Abstract
Polyarteritis nodosa (PAN) is a rare systemic necrotizing vasculitis affecting small- to medium-sized arteries. The most common gastrointestinal manifestation of PAN is postprandial abdominal pain from mesenteric arteritis causing bowel ischemia. When transmural ischemia develops, there may be ischemic necrosis and perforation of the bowel wall, which are life-threatening. Severe, life-threatening gastrointestinal involvement is relatively rare in pediatric PAN and may require different management in adult patients. We report a pediatric PAN case in a patient who presented with acute abdominal pain and superimposed cytomegalovirus enteritis with jejunoileal perforation. The patient improved with emergency small intestinal resection followed by conventional immunosuppressive drugs of a corticosteroid and cyclophosphamide, and anti-viral drugs. Before increasing the immunosuppressive drug dosage, initial screening of infectious cytomegalovirus and comprehensive evaluation for surgical conditions are essential in pediatric PAN with severe gastrointestinal involvement. Early aggressive treatment for acute abdomen is useful in reducing morbidity and mortality in pediatric PAN.
Keywords: polyarteritis nodosa, cytomegalovirus enteritis, abdominal pain, bowel perforation
Introduction
Systemic polyarteritis nodosa (PAN) is a rare necrotizing vasculitis predominantly affecting small- to medium-sized blood vessels.1 Kussmaul and Maier first described an autopsy case of PAN in 1866.2 The diagnostic criteria of PAN are evidence of a necrotizing vasculitis or an angiographic abnormality of a medium- or small-sized artery and one of the following: skin involvement, myalgia/muscle tenderness, hypertension, peripheral neuropathy, and renal involvement.1,3–6 The annual incidence of PAN is estimated to be 0.9–8.0/million in European countries and it has a prevalence of 31/million. PAN can affect all ethnicities and often occurs in patients aged 40–60 years. Unlike other types of vasculitis, PAN has a male preponderance (male-to-female ratio of 1.5:1).3–5
PAN affects medium and small vessels, such as capillaries and small arterioles, but spares large vessels, such as the aorta and its major branches.7 Vascular lesions arise at the muscular arterial wall, mainly at bifurcations and branch points. Inflammation can start in the intima and progress to the entire arterial wall, including the internal and external elastic lamina, resulting in fibrinoid necrosis.7 When vessel walls are weak, aneurysms can develop, increasing the subsequent risk for rupture, hemorrhage, and thrombosis. As PAN progress, proliferation of the intima or media may result in obstruction, causing tissue ischemia or infarction.8 Approximately 50% of patients with PAN have gastrointestinal (GI) involvement, which indicates that it is a major morbidity and cause of mortality.9–11
Postprandial abdominal pain is the most common symptom of GI manifestations. This pain is characteristically intermittent and worsens after meals. The pain is caused by mesenteric arteritis and results in bowel ischemia, ulceration, or perforation.12 Adult PAN is associated with various viral infection, such as hepatitis B virus, but is less common in human immunodeficiency virus, cytomegalovirus (CMV), and parvovirus B19 infections.13,14 The cause of pediatric PAN is unknown and is rarely associated with hepatitis B virus owing to hepatitis immunization.7 A standard treatment of conventional PAN requires a combination of a corticosteroid and cyclophosphamide. Optional therapeutics for refractory PAN include intravenous immunoglobin, plasma exchange, infliximab, and rituximab (anti-CD20 monoclonal antibody).1,3–6 We report the case of a 6-year-old girl who was diagnosed with PAN. She developed recurrent acute abdominal pain with superimposed CMV enteritis and jejunoileal perforation. She successfully recovered after surgical intervention and a standard regimen of immunosuppressive drugs. Early recognition and appropriate decisions are essential for the management of pediatric PAN.
Case Report
A 6-year-old girl was referred to our tertiary care hospital with abdominal pain, hypertension, and recurrent stroke as shown by computed tomography (CT) and magnetic resonance imaging of the brain. At a previous hospital, she was diagnosed with PAN by CT angiography of the whole aorta. CT angiography showed multiple aneurysms at the spleen, both kidney vessels, the inferior gastroepiploic artery, the superior mesenteric artery, and the internal iliac artery. She had an elevated erythrocyte sediment rate of 42 mm/h. She received a high dose of methylprednisolone for 3 days as an initial induction therapy before being referred to our hospital.
On the first day of admission (D1), she was afebrile, partially cooperative, and had a blood pressure of 130/94 mmHg (no difference in blood pressure in the extremities), pulse rate of 100 beats/min, body weight of 13.5 kg, and height of 105 cm on a physical examination. An abdominal examination showed no distension, tenderness, or organomegaly. A neurological examination showed limited movements of the extraocular muscles of both eyes, limitation and ptosis of the left eye, and right hemiparesis with a muscle power grade of at least II/V. Pertinent laboratory values included a hemoglobin concentration of 10.6 g/dL, white blood cell count of 7900 cells/mm3 (neutrophils, 79%; lymphocytes, 20%), platelet count of 507,000/mm3, erythrocyte sediment rate of 5 mm/h, C-reactive protein concentration of 2.79 mg/L, lipase concentration of 40 U/L, amylase concentration of 78 U/L, and normal urine analysis. Additionally, antinuclear antibodies, antineutrophil cytoplasmic antibody, and antiphospholipid antibody were within the normal range. She received the first dose of cyclophosphamide 500 mg/body surface area by infusion. The methylprednisolone was tapered to 2 mg/kg/day. The abdominal pain improved after she received immunosuppressive treatment.
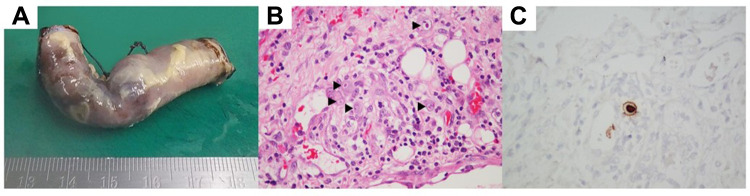
On D15 after admission, her abdominal pain worsened with a new onset of fever, but no diarrhea or GI bleeding was observed. A laboratory examination showed leukopenia, which included a hemoglobin concentration of 8.2 g/dL, white blood cell count of 3,600 cells/mm3 (neutrophils, 86%; lymphocytes, 4%), platelet count of 135,000/mm3, erythrocyte sediment rate of 24 mm/h, C-reactive protein concentration of 55.5 mg/L, and normal urine analysis. A repeated CT angiography of the whole aorta showed a slightly decreased size of some intra-abdominal arterial aneurysms with segments VI and VII of the liver and splenic infarctions. Therefore, an additional drug was prescribed as a single dose of infliximab. Six hours later, the patient had progressive severe abdominal pain, abdominal distension, decreased bowel sounds, generalized tenderness, and abdominal guarding, with the presence of pneumoperitoneum on supine abdominal plain film. An emergency pediatric surgery was performed, and it showed multiple hemorrhagic spots at the serosa of the proximal jejunum, which measured 60 cm in length, and a perforated ulcer (0.5 x 0.5 cm) at the terminal ileum 15 cm from the ileocecal valve. Pathological findings showed transmural suppurative enteritis with perforation and viral cytopathic change with typical “owl eye appearance” inclusion bodies, which was compatible with perforated CMV enteritis. No definite evidence of active vasculitis or thrombosis was identified (Figure 1).
Figure 1.
Cytomegaloviral enteritis with jejunoileal perforation. (A) Macroscopic findings. Serosal fibrinous exudate with perforation can be seen in the jejunoileum. (B) Microscopic findings. Cytomegalic cells with basophilic intranuclear inclusions can be seen (arrowheads, hematoxylin and eosin stain, 40×). (C) CMV-infected cells are shown by in situ hybridization.
The patient’s blood CMV viral load examination showed 338,219 copies/mL. The patient’s clinical conditions improved postoperatively after a complete course of an anti-viral drug therapy (ganciclovir). The induction therapy was continued monthly with cyclophosphamide, but infliximab infusion for refractory PAN was stopped. Weaning of the steroid was planned according to a standard regimen.
Discussion
Our patient with PAN developed recurrent abdominal pain and a new episode of fever without diarrhea or GI bleeding on D15 after receiving immunosuppressive drugs. A complete blood count showed leukopenia and thrombocytopenia. She developed bowel perforation and underwent an emergency small intestinal resection. The pathological findings of the intestine showed CMV enteritis with jejunoileal perforation, but no evidence of active vasculitis. The patient likely developed CMV enteritis after receiving the high dose of immunosuppressive medications, which led to the recurrent abdominal pain. Even though the erythrocyte sedimentation rate was not high, the new onset of fever along with cytopenia were additional indicators of CMV infection in this patient. The lowered erythrocyte sedimentation rate after referral to our hospital was likely due to the administration of immunosuppressive drugs in the previous hospital. Conventional cases of PAN can be treated by initial therapy of a corticosteroid and cyclophosphamide, followed by maintenance therapy of azathioprine or mycophenolate mofetil.15
Approximately half of PAN cases present with GI involvement.16 In our case, the bowel perforation needed to be treated by surgery. This finding is similar to that in a study by Levine et al, who reported 24 patients diagnosed with PAN.17 In their study, 50% of all patients with GI involvement had acute abdominal pain, and most of the 13 patients with acute abdomen underwent surgery. There were eight episodes of a patient developing bowel infarction or perforation, and four had aneurysmal ruptures.17
Several previous cases of PAN presented with abdominal pain and multiple intestinal perforations of the small intestine, jejunum, or ileum.18–20 In a previous report, a 3-year-old girl had a prolonged fever without abdominal pain.21 A physical examination showed abdominal distension and rebound tenderness, and multiple free air fluid levels were detected on an abdominal X-ray. At surgery, there was fecal peritonitis with multiple gangrenous patches in the jejunoileum. The common sites of perforation of patients with PAN are the small intestine, jejunum, and ileum.22.
A review of 11 reports showed that severe, life-threatening GI involvement was found as small intestinal stricture, enteritis, colitis, ischemic necrosis, and its complications, such as obstruction, perforation, peritonitis, and mesenteric infarction.21,23–32 These pediatric patients with PAN who presented with aneurysms with or without rupture are shown in Table 1.
Table 1.
Pediatric PAN Cases with Life-Threatening Gastrointestinal Involvement
| Series | Reference | Age | Sex | Location | Pathological Findings | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Chattopadhyay21 | 3 y | F | Jejunoileum | Gangrenous enteritis with perforation and peritonitis | Jejunoileal resection, medication: corticosteroid | Survived |
| 2 | Seifarth et al23 | 1 y | M | Small intestine | Ischemia and stricture of the small intestine | Segmental resection | Survived |
| 3 | Beckum et al24 | 2 y, 8 mo | M | Jejunum and colon | Ischemic jejunal enteritis with multiple ulcers and two areas of colonic perforations | Small intestine and colon resection, medication: infliximab, corticosteroid, cyclophosphamide, mycophenolate mofetil, and methotrexate | Survived |
| 4 | Venuta et al25 | 1 y, 1 mo | M | Jejunum | Jejunal obstruction owing to ischemic necrosis | Jejunal resection | Survived |
| 5 | Kendirli et al26 | 15 y | M | Hepatic and superior mesenteric arteries, jejunum | Multiple aneurysms in the hepatic and superior mesenteric arteries, and jejunal enteritis with massive intestinal bleeding | Jejunal resection, medication: corticosteroid, Intravenous immunoglobulin, and cyclophosphamide | Deceased |
| 6 | Crankson et al27 | 10 y | M | Jejunum | Ischemic necrosis with peritonitis | Partial resection of the jejunum, medication: corticosteroid and cyclophosphamide | Survived |
| 7 | Park et al28 | 5 y | M | Renal, hepatic, and superior mesenteric arteries | Multiple microaneurysms | Medication: corticosteroid, cyclophosphamide, and intravenous immunoglobulin | Survived |
| 8 | Bakkaloğlu et al29 | 7 y | M | Kidney | Renal infarction | Medication: corticosteroid and colchicine | Survived |
| 9 | Mocan et al30 | 10 y | M | Mesenteric arteries | Mesenteric arteritis with large ischemic segments, resulting in infarction and perforation | Appendectomy, medication: corticosteroid and cyclophosphamide | Deceased |
| 10 | Almgren et al31 | 9 y | M | Visceral arteries | Multiple aneurysms of visceral arteries with a ruptured ileocolic arterial aneurysm | Ileocolic, inferior mesenteric and common hepatic artery ligation, medication: corticosteroid | Survived |
| 11 | Lerkvaleekul et al32 | 9 y | F | Visceral arteries | Recurrent ruptured abdominal aneurysm, lower GI bleeding | Arterial embolization, medication: infliximab | Survived |
| 12 | Present case | 6 y | F | Jejunoileum | Perforation | Jejunoileal resection, medication: corticosteroid, cyclophosphamide, and infliximab (single dose) | Survived |
Abbreviations: y, years; mo, months; M, male; F, female; GI, gastrointestinal.
Uncommon GI involvement in these pediatric patients was treated with immunosuppressive drugs, intervention, or surgical resection. The outcome was survival in 10 out of 12 cases. Two of the patients were deceased. Kendirli et al reported catastrophic GI involvement in a 15-year-old boy with PAN and multiple aneurysms involving the hepatic and superior mesenteric arteries and jejunal enteritis with massive intestinal bleeding.26 This boy died from sepsis despite aggressive therapy. Mocan reported a fatal PAN in a 10-year-old boy who presented with acute abdomen and severe GI bleeding due to massive mesenteric arteritis, necrosis, and perforation.30 With regard to patients who survived PAN, Bakkaloğlu et al described a 7-year-old boy with familial Mediterranean fever complicated by PAN.29 Definite familial Mediterranean fever was diagnosed on the basis of typical attacks characterized by abdominal pain, fever, and an acute-phase response, including a homozygous MEFV mutation (M694 V/M694 V). Non-aneurysmal angiographic signs of PAN were present under renal angiography. The patient developed renal infarction and his condition was controlled with colchicine and a corticosteroid. Other PAN cases were improved after surgery, followed by corticosteroid, cyclophosphamide, or optional immunosuppressive drug therapy. Cases of pediatric PAN with life-threatening GI involvement are relatively rare and may require different management from adult patients.
Generally, GI CMV infection can occur in a healthy or an immunocompromised host. The most common involved site is the colon. The most common manifestation is diarrhea, followed by abdominal pain and rectal bleeding.33 However, the most frequent site of intestinal perforation in an immunocompromised patient is the distal ileum.34 A negative blood test for CMV does not exclude CMV colitis. Therefore, the gold standard for diagnosing CMV colitis is through histopathology. According to a study by Arnold et al, approximately 18% of patients with CMV infection showed peritonitis from multiple jejunoileal perforations, and the macroscopic manifestation was heterogeneous and varied according to the site of involvement.35 CMV can cause duodenal ulcers, antimesenteric perforations, and hemorrhagic ulceration.
In our study, the patient developed progressive severe abdominal pain with clinical signs of a systemic inflammatory response and peritonitis, and immediately underwent surgery. The operative findings were multiple hemorrhagic spots at the serosa of the proximal jejunum and a perforated ulcer at the terminal ileum. Additionally, the serology and histopathology were positive for CMV infection. Acute abdominal pain leading to perforation in this patient with PAN was caused by superimposed CMV infection.
In patients with PAN who receive conventional treatment, including corticosteroid and cyclophosphamide administration, the condition of the refractory disease or opportunistic infection should be evaluated when progressive abdominal pain develops. Therefore, infection should be excluded before increasing immunosuppressive medications. In refractory disease, anti-tumor necrosis factor therapy might be another treatment option for the refractory condition to prevent ongoing inflammation. However, early recognition of the patient’s condition with aggressive treatment is vital to reduce morbidity and mortality.
Conclusion
In conclusion, we report a 6-year-old girl with PAN who presented with abdominal pain, hypertension, and recurrent stroke. Initially, she improved with induction therapy and then developed recurrent acute abdominal pain with superimposed CMV enteritis and jejunoileal perforation. She underwent emergency jejunoileal resection, including an anti-viral drug, followed by the standard dosage of immunosuppressive drugs. The patient successfully recovered and was allowed to be discharged. Critical evaluation for secondary infection and early aggressive treatment for acute abdomen are important in reducing morbidity and mortality in pediatric PAN with severe life-threatening GI involvement.
Acknowledgments
We wish to thank the patient’s parents who provided consent for us to study their daughter for medical academic purposes. We acknowledge Mr. Phu Waisayarat, a medical student of the Faculty of Medicine, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, Thailand for providing English proofreading services. We thank Ellen Knapp, PhD, from Edanz (https://edanz.com/ac) for editing a draft of this manuscript.
Funding Statement
The authors received no financial support for the research, authorship, or publication of this article.
Abbreviations
PAN, polyarteritis nodosa; GI, gastrointestinal; CMV, cytomegalovirus.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by the Institutional Review Board of Mahidol University, in full compliance with International Guidelines for Human Research Protection, such as the Declaration of Helsinki, The Belmont Report, CIOMS Guidelines, and the International Conference on Harmonization in Good Clinical Practice (COA. MURA2022/10).
Consent for Publication
Written informed consent was obtained from the patient’s parents for this case report and any accompanying images.
Author Contributions
All authors contributed toward data analysis, and drafting and revising the manuscript. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715 [DOI] [PubMed] [Google Scholar]
- 2.Kussmaul A, Maier R. Ueber eine bisher nicht beschriebene eigenthümliche Arterienerkrankung (Periarteritis nodosa), die mit Morbus Brightii und rapid fortschreitender allgemeiner Muskellähmung einhergeht. Dtsch Arch Klin Med. 1866;1:484–518. German. [Google Scholar]
- 3.Stanton M, Tiwari V. Polyarteritis Nodosa. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 4.De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15(6):564–570. doi: 10.1016/j.autrev.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 5.Ozen S. The changing face of polyarteritis nodosa and necrotizing vasculitis. Nat Rev Rheumatol. 2017;13(6):381–386. doi: 10.1038/nrrheum.2017.68 [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Rodríguez J, Alba MA, Prieto-González S, Cid MC. Diagnosis and classification of polyarteritis nodosa. J Autoimmun. 2014;14:4849, 84–89. doi: 10.1016/j.jaut.2014.01.029 [DOI] [PubMed] [Google Scholar]
- 7.Stone JH. Polyarteritis nodosa. JAMA. 2002;288(13):1632–1639. doi: 10.1001/jama.288.13.1632 [DOI] [PubMed] [Google Scholar]
- 8.Colmegna I, Maldonado-Cocco JA. Polyarteritis nodosa revisited. Curr Rheumatol Rep. 2005;7(4):288–296. doi: 10.1007/s11926-005-0039-2 [DOI] [PubMed] [Google Scholar]
- 9.Ebert EC, Hagspiel KD, Nagar M, Schlesinger N. Gastrointestinal involvement in polyarteritis nodosa. Clin Gastroenterol Hepatol. 2008;6(9):960–966. doi: 10.1016/j.cgh.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 10.Lee JS, Kim JG, Lee S. Clinical presentations and long term prognosis of childhood onset polyarteritis nodosa in single centre of Korea. Sci Rep. 2021;11(1):8393. doi: 10.1038/s41598-021-87718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merlin E, Mouy R, Pereira B, et al. Long-term outcome of children with pediatric-onset cutaneous and visceral polyarteritis nodosa. Joint Bone Spine. 2015;82(4):251–257. doi: 10.1016/j.jbspin.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 12.Lhote F, Cohen P, Guillevin L. Polyarteritis nodosa, microscopic polyangiitis and Churg-Strauss syndrome. Lupus. 1998;7(4):238–258. doi: 10.1191/096120398678920055 [DOI] [PubMed] [Google Scholar]
- 13.Finkel TH, Török TJ, Ferguson PJ, et al. Chronic parvovirus B19 infection and systemic necrotising vasculitis: opportunistic infection or aetiological agent? Lancet. 1994;343(8908):1255–1258. doi: 10.1016/S0140-6736(94)92152-0 [DOI] [PubMed] [Google Scholar]
- 14.Fernandes SR, Bértolo MB, Rossi CL, et al. Polyarteritis nodosa and cytomegalovirus: diagnosis by polymerase chain reaction. Clin Rheumatol. 1999;18(6):501–503. doi: 10.1007/s100670050148 [DOI] [PubMed] [Google Scholar]
- 15.Eleftheriou D, Dillon MJ, Tullus K, et al. Systemic polyarteritis nodosa in the young: a single-center experience over thirty-two years. Arthritis Rheum. 2013;65(9):2476–2485. doi: 10.1002/art.38024 [DOI] [PubMed] [Google Scholar]
- 16.de Carpi JM, Castejón E, Masiques L, Vilar P, Antón J, Varea V. Gastrointestinal involvement in pediatric polyarteritis nodosa. J Pediatr Gastroenterol Nutr. 2007;44(2):274–278. doi: 10.1097/01.mpg.0000235753.37358.72 [DOI] [PubMed] [Google Scholar]
- 17.Levine SM, Hellmann DB, Stone JH. Gastrointestinal involvement in polyarteritis nodosa (1986–2000): presentation and outcomes in 24 patients. Am J Med. 2002;112(5):386–391. doi: 10.1016/S0002-9343(01)01131-7 [DOI] [PubMed] [Google Scholar]
- 18.Miller DR, O’farrell TP. Perforation of the small intestine secondary to necrotizing vasculitis (periarteritis nodosa). Ann Surg. 1965;162(1):81–90. doi: 10.1097/00000658-196507000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mousavie SH, Mirzaei R, Mahjoubi B, Azizi R. Successful management of multiple small bowel perforations due to polyarteritis nodosa. J Res Med Sci. 2014;19(3):276–278. [PMC free article] [PubMed] [Google Scholar]
- 20.Buldukoglu OC, Koklu S, Koklu H, Uluoglu O, Kulduk G. Intestinal perforation as the initial presentation of polyarteritis nodosa in an older adult. Geriatr Gerontol Int. 2015;15(1):121–122. doi: 10.1111/ggi.12266 [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay A. Intestinal perforation due to polyarteritis nodosa. Indian J Pediatr. 2001;68(3):297–298. doi: 10.1007/BF02723211 [DOI] [PubMed] [Google Scholar]
- 22.Takada T, Yoshida H, Tsukuda M, Katoh H. A case of classic polyarteritis nodosa presenting as acute abdomen. A review of 50 patients with PN involving small intestine undergone laparotomy in Japanese literature. Jpn J Gastroenterol Surg. 2003;36(1):51–56. doi: 10.5833/jjgs.36.51 [DOI] [Google Scholar]
- 23.Seifarth FG, Ibrahim S, Spalding SJ, Reid JR. Intestinal obstruction secondary to infantile polyarteritis nodosa. Afr J Paediatr Surg. 2014;11(3):264–266. doi: 10.4103/0189-6725.137339 [DOI] [PubMed] [Google Scholar]
- 24.Beckum KM, Kim DJ, Kelly DR, et al. Polyarteritis nodosa in childhood: recognition of early dermatologic signs may prevent morbidity. Pediatr Dermatol. 2014;31(1):e6–e9. doi: 10.1111/pde.12207 [DOI] [PubMed] [Google Scholar]
- 25.Venuta A, Ceccarelli PL, Biondini D, Montanari F. Jejunal obstruction as initial presentation of polyarteritis nodosa in a 13-month-old boy. J Pediatr Surg. 2011;46(7):E27–E29. doi: 10.1016/j.jpedsurg.2011.03.084 [DOI] [PubMed] [Google Scholar]
- 26.Kendirli T, Yüksel S, Oral M, et al. Fatal polyarteritis nodosa with gastrointestinal involvement in a child. Pediatr Emerg Care. 2006;22(12):810–812. doi: 10.1097/01.pec.0000245172.38967.d0 [DOI] [PubMed] [Google Scholar]
- 27.Crankson SJ, Oda O, Al-Zaben AA, Suwairi WAI, Makanjoula D. Intestinal ischamemia in a child due to polyarteritis nodosa: a case report. Trop Gastroenterol. 2006;27(1):41–43. [PubMed] [Google Scholar]
- 28.Park H-J, Choi Y-J, Kim J-E, Ye Y-M, Park H-S, Suh C-H. Successful treatment of pediatric systemic polyarteritis nodosa with cholestatic hepatitis. Clin Rheumatol. 2007;26(1):122–124. doi: 10.1007/s10067-005-0121-1 [DOI] [PubMed] [Google Scholar]
- 29.Bakkaloğlu SA, Muzaç S, Akpek S, Söylemezoğlu O, Buyan N, Hasanoğlu E. Polyarteritis nodosa in a case of familial Mediterranean fever. Pediatr Nephrol. 2004;19(5):536–538. doi: 10.1007/s00467-003-1390-z [DOI] [PubMed] [Google Scholar]
- 30.Mocan H, Mocan MC, Sen Y, Kuzey G, Civiloglu C. Fatal polyarteritis nodosa with massive mesenteric necrosis in a child. Clin Rheumatol. 1999;18(1):88–90. doi: 10.1007/s100670050063 [DOI] [PubMed] [Google Scholar]
- 31.Almgren B, Eriksson I, Foucard T, Lörelius LE, Olsen L. Multiple aneurysms of visceral arteries in a child with polyarteritis nodosa. J Pediatr Surg. 1980;15(3):347–348. doi: 10.1016/S0022-3468(80)80156-4 [DOI] [PubMed] [Google Scholar]
- 32.Lerkvaleekul B, Treepongkaruna S, Ruangwattanapaisarn N, Treesit T, Vilaiyuk S. Recurrent ruptured abdominal aneurysms in polyarteritis nodosa successfully treated with infliximab. Biologics. 2019;13:111–116. doi: 10.2147/BTT.S204726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le PH, Lin WR, Kuo CJ, et al. Clinical characteristics of cytomegalovirus colitis: a 15-year experience from a tertiary reference center. Ther Clin Risk Manag. 2017;13:1585–1593. doi: 10.2147/TCRM.S151180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334–342. doi: 10.1007/s11894-012-0266-4 [DOI] [PubMed] [Google Scholar]
- 35.Arnold M, Itzikowitz R, Young B, et al. Surgical manifestations of gastrointestinal cytomegalovirus infection in children: clinical audit and literature review. J Pediatr Surg. 2015;50:1874–1879. [DOI] [PubMed] [Google Scholar]