Abstract
Objectives. To understand the frequency, magnitude, geography, and characteristics of tuberculosis outbreaks in US state prisons.
Methods. Using data from the National Tuberculosis Surveillance System, we identified all cases of tuberculosis during 2011 to 2019 that were reported as occurring among individuals incarcerated in a state prison at the time of diagnosis. We used whole-genome sequencing to define 3 or more cases within 2 single nucleotide polymorphisms within 3 years as clustered; we classified clusters with 6 or more cases during a 3-year period as tuberculosis outbreaks.
Results. During 2011 to 2019, 566 tuberculosis cases occurred in 41 state prison systems (a median of 3 cases per state). A total of 19 tuberculosis genotype clusters comprising 134 cases were identified in 6 state prison systems; these clusters included a subset of 5 outbreaks in 2 states. Two Alabama outbreaks during 2011 to 2017 totaled 20 cases; 3 Texas outbreaks during 2014 to 2019 totaled 51 cases.
Conclusions. Only Alabama and Texas reported outbreaks during the 9-year period; only Texas state prisons had ongoing transmission in 2019. Effective interventions are needed to stop tuberculosis outbreaks in Texas state prisons. (Am J Public Health. 2022;112(8):1170–1179. https://doi.org/10.2105/AJPH.2022.306864)
The inherent social vulnerability of incarcerated individuals entitles them to certain protections.1 Protecting them from harmful pathogens is both a public health and social justice issue.2,3 Because weekly turnover is above 50% in local jails,4 the incidence of infectious diseases in jails largely derives from background community epidemiology. In contrast, an average state prison sentence is 2.6 years.5 For the approximately 1.2 million individuals currently incarcerated in US state prison systems,6 this longer sentence duration means that the natural history of an infectious disease—from initial exposure to illness onset—is more likely to occur during incarceration.
Following the 1980s-to-1990s sharp increase in the number of incarcerated individuals in the United States, which co-occurred with the onset of the HIV/AIDS epidemic,7 multiple tuberculosis (TB) outbreaks in correctional facilities were documented.8–11 Few state prison TB outbreaks appeared in the subsequent literature,12–15 but each one involved Mycobacterium tuberculosis spread beyond the prison and into the broader community, including correctional officers and children. The recent dearth of articles describing TB outbreaks in state prisons could be a result of a true decline in such outbreaks. However, before the analysis described here, the nationwide incidence of TB outbreaks in state prisons was unknown. In this analysis, we used established national TB surveillance and next-generation whole-genome sequencing methods to estimate the frequency and magnitude of TB outbreaks in state prisons, describe their geographic distribution, and summarize characteristics of individuals associated with those outbreaks.
METHODS
Public health departments report all verified cases of TB in the United States to the National Tuberculosis Surveillance System.16 Each case report includes demographic, clinical, and programmatic variables such as whether a patient was diagnosed with TB while incarcerated and, if so, the incarceration facility type (federal, state, local, juvenile, other, or unknown). Case reports also include employment type, which facilitates identification of TB cases among correctional workers. M. tuberculosis isolates from culture-confirmed cases are routinely genotyped.
We included all verified TB cases that the 50 US states reported during 2011 to 2019 as occurring in a person incarcerated in a state prison at the beginning of the diagnostic evaluation that led to the TB diagnosis. Data on incarceration length and history were unavailable. To generate annual TB incidence in state prisons, we used each state’s year-end estimates from the US Bureau of Justice Statistics as denominators.5,17,18
To identify TB cases that might represent M. tuberculosis transmission within state prison facilities, we first identified all clusters of 3 or more TB cases in a single state prison system during any 3-year period from 2011 to 2019 that involved 2-locus or fewer differences on spacer oligonucleotide typing (spoligotyping) and 24-locus mycobacterial interspersed repetitive unit–variable number tandem repeat typing results; these clusters represented the top 10% of cluster sizes in our data set. Then, to increase our molecular resolution, we performed whole-genome sequencing for all isolates from cases in those initially identified clusters. We used whole-genome single nucleotide polymorphism comparisons to measure the genetic distance between isolates. A conservative threshold of 2 or fewer single nucleotide polymorphisms was used to define cases as closely related (i.e., signifying evidence of recent transmission).
Only the initially identified clusters with 3 or more closely related cases during a 3-year period remained in the analysis; we added other cases in the same state prison system from other years during 2011 to 2019 if those other cases’ isolates were within 2 single nucleotide polymorphisms of an isolate from a case in the cluster. Finally, we classified the subsets of clusters with 6 or more closely related cases during a 3-year period as TB outbreaks.19,20
We compared demographic, programmatic, and clinical characteristics of cases among incarcerated individuals in clusters (“clustered cases”) with cases among incarcerated individuals who were not in clusters (“nonclustered cases”) of 3 or more cases and cases for which a cluster designation could not be made (“nondesignated cases”; i.e., nongenotyped cases, cases for which an isolate could not be analyzed, and cases in a cluster with less than 3 cases with an analyzable sequence). Demographic characteristics included sex, age, race/ethnicity, and country of birth. Programmatic characteristics included elements of the standard diagnostic evaluation for TB (e.g., chest radiograph performed and sputum smear examined) and identification of known risk factors (e.g., whether the patient was documented as having had an infectious TB exposure in the past 2 years). Clinical characteristics included acid-fast bacilli sputum smear and chest radiograph results, drug resistance, and patient outcome (e.g., treatment completion, death).
We used SAS version 9.4 (SAS Institute Inc, Cary, NC) to conduct our analysis. All data were collected as part of routine TB surveillance activities.
RESULTS
Of the 85 161 verified TB cases reported to the National Tuberculosis Surveillance System during 2011 to 2019, 566 (0.66%) occurred among individuals who were incarcerated in a state prison at the time of diagnosis. The total number of TB cases in state prisons nationally ranged from 107 cases in 2011 to 41 cases in 2019 (a national annual median of 59 cases).
Per state, a median total of 3 TB cases occurred in state prisons during 2011 to 2019. Iowa, Maine, Montana, Nebraska, New Hampshire, Utah, Vermont, West Virginia, and Wyoming reported no cases in state prisons; 10 states reported only 1 case in a state prison during the 9-year period (Table 1). Fourteen states reported 8 or more cases (75th percentile), and 3 states reported 48 or more cases (95th percentile): California (48 cases), Florida (61 cases), and Texas (201 cases).
TABLE 1—
Tuberculosis (TB) Cases, Median Incidence, and Clusters Among Individuals Incarcerated in a State Prison at the Time of Diagnosis: United States, 2011–2019
| Statea | No. TB Cases | Median TB Incidenceb | No. Clusters With ≥ 3 TB Cases |
| Texas | 201 | 14.0 | 11 |
| Florida | 61 | 4.9 | 2 |
| California | 48 | 3.8 | 0 |
| Alabama | 39 | 6.5 | 2 |
| Georgia | 39 | 5.6 | 1 |
| North Carolina | 20 | 8.2 | 2 |
| Oklahoma | 13 | 3.6 | 0 |
| Louisiana | 12 | 2.7 | 0 |
| Arizona | 11 | 2.4 | 0 |
| Indiana | 10 | 3.7 | 1 |
| Missouri | 9 | 0.0 | 0 |
| South Carolina | 9 | 0.0 | 0 |
| Arkansas | 8 | 5.6 | 0 |
| New Jersey | 8 | 4.9 | 0 |
| Alaska | 7 | 19.7 | 0 |
| Illinois | 7 | 2.2 | 0 |
| Massachusetts | 7 | 0.0 | 0 |
| Mississippi | 7 | 5.2 | 0 |
| Kentucky | 6 | 0.0 | 0 |
| New York | 4 | 0.0 | 0 |
| Tennessee | 4 | 0.0 | 0 |
| Virginia | 4 | 0.0 | 0 |
| Delaware | 3 | 0.0 | 0 |
| Hawaii | 3 | 0.0 | 0 |
| Minnesota | 3 | 0.0 | 0 |
| New Mexico | 3 | 0.0 | 0 |
| Idaho | 2 | 0.0 | 0 |
| North Dakota | 2 | 0.0 | 0 |
| Ohio | 2 | 0.0 | 0 |
| Oregon | 2 | 0.0 | 0 |
| Washington | 2 | 0.0 | 0 |
| Colorado | 1 | 0.0 | 0 |
| Connecticut | 1 | 0.0 | 0 |
| Kansas | 1 | 0.0 | 0 |
| Maryland | 1 | 0.0 | 0 |
| Michigan | 1 | 0.0 | 0 |
| Nevada | 1 | 0.0 | 0 |
| Pennsylvania | 1 | 0.0 | 0 |
| Rhode Island | 1 | 0.0 | 0 |
| South Dakota | 1 | 0.0 | 0 |
| Wisconsin | 1 | 0.0 | 0 |
States without any TB cases in a state prison system during 2011 to 2019 are excluded (Iowa, Maine, Montana, Nebraska, New Hampshire, Utah, Vermont, West Virginia, Wyoming).
Median TB incidence per 100 000 individuals incarcerated within state prisons.
TB incidence among people incarcerated in state prisons ranged from 7.7 cases per 100 000 individuals in 2011 to 3.1 cases per 100 000 in 2017 (median = 5.0 cases per 100 000), as compared with 3.4 per 100 000 and 2.7 per 100 000 in 2011 and 2019, respectively, in the general US population. Seven states had a median incidence of more than 5 cases per 100 000 individuals in state prisons during 2011 to 2019 (Table 1): Alabama (6.5), Alaska (19.7), Arkansas (5.6), Georgia (5.6), Mississippi (5.2), North Carolina (8.2), and Texas (14.0).
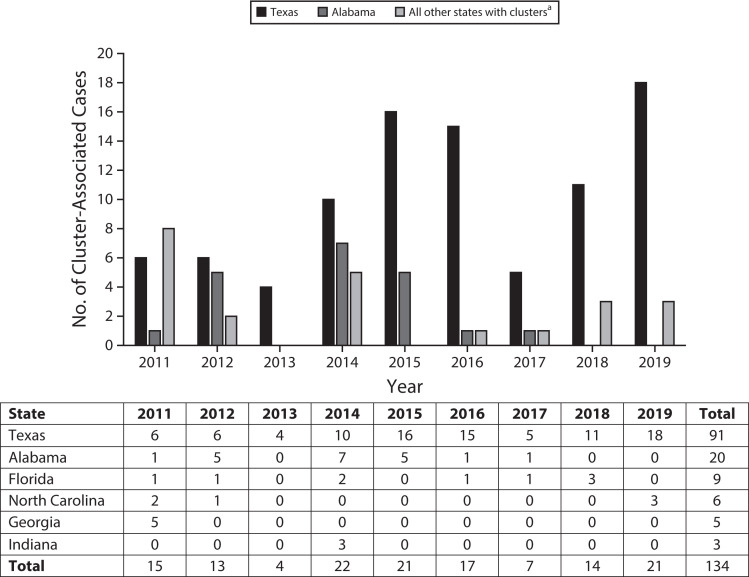
A cluster designation could be made for 422 (74.6%) of the 566 cases among individuals incarcerated in a state prison at diagnosis. Among those, we identified a total of 19 TB clusters comprising 134 cases (Figure 1). States with TB clusters of 3 or more cases in state prisons included Alabama (2 clusters), Florida (2 clusters), Georgia (1 cluster), Indiana (1 cluster), North Carolina (2 clusters), and Texas (11 clusters; Figure 1).
FIGURE 1—
Cluster-Associated Cases of Tuberculosis Among Individuals Incarcerated in a State Prison System at the Time of Diagnosis, by State: United States, 2011–2019
aIncludes a combined count of clustered cases in Georgia, Florida, Indiana, and North Carolina. The table portion shows cluster-associated case counts by state and year of diagnosis.
Clustered cases (i.e., all 134 cases in 19 clusters in 6 states) occurred predominantly among US-born (98.5%) and non-Hispanic Black (56.7%) individuals. As a comparison, nonclustered cases (n = 288) were associated with proportionately fewer US-born (78.8%) and non-Hispanic Black (39.2%) individuals (Table 2). Pulmonary TB percentages were similar among individuals who were (80.6%) and were not (83.7%) part of a cluster, as was the prevalence of acid-fast bacilli smear-positive disease in cases among people reported as receiving a sputum smear examination (51.1% and 53.0%, respectively). However, sputum smear examinations were reported less frequently in surveillance data for TB cases among individuals diagnosed within the Texas state prison system (56.2%) than for cases among incarcerated individuals in the reminder of the United States (mean = 92.9%); this incomplete reporting of a clinical evaluation element primarily affected people who were part of clusters (data not shown).
TABLE 2—
Characteristics of Individuals Incarcerated in a State Prison When Diagnosed With Tuberculosis (TB), by Cluster Status: United States, 2011–2019
| Characteristic | Nondesignated Casesa (n = 144), No. (%) | Nonclustered Cases (n = 288), No. (%) | Clustered Cases (n = 134), No. (%) |
| Male sex | 136 (94.4) | 274 (95.1) | 134 (100.0) |
| US-born | 125 (86.8) | 227 (78.8) | 132 (98.5) |
| Age group, y | |||
| 15–24 | 12 (8.3) | 31 (10.8) | 10 (7.5) |
| 25–44 | 68 (47.2) | 132 (45.8) | 71 (53.0) |
| 45–64 | 52 (36.1) | 104 (36.1) | 47 (35.1) |
| ≥ 65 | 12 (8.3) | 21 (7.3) | 6 (4.5) |
| Race/ethnicity | |||
| Hispanic | 32 (22.2) | 84 (29.2) | 35 (26.1) |
| Non-Hispanic Black | 67 (46.5) | 113 (39.2) | 76 (56.7) |
| Non-Hispanic White | 32 (22.2) | 66 (22.9) | 23 (17.2) |
| Non-Hispanic other | 13 (9.0) | 25 (8.7) | 0 (0.0) |
| Social characteristicsb | |||
| Excessive alcohol use | 15 (10.4) | 47 (16.4) | 2 (1.5) |
| Injection drug use | 8 (5.6) | 20 (7.0) | 3 (2.2) |
| Noninjection drug use | 25 (17.4) | 55 (19.2) | 12 (9.0) |
| Homelessness | 10 (6.9) | 21 (7.3) | 1 (0.8) |
| Known TB risk factors | |||
| Previous episode of TB | 6 (4.2) | 15 (5.2) | 3 (2.2) |
| Incomplete treatment of latent TB infection | 6 (4.2) | 19 (6.6) | 2 (1.5) |
| Documented TB exposure in past 2 y | 18 (12.5) | 30 (10.4) | 22 (16.4) |
| Method of diagnosis | |||
| Culture, NAAT, or acid-fast bacilli smear | 51 (35.4) | 288 (100.0) | 134 (100.0) |
| Clinical or provider diagnosis | 93 (64.6) | 0 (0.0) | 0 (0.0) |
| Chest radiograph performed | 138 (95.8) | 270 (93.8) | 123 (91.8) |
| Clinical characteristics | |||
| Lung cavity visible on chest radiographc | 36 (30.8) | 64 (23.7) | 26 (21.1) |
| Pulmonary TB involvement | 116 (80.6) | 241 (83.7) | 108 (80.6) |
| Sputum smear tested for acid-fast bacilli | 122 (84.7) | 236 (81.9) | 94 (70.2) |
| Positive acid-fast bacilli smeard | 21 (14.6) | 125 (53.0) | 48 (51.1) |
| HIV coinfection | 13 (9.0) | 30 (10.4) | 2 (1.5) |
| Multidrug-resistant TB | 0 (0.0) | 4 (1.5) | 0 (0.0) |
| Patient outcomee | |||
| Treatment completedf | 104 (85.3) | 207 (88.1) | 87 (89.7) |
| Deceasedg | 10 (7.9) | 12 (5.0) | 3 (3.0) |
Note. NAAT = nucleic acid amplification test.
Characteristic counts and percentages for 144 cases in which a cluster designation could not be made.
Counts and percentages are for presence of a risk factor and are based on self-report of the risk factor during the 12 months before diagnosis.
Percentages are based on individuals with chest radiographs performed.
Percentages are based on cases reported to surveillance as involving a sputum smear test. After rereviewing medical records during 2021, the Texas Department of State Health Services and the Texas Department of Criminal Justice identified sputum smear results for 13 additional cases initially reported to surveillance during 2015–2019 as not involving a smear test.
Numbers and percentages are based on cases with complete data on patient outcome and 2 years of follow-up (i.e., cases reported during 2011–2017) so that patient outcome could be documented in the 2011–2019 data set.
Denominators exclude 53 nonclustered cases and 37 clustered cases with incomplete data on treatment outcome.
Denominators exclude 47 nonclustered cases and 35 clustered cases with incomplete data on death during treatment. An additional 2 nonclustered cases among patients who died before diagnosis were also excluded because they were reported during 2018–2019.
HIV coinfection was present among fewer incarcerated individuals who were part of clusters (1.5%) than among those who were not (10.4%). The 4 individuals with multidrug-resistant TB that occurred in state prisons were not part of clusters.
Of the 19 TB clusters, 5 clusters in 2 state prison systems met the outbreak definition of 6 or more cases: both clusters in Alabama and 3 of the 11 clusters in Texas. Case counts for the 5 outbreaks ranged from 9 to 32 cases in these 2 states. No additional cases occurred in the 2 Alabama outbreaks after 2017, but all 3 outbreaks in Texas continued to accumulate cases through 2019.
All outbreak-associated cases in Alabama and 72.5% of outbreak-associated cases in Texas were reporting as having pulmonary involvement. All of Alabama’s 20 outbreak-associated cases had a chest radiograph performed and sputum smear examination reported. In Texas, 49 (96.1%) of the total 51 outbreak-associated cases had a chest radiograph performed, and 30 (58.8%) had a sputum smear result reported (Table 3).
TABLE 3—
Characteristics of Individuals Associated With the 5 Tuberculosis (TB) Outbreaks in State Prison Systems, by State: United States, 2011–2019
| Characteristic | Alabama (n = 20), No. (%) or Median (Range) | Texas (n = 51), No. (%) or Median (Range) |
| Age, y | 37.5 (22–71) | 35 (19–70) |
| US-born | 20 (100.0) | 50 (98.0) |
| Race/ethnicity | ||
| Hispanic | 0 (0.0) | 20 (39.2) |
| Non-Hispanic Black | 14 (70.0) | 24 (47.1) |
| Non-Hispanic White | 6 (30.0) | 7 (13.7) |
| Documented TB exposure in past 2 y | 13 (65.0) | 3 (5.9) |
| Social characteristicsa | ||
| Excessive alcohol use | 0 (0.0) | 2 (3.9) |
| Injection drug use | 0 (0.0) | 3 (5.9) |
| Noninjection drug use | 1 (5.0) | 7 (13.7) |
| Clinical characteristics and disease evaluation | ||
| Chest radiograph done | 20 (100.0) | 49 (96.1) |
| Lung cavity visible on chest radiographb | 6 (30.0) | 10 (20.4) |
| Pulmonary TB involvement | 20 (100.0) | 37 (72.5) |
| Sputum smear tested for acid-fast bacilli | 20 (100.0) | 30 (58.8) |
| Positive acid-fast bacilli smearc | 11 (55.0) | 15 (50.0) |
| HIV coinfection | 0 (0.0) | 0 (0.0) |
| Patient outcomed | ||
| Completed treatmente | 20 (100.0) | 25 (78.1) |
| Deceased | 0 (0.0) | 0 (0.0) |
Counts and percentages are for presence of the risk factor and are based on self-report of the risk factor during the 12 months before diagnosis.
Percentages are based on individuals with chest radiographs done.
Percentages are based on cases reported to surveillance as involving a sputum smear test. After rereviewing medical records during 2021, the Texas Department of State Health Services and the Texas Department of Criminal Justice identified sputum smear results for 13 additional cases initially reported to surveillance during 2015–2019 as not involving a smear test.
Numbers and percentages are based on cases with complete data on patient outcome and 2 years of follow-up (i.e., cases reported during 2011–2017) so that patient outcome could be documented in the 2011–2019 data set.
Denominator excludes 19 Texas cases with incomplete data on treatment outcome.
Compared with 65% of outbreak-associated cases among incarcerated persons in Alabama, relatively few (5.9%) of the outbreak-associated cases among incarcerated persons in Texas were reported as recent contacts of infectious TB cases in surveillance data. Of the outbreak-associated cases among incarcerated individuals with the opportunity to complete treatment by the end of the surveillance monitoring period, 25 (78.1%) of 32 in Texas and all 20 in Alabama involved completion of treatment.
Although not included in TB case counts involving incarcerated people, there was 1 case in a correctional employee for each outbreak-associated genotype: 2 correctional employees in Alabama and 3 correctional employees in Texas.
DISCUSSION
In this first national analysis of TB clustering in US state prisons, we found that outbreaks of TB are rare. Two states reported 5 outbreaks of 6 or more cases during 2011 to 2019. In Alabama, the last outbreak-associated case was reported in 2017. In Texas, all 3 identified outbreaks continued to add new outbreak-associated cases through the end of 2019.
In contrast to TB outbreaks in correctional settings in the 1990s,8,10,12 multidrug-resistant TB, HIV coinfection, and deaths did not characterize any of these outbreaks. The momentous strides in management and treatment of HIV have made this dangerous coinfection relatively infrequent.16 None of the outbreak-associated cases among incarcerated individuals in this analysis involved HIV coinfection, demonstrating that HIV is no longer fueling TB outbreaks in US state prisons. Conversely, 10.4% of cases among incarcerated individuals who were not part of a genotype cluster involved HIV coinfection, indicating that this strong risk factor for progression from M. tuberculosis infection to TB21 may have contributed to TB incidence among individuals in state prisons who were not part of a cluster.
The Centers for Disease Control and Prevention’s guidance on TB control in correctional facilities22 focuses on the importance of testing people for both latent TB infection and TB disease at the time of admission and at least annually thereafter if they remain incarcerated. Individuals who have signs or symptoms suggestive of TB should be housed separately in an airborne infection medical isolation room until a TB diagnosis has been excluded or treatment has rendered them noninfectious. Newly admitted individuals with latent TB infection benefit from treatment that prevents later progression to TB.
Short-course regimens for latent TB infection have demonstrated better treatment completion rates23 and decreased costs24 for correctional facilities than the older 9-month isoniazid regimen. As a result of these logistical advantages, the Federal Bureau of Prisons uses the 12-week, once-weekly dosing regimen of isoniazid and rifapentine as the standard treatment of latent TB infection.25 Latent TB infection identified before progression to TB can be treated for approximately $500.26 By contrast, the direct treatment cost for a single case of drug-susceptible TB in 2020 was approximately $20 000.27,28 State prison systems that implement treatment protocols similar to that of the Federal Bureau of Prisons could decrease costs, both in facilities and in the communities where people return upon release.
Any evidence of person-to-person transmission within correctional facilities also warrants additional investigation and interventions.22 To prevent widespread and ongoing waves of M. tuberculosis transmission, there should be rapid and thorough contact investigations of potentially infectious TB whenever there is a suspected or confirmed case of pulmonary, laryngeal, or pleural disease. Sputum smear and chest radiograph results can help determine the patient’s infectiousness, location of disease, and the extent of the contact investigation. For this reason, every patient with suspected TB, including those with suspected extrapulmonary TB only, should undergo a chest radiograph and provide sputum for acid-fast bacilli smears and cultures. However, surveillance records documented sputum smear results for 58.8% (i.e., 30 of 51) of Texas’s outbreak-associated cases (Table 3). The reasons for incomplete reporting—which could lead to underascertainment of pulmonary TB status and underestimation of patient infectiousness—are unknown but should be addressed.
Contact investigations can be accomplished effectively as a collaborative process with state or local health departments.22 Contacts at highest risk should be screened first. Early detection of additional cases is an important TB control aspect of contact investigations, particularly in congregate settings; initiating treatment not only benefits the individual contact but also halts infectiousness to other incarcerated individuals and correctional employees, breaking the chain of transmission and potentially averting an outbreak.
Reporting new cases as recent contacts of an infectious individual demonstrates that epidemiological links between incarcerated people are known, which can facilitate interventions for interrupting transmission. In Alabama, 65% of individuals associated with outbreaks were listed as known recent contacts, suggesting that these outbreaks were effectively halted through active case finding (i.e., enabling early detection and treatment). In Texas, less than 10% of outbreak-associated cases were reported this way. Whether this was the result of incomplete contact investigations or incomplete reporting is unknown. When contact investigations are inadequate, opportunities to break the chain of transmission are lost, and cluster growth is expected.
Many of the challenges associated with executing effective contact investigations outside correctional settings, such as obtaining names of potentially exposed and infected individuals, locating them, and arranging for testing and treatment, are negated by the fixed and detained position of incarcerated individuals. Therefore, identifying and halting transmission in a prison should be a swift and obtainable objective. Prisons that experience ongoing transmission should review their administrative infection control and contact investigation policies and procedures. Health service staff in correctional facilities should work closely with their local or state health department to investigate potential transmission as soon as a diagnosis of TB in a congregate setting is suspected and to stop outbreaks when they occur.
Outbreaks in correctional settings are not only detrimental to the health of incarcerated populations, they also threaten the health of correctional workers and the surrounding community.12–15,29,30 According to estimates from a previous report, approximately one third of new M. tuberculosis infections among prison employees are due to occupational exposures.29 Not all corrections institutions, however, require TB testing of employees,31 so the extent of this occupational risk is difficult to ascertain. Each of the 5 outbreak-associated genotypes in our analysis involved at least one diagnosed TB case in a correctional employee. Although beyond the scope of our study, other reports have shown substantial circulation of outbreak strains in the community in the years following an outbreak in a correctional institution.10,12–15,30
Furthermore, M. tuberculosis transmission in correctional facilities hampers progress toward the national goal of TB elimination. Worldwide, the fraction of TB in the general population that can be attributed to exposure in prisons has been estimated as 8.5%.32 Although it is difficult to draw conclusions from an international systematic review that includes both high- and low-burden TB countries, a US-based analysis in an urban area also revealed substantial overlap between incarceration and TB: 46% of US-born adults with TB had documented histories of being incarcerated in a jail or a prison, including 16% during the year before diagnosis.33 According to our analysis, if Texas state prisons reduced TB clustering to match clustering levels in other state prison systems (i.e., typically 0, but at most 2, rather than 11 clusters of 3 or more closely related cases), their overall TB case counts would be reduced by up to 45%, and the national total number of TB cases among people incarcerated in state prisons each year would decrease by about 15%.
Finally, and importantly, preventing transmission of infectious diseases among prisoners is an ethical and social justice obligation.2,3 The United States has one of the highest incarceration rates in the world.34 The loss of autonomy associated with confinement uniquely compromises incarcerated people’s ability to protect themselves1 from airborne diseases. Responsibility for the health and safety of state prisoners belongs to the state’s department of corrections (or equivalent organization), with opportunities for additional resources from and interventions by state government officials when current procedures are inadequate to prevent outbreaks from occurring or persisting.
Strengths and Limitations
In this study, we used established national surveillance data and next-generation whole-genome sequencing methods to describe M. tuberculosis transmission and TB outbreaks in state prisons in the United States. Strengths of our analysis include its unique national scope, with 9 years of data and the specificity of the outbreak classification used (i.e., a conservative threshold of 2 or fewer single nucleotide polymorphisms with whole-genome sequencing methods). However, we likely undercounted the number of cases associated with recent M. tuberculosis transmission in state prisons. This underestimate would be a result of not only the high specificity of our whole-genome sequencing approach but also our inability to include nongenotyped cases. We also lacked information about previous incarceration, so any matching cases diagnosed among individuals after release from prison would have been excluded.
Another limitation is our inability to determine disease timing relative to duration of incarceration; individuals who were infected just prior to incarceration (e.g., by the same state prison strain circulating in the community) may have been misclassified as part of a prison cluster. In addition, although using surveillance data facilitated a standard approach to state prisons throughout the United States, we may have mischaracterized outbreaks if actual patient characteristics were different than those reported to surveillance (e.g., sputum smear results reported to surveillance as not available when smear tests were in fact performed with results documented elsewhere).
Finally, standard surveillance records provide an incomplete characterization of factors associated with transmission and outbreaks in state prisons. Reviews of entry screenings, infectious periods, diagnostic delays, sentence lengths, epidemiological links, and infection control policies and procedures in affected facilities would be needed to provide better targeted recommendations.
Public Health Implications
This first nationwide analysis describing the epidemiology of TB outbreaks in US state prisons demonstrates that TB transmission and outbreaks were rare in most state prison systems during 2011 to 2019. Given the numerous case reports of TB outbreaks in correctional settings in the 1990s,8–11 this finding is reassuring and affirms the effectiveness of TB prevention and control practices22 in most state prisons. However, the large and ongoing outbreaks in Texas state prisons warrant additional investigation. A better understanding of policies and practices facilitating transmission is needed to inform the targeted public health actions needed to stop these outbreaks, reduce morbidity in a vulnerable population, and substantially reduce the TB burden in the Texas state prison system.
ACKNOWLEDGMENTS
We thank Shanica Railey, Sarah Talarico, and Clint McDaniel.
Note. The findings and conclusions are those of the authors and not necessarily those of the Centers for Disease Control and Prevention.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
HUMAN PARTICIPANT PROTECTION
All data were collected as part of routine tuberculosis surveillance activities. As such, the Centers for Disease Control and Prevention determined that this analysis did not constitute research involving human participants and was not subject to institutional board review.
See also Chang, p. 1084.
REFERENCES
- 1.Code of Federal Regulations. 2021. https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=83cd09e1c0f5c6937cd9d7513160fc3f&pitd=20180719&n=pt45.1.46&r=PART&ty=HTML#sp45.1.46.c
- 2.Bowleg L. Reframing mass incarceration as a social-structural driver of health inequity. Am J Public Health. 2020;110(suppl 1):S1–S12. doi: 10.2105/AJPH.2019.305464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobor G, Plough A. Addressing mass incarceration to achieve health equity. Am J Public Health. 2020;110(suppl 1):S13. doi: 10.2105/AJPH.2019.305433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng Z, Minton TD.2021. https://bjs.ojp.gov/library/publications/jail-inmates-2019
- 5.US Bureau of Justice Statistics. 2021. https://www.bjs.gov
- 6.Carson EA.2021. https://bjs.ojp.gov/content/pub/pdf/p19.pdf
- 7.Spaulding A, Stephenson B, Macalino G, Ruby W, Clarke J, Flanigan T. Human immunodeficiency virus in correctional facilities: a review. Clin Infect Dis. 2002;35(3):305–312. doi: 10.1086/341418. [DOI] [PubMed] [Google Scholar]
- 8.Valway SE, Greifinger RB, Papania M, et al. Multidrug-resistant tuberculosis in the New York State prison system, 1990–1991. J Infect Dis. 1994;170(1):151–156. doi: 10.1093/infdis/170.1.151. [DOI] [PubMed] [Google Scholar]
- 9.Bergmire-Sweat D, Barnett BJ, Harris SL, Taylor JP, Mazurek GH, Reddy V. Tuberculosis outbreak in a Texas prison, 1994. Epidemiol Infect. 1996;117(3):485–492. doi: 10.1017/S095026880005915X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Tuberculosis outbreaks in prison housing units for HIV-infected inmates—California, 1995–1996. MMWR Morb Mortal Wkly Rep. 1999;48(4):79–82. [PubMed] [Google Scholar]
- 11.Jones TF, Craig AS, Valway SE, Woodley CL, Schaffner W. Transmission of tuberculosis in a jail. Ann Intern Med. 1999;131(8):557–563. doi: 10.7326/0003-4819-131-8-199910190-00002. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin SI, Spradling P, Drociuk D, Ridzon R, Pozsik CJ, Onorato I. Extensive transmission of Mycobacterium tuberculosis among congregated, HIV-infected prison inmates in South Carolina, United States. Int J Tuberc Lung Dis. 2003;7(7):665–672. [PubMed] [Google Scholar]
- 13.Lambert LA, Espinoza L, Haddad MB, et al. Transmission of Mycobacterium tuberculosis in a Tennessee prison, 2002–2004. J Correct Health Care. 2007;14(1):39–47. doi: 10.1177/1078345807308847. [DOI] [Google Scholar]
- 14.Sosa LE, Lobato MN, Condren T, Williams MN, Hadler JL. Outbreak of tuberculosis in a correctional facility: consequences of missed opportunities. Int J Tuberc Lung Dis. 2008;12(6):689–691. [PubMed] [Google Scholar]
- 15.Séraphin MN, Didelot X, Nolan DJ, et al. Genomic investigation of a Mycobacterium tuberculosis outbreak involving prison and community cases in Florida, United States. Am J Trop Med Hyg. 2018;99(4):867–874. doi: 10.4269/ajtmh.17-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 2019. https://www.cdc.gov/tb/statistics/reports/2019/default.htm
- 17.MacNeil JR, Lobato MN, Moore M. An unanswered health disparity: tuberculosis among correctional inmates, 1993 through 2003. Am J Public Health. 2005;95(10):1800–1805. doi: 10.2105/AJPH.2004.055442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert LA, Armstrong LR, Lobato MN, Ho C, France AM, Haddad MB. Tuberculosis in jails and prisons: United States, 2002–2013. Am J Public Health. 2016;106(12):2231–2237. doi: 10.2105/AJPH.2016.303423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuen CM, Kammerer JS, Marks K, Navin TR, France AM. Recent transmission of tuberculosis—United States, 2011–2014. PLoS One. 2016;11(4):e0153728. doi: 10.1371/journal.pone.0153728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Althomsons SP, Kammerer JS, Shang N, Navin TR. Using routinely reported tuberculosis genotyping and surveillance data to predict tuberculosis outbreaks. PLoS One. 2012;7(11):e48754. doi: 10.1371/journal.pone.0048754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. TB and HIV coinfection. Available. 2021. https://www.cdc.gov/tb/topic/basics/tbhivcoinfection.htm
- 22.Centers for Disease Control and Prevention. Prevention and control of tuberculosis in correctional and detention facilities: recommendations from the CDC. Available at. 2021. https://www.cdc.gov/mmwr/PDF/rr/rr5509.pdf
- 23.Schmit KM, Lobato MN, Lang SG, Wheeler S, Kendig NE, Bur S. High completion rate for 12 weekly doses of isoniazid and rifapentine as treatment for latent Mycobacterium tuberculosis infection in the Federal Bureau of Prisons. J Public Health Manag Pract. 2019;25(2):E1–E6. doi: 10.1097/PHH.0000000000000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheeler C, Mohle-Boetani J. Completion rates, adverse effects, and costs of a 3-month and 9-month treatment regimen for latent tuberculosis infection in California inmates, 2011–2014. Public Health Rep. 2019;134(suppl 1):71S–79S. doi: 10.1177/0033354919826557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federal Bureau of Prisons. Tuberculosis. Available. 2021. https://www.bop.gov/resources/pdfs/TB_CPG.pdf
- 26.Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/tb/publications/infographic/ltbi-treatment-costs.htm
- 27.Centers for Disease Control and Prevention. CDC estimates for TB treatment costs. 2020. https://www.cdc.gov/tb/publications/infographic/default.htm
- 28.Marks SM, Flood J, Seaworth B, et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis. 2014;20(5):812–821. doi: 10.3201/eid2005.131037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steenland K, Levine AJ, Sieber K, Schulte P, Aziz D. Incidence of tuberculosis infection among New York State prison employees. Am J Public Health. 1997;87(12):2012–2014. doi: 10.2105/AJPH.87.12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones TF, Woodley CL, Fountain FF, Schaffner W. Increased incidence of the outbreak strain of Mycobacterium tuberculosis in the surrounding community after an outbreak in a jail. South Med J. 2003;96(2):155–157. doi: 10.1097/01.SMJ.0000053678.62096.6F. [DOI] [PubMed] [Google Scholar]
- 31.Wilcock K, Hammett TM, Parent DG.2021. https://www.ojp.gov/pdffiles/ctub.pdf
- 32.Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, Scano F. Tuberculosis incidence in prisons: a systematic review. PLoS Med. 2010;7(12):e1000381. doi: 10.1371/journal.pmed.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haddad MB, Foote MK, Ray SM, et al. Substantial overlap between incarceration and tuberculosis in Atlanta, Georgia, 2011. Open Forum Infect Dis. 2014;1(1):ofu041. doi: 10.1093/ofid/ofu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Institute for Crime & Justice Policy Research. 2021. https://www.prisonstudies.org/highest-to-lowest/prison_population_rate