 3 CCs were highly similar; Type 1 and Type 2 GCs were highly similar; Type 4 CCs diverged markedly from Type 1 to 3 CCs; and Type 3 and 4 GCs diverged noticeably from Type 1 and 2 GCs, indicating a stronger deviation with respect to luteinization marker gene expression with more severe follicular abnormality (Fig. 2B).
Figure 2.
Overview of gene expression changes for manually curated luteinization markers identified from the literature. (A) Five columns exploring the changes in luteinization marker expression in cumulus and granulosa cell samples. (A) Column 1 depicts the expected change in expression during luteinization, wherein red fill denotes an increase in expression, blue a decrease. (A) Columns 2 and 3 depict the changes in expression of markers in cumulus and granulosa cells, respectively, from different follicle types; fill of any color denotes log2 (fold-change), red equating to an increase and blue a decrease in expression. (A) Column 4 depicts the difference in marker expression when comparing cumulus and granulosa cells within follicle type; yellow fill denotes higher expression in cumulus cells and green higher expression in granulosa cells. (A) Column 5 identifies which of the markers are targets of hormones per the IPA database. (A) Columns 2-4, DEG status is denoted by **FDR < 0.01 and *FDR < 0.05. (B) A correlation heatmap with hierarchical clustering based on the average FPKM of the luteinization markers for both cumulus and granulosa cells among the 4 follicle types. Red fill indicates a higher correlation of expression values, while blue denotes less correlation. CC, cumulus cell; GC, granulosa cell. Data were generated from Tables S2 and S3 (32).
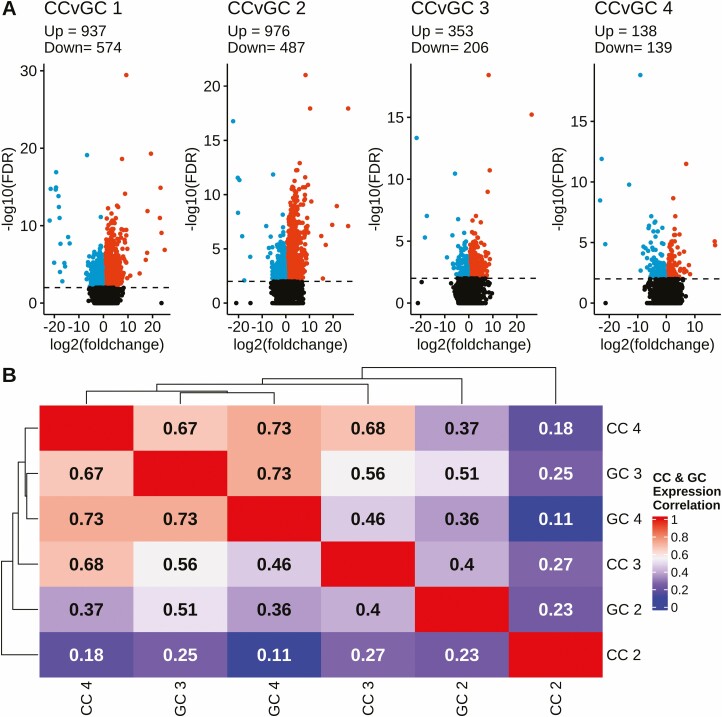
CCs and GCs Progressively Become More Alike as Follicular Phenotype Becomes More Abnormal
An interesting relationship between CCs and GCs was seen wherein CCs and GCs became more like each other across this progression from less to more severe follicular abnormality. This relationship emerged in multiple analyses. The volcano plots (Fig. 3A) revealed similar numbers of GC-CC DEGs for Type 1 and 2 follicles (1511 and 1463, respectively) but fewer for Type 3 and 4 follicles (559 and 277, respectively). We performed a correlation analysis of all expressed genes for CC and GC normalized against CC and GC in Type 1 follicles (Fig. 3B) and found increased similarities between CC4 and both GC3 and GC4. This was also evident for the luteinization markers; progressively fewer differences between CC and GC were seen moving across Type 2, 3, and 4 follicles (Fig. 2A, fourth column). We also performed a hierarchical clustering of correlation between the luteinization markers for CC and GC. Although CC and GC clustered separately, increased correlations were seen comparing CC4 with GC4, and comparing CC3 with GC1 and GC3 (Fig. 2B). We also noted that out of a group of 15 luteinization markers reported for GCs at 22 hours post-hCG in cattle (52), 13 were detected in CCs and in GCs in our study. Of these, 4 were upregulated in Type 4 GCs, and, importantly, 9 were upregulated in Type 4 CCs (Fig. 2). This further illustrates that the Type 4 CCs acquire increased resemblance to GCs.
Figure 3.
Volcano plots comparing DEGs in cumulus and granulosa cells for each follicle type (1-4) and correlation heatmap with applied hierarchical clustering of expression values for cumulus and granulosa cells. (A) Each plot displays DESeq2 results (–log10 [FDR]) and represents the DEGs in the cumulus and granulosa cells within a follicle type. DEGs were called at a significance threshold greater than 2.0 (FDR < 0.01), denoted by horizontal dashed line. Point color key: Black = non-DEG, Red = upregulated DEGs, Blue = downregulated DEGs. CC = cumulus cells and GC = granulosa cells. (B) Within cumulus and granulosa cell types, FPKM values for follicle Types 2 to 4 were normalized against those for Type 1 as the denominator. The correlation between the types were calculated via the Pearson method. Color denotes correlation coefficient: red equating to more similar, and blue less similar. All data were generated from Tables S2 and S3 (32).
More Severe Follicular Phenotypes are Associated With Progressively Increased Effects on Hormone-responsive Genes
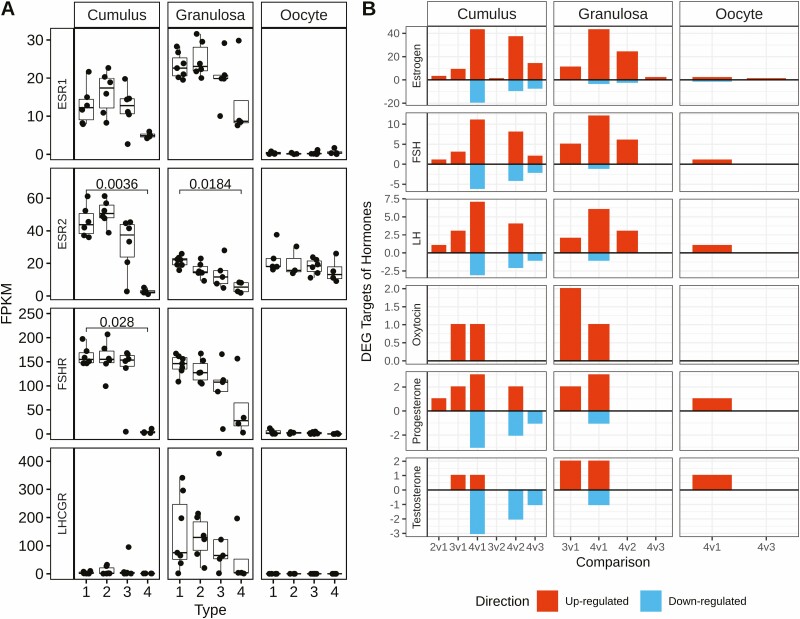
Our analysis of luteinization markers revealed that many of the markers that were affected in CCs and GCs are regulated by either estrogen-, FSH-, or LH signaling (14, 14, and 13, luteinization markers, respectively) or all 3 of these signaling pathways (6 luteinization markers), 13 by progesterone, some by testosterone (6 luteinization markers) or oxytocin (3 luteinization markers), and 1 (PTGS2) that is regulated by all of these hormones (Fig. 2A Column 5). To explore potential endocrine regulation of these and other DEGs across cell and follicle types, we examined expression levels of mRNAs encoding the relevant hormone receptors (Fig. 4A). Estrogen receptor, ESR1 mRNA trended toward reduced expression and ESR2 mRNA was significantly reduced in both CC (P = .0036) and GC (P = .0184) of Type 4 follicles. FSH receptor mRNA expression was significantly downregulated in Type 4 CCs (P = .028) and appeared to be variably diminished in Type 4 GCs but failing to reach significance. LH receptor mRNA was expressed more highly in GCs than CCs, as expected, with a trend toward downregulation in Type 4 GCs, although not statistically significant.
Figure 4.
Expression of hormone receptor mRNAs and the number of DEGs potentially affected by hormones. (A) Boxplots portray the FPKM values of ESR1, ESR2, FSHR, and LHCGR for each cell and follicle type. The x-axis denotes follicle Types 1 to 4 and the y-axis denotes the FPKM values for each sample. Significant differences between types; within cell type were overlayed from the DESeq2 analysis. Horizontal lines denote those comparisons with an adjusted P < .05. (B) Barplots show the number of DEGs targeted by each hormone indicated, as predicted by the IPA database, for each cell type and for each comparison indicated on the x-axis. The y-axis quantifies the number of DEGs, with the color denoting the direction of regulation: red = upregulated, blue = downregulated, bifurcated by horizontal line at y = 0. Data generated from Tables S2-S4 (32).
Analysis of the number of DEGs potentially regulated by the different hormones (Fig. 4B) revealed progressively more target DEGs across the series of Type 2, 3, and 4 follicles, consistent with the increasing number of total DEGs. The largest number of target DEGs in CC and GC was seen for estrogen, followed in order of magnitude, with fewer effects elicited by FSH, LH, oxytocin, progesterone, and testosterone. The majority of estrogen, FSH, LH, and oxytocin effects were increases in DEG expression. Progesterone yielded an enhanced number of decreases in DEG expression for CC.
IPA Reveals Affected Pathways and Processes and Implicates Relevant Upstream Regulators
To identify the major CPs and BFs impacted by the transcriptome alterations in follicle Types 2 to 4 compared with Type 1 within each cell type, and to identify potential causative URs directing major components of the transcriptome changes, we subjected DEG lists to IPA.
Oocyte
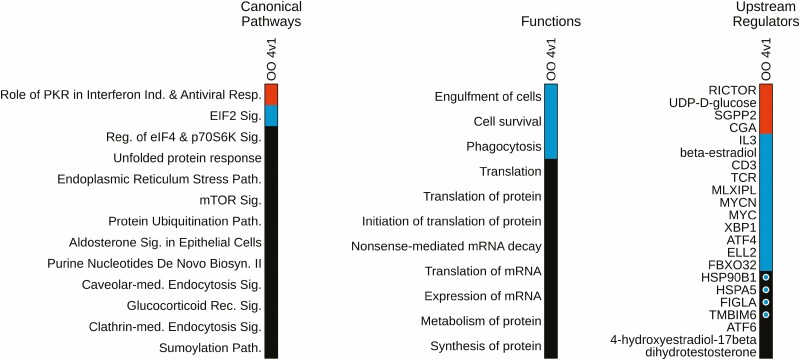
IPA for oocytes was limited to the Type 4 vs 1 comparison, as only this comparison yielded a sufficient number (283, FDR < 0.01) of DEGs for analysis. IPA identified 13 affected CPs (Fig. 5; Table S5 (32)) 1 of which (Role of PKR in Interferon Induction and Antiviral Response) was predicted to be activated (z = 2.45), and another (EIF2 signaling) predicted to be inhibited (z = –2.33). Other prominent affected CPs included the unfolded protein response, endoplasmic reticulum stress pathway, mTOR signaling, protein ubiquitination, clathrin-mediated endocytosis, and the sumoylation pathway. IPA also identified 115 affected BFs (Fig. 5; Table S6 (32)), including 1 predicted as activated (regeneration of axons) and 5 identified as inhibited (phagocytosis, cell survival, cell viability of leukemia cells, engulfment of cells, proliferation of kidney cells). A total of 108 URs showed significant effects with 4 identified as activated (Fig. 5; Table S7 (32)), including RICTOR, UDP-D-glucose, CGA, and SGPP2. A further 11 URs were identified as being inhibited (z ≤ –1.96) including MYCN, MLXIPL, MYC, TCR, CD3, XBP1, ATF4, ELL2, FBXO32, β-estradiol, and IL3. Additional URs were identified as affected, 4 of which were reduced in expression (HSP90B1, HSPA5, FIGLA, and TMBIM6).
Figure 5.
Oocyte IPA pathway, regulator, and function heatmap. Heatmaps depict significantly affected canonical pathways, biological functions, and upstream regulators for the follicle Type 4 vs 1 comparison in oocytes. Colored fill for cells in heat maps denote z-score: red = activated, blue = inhibited, black = no significant z-score. Within the Upstream Regulator panel, the circles within colored fills denote regulators that are also differentially expressed: blue = downregulated. Data generated from Tables S5-S7 (32).
Cumulus Cells
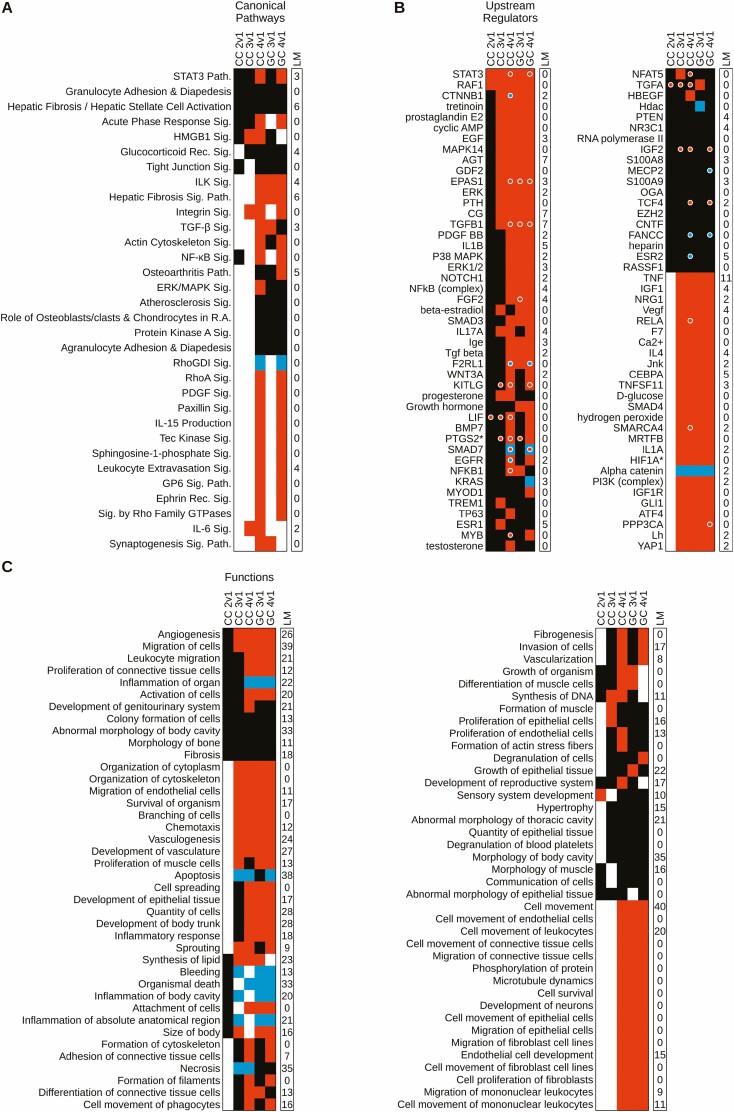
IPA for CC revealed 14 affected CPs for Type 2 CC, none of which were predicted as activated or inhibited (Fig. 6A; Table S5 (32)). For Type 3 CC, there were 15 affected CP (P < .05; Table S5) (32), of which 3 were activated (z ≥ 1.96; Table S5 (32)) and none were inhibited. For Type 4 CCs, 240 CPs were significantly affected, with 152 activated (P < .05; z ≥ 1.96) and 5 inhibited (z ≤ –1.96; Table S5 (32)). There was considerable overlap in affected CPs for Type 4 CCs and Type 4 GCs (Fig. 6A; Table S5 (32)), echoing the other similarities described above for these 2 cell populations. Prominent overlaps included 17 activated CPs (eg, STAT3, ILK, integrin, fibrosis, RHOA, among others) and 1 prominent inhibited CP (RHOGDI signaling) shown in Fig. 6A. Overall, there were 18 shared activated CPs and 46 shared inhibited CPs (Table S5 (32)).
Figure 6.
Cumulus and granulosa cell IPA pathway, regulator, and function heatmap. Heatmap depicts IPA canonical pathways (A), upstream regulators (B), and biological functions (C) for cumulus and granulosa cells. All panels consist of 2 columns (wrapped in 2 portions for B and C): left column denotes dataset of origin with the cell type and follicle type comparison indicated, right column (“LM”) denotes the number of luteinization markers (Fig. 2) present in the pathway. Along the y-axis are the identified, canonical pathways, upstream regulators, and biological functions. Colored fill for cells in heat maps denote z-score: red = activated, blue = inhibited, black = no significant z-score. Level of significant overlap was set at P < .05, and cells with white fill denote those not meeting significance. (A) Entries were limited to those with significance in at least 3 datasets and/or significant z-scores in at least 2; data generated from Table S5 (32). (B) Entries were limited to those with significance in at least 4 datasets and/or significant z-scores in at least 3 datasets; data generated from Table S6 (32). (C) Entries were limited to those with significance in at least 5 datasets and/or significant z-scores in at least 4 datasets; data generated from Table S7 (32).
Despite the limited number of affected CPs for Type 2 CCs, there were 171 BFs that showed significant effects relative to Type 1 CCs (P < .05). One BF, sensory system development, was activated (z = 1.97; Table S6 (32)) and none were inhibited. For Type 3 CCs, there were 310 BFs that showed significant effects (P < .05; Table S6) (32), with 52 identified as being activated (z ≥ 1.96) and 8 identified as being inhibited (z ≤ –1.96). For Type 4 CCs there were 261 affected BFs (P < .05; Table S6 (32)), of which 138 were activated (z ≥ 1.96) and 9 were inhibited (z ≤ -1.96; Table S6 (32)). There was considerable overlap between Type 3 and 4 CCs and Type 3 and 4 GCs for affected BFs, with 54 shared across all 4 cell types (e.g., angiogenesis, migration of cells, organization of cytoskeleton, several entries related to cell morphology, and several entries related to survival), and 98 shared across Type 4 CCs and Type 3 and 4 GCs, which include many entries related to cell movement (Fig. 6C; Table S6 (32)). Additional BF were shared between Type 3 CCs and Type 4 GCs, further echoing the similarities between these cell populations. Apoptosis was predicted to be an inhibited BF in Type 3 and 4 CCs and Type 4 GCs (Fig. 6C; Table S6 (32)).
For Type 2 CCs, 100 URs showed significant effects (P < .05) with 2 activated (z ≥ 1.96; Table S7 (32)) and none inhibited. For Type 3 CCs, 522 URs were affected (P < .05) with 79 identified as activated (z ≥ 1.96; Table S7 (32)) and 4 identified as inhibited (z ≤ –1.96). For Type 4 CCs, 1,044 URs were affected (P < .05) with 240 activated (z ≥ 1.96; Table S7 (32)) and 76 inhibited (z ≤ –1.96). Many URs showed significant effects, with predicted activation across Type 2 to 4 CCs and Type 3 and 4 GCs, and many of these were predicted to be activated. Two URs (STAT3, RAF1) were predicted to be activated across all CC and GC populations, and 40 were predicted to be activated across Type 3 and 4 CCs and GCs (eg, CTNNB1, EGF, TGFB1, TNF, IGF1, VEGF, SMAD4) (Fig. 6B; Table S7 (32)). Beta-estradiol was affected across all 5 cell populations and predicted to be activated in Type 3 CCs and Type 3 and 4 GCs, with positive z-scores (but z < 1.96) for the other cell populations. Progesterone was classified as activated for Type 3 and 4 CCs. Additional affected URs were shared between 2 luteinization markers (PTGS2 and HIF1A). PTGS2 was activated in Type 4 CCs and GCs and upregulated in Type 2 and 3 CCs and Type 3 GCs. HIF1A was activated in Type 3 and 4 CCs and GCs. Type 4 CCs and GCs again echoed the similarities between these 2 cell populations described above. Two URs (EPAS1, TGFB1) were themselves upregulated in Type 3 CCs and Type 3 and 4 GCs (Fig. 6B; Table S7) (32). Others were upregulated (STAT3, KITLG, SMAD7, IGF2, TCF4) or downregulated (F2RL1, FANCC) in Type 3 CCs and Type 4 GCs (Fig. 6B; Table S7) (32). Alpha-catenin was predicted to be inhibited across all Type 2 to 4 CC and GC populations.
Granulosa Cells
IPA for GCs yielded significant results for comparisons of Type 2 to 4 follicles with Type 1 follicles. Due to the limited number of mRNAs identified, IPA was not performed for Type 2 GCs. For Type 3 GCs, there were 45 affected CPs (P < .05), with 4 CPs identified as being activated (z ≥ 1.96; Fig. 6A; Table S5 (32)) including ILK signaling, hepatic fibrosis signaling, TGF-β signaling, and synaptogenesis signaling, and none were inhibited. For Type 4 follicles, 69 CPs were affected (P < .05), with 23 activated (including STAT3, ILK, hepatic fibrosis, integrin, TGF-β, actin cytoskeleton, NF-kB, RHOA, PDGF, paxillin, spingosine-1-phosphate, and other signaling pathways) and 1 (RHOGDI signaling) inhibited (Fig. 6A; Table S5 (32)).
There were 351 significantly affected BFs (P < .05) for Type 3 GC, with 102 identified as activated (z ≥ 1.96; Fig. 6C; Table S6 (32)) and 8 inhibited (z ≤ –1.96). For Type 4 GC, there were 263 BFs that showed significant effects (P < .05; Table S6 (32)), with 153 identified as activated (z ≥ 1.96) and 12 identified as inhibited (z ≤ –1.96). There was considerable overlap between Type 3 and Type 4 affected BFs (Fig. 6C; Table S6 (32)), including activation of angiogenesis, cell migration, vasculogenesis, and various entries related to cell movement and cell/organismal survival, as well as inhibition of several entries related to inflammation. Type 4 GCs additionally displayed inhibition of apoptosis, which was also affected in Type 3 GCs but without a significant z-score.
Type 3 GCs yielded 1235 affected URs (P < .05). Of these, 201 URs were identified as activated (z ≥ 1.96; Table S7 (32)) and a further 26 were identified as being inhibited (z ≤ –1.96). For Type 4 GCs, 1549 URs showed significant effects (P < .05), 391 URs were activated (z ≥ 1.96), and 118 were inhibited (z ≤ 1.96; Table S7 (32)). Activated URs included many prominent reproductive or ovarian factors such as β-estradiol, TGFβ1, STAT3, YAP1, and others. There was once again considerable overlap between Type 3 and 4 GCs for affected URs (Fig. 6B; Table S7 (32)). These included predicted activation of STAT3, RAF1, CTNNB1, TGFβ1, among many others, and inhibition of alpha catenin. A number of affected URs were themselves among the DEG lists, including STAT3, KITLG, SMAD7, IGF2, and TCF4, which were upregulated, and F2RL1, FANCC, and MECP2, which were downregulated in Type 4 GCs, and EPAS1 and TGFβ1, which were upregulated in Type 3 and 4 GCs. SMAD7 downregulation contrasted with its predicted activation in Type 4 GCs.
FSH Impacts Major CPs and BFs Downstream of β-Estradiol and KIT Ligand
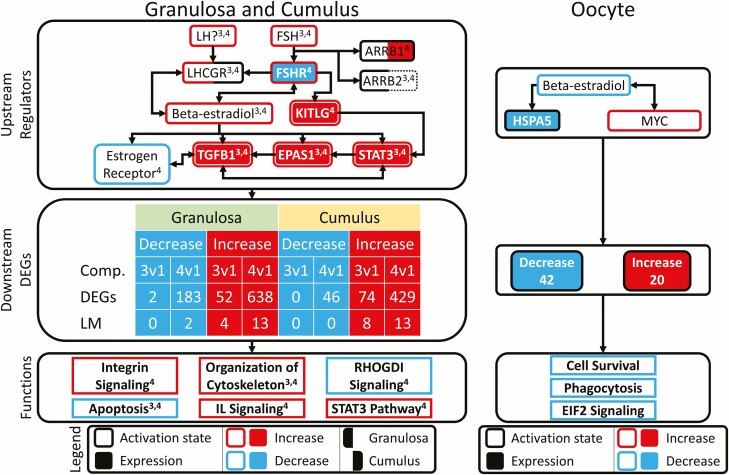
The above IPA revealed significant effects on many CPs, BFs, and URs. To connect these effects to actions of FSH, we used the IPA Path Explorer tool to connect identified URs from Type 3 and 4 CCs and GCs and to identify additional URs that connect FSH stimulation to downstream effects on CPs and BFs in CCs. This analysis identified a system of extended networks shared in both CCs and GCs along with an affected extended network in oocytes (Fig. 7). In CCs and GCs, the predominant effect was initiated through FSH and the FSHR. Although IPA indicated predicted activation of LH signaling in GCs, this could reflect instead the extensive overlaps in downstream actions of the 2 hormones. FSHR was downregulated in Type 4 cells, but its activity was predicted to be activated in both CCs and GCs (Figs. 4 and 7; Table S6 (32)). FSHR activation was connected to increased β-estradiol signaling and to increased expression and activity of TFGβ1, EPAS1, and STAT3 in both Type 3 and 4 cells, and to inhibited activity of estrogen receptor in Type 4 cells. FSHR was also connected to increased KITL expression and activation in Type 4 cells. Additional actions were predicted to be mediated via significant changes (no z-score) in arrestins: ARRB1 (Type 4 GCs and CCs) and ARRB2 (Type 3 and 4 GCs) with increased expression of ARRB1 in Type 4 CCs. These effects on URs then worked via effects on increased and decreased DEG expression ultimately to elicit predicted increases in CPs and BFs (integrin signaling, cytoskeleton organization, STAT3 signaling, and IL1 signaling), along with reduced RHOGDI signaling and apoptosis in Type 4 CCs and GCs. The effects on cytoskeleton organization and apoptosis were also seen for Type 3 CCs and GCs.
Figure 7.
Expanded network analysis of IPA results. Custom expanded network figure linking significant upstream regulators, their downstream DEG targets, and enriched pathways and functions, derived from the IPA software. Figure is split into 2 panels: left panel contains joint information for granulosa and cumulus cell (3v1 and 4v1, comparisons), right panel contains oocyte (4v1 comparison). Each panel is further divided into 3 sections: (1) upstream regulators, (2) downstream DEGs, and (3) functions. Section 1 contains upstream regulators, connected in a network, which were identified with significant z-scores, P values of overlap, and/or were differentially expressed. The downstream DEG section tabulates the number of DEGs for each cell type, bifurcated by direction of regulation, and source of comparison. The function section identifies the pathways and functions significantly enriched by the above DEGs and upstream regulators. For the granulosa and cumulus cell section, superscripts denote which comparison of origin: 3 = 3v1, 4 = 4v1. For both vertical sections, outer border colors for molecules denote significant predicted z-score (|z| > 1.96): blue = inhibited, red = activated. Interior solid coloring denotes molecules for which mRNAs are differentially expressed (FDR < 0.05): blue = downregulated, red = upregulated. Outer borders in solid black indicate a significant effect without z score, while dashed outer borders denote instances of nonsignificant P values. Left and right portions of borders and inner fills denote results for granulosa and cumulus cells, respectively. The “?” symbol by LH indicates that, although IPA suggests LH as a possible affected UR, there is substantial overlap between FSH and LH signaling (about 2/3 of pathway members).
In Type 4 oocytes, the IPA extended network analysis highlighted effects initiated by a predicted decrease in β-estradiol signaling with reduced expression of HSPA5 and increased activation of MYC signaling. These URs impacted 62 DEGs that were associated with predicted downstream effects including reduced cell survival, phagocytosis and EIF2 signaling (Fig. 7).
Assessment of Possible Atretic State in Type 4 Follicles
One possible impact of excessive FSH could be to induce follicular atresia. To assess whether this is occurring, a previously defined set of bovine ovarian follicular atresia markers totaling 197 were downloaded from a meta-analysis on bovine granulosa cells (55). The reported 197 marker genes were filtered here to include only those with an average P < .05 (n = 183), and, of these, 149 displayed detected expression in our dataset. These 149 genes were intersected with the DEG lists for both CCs and GCs, comparing follicle Type 2 to 4 with Type 1 (Figure S2 (32)). Only 54 of the 149 were identified as DEGs in at least 1 of the 6 comparisons. Twenty were affected in both GCs and CCs for Type 4 vs 1 and in the expected direction. Of the top 30 DEGs reported by (55), 9 were not detected (NMB, OSAP, CRISPLD2, CLCA2, DEFB4, CD5L, SWAP70, DEFBS, FABP4), 5 displayed expected increased expression in both CCs and GCs, (TRIB1, SH3RF1, CKB, ETS2, PLAT), 4 displayed expected decreased expression (CALB2, SUSD4, TRC1D8, TOX2), 3 were affected only in GCs (FOLR2, GSDMB, CTSS), 6 were unaffected (INHBA, GRB14, NAPIL5, CITED1, LCRRC17, FDFT1, IL1A), and 1 displayed the opposite to expected effect (GSTA5).
Effects of cpFSH Dose on Genes Related to Progesterone Synthesis and Oxytocin Signaling
A prominent feature of the cpFSH effect is an increase in follicular levels of progesterone and oxytocin, particularly in Type 4 follicles. Relative to Type 1 follicles, we observed increased expression of mRNAs encoding STAR in CC from Type 4 follicles (Row 2860; Table S2 (32)) and GC from Type 3 and 4 follicles (Row 1213; Table S3 (32)), and HSD3B1 in CC from Type 4 follicles (Row 788; Table S2 (32)). We also noted significant positive IPA results for “Steroidogenesis of hormone” and “Synthesis of steroid” in Type 3 CCs and GCs, with a significant positive z-score for Type 3 CCs (Rows 255 and 385, respectively; Table S6 (32)). Likewise, relative to Type 1 follicles expression of the mRNA encoding oxytocin, OXT, was increased in CCs from Type 4 follicles (Row 926, Table S2 (32)) and GCs from Type 3 and 4 follicles (Row 406; Table S3 (32)). We did not observe an increase in oxytocin receptor mRNA expression in CC or GC.
Discussion
The main discovery from this study is that excessive cpFSH doses (3-fold above industry standard), associated with a premature luteinization phenotype (based on intrafollicular steroid hormone concentrations and COC morphology (24)) in most ovulatory-size follicles, lead to progressively more severe changes in the transcriptomes of CCs and GCs as follicular abnormalities increased. These 2 cell types become more alike with the severity of follicular abnormality, while also diverging substantially from their normal phenotypes seen in follicles from animals receiving the industry standard cpFSH dose. These changes are accompanied by less severe changes in the oocyte transcriptome; however, the oocyte transcriptome changes suggest significant alterations in cellular physiology that may compromise oocyte quality. We recognize that oocytes execute major changes in their proteomes via temporally regulated translation and degradation of stored mRNAs as well as post-translational processes. However, observed changes in the relative abundances in abnormal follicles, particularly deficiencies in essential mRNAs or mRNAs related to essential oocyte processes, can reasonably be interpreted as indicating potential compromise in oocyte quality, either through deficient mRNA content or through abnormal regulation of stabilization/destabilization impacting the capacity to produce key proteins when needed. Thus, although oocytes from Type 2 and 3 follicles display very minor transcriptome changes, the more extensive changes exhibited in oocytes from Type 4 follicles indicate a potential compromise in oocyte quality. Further studies to assess oocyte quality would be valuable, but given complexity and cost are beyond the scope of this report.
Overall, about half (Types 3 and 4) of the ovulatory-size follicles in animals receiving the excessive cpFSH dose display substantial transcriptome changes, which may reflect loss of FSH sensitivity during folliculogenesis, for example through downregulation of FSH receptor signaling pathways. Such a loss of sensitivity would disrupt gene regulation, leading to the previously reported reduction in ovarian function during ovarian stimulation with excessive cpFSH doses in the SORH model (3, 24). Moreover, our analysis indicates that the orderly progression of cellular and molecular changes typical of the final stages of folliculogenesis are severely disrupted, with aspects of ovulation, inhibited apoptosis and stress, and partial luteinization occurring together, and are associated with compromised oocyte health in the most severely affected follicles. Rather than simply reflecting a shift in the intrafollicular endocrine milieu characteristic of premature luteinization, the transcriptome data indicate even more drastic alterations in ovulatory follicle function that do not compare readily to the normal follicular developmental progression. Collectively, these observations indicate that excessive cpFSH doses lead to follicular hyperstimulation dysgenesis negatively impacting ovulatory follicle function and oocyte viability. Previous studies in cattle found that prolonged FSH doses led to altered GC transcriptomes (56, 57). The results here greatly extend our understanding of adverse effects of excess cpFSH on ovarian cells, and further indicate a significant risk of using FSH doses greater than necessary to achieve a maximum response during ovarian stimulation protocols (i.e., excessive doses), with the potential to reduce desired ART outcomes by diminishing ovarian function and compromising oocyte quality.
The mechanisms by which excessive FSH doses cause ovarian follicle dysfunction resulting in poor oocyte quality and ART outcomes have been unknown. Results from an earlier meta-analysis revealed a novel set of follicular cell biomarker genes that could be predictive of oocyte quality, and suggested that correct synchrony of the developmental transition in gene expression among the CCs, GCs, and oocytes may be disrupted by high FSH doses, which could negatively impact oocyte quality (58). Another meta-analysis of array studies examining developmental GC or CC transcriptome changes and oocyte transcriptome changes associated with FSH coasting also indicate effects on oocyte quality (59). Using the SORH model, key changes in the different cell types that comprise an ovulatory-size follicle (CCs, GCs, and oocytes) following ovarian stimulation with an excessive 210 IU cpFSH dose were evaluated in detail using RNA-seq and provide new mechanistic insights into the effects of excessive FSH doses during ovarian stimulation.
Building on our previous work characterizing ovulatory-sized follicles according to follicular fluid E:P ratios and COC morphology (24), we show herein that the increasing severity in follicle phenotypes with excessive cpFSH doses in the SORH model (i.e., progressing from Type 2 to 4) was accompanied by a similarly intensifying disruption in transcriptomes. This was particularly evident in the somatic (CCs and GCs) cells of the follicle. We also observed a significant downregulation of FSHR mRNA in CCs and a nonsignificant decrease in GCs of Type 4 follicles, indicating a potential desensitization of these follicles to FSH. This suggests either an intrinsic difference in the capacity to respond to cpFSH stimulation (60) or that individual follicles were exposed to different amounts of cpFSH as a result of differential vascular supply (61, 62). Either possibility may explain the observed variation in EA and EI states in follicles from the same heifer, given that β-estradiol and progesterone production by GCs are induced by FSH (63). Additionally, high cpFSH doses were associated with predicted disruptions in cell–cell communication and cellular physiology that may reduce oocyte quality. Overall, these data indicate that excessive FSH doses during ovarian stimulation result in varying degrees of abnormality in most ovulatory-size follicles, with significant negative impacts on key molecular and cellular processes.
Antrum formation is typically associated with divergence of CC and GC phenotypes and function, as well as spatial separation within the follicle, with CCs, but not GCs, remaining in direct contact with the oocyte (64-66). The AFC for experimental animals in our study was monitored by ultrasonography prior to and throughout the ovarian stimulation period. We observed that the majority of follicles responding to stimulation with either cpFSH dose were antral follicles (≥3 mm) present on the ovary prior to initiation of the ovarian stimulation protocol. Thus, the divergence of CCs and GCs prior to ovarian stimulation is inferred. With the high cpFSH dose, CC and GC transcriptomes became progressively more different from normal transcriptome profiles, and became more alike with greater follicular abnormality, a relationship reflected in the markers of luteinization and in the IPA results, where there were a large proportion of shared URs and BFs. For example, in the luteinization markers analysis, the transcriptomes of CCs from Type 3, and GCs from Type 3 and 4 follicles, were similar to each other but distinct from the control Type 1 CCs or GCs. This change signifies an increasingly severe and profound disruption in follicular biology with high cpFSH doses. Several possibilities could explain such a change including, but not limited to, atresia, premature luteinization or differentiation into an unidentified cell type. The features of EI (e.g., Type 4) follicles are generally thought to reflect atresia (25, 26, 67). The limited effects observed on the expression of bovine atresia marker genes could be interpreted as indicating a mild early atretic phenotype. However, the IPA indicated that apoptosis and necrosis, both hallmarks of atresia, were inhibited in CCs and GCs of Type 3 and 4 follicles, and thus do not support this interpretation. Instead, IPA revealed a novel and complex phenotype, wherein increasing severity of follicle abnormality was accompanied by activation of URs (e.g., EGF, TGFB1, TNF) known to influence GC differentiation and proliferation (68-70) and numerous changes indicative of tissue remodeling (eg, angiogenesis, cell movement/migration, proliferation and differentiation of cells). Many of these are hallmarks of the complex and tightly regulated changes that occur in ovarian follicles in preparation for ovulation and resultant luteinization (71).
We presented evidence that the phenotypic differences (eCOCs and follicular fluid E:P ratio) were indicative of premature luteinization, particularly in the Type 4 follicles (24). However, premature luteinization refers to mostly clinical observations following ovarian stimulation with high FSH doses, where peripheral E:P concentrations were shifted favoring progesterone production and suggesting that follicles were undergoing luteinization without an ovulatory (LH or hCG) stimulus (11-13, 72). Key steroidogenic enzymes involved in progesterone synthesis were also upregulated including STAR (increased in Type 4 CCs and GCs) and HSD3B1 (increased in Type 4 GCs). Examining a manually curated list of luteinization markers (44-52) revealed that a majority of luteinization markers were not affected in any cell type from any of the follicle types. These observations indicate that premature luteinization in this system is not due to a simple conversion of GCs or CCs to a corpus luteum–like state. Rather, both GCs and CCs progressively deviate toward a cell state characterized by a loss of cell–cell communication within the follicle, as evidenced by the morphological expansion of the COC and shared upregulation of BFs relating to changes in cell morphology (including branching of cells and organization of cytoskeleton) and cell migration (including migration of cells and cell movement). Moreover, many of these changes predicted by the IPA, including key prodifferentiation URs shared by GCs and CCs in Type 3 and 4 follicles (depicted in Figs. 6 and 7; eg, TGFB1, EGF, TNF, EPAS1) and BFs (including interleukin signaling, integrin signaling, reorganization of the cytoskeleton, angiogenesis, and immune or inflammatory responses) support the idea that molecular changes within these cells are associated with reorganization of the follicles characteristic of ovulation (68, 71, 73). This suggests a temporal dysregulation of the final stages of folliculogenesis, whereby a chaotic combination of processes characteristic of ovulation and luteinization occur concurrently, rather than sequentially. This may explain the increased incidence of anovulation observed in our previous study with the SORH model in response to ovarian stimulation with the excessive cpFSH dose (3).
An unaddressed concern in the field of bovine ART has been the impact of LH contamination present in the cpFSH preparations used for ovarian stimulation. Gonadotropin preparations with significant LH activity or contamination were utilized in many of the foundational studies leading to the development of ovarian stimulation in cattle (e.g., pregnant mare serum gonadotropin; PMSG (74)) and women (e.g., human pituitary gonadotropin; hPG (75)). Despite widespread use of recombinant hFSH in human ART, commercially available recombinant bovine equivalents are not currently available. Using the SORH model and Folltropin-V, a commonly used cpFSH preparation, we have shown the LH contamination contained in this preparation was not sufficient to increase circulating progesterone concentrations or induce premature ovulation, even in excessive dose treated animals (3). The only differences in gonadotropin receptor expression were observed in the CCs where FSHR expression in Type 4 follicles was reduced, a pattern also observed in the GCs, implicating FSH signaling as the major driver for differences in follicle types. Moreover, the convergence of CC and GC transcriptomes suggests excessive cpFSH, mimics some (but not all) aspects of LH signaling in a subset (Type 3 and 4) of follicles. As reflected in the expanded network analysis, the downstream impacts of FSH and LH on CCs and GCs are inextricably linked. For instance, EGF-like factors, key intermediaries of LH signaling in the follicle and with key roles inducing CC expansion and oocyte maturation, are also produced in response to FSH (76-78). In in vitro maturation experiments with recombinant FSH, LH was still identified as a key UR supporting the argument that in vitro FSH may induce signaling events in the COC similar to those observed in response to LH on in vivo follicle development (79, 80). Based on the data herein, it could also be argued that excessive FSH doses in vivo also induce changes in a proportion of follicles that previously have been attributable to LH.
Despite the oocyte yielding the fewest DEGs, IPA of the Type 4 vs 1 oocyte data indicated potentially deleterious changes affecting key processes relating to oocyte function during maturation. In contrast to the CCs and GCs, the activity of the key UR, β-estradiol (reduced in follicular fluid in Type 4 follicles), appeared to be inhibited in the oocyte. This was accompanied by predicted inhibition of cell survival, phagocytosis and EIF2 signaling. Although estrogen signaling is not essential for oocyte maturation in vivo (81), estradiol and oocyte secreted factors have indispensable roles in maintaining the CC phenotype and function (66, 82). Whether loss of CC communication with the oocyte preceded and/or induced the reported changes in oocyte function could not be determined. The EIF2 signaling pathway has a role during oocyte nuclear maturation, particularly at the germinal vesicle to metaphase II transition during meiosis (83, 84). Thus, reduced EIF2 signaling may be indicative of compromised endoplasmic reticulum stress and unfolded protein response pathways, increasing oocyte susceptibility to stressors such as oxidative stress (85, 86). Likewise, both engulfment of cells and phagocytosis were downregulated in Type 4 oocytes, potentially signifying a reduced capacity for fertilization (87). However, in combination with the reduced cell survival, the decreased phagocytosis may instead reflect a shift in the balance between apoptosis and autophagy, known to be characteristic of oocyte cell death, reflecting an emerging atretic state (88-90).
This study has demonstrated that ovarian stimulation with an excessive cpFSH dose resulted in a convergence of CC and GC transcriptomes as phenotypic follicle abnormalities increased. We cannot state whether CCs became more GC-like or vice versa, or whether both cell types acquired a different abnormal phenotype as follicular abnormalities increased. Moreover, it does not appear that either GCs or CCs simply convert to a corpus luteum-like state, as would be expected for simple premature luteinization. Instead, it appears that the changes in GCs and CCs reflect an overall loss of cell–cell communication within the follicle that resulted in the dysregulation of maturational processes in both CCs and GCs, associated with altered oocyte functions that could compromise oocyte quality. Together with our previous observations of reduced ovulation rates in SORH heifers treated with an excessive cpFSH dose during ovarian stimulation, and the fact that Type 3 and 4 follicles represented ~50% of ovulatory-sized follicles produced, the dysfunction observed in the CCs, GCs, and the oocyte likely impair ovulation and would likely result in poor ART outcomes due to reduced oocyte quality. Overall, given that the observed hyperstimulation dysgenesis affected the majority of follicles produced, these results provide a compelling argument against the use of excessive FSH doses during ovarian stimulation in SORH cattle and in small ovarian reserve patients seeking ART.
Glossary
Abbreviations
- AFC
antral follicle count
- ART
assisted reproductive technology
- BF
biological function
- CC
cumulus cell
- cCOC
compact cumulus–oocyte complex
- CP
canonical pathway
- DEG
differentially expressed gene
- DF
disease and function
- DPBS
Dulbecco’s phosphate-buffered saline
- EA
estrogen-active
- eCOC
expanded COC
- EI
estrogen-inactive
- E:P
estradiol:progesterone
- FDR
false discovery rate
- FSH
follicle-stimulating hormone
- GC
granulosa cell
- hCG
human chorionic gonadotropin
- hFSH
human FSH
- IPA
Ingenuity Pathway Analysis
- LH
luteinizing hormone
Contributor Information
Zaramasina L Clark, Reproductive and Developmental Sciences Program and the Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA; School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand.
Meghan L Ruebel, Reproductive and Developmental Sciences Program and the Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA; USDA-ARS Arkansas Children’s Nutrition Center 15 Children’s Way Little Rock, AR 72202, USA.
Peter Z Schall, Reproductive and Developmental Sciences Program and the Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA; University of Michigan Medical School, Department of Human Genetics, Ann Arbor, Michigan, USA.
Kaitlin R Karl, Reproductive and Developmental Sciences Program and the Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA.
James J Ireland, Reproductive and Developmental Sciences Program and the Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA.
Keith E Latham, Reproductive and Developmental Sciences Program and the Department of Animal Science, Michigan State University, East Lansing, MI 48824, USA.
Funding
This study was supported by the Agriculture and Food Research Initiative Competitive USDA-NIH Dual Purpose Program (grant no. 2017-67015-26084), the USDA National Institute of Food and Agriculture, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number T32HD087166, and AgBioResearch at Michigan State University.
Disclosure Summary
The authors have nothing to disclose.
Data Availability
Supplemental data files are available at Figshare https://figshare.com/search?q=1920860410.6084%2Fm9.figshare.19208604, doi: 10.6084/m9.figshare.19208604 (32). RNA sequencing data are available at the Gene Expression Omnibus (accession number GSE197116) (38).
References
- 1. Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ. Gonadotropin dose is negatively correlated with live birth rate: analysis of more than 650,000 assisted reproductive technology cycles. Fertil Steril. 2015;104(5):1145-52.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clark ZL, Thakur M, Leach RE, Ireland JJ. FSH dose is negatively correlated with number of oocytes retrieved: analysis of a data set with ~650,000 ART cycles that previously identified an inverse relationship between FSH dose and live birth rate. J Assist Reprod and Genet. 2021;38(7):1787-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karl KR, Jimenez-Krassel F, Gibbings E, et al. Negative impact of high doses of follicle-stimulating hormone during superovulation on the ovulatory follicle function in small ovarian reserve dairy heifers. Biol Reprod. 2020;104(3):695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGowan MR, Braithwaite M, Jochle W, Mapletoft RJ. Superovulation of beef heifers with Pergonal (HMG): a dose response trial. Theriogenology. 1985;24(2):173-184. [DOI] [PubMed] [Google Scholar]
- 5. Pawlyshyn V, Lindsell CE, Braithwaite M, Mapletoft RJ. Superovulation of beef cows with FSH-P: a dose-response trial. Theriogenology. 1986;25(1):179. [Google Scholar]
- 6. Donaldson LE. Dose of FSH-P as a source of variation in embryo production from superovulated cows. Theriogenology. 1984;22(2):205-212. [DOI] [PubMed] [Google Scholar]
- 7. Greve T, Lehn-Jensen H, Rasbech NO. Morphological evaluation of bovine embryos recovered non-surgically from superovulated dairy cows on days 6 1/2 to 7 1/2: a field study. Ann Biol Anim Biochim Biophys. 1979;19(5):1599-1611. [Google Scholar]
- 8. Escudero E, Bosch E, Crespo J, Simon C, Remohi J, Pellicer. A. Comparison of two different starting multiple dose gonadotropin-releasing hormone antagonist protocols in a selected group of in vitro fertilization-embryo transfer patients. Fertil Steril. 2004;81(3):562-566. [DOI] [PubMed] [Google Scholar]
- 9. Ubaldi F, Camus M, Smitz J, Bennink HC, Van Steirteghem A, Devroey P. Premature luteinization in in vitro fertilization cycles using gonadotropin-releasing hormone agonist (GnRH-a) and recombinant follicle-stimulating hormone (FSH) and GnRH-a and urinary FSH. Fertil Steril. 1996;66(2):275-280. [DOI] [PubMed] [Google Scholar]
- 10. Tapanainen JS, Lapolt PS, Perlas E, Hsueh AJ. Induction of ovarian follicle luteinization by recombinant follicle-stimulating hormone. Endocrinology. 1993;133(6):2875-2880. [DOI] [PubMed] [Google Scholar]
- 11. Fanchin R, de Ziegler D, Taieb J, Hazout A, Frydman R. Premature elevation of plasma progesterone alters pregnancy rates of in vitro fertilization and embryo transfer. Fertil Steril. 1993;59(5):1090-1094. [DOI] [PubMed] [Google Scholar]
- 12. Hofmann GE, Bentzien F, Bergh PA, et al. Premature luteinization in controlled ovarian hyperstimulation has no adverse effect on oocyte and embryo quality. Fertil Steril. 1993;60(4):675-679. [DOI] [PubMed] [Google Scholar]
- 13. Mascarenhas M, Kamath MS, Chandy A, Kunjummen AT. Progesterone/Estradiol ratio as a predictor in the ART cycles with premature progesterone elevation on the day of hCG trigger. J Reprod Infertil. 2015;16(3):155-161. [PMC free article] [PubMed] [Google Scholar]
- 14. Schoolcraft W, Sinton E, Schlenker T, Huynh D, Hamilton F, Meldrum DR. Lower pregnancy rate with premature luteinization during pituitary suppression with leuprolide acetate. Fertil Steril. 1991;55(3):563-566. [PubMed] [Google Scholar]
- 15. Stanger JD, Yovich JL. Reduced in-vitro fertilization of human oocytes from patients with raised basal luteinizing hormone levels during the follicular phase. Br J Obstet Gynaecol. 1985;92(4):385-393. [DOI] [PubMed] [Google Scholar]
- 16. Younis JS, Haddad S, Matilsky M, Ben-Ami M. Premature luteinization: could it be an early manifestation of low ovarian reserve? Fertil Steril. 1998;69(3):461-465. [DOI] [PubMed] [Google Scholar]
- 17. Lawrenz B, Melado L, Fatemi H. Premature progesterone rise in ART-cycles. Reprod Biol. 2018;18(1):1-4. [DOI] [PubMed] [Google Scholar]
- 18. Kaponis A, Chronopoulou E, Decavalas G. The curious case of premature luteinization. J Asst Reprod Gen. 2018;35(10):1723-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burns DS, Jimenez-Krassel F, Ireland JLH, Knight PG, Ireland JJ. Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol Reprod. 2005;73(1):54-62. [DOI] [PubMed] [Google Scholar]
- 20. Ireland JLH, Scheetz D, Jimenez-Krassel F, et al. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol Reprod. 2008;79(6):1219-1225. [DOI] [PubMed] [Google Scholar]
- 21. Ireland JJ, Ward F, Jimenez-Krassel F, et al. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum Reprod. 2007;22(6):1687-1695. [DOI] [PubMed] [Google Scholar]
- 22. Oudendijk JF, Yarde F, Eijkemans MJC, Broekmans FJM, Broer SL. The poor responder in IVF: is the prognosis always poor? A systematic review. Hum Reprod Update. 2011;18(1):1-11. [DOI] [PubMed] [Google Scholar]
- 23. Broer SL, van Disseldorp J, Broeze KA, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update. 2013;19(1):26-36. [DOI] [PubMed] [Google Scholar]
- 24. Clark ZL, Karl KR, Ruebel ML, Latham KE, Ireland JJ. Excessive follicle-stimulating hormone during ovarian stimulation of cattle may induce premature luteinization of most ovulatory-size follicles. Biol Reprod. 2022;106(5):968-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ireland JJ, Roche JF. Development of antral follicles in cattle after prostaglandin-induced luteolysis: changes in serum hormones, steroids in follicular fluid, and gonadotropin receptors. Endocrinology. 1982;111(6):2077-2086. [DOI] [PubMed] [Google Scholar]
- 26. Ireland JJ, Roche JF. Growth and differentiation of large antral follicles after spontaneous luteolysis in heifers: changes in concentration of hormones in follicular fluid and specific binding of gonadotropins to follicles. J Anim Sci. 1983;57(1):157-167. [DOI] [PubMed] [Google Scholar]
- 27. Assidi M, Dieleman SJ, Sirard MA. Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: potential early markers of oocyte competence. Reproduction. 2010;140(6):835-852. [DOI] [PubMed] [Google Scholar]
- 28. Ireland JJ, Roche JF. Development of nonovulatory antral follicles in heifers: changes in steroids in follicular fluid and receptors for gonadotropins. Endocrinology. 1983;112(1):150-156. [DOI] [PubMed] [Google Scholar]
- 29. Folltropin-V. Vol 2021: VETOQUINOL N.-A. INC., Commerical Division, 2000, CHEMIN GEORGES, LAVALTRIE, QC, J57 3S5. [Google Scholar]
- 30. Price CA, Carrière PD, Gosselin N, Kohram H, Guilbault LA. Effects of superovulation on endogenous LH secretion in cattle, and consequences for embryo production. Theriogenology. 1999;51(1):37-46. [DOI] [PubMed] [Google Scholar]
- 31. Krämer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2013;30(4):523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clark ZL, Ruebel ML, Schall PZ, Karl KR, Ireland JJ, Latham KE. Follicular hyperstimulation dysgenesis: a new explanation for adverse effects of excessive FSH doses during ovarian stimulation. Figshare. https://figshare.com/search?q=1920860410.6084%2Fm9.figshare.19208604. Doi: April 7, 2022.10.6084/m9.figshare.19208604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scheetz D, Folger JK, Smith GW, Ireland JJ. Granulosa cells are refractory to FSH action in individuals with a low antral follicle count. Reprod Fertil Dev. 2012;24(2):327-336. [DOI] [PubMed] [Google Scholar]
- 34. Voss AK, Fortune JE. Oxytocin secretion by bovine granulosa cells: effects of stage of follicular development, gonadotropins, and coculture with theca interna. Endocrinology. 1991;128(4):1991-1999. [DOI] [PubMed] [Google Scholar]
- 35. Voss AK, Fortune JE. Oxytocin/neurophysin-I messenger ribonucleic acid in bovine granulosa cells increases after the luteinizing hormone (LH) surge and is stimulated by LH in vitro. Endocrinology. 1992;131(6):2755-2762. [DOI] [PubMed] [Google Scholar]
- 36. Meidan R, Wolfenson D, Thatcher WW, et al. Oxytocin and estradiol concentrations in follicular fluid as a means for the classification of large bovine follicles. Theriogenology 1993;39(2):421-432. [DOI] [PubMed] [Google Scholar]
- 37. Inc. SI. SAS/SHARE® 9.4: User’s Guide, 2nd ed. SAS Institute Inc; 2019. https://www.sas.com/en_us/legal/editorial-guidelines.html [Google Scholar]
- 38. Latham KE, Clark ZL, Ruebel ML, Karl KR, Schall PZ, Ireland JJ. Effects of excessive FSH dose on granulosa cell, cumulus cell and oocyte transcriptomes in small ovarian reserve heifers. Gene Expression Omnibus. 2022. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE197116 [Google Scholar]
- 39. Krueger F, James F, Ewels P, Afyounian E, Schuster-Boeckler B.. FelixKrueger/TrimGalore: v0.6.7 – DOI via Zenodo (0.6.7). July23, 2021. https://zenodo.org/record/5127899#.YtS0RsHMJ44 [Google Scholar]
- 40. Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525-527. [DOI] [PubMed] [Google Scholar]
- 41. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protocols. 2009;4(8):1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chorostecki U, Molina M, Pryszcz LP, Gabaldon T. MetaPhOrs 2.0: integrative, phylogeny-based inference of orthology and paralogy across the tree of life. Nucleic Acids Res. 2020;48(W1):W553-W557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Christenson LK, Gunewardena S, Hong X, Spitschak M, Baufeld A, Vanselow J. Research resource: preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol Endocrinol. 2013;27(7):1153-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hamel M, Dufort I, Robert C, Léveillé M-C, Leader A, Sirard M-A. Identification of follicular marker genes as pregnancy predictors for human IVF: new evidence for the involvement of luteinization process. Mol Hum Reprod. 2010;16(8):548-556. [DOI] [PubMed] [Google Scholar]
- 46. Romereim SM, Summers AF, Pohlmeier WE, et al. Gene expression profiling of bovine ovarian follicular and luteal cells provides insight into cellular identities and functions. Mol Cell Endocrinol. 2017;439(12017):379-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baddela VS, Sharma A, Viergutz T, Koczan D, Vanselow J. Low oxygen levels induce early luteinization associated changes in bovine granulosa cells. Front Physiol. 2018;9(1066). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee HJ, Jee BC, Kim SK, et al. Expressions of aquaporin family in human luteinized granulosa cells and their correlations with IVF outcomes. Hum Reprod. 2016;31(4):822-831. [DOI] [PubMed] [Google Scholar]
- 49. Fan H-Y, Liu Z, Johnson PF, Richards JS. CCAAT/Enhancer Binding Protein (C/EBP)-alpha and -beta are essential for ovulation and luteinization by regulating the expression of novel target genes. Biol Reprod. 2010;83(Suppl 1):151-151. [Google Scholar]
- 50. Bertolin K, Gossen J, Schoonjans K, Murphy BD. The orphan nuclear receptor Nr5a2 is essential for luteinization in the female mouse ovary. Endocrinology. 2014;155(5):1931-1943. [DOI] [PubMed] [Google Scholar]
- 51. Lee-Thacker S, Jeon H, Choi Y, et al. Core Binding Factors are essential for ovulation, luteinization, and female fertility in mice. Sci Rep. 2020;10(1):9921-9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gilbert I, Robert C, Dieleman S, Blondin P, Sirard MA. Transcriptional effect of the LH surge in bovine granulosa cells during the peri-ovulation period. Reproduction. 2011;141(2):193-205. [DOI] [PubMed] [Google Scholar]
- 53. Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847-2849. [DOI] [PubMed] [Google Scholar]
- 54. Wilke C, Fox SJ, Bates T, et al. Bugfix release for changes in testing infrastructure cowplot (v1.1.1) wilkelab/cowplot: 1.1.1 (Version 1.1.1). January 2, 2021. https://zenodo.org/record/4411966#.YtS3kMHMJ44 [Google Scholar]
- 55. Landry DA, Rossi-Perazza L, Lafontaine S, Sirard MA. Expression of atresia biomarkers in granulosa cells after ovarian stimulation in heifers. Reproduction. 2018;156(3):239-248. [DOI] [PubMed] [Google Scholar]
- 56. Dias FC, Khan MI, Adams GP, Sirard MA, Singh J. Granulosa cell function and oocyte competence: super-follicles, super-moms and super-stimulation in cattle. Anim Reprod Sci. 2014;149(1-2):80-89. [DOI] [PubMed] [Google Scholar]
- 57. Dias FCF, Khan MIR, Sirard MA, Adams GP, Singh J. Transcriptome analysis of granulosa cells after conventional vs long FSH-induced superstimulation in cattle. BMC Genomics. 2018;19(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sirard MA. Folliculogenesis and acquisition of oocyte competence in cows. Anim Reprod. 2019;16(3):449-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Khan DR, Landry DA, Fournier E, Vigneault C, Blondin P, Sirard MA. Transcriptome meta-analysis of three follicular compartments and its correlation with ovarian follicle maturity and oocyte developmental competence in cows. Physiol Genomics. 2016;48(8):633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McNatty KP, Heath DA, Hudson NL, et al. The conflict between hierarchical ovarian follicular development and superovulation treatment. Reproduction. 2010;140(2):287-294. [DOI] [PubMed] [Google Scholar]
- 61. Acosta TJ, Hayashi KG, Matsui M, Miyamoto A. Changes in follicular vascularity during the first follicular wave in lactating cows. J Reprod Dev. 2005;51(2):273-280. [DOI] [PubMed] [Google Scholar]
- 62. Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rouillier P, Matton P, Sirard M-A, Guilbault LA. Follicle-stimulating hormone-induced estradiol and progesterone production by bovine antral and mural granulosa cells cultured in vitro in a completely defined medium. J Anim Sci. 1996;74(12):3012-3019. [DOI] [PubMed] [Google Scholar]
- 64. Brower PT, Schultz RM. Intercellular communication between granulosa cells and mouse oocytes: existence and possible nutritional role during oocyte growth. Dev Biol. 1982;90(1):144-153. [DOI] [PubMed] [Google Scholar]
- 65. Diaz FJ, O’Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol. 2006;299(1):91-104. [DOI] [PubMed] [Google Scholar]
- 66. Wigglesworth K, Lee K-B, Emori C, Sugiura K, Eppig JJ. Transcriptomic diversification of developing cumulus and mural granulosa cells in mouse ovarian follicles. Biol Reprod. 2015;92(1)23,1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sunderland SJ, Crowe MA, Boland MP, Roche JF, Ireland JJ. Selection, dominance and atresia of follicles during the oestrous cycle of heifers. J Reprod Infertil. 1994;101:547-555. [DOI] [PubMed] [Google Scholar]
- 68. Silva JRV, Lima FEO, Souza ALP, Silva AWB. Interleukin-1β and TNF-α systems in ovarian follicles and their roles during follicular development, oocyte maturation and ovulation. Zygote. 2020;28(4):270-277. [DOI] [PubMed] [Google Scholar]
- 69. Darbon JM, Oury F, Laredo J, Bayard F. Tumor necrosis factor -α inhibits follicle-stimulating hormone-induced differentiation in cultured rat granulosa cells. Biochem Biophys Res Commun. 1989;163(2):1038-1046. [DOI] [PubMed] [Google Scholar]
- 70. Puttabyatappa M, Brogan RS, VandeVoort CA, Chaffin CL. EGF-like ligands mediate progesterone’s anti-apoptotic action on macaque granulosa cells. Biol Reprod. 2013;88(1) 18, 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE Jr. Ovulation: parallels with inflammatory processes. Endocr Rev. 2018;40(2):369-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hofmann GE, Khoury J, Johnson CA, Thie J, Scott RT. Premature luteinization during controlled ovarian hyperstimulation for in vitro fertilization-embryo transfer has no impact on pregnancy outcome. Fertil Steril. 1996;66(6):980-986. [DOI] [PubMed] [Google Scholar]
- 73. Kitasaka H, Kawai T, Hoque SAM, Umehara T, Fujita Y, Shimada M. Inductions of granulosa cell luteinization and cumulus expansion are dependent on the fibronectin-integrin pathway during ovulation process in mice. PLoS One. 2018;13(2):e0192458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hafez E, Sugie T, Gordon I. Superovulation and related phenomena in the beef cow. Reproduction. 1963;5(3):359-379. [DOI] [PubMed] [Google Scholar]
- 75. Gemzell CA, Diczfalusy E, Tillinger G. Clinical effect of human pituitary follicle-stimulating hormone (FSH). J Clin Endocrinol Metab. 1958;18(12):1333-1348. [DOI] [PubMed] [Google Scholar]
- 76. Richani D, Gilchrist RB. The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update. 2017;24(1):1-14. [DOI] [PubMed] [Google Scholar]
- 77. Caixeta ES, Machado MF, Ripamonte P, Price C, Buratini J. Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod Fertil Dev. 2013;25(6):890-899. [DOI] [PubMed] [Google Scholar]
- 78. Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20(6):1352-1365. [DOI] [PubMed] [Google Scholar]
- 79. Assidi M, Richard FJ, Sirard M-A. FSH in vitro versus LH in vivo: similar genomic effects on the cumulus. J Ovarian Res. 2013;6(1):68-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khan DR, Guillemette C, Sirard MA, Richard FJ. Characterization of FSH signalling networks in bovine cumulus cells: a perspective on oocyte competence acquisition. Mol Hum Reprod. 2015;21(9):688-701. [DOI] [PubMed] [Google Scholar]
- 81. Huynh K, Jones G, Thouas G, Britt KL, Simpson ER, Jones MEE. Estrogen is not directly required for oocyte developmental competence. Biol Reprod. 2004;70(5):1263-1269. [DOI] [PubMed] [Google Scholar]
- 82. Sugiura K, Su Y-Q, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15. Mol Endocrinol. 2010;24(12):2303-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Christou-Kent M, Kherraf Z-E, Amiri-Yekta A, et al. PATL2 is a key actor of oocyte maturation whose invalidation causes infertility in women and mice. EMBO Mol Med. 2018;10(5):e8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ruebel ML, Schall PZ, Midic U, et al. Transcriptome analysis of rhesus monkey failed-to-mature oocytes: deficiencies in transcriptional regulation and cytoplasmic maturation of the oocyte mRNA population. Mol Hum Reprod. 2018;24(10):478-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alves VS, Motta FL, Roffé M, Delamano A, Pesquero JB, Castilho BA. GCN2 activation and eIF2α phosphorylation in the maturation of mouse oocytes. Biochem Biophys Res Commun. 2009;378(1):41-44. [DOI] [PubMed] [Google Scholar]
- 86. Latham KE. Stress signaling in mammalian oocytes and embryos: a basis for intervention and improvement of outcomes. Cell Tissue Res. 2016;363(1):159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bronson R. Is the oocyte a non-professional phagocyte? Hum Reprod Update. 1998;4(6):763-775. [DOI] [PubMed] [Google Scholar]
- 88. Lobascio AM, Klinger FG, Scaldaferri ML, Farini D, De Felici M. Analysis of programmed cell death in mouse fetal oocytes. Reproduction. 2007;134(2):241-252. [DOI] [PubMed] [Google Scholar]
- 89. Escobar ML, Echeverría OM, Ortíz R, Vázquez-Nin GH. Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis. 2008;13(10):1253. [DOI] [PubMed] [Google Scholar]
- 90. Rodrigues P, Limback D, McGinnis LK, Plancha CE, Albertini DF. Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction. 2009;137(4):709-720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplemental data files are available at Figshare https://figshare.com/search?q=1920860410.6084%2Fm9.figshare.19208604, doi: 10.6084/m9.figshare.19208604 (32). RNA sequencing data are available at the Gene Expression Omnibus (accession number GSE197116) (38).