Abstract
Pituitary apoplexy (PA) is a rare clinical syndrome due to pituitary hemorrhage or infarction. It is characterized by the sudden onset of one or more of the following: severe headache, visual disturbance, nausea/vomiting, and or altered mental status. Most commonly, PA occurs in an underlying pituitary adenoma. The pathophysiology is not fully understood, but it is thought to involve elements of increased metabolic demand and/or compromise to the vasculature of the pituitary or pituitary tumor. Several risk factors have been described. Stabilization of the patient on presentation, replacement of hormonal deficiencies, and reversal of electrolyte abnormalities are the recommended initial steps in the management of patients with PA. Surgical decompression of the mass effect had been the recommended treatment for patients with PA; however, retrospective studies of patients with PA have demonstrated similar outcomes when a conservative approach is applied. This suggests that in highly selected clinical scenarios (mild visual deficit and improving symptoms), conservative management is possible. Further studies, however, are necessary to better stratify patients but are limited by the rarity of the condition and the acuity.
Keywords: pituitary apoplexy, pituitary hemorrhage, pituitary infarction, pituitary necrosis, hypopituitarism, pituitary tumor
Classically, pituitary apoplexy (PA) is defined as a clinical syndrome consisting of sudden onset of a severe headache, vomiting, and visual deterioration with or without altered mental status [1]. This constellation of symptoms is caused by acute/subacute hemorrhage or infarction, most commonly of a pituitary tumor or occasionally other pathology [2]. Changing the nomenclature to pituitary tumor apoplexy has been suggested to provide a more accurate description of this condition [3]. Although this condition was first described in 1898 [4], the term PA was not used until the 1950s by Broughman et al [5]. Pituitary hemorrhage or infarction may also occur in the absence of the symptoms and is detected on radiological or on pathological assessment. This phenomenon has been described and will be referred to as subclinical or asymptomatic and is not considered under the umbrella definition of PA. Sheehan syndrome, on the other hand, is pituitary infarction typically following massive peripartum hemorrhage, which can lead to hypopituitarism. Despite PA being a well-established entity and often considered to be a medical emergency, optimal treatment remains uncertain given the rarity of the condition. In this minireview we will discuss the epidemiology, hypothesized pathophysiology, and clinical presentation, and will summarize the most recent evidence surrounding treatment options. Unless otherwise specified, PA refers to the acute clinical syndrome in the presence of pituitary hemorrhage or infarction.
Materials and Methods
We searched PubMed using the search terms “pituitary apoplexy” (MeSH terms) OR (“pituitary”[all fields] AND “apoplexy”[all fields]) OR “pituitary apoplexy”[all fields] and using the filter “humans.” This initial search yielded 1313 references. Additional relevant references were also identified in the references list of articles obtained. With regard to treatment outcomes, articles published within the last 10 years where comparisons were according to timing of surgery or type of treatment used were prioritized. A formal systematic review with grading of the evidence was not conducted for the purpose of this article.
Epidemiology
PA is rare; however, the estimated prevalence varies depending on the cohort assessed, such as nonfunctioning pituitary adenomas (NFPA) or pathological specimens. In a population-based study assessing the prevalence of pituitary adenomas in a defined geographical area, the prevalence of PA was 6.2 out of 100 000 inhabitants in the United Kingdom [6]. In a retrospective, population-based regional cohort in Finland, the incidence of PA was 0.17 out of 100 000 per year [7]. The event rate of PA among patients observed with pituitary adenomas assessed in a meta-analysis reported an event rate per 100 person-years of 0.2 (95% CI, 0.0-0.5) [8]. Similarly, a systematic review that assessed the natural history pituitary incidentalomas reported the incidence of PA to be 0.6 per 100 person-years [9]. However, in a more specific cohort, for example, among surgically operated pituitary adenomas, the prevalence of classic PA varies and ranges from 0.6% to 9.1% [10-12] with even higher prevalence noted when restricted to NFPAs (21%) [13].
PA most commonly occurs in a pituitary adenoma, with the majority being previously undiagnosed (~ 80%). It predominantly occurs in macroadenomas, and NFPAs are reported to be the most common subtype [2]. PA has been reported in giant adenomas, the majority of which were known (60%) before the apoplectic event [14]. Among functional pituitary tumors, prolactinomas are the most common [15]. It is suggested, however, that functional tumors may be underestimated as a proportion of pathological specimens are unable to be subtyped in the setting of significant hemorrhage or infarction [16]. There appears to be a male predominance with an average age at presentation of 50 to 60 years. In addition to pituitary adenomas, PA has been reported in association with a Rathke cleft cyst, craniopharyngioma, pituitary metastasis, or a primitive neuroectodermal tumor [17].
Incidence of pituitary hemorrhage and subclinical PA (which is not synonymous with classic PA) is not well known. In a UK study assessing the prevalence of pituitary hemorrhage in patients with prolactinoma, 6.3% (23/368) had evidence of pituitary hemorrhage without the classic features of PA [18]. Most of these patients had macroprolactinomas and were female. The prevalence of subclinical hemorrhage and PA among 328 patients with NFPAs who had surgery was reported to be 14.3% and 3.4%, respectively [19]. Similarly, in another study of 385 patients with NFPAs 13.2% and 9.6% had subclinical hemorrhage or PA, respectively [20]. Overall, these studies indicated that subclinical PA/hemorrhage is more prevalent than classic PA.
Pathophysiology
Several hypotheses have been suggested as to the pathogenesis of PA. Pituitary tumors demonstrate high metabolic needs based on increased [18F]-fluorodeoxyglucose as well as [11C]-L-methionine uptake on positron emission tomography imaging and therefore anything that threatens delivery of this demand can lead to PA [21]. Although the normal pituitary gland is predominantly supplied via the hypophyseal portal system and to a lesser degree by direct arterial supply, the opposite has been observed in adenomas [3, 22, 23]. In fact, the blood supply in adenomas might originate from the inferior hypophyseal artery, which is more sensitive to changes in systemic blood pressure and therefore alterations in blood flow or blood pressure could precipitate PA. Indeed, intrasellar pressure is measured to be high in patients with pituitary tumor and even higher in those with PA [24-26]. Another hypothesis includes the presence or development of abnormal blood vessels and decreased microvascular density. Tumoral blood vessels are believed to be more fragile [27] with incomplete maturation and ruptured basal membranes [28, 29]. Pathological comparison of pituitary adenomas (n = 19) to that of pituitary at autopsy (n = 1) suggests that pituitary adenomas are less vascularized, and capillaries present have a disorganized appearance with changes similar to those seen in ischemia [30]. Furthermore, vascular endothelial growth factor is reduced in the majority of pituitary tumors assessed when compared to different types of central nervous system tumors based on messenger RNA quantification, suggesting decreased angiogenesis in pituitary tumors [21]. Microvascular density is also found to be reduced compared to normal pituitary [30, 31]. Therefore hemorrhage, infarction, and necrosis can be seen on pathology either independently or in combination. Lastly, tumor outgrowing its blood supply has been considered as a cause for PA given that most tumors are macroadenomas, although PA has been described in microadenomas [32]. In a meta-analysis reporting the incidence of adverse events in those with pituitary adenomas, the incidence of PA did not differ between macroadenomas and microadenomas although there was a trend for increased incidence among macroadenomas. PA incidence, however, was highest in those tumors with greatest average growth (> 3.5 mm) and tumor growth was more likely among macroadenomas [8].
Recognized risk factors associated with the occurrence of PA included surgical procedures, anticoagulation, pituitary tumor medical treatment, dynamic pituitary testing/hormonal treatment, and head trauma, although in 10% to 40% no predisposing factor can be identified [2, 33]. In a review by Briet et al [2], predisposing or precipitating factors of PA were assessed with 35 cases of PA associated with dynamic testing (including growth hormone–releasing hormone, thyroid-releasing hormone, corticotropin-releasing hormone, and gonadotropin-releasing hormone, insulin tolerance test, dexamethasone, and chlorpromazine), 11 cases associated with gonadotropin-releasing hormone agonists, and 14 cases associated with dopamine agonists. Events that contribute to alterations in blood pressure that impose metabolic stress on pituitary tumors have been implicated. Such events include orthopedic and cardiovascular surgery, interventional radiological procedures, and head trauma. Several studies have suggested that hypertension may be a contributor [34-36]; however, in a study of 42 patients with PA and 84 controls, PA was not associated with the presence of hypertension (21% vs 23%; P = ≥.999) [37]. Cases of apoplexy have been described rarely during pregnancy, which may be related to lactotroph hyperplasia [38, 39]. More recently, PA has been reported in several patients with COVID-19 [40-42] and several reports in relation to COVID-19 vaccination [43-45]. The postulated mechanism is increased coagulopathy associated with SARS-CoV-2 and expression of ACE2 in the cerebral vasculature [46].
Presentation
By definition, the most common presenting symptom of PA is sudden severe frontal or retro-orbital headache seen in 80% to 90% cases [47]. Typically, the headache is thunderclap in character, but status migraine or paroxysmal hemicrania have been reported and only a few reports of insidious headache have been published. Acute visual abnormalities as a consequence of mass effect related to the sudden distention of sellar contents around the cranial nerves have been reported in 47% to 68% of patients, with blurred vision present in 20% in a study of 87 patients with PA [48]. Cranial nerve palsies (III, IV, and VI) have been described in 39% to 52% of cases, with palsy of the third cranial nerve being the most predominant. Optic nerve compromise resulting in bitemporal hemianopsia can occur in 30% to 71%, while facial nerve weakness is observed infrequently in 9% of cases. Reduced consciousness can complicate presentation in up to 42% of patients [49]. Nausea, vomiting, and photophobia may also occur, mimicking meningitis [47, 48, 50-52].
To objectively quantify the severity of neuro-ophthalmic signs in patients with PA at presentation and to assess subsequent clinical course, a pituitary apoplexy score (PAS) was proposed by the Pituitary Apoplexy Guidelines Development Group in the United Kingdom [1]. The PAS is a sum of points allocated for presence of reduced visual acuity, visual field deficits, ocular paresis, and level of consciousness determined using the Glasgow Coma Scale.
In addition to PA, major central nervous system disorders should be considered in the differential diagnosis in patients presenting with similar dramatic symptoms including meningitis, hypophysitis, subarachnoid hemorrhage, infarction, cavernous sinus thrombosis, and carotid dissection. While patients might present with the symptoms discussed within 24 to 72 hours of the initial event (acutely), a subgroup of patients might present subacutely when the diagnosis is established after 72 hours. The timing becomes relevant for interpretation of imaging results.
Given the presence of an undiagnosed underlying pituitary tumor in the majority of cases (secreting or nonsecreting), a certain degree of endocrine dysfunction and therefore symptoms related to this might be present by the time of presentation. Pituitary hormonal evaluation at presentation frequently reveals hypopituitarism. Presence of secondary adrenal insufficiency has been described in 45% to 70% cases and might be accompanied by hyponatremia, hypotension, and even acute hypoglycemia [2, 33, 48, 51, 52]. Serum cortisol levels should be interpreted in the context of severity and acuity of disease as in critically ill patients. Secondary hypothyroidism can be found in 35% to 70%, and about 60% of men are diagnosed with hypogonadism whereas hypogonadism in women is reported in 50% to 75% [2, 33, 48, 51, 52]. Growth hormone deficiency is not usually evaluated in the acute setting and therefore the true prevalence of cases is not known. Hyperprolactinemia due to stalk compression or hypoprolactinemia may also be seen. A small subset of patients may present with acute symptoms of diabetes insipidus [52]. Patients may describe onset of symptoms suggestive of hormonal excess before presentation if the underlying tumor was functional.
Imaging
Given the severity of the symptomatology at presentation, patients are evaluated initially in emergency departments and the majority (79%) of patients undergo initial computed tomography (CT) head imaging, although over the last few decades there has been a shift toward magnetic resonance imaging (MRI). While CT detects hemorrhage in 20% to 40% of cases, MRI shows presence of blood in 89% of cases. Infarct of the tumor can be seen in 8% of cases. Cavernous sinuses might be involved in 41% of cases. As discussed previously, PA most commonly occurs in pituitary adenomas, which are more commonly macroadenomas (22-27 mm) [48, 53].
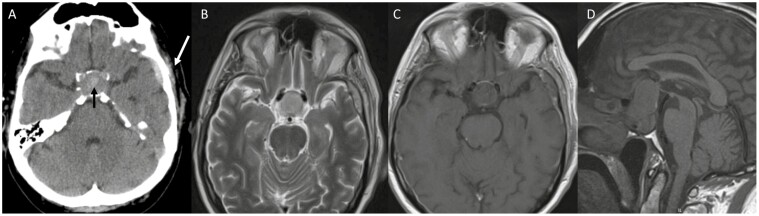
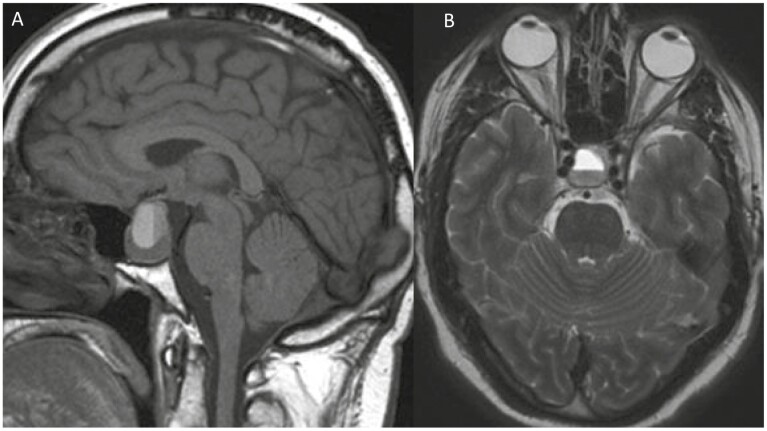
Of importance for clinicians is the timing of imaging studies performed in relation to the clinical event because of the changing chemical characteristics of hemoglobin and degradation of blood over time (Fig. 1A-1D). On CT, blood products are initially hyperdense making it an ideal imagining option at least acutely (< 6 hours from symptom onset), though hyperacute blood can be isointense and infarction may be difficult to discern. Over time, the density declines approaching similar densities to that of water. On MRI in the acute phase, blood can be difficult to characterize in the presence of deoxyhemoglobin (blood is isointense on T1 and bright on T2 in the hyperacute stage). However, within 24 to 48 hours, T1 hyperintensity signal usually increases as a result of conversion to methemoglobin present intracellularly, which persists for 7 to 28 days. As hemosiderin is formed, the T1 signal intensity drops (> 14-28 days). T2 sequences demonstrate brightness in the hyperacute phase, while hypointensity persists through the acute and early subacute phases (1-7 days), which become hyperintense with extracellular methemoglobin formation (7-28 days). During the chronic phase, T2 signal drops once again (> 14-28 days) as it becomes hemosiderin. The changes seen are reviewed in detail by Goyal et al [54]. As phases change, there can be fluid-fluid levels reflecting the different intensities of the different stages (eg, upper part hyperintense, lower hypointense on T1 sequences; Fig. 2A and 2B). MRI technology also allows for better characterization and proximity to adjacent vital structures. An interesting feature suggestive of PA is thickening of the sphenoid sinus mucosa in the acute phase [55]. In both hemorrhagic and nonhemorrhagic apoplexy, administration of contrast leads characteristically to peripheral rim enhancement.
Figure 1.
Pituitary apoplexy following head trauma in a 61-year-old cyclist on aspirin who developed acute loss of vision in the left eye. A, Noncontrast axial computed tomography (CT) image demonstrating left temporal bone fracture with associated subdural hematoma (white arrow). The sella is expanded because of the presence of a pituitary adenoma. Within the center of pituitary adenoma there is hyperintense material as a result of hemorrhage (black arrow). B, T2 axial magnetic resonance imaging (MRI); C, T1 axial MRI; and D, T1 sagittal MRI performed shortly afterward also demonstrate the pituitary tumor and hemorrhage, extending suprasellar and leading to visual symptoms. In the acute phase hemorrhage is hypointense on T1 and can therefore be difficult to visualize compared to CT.
Figure 2.
Hemorrhagic pituitary apoplexy within a pituitary adenoma demonstrating a fluid-fluid level seen on A, sagittal T1 and B, axial T2 imaging 1 month after initial event. The upper layer of fluid is hyperintense on T1 containing extracellular met hemoglobin whereas the lower layer contains blood remnants.
Management
PA can lead to acute and severe anterior pituitary hormonal deficiencies as outlined previously. This may result in considerable electrolyte abnormalities and hemodynamic instability, and therefore initial management is aimed at appropriate resuscitation to reverse electrolyte abnormality and ensure patient stabilization. Of greatest concern is cortisol and thyroid hormone deficiency. Glucocorticoid replacement (eg, intravenous hydrocortisone 200 mg/24 hours either continuously or intermittently as 50 mg every 6 hours) should be prioritized, and investigations should not delay treatment [56]. Thyroid hormone replacement, if needed, should be administered only after glucocorticoids have been given. However, once stabilized, subsequent optimal management remains uncertain. Studies have assessed outcomes according to type of surgery (microscopic vs endoscopic transsphenoidal resection) and timing of surgery (early vs delayed), whereas others have assessed outcomes according to intervention (conservative vs surgical management). Guijt et al [57] recently proposed a PA classification according to symptom duration (type A: hours-3 days, type B: 3 days-2 weeks, and type C: > 2 weeks); however, outcomes according to this classification have yet to be assessed. Comparative studies published in the last 10 years are presented in Table 1. Several meta-analyses have been performed with notable heterogeneity [66, 73, 74].
Table 1.
List of published studies comparing surgical timing and or surgical vs conservative treatment over last 10 years (2011-2022)
| Author, y | Country, y included | Total N | Definition of early surgery | Total in each surgical time frame (N) | Type of surgery | Comparison of surgical timing | Comparison of conservative vs surgical treatment |
|---|---|---|---|---|---|---|---|
| Budohoski et al, 2022 [58] | UK, 2003-2020 | N = 160, surgery (N = 96), conservative (N = 64) | Group 1 (< 7 d), group 2 (> 7 and < 30 d), group 3 (no surgery) |
Group 1 (N = 61), group 2 (N = 35) | ETSS | Although differences in VF and VA outcomes were seen between groups 1-3, authors note that chiasm compression and VA change were independent risk factors for urgent surgery | Although differences in VF and VA outcomes were seen between groups 1-3, authors note that chiasm compression and VA change were independent risk factors for urgent surgery |
| Cabuck et al, 2021 [59] | Turkey 1997-2019 | N = 91 | Early (1-7 d), delayed (8-21), late (> 21) | Early (N = 26), delayed (N = 29), late (N = 36) | ETSS | Surgery improved headache, neuro-ophthalmic abnormalities, but hormonal recovery less likely with delayed surgery | NA |
| Cavalli et al 2021 [60] | UK, 2009-2017 | N = 30, surgery (N = 18), conservative (N = 12) | Emergency (< 7 d), delayed (> 7 d) | Emergency surgery (N = 10), delayed surgery (N = 8) | ETSS | No difference between VF and CN palsy recovery between emergency and delayed surgery | No difference in VF, VA, hormonal, or CN palsy improvement on early follow-up. Need for thyroid hormone and gonadotropin replacement was lower in conservative group on long-term follow-up and VF defect was lower in emergency surgery group on long-term follow-up |
| Marx et al 2021 [61] | France 2007-2018 | N = 46, surgery (19), conservative (27) | Early (< 7 d), delayed (> 7 d) | Early (N = 13), delayed (N = 6) | NR | No difference in neuro-ophthalmic or endocrine outcome according to surgical timing | VA and VF deficits were greater in surgical cohort. Mean number of pituitary deficits was higher in surgical group (2.47 vs 1.45, P = .029), no difference if VF, VA, or CN palsy |
| Nakhleh et al, 2021 [62] | Israel, 2001-2017 | N = 27, surgery (17), conservative (10) | Early (< 7 d), delayed (> 7 d) | Early (N = 11), delayed (N = 6) | ETSS | No difference between early and late surgical intervention | No difference regarding VF defect, CN palsy, or hypopituitarism |
| Shepard et al, 2021 [63] | USA, 2007-2019 | N = 64, surgery (N = 17), conservative (N = 47) |
Early surgery (< 7 d), delayed surgery (failed conservative management > 7 d but < 3 mo after presentation) | Early (N = 17), delayed (N = 7) | ETSS | NR | No difference between VA/VF recovery. CN improvement was higher in conservative group (100% vs 60%) and median time to recovery was faster |
| Zhu et al, 2021 [64] | China, 2012-2020 | N = 46 | Early (< 7 d), delayed (> 7 d) | Early (N = 12), delayed (N = 33)a | ETSS | No difference in visual disturbance or hormonal recovery according to surgical timing | NA |
| Lammert et al, 2020 [65] | Germany, 2013-2016 | N = 21 | Group A (0-7 d), group B (1-4 wk), group C (> 4 wk) | Group A (N = 7), group B (N = 8), group C (N = 6) | MTSS | No patient in group A had full recovery of pituitary abnormalities, 3/8 in group B and 2/6 in group C | NA |
| Pangal et al, 2020 [53] | USA, 2012-2018 | N = 50 | Early (< 7 days), delayed (> 7 d) | Early (N = 10), delayed (N = 11)b |
ETSS | Postoperative panhypopituitarism was significantly more common (70% vs 18%; P = .0189) in group of patients with delayed surgery vs earlier surgery. No difference in headache resolution or vision | NA |
| Almeida et al, 2019 [66] | Canada, 2007-2017 | N = 67, surgery (N = 49), conservative (N = 18) |
Early (≤ 3 d), delayed (> 3 d) | Early (N = 22), delayed (N = 27) | ETSS | No difference in visual recovery according to surgical timing | Those managed conservatively had fewer visual deficits, but similar outcome between surgery and conservative management in terms of VF, pituitary dysfunction, and CN deficits |
| Kim et al, 2018 [67] | Korea, 1998-2014 | N = 41 | Early (< 7 d), delayed (> 7 d) | Early (N = 17), delayed (N = 24) |
TSS not specified | Early surgical group had more ocular paresis before surgery, but ocular paresis, VA and VF recovery did not differ between groups | NA |
| Rutkowski et al, 2018 [68] | USA, 2003-2014 | N = 32 | Early (< 72 h of symptom onset) | Early (N = 13), Delayed (N = 19) |
ETSS | No difference in improvement of vison, CN, endocrinopathy, or nonneurological symptoms or signs | NA |
| Abbara et al, 2018 [69] | UK, 1991-2015 | N = 52, surgery (N = 19), conservative (N = 33) |
NA | NA | NA | NA | More patients with VF defect and VA abnormality were treated surgically. No difference in No. of patients who had full recovery of pituitary deficits, and all had at least some improvement in visual disturbance |
| Teixeira et al, 2018 [70] | Portugal, 2005-2015 | N = 23, surgery (N = 14), conservative (N = 9) |
NA | NA | Internasal transsphenoidal surgery Endoscopic (9), Microscopic (5) | No difference between microscopic and endoscopic surgical approach and visual outcomes at 6 mo. Recovery of pituitary dysfunction was greater in endoscopic group | Surgery associated with longer length of hospital stay (15 vs 6 d). Neurological deficits were worse in surgery group, but recovery was similar. Endocrine dysfunction was greater in conservative group (88 vs 35%) at 6-mo follow-up |
| Giritharan et al, 2016 [71] | UK, 2005-2014 | N = 31 surgery (N = 20), conservative (N = 11) |
Elective (< 7 d), emergency (> 7 d) | Elective (N = 11), emergency (N = 9) |
ETSS | No difference in rates of hypopituitarism and visual recovery | No difference in visual or hormonal outcomes. Differences in median PAS score at presentation (emergency 3, elective 2, and conservative 0) |
| Singh et al, 2015 [48] | USA, 1992-2013 | N = 87, surgery (N = 69), conservative (N = 18) |
Early during hospital stay median hospital stay 5 d (IQR 3-10 d) | Early (N = 61), delayed (N = 8), | TSS not specified | No difference in any of the outcome measures across treatment groups | No difference in outcome measures across groups |
| Bujawansa et al, 2014 [15] | UK, 1985-2010 | N = 55, Surgery (N = 32), Conservative (N = 23) |
Early (< 7 d) and Elective (> 7 d) | Early (N = 23) and Elective (N = 10) | NR | Patients who proceeded to early surgery had higher PAS scores. No difference in VF, VA deficits, or CN palsies | No difference in complete/near-complete VF defect, complete/near-complete CN palsy or need for hormone replacement between groups |
| Leyer et al, 2011 [72] | France, 1996-2008 | N = 44, surgery (N = 19), conservative (N = 24), radiotherapy (N = 1) | Early surgery < 1 mo, delayed > 1 mo | Early (N = 19), delayed (N = 4) (who were initially managed conservatively) | ETSS | No difference in ophthalmic symptoms among those operated < 8 d compared to > 8 d | No difference in pituitary function or visual symptoms between surgically or conservatively managed patients |
Studies are presented in chronological order with most recent first.
Abbreviations: CN, cranial nerve; ETSS, endoscopic transsphenoidal surgery; IQR, interquartile range; MTSS, microscopic transsphenoidal surgery; NA, not available; PAS, pituitary apoplexy score; TSS, transsphenoidal; UK, United Kingdom; USA, United States of America; VA, visual acuity; VF, visual fields.
a A patient was excluded in comparative analysis as authors indicate this patient had “asymptomatic apoplexy.”
b Follow-up data according to surgical timing was only available in 22.
Early vs Delayed Surgery
Surgery is reported to provide rapid relief of headache; however, the time to recovery of other sequelae of PA can vary. In a study in which the mean time to surgery from symptom onset was 15 days, the time to recovery of hormonal abnormalities (2 ± 1.8 weeks) was the quickest compared to visual field deficits (8.0 ± 9.9 weeks), whereas cranial nerve abnormalities were reported to take the longest (2.4 ± 2.2 months) [75]. It is hypothesized that the differences in recovery may be related to differing tissue sensitivities to anatomic compression [75]. Several studies have examined the timing of surgery with respect to outcomes, including neurological symptoms (eg, headache, visual fields, visual acuity, and ophthalmoplegia) and pituitary dysfunction. The definition of early surgical intervention, however, has varied between studies with studies defining early surgery as immediate, fewer than 3 days [64, 68], fewer than 4 days [76], or less than 1 week [67, 71]. These cutoffs are arbitrary. Regardless of the surgical timing, overall, a substantial proportion of patients have improvement in visual deficits (57%-95%) and cranial nerve palsies (63%-100%); however, the proportion who have pituitary function improvement is lower (19%-57%) [33]. A small retrospective study of 24 patients with PA suggested that preoperatively prolactin levels greater than 8.8 ng/mL may be predictive of pituitary endocrine function recovery [65], but this has not been replicated. Another study proposed that normal or high prolactin levels may indicate viable pituitary tissue with a higher chance of hypopituitarism recovery following surgery [26].
Recovery of visual acuity as measured by logMAR improved after surgery (0.35 vs 0.1); however, with respect to surgical timing there was no association between visual acuity improvement and time between symptom onset and surgery (P = .49) [77]. A study that included 186 cases of monocular or binocular blindness did not find a difference in outcomes between emergent (< 3 days) vs early surgery (< 7 days) (78 vs 71%; P = ≥ .999), but recovery was less likely the worse the visual deficit was on presentation, for example, the proportion who have improved visual deficits in binocular vs monocular (77% vs 45%; P = .014) [78]. Additionally, a recent meta-analysis that included 12 studies with 200 patients, of whom 86% had visual deficits, noted that 97.8% had an improvement in visual field deficits when the time to surgery was fewer than 7 days compared to 84.8% when the time to surgery was more than 7 days (odds ratio [OR] 2.6; 95% CI, 0.94-7.31; P = .07) [79]. Other studies have reported no difference not only in visual outcomes, but also in pituitary hormone recovery and cranial neuropathy when comparing “early” vs “delayed” surgical intervention [48, 67, 68, 71]. However, it is worth noting that patients who had emergent surgery were more likely to have worse visual symptoms at presentation [67] or have a larger tumor [66]. Therefore, selection bias has contributed to the treatment patients with PA receive and the results must be interpretated with caution within these confines. As a result, early or emergent surgical intervention is often considered in those with substantial visual deficits, but only after electrolyte and hemodynamic abnormalities are addressed.
Conservative vs Surgical Management
More recently, comparisons between surgical intervention and conservative management regarding outcomes have been reported in which none of the patients with PA were randomly allocated to either treatment group. Again, inclusion criteria for those patients considered to be part of the surgical group have varied with some considering those allocated surgery to include patients who had surgery up to 173 days after the acute apoplectic event [12] or those who had surgery for recurrent tumor. Furthermore, medical interventions administered in those allocated to the conservative treatment group have differed across studies, specifically, the type, dose, and duration of glucocorticoids administered vary as well as their indication as hormonal replacement or anti-inflammatory effect. Additionally, treatments such as diuresis and mannitol have been used in patients managed conservatively [12]. These varying definitions and treatments may make comparisons across studies challenging.
A further factor in outcome assessment is the inherent allocation bias in these retrospective studies. When assessed, patients allocated to surgery instead of observation have a more severe presentation [61, 64]. Studies using the PAS demonstrate that the average PAS scores for those who have surgery compared to those managed conservatively is significantly higher [15, 71]. In another study, patients who had surgery had larger tumors, reduced visual acuity, or were noted to have compression of the optic chiasm [66]. Similarly, in a study by Singh et al [48] in which 61 patients with PA had surgery and 18 were observed, more patients who were treated surgically had optic chiasm compression whereas those managed conservatively had a reduced level of consciousness.
There have been some conflicting results regarding outcomes in patients managed surgically compared to conservatively. In a study of 14 patients with PA treated surgically and 8 patients managed medically, surgery was associated with a longer length of hospital stay (15 vs 6 days). Conservative management was associated with a greater long-term prevalence of anterior pituitary deficits (88% vs 35%) [70]. Similarly, a study of 23 patients (14 surgical and 9 conservatively treated) noted that endocrine outcomes were better in those who were treated surgically (P = .017) [70]. Other studies have not noted such differences in pituitary hormone deficit improvement [48].
Patients managed conservatively had improvement in cranial neuropathy (100% vs 60%; P = .02), and median time to cranial neuropathy improvement was shorter (1.5 vs 35.5 months; P < .01) in a study of 64 patients in which 47 were managed conservatively and 17 had early surgery. It is worth noting that 7 of the 47 patients managed conservatively eventually proceeded to surgery (median time to surgery 50 days) due to recurrent apoplexy in 1 patient, worsening vision in 3, and lack of radiological improvement in 3 [63]. Another study of 12 patients initially managed medically (dexamethasone 2-16 mg in 11 patients) demonstrated full recovery of ophthalmoplegia in 6 patients and partial in 1. Surgery was performed in 5 patients because of recurrence of symptoms on glucocorticoid taper, or failure to improve or change in mentation as assessed by Glasgow Coma Scale. Surgery led to improvement in neurological and visual function, but only complete visual recovery in one individual [80]. Contrary to this, a meta-analysis that included 5 studies between 1992 and 2014 comparing outcomes in patients with PA treated conservatively to those treated surgically, a significant difference in recovery of visual field deficits (OR 0.32; 95% CI, 0.10-0.97) and ocular palsy (OR 0.17; 95% CI, 0.03-0.79) was reported following surgery [74]. However, in another meta-analysis that included a larger number of studies (n = 14 with 457 cases) between 1988 and 2018 comparing outcomes in surgically vs medically patients with PA [73], outcomes did not differ between those treated surgically vs medically regarding endocrine dysfunction, visual acuity, visual field abnormality, and ophthalmoplegia. Similarly, a pooled analysis of 11 studies by Almeida et al [66] did not identify a difference in complete recovery of pituitary hormone, visual deficits, or cranial nerve abnormalities in those managed surgically vs conservatively .
Despite the inherent differences between patients with PA treated surgically or managed conservatively, the majority of studies do not reveal a difference in reported outcomes between these 2 treatment modalities regarding visual acuity, visual field deficits, ocular paresis, or anterior pituitary hormonal abnormalities. This suggests that appropriate patients are currently being selected for conservative management and those highly selected patients (those with mild visual deficit, improving symptoms, prolactinomas, or low PAS scores) are likely to have similar outcomes to patients with PA who are surgically managed. Consequently, the number of patients with PA being managed conservatively has increased [48]. Taking into account this information, patients with severe visual deficits, worsening symptoms, or a high PAS score should be considered for surgery if deemed to be surgical candidates.
Follow-up
Following initial management, whether surgical or medical, ongoing clinical and radiological surveillance is recommended. Reports of complete regression of pituitary adenoma, including functioning adenomas, have been described, but so has growth or regrowth of the underlying tumor. Among 32 patients with PA managed conservatively, median initial tumor volume was 2.75 cm3 (range, 0.32-10.7 cm3) whereas on follow-up it was 0.65 cm3 (range, 0-8.74 cm3) [81]. Complete regression was seen in 28% and the median time to regression was 18 months. Tumor progression or recurrence is seen in up to 20% of patients [51], but has varied between studies and is possibly related to length of follow-up. The tumor recurrence rate was similar for those who had surgery compared to those who were managed conservatively [66], although other studies have reported increased [82] and reduced [72] recurrence with surgery, but is less likely after radiation therapy [83]. Collectively, this suggests that recurrence rates are low, but long-term surveillance is needed irrespective of treatment modality.
Following PA, anterior pituitary hormone deficits are common. Partial or complete recovery has been reported in up to 50% of cases; however, completely normal pituitary function is reported in only 5% to 37% of patients following PA [2]. It was postulated that rapid surgical decompression of the mass effect might alleviate pressure imposed and allow for greater recovery than with conservative or indeed delayed surgical treatment [3, 26]. In addition, in cases in which the acute event caused severe anterior pituitary damage, the recovery of function is unlikely possible. Although recovery was more common at 6 months in those who had endoscopic transsphenoidal surgery compared to conservative management or microscopic transsphenoidal surgery [70], such differences have not been demonstrated in other studies [15, 60, 62, 66]. Similarly, diabetes insipidus may be transient (either following PA or surgery) and therefore should be reevaluated on follow-up.
Conclusion
PA due to hemorrhage or infarction is a rare acute event but can lead to considerable morbidity and possibility mortality. Recognition and prompt initiation of hormonal replacement are the first and undisputed steps in the management of PA. Severity of presentation in addition to multidisciplinary discussion with neurology, neurosurgery, and intensive care teams have guided subsequent management of patients with PA. Those with stable or resolving neuro-ophthalmic symptoms are considered candidates for conservative management. Further studies, however, are necessary to more objectively categorize patients who would benefit from surgery or observation. Given that pituitary tumors are frequently identified at presentation, long-term follow-up is recommended to detect progression or recurrence, which may warrant treatment.
Acknowledgments
The authors would like to thank Bradley J. Erickson, MD, PhD, for providing medical images from the Department of Radiology. We would also like to thank Larry Prokop.
Glossary
Abbreviations
- CT
computed tomography
- MRI
magnetic resonance imaging
- NFPA
nonfunctioning pituitary adenoma
- OR
odds ratio
- PA
pituitary apoplexy
- PAS
pituitary apoplexy score
Contributor Information
Diane Donegan, Division of Endocrinology, Diabetes and Metabolism, Indiana University, Indianapolis, Indiana 46220, USA; Division of Endocrinology, Diabetes and Metabolism, Mayo Clinic Minnesota, Rochester, Minnesota 55905, USA.
Dana Erickson, Email: Erickson.dana@mayo.edu, Division of Endocrinology, Diabetes and Metabolism, Mayo Clinic Minnesota, Rochester, Minnesota 55905, USA.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosures
The authors have nothing to declare.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the present study.
References
- 1. Rajasekaran S, Vanderpump M, Baldeweg S, et al. UK guidelines for the management of pituitary apoplexy. Clin Endocrinol (Oxf). 2011;74(1):9-20. doi: 10.1111/j.1365-2265.2010.03913.x [DOI] [PubMed] [Google Scholar]
- 2. Briet C, Salenave S, Bonneville JF, Laws ER, Chanson P. Pituitary apoplexy. Endocr Rev. 2015;36(6):622-645. doi: 10.1210/er.2015-1042 [DOI] [PubMed] [Google Scholar]
- 3. Nawar RN, AbdelMannan D, Selman WR, Arafah BM. Pituitary tumor apoplexy: a review. J Intensive Care Med. 2008;23(2):75-90. doi: 10.1177/0885066607312992 [DOI] [PubMed] [Google Scholar]
- 4. Bailey P. Pathological report of a case of acromegaly, with special reference to the lesions in the hypophysis cerebri and in the thyroid gland; and a case of haemorrhage into the pituitary. Philadelphia Med J. 1898;1:789-792. [Google Scholar]
- 5. Brougham M, Heusner AP, Adams RD. Acute degenerative changes in adenomas of the pituitary body—with special reference to pituitary apoplexy. J Neurosurg. 1950;7(5):421-439. doi: 10.3171/jns.1950.7.5.0421 [DOI] [PubMed] [Google Scholar]
- 6. Fernandez A, Karavitaki N, Wass JAH. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010;72(3):377-382. doi: 10.1111/j.1365-2265.2009.03667.x [DOI] [PubMed] [Google Scholar]
- 7. Raappana A, Koivukangas J, Ebeling T, Pirilä T. Incidence of pituitary adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab. 2010;95(9):4268-4275. doi: 10.1210/jc.2010-0537 [DOI] [PubMed] [Google Scholar]
- 8. Fernández-Balsells MM, Murad MH, Barwise A, et al. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2011;96(4):905-912. doi: 10.1210/jc.2010-1054 [DOI] [PubMed] [Google Scholar]
- 9. Sivakumar W, Chamoun R, Nguyen V, Couldwell WT. Incidental pituitary adenomas. Neurosurg Focus. 2011;31(6):E18. doi: 10.3171/2011.9.FOCUS11217 [DOI] [PubMed] [Google Scholar]
- 10. Wakai S, Fukushima T, Teramoto A, Sano K. Pituitary apoplexy: its incidence and clinical significance. J Neurosurg. 1981;55(2):187-193. doi: 10.3171/jns.1981.55.2.0187 [DOI] [PubMed] [Google Scholar]
- 11. Bills DC, Meyer FB, Laws ER Jr, et al. A retrospective analysis of pituitary apoplexy. Neurosurgery. 1993;33(4):602-608; discussion 608-609. doi: 10.1227/00006123-199310000-00007 [DOI] [PubMed] [Google Scholar]
- 12. Bonicki W, Kasperlik-Załuska A, Koszewski W, Zgliczyński W, Wisławski J. Pituitary apoplexy: endocrine, surgical and oncological emergency. Incidence, clinical course and treatment with reference to 799 cases of pituitary adenomas. Acta Neurochir (Wien). 1993;120(3-4):118-122. doi: 10.1007/BF02112028 [DOI] [PubMed] [Google Scholar]
- 13. Nielsen EH, Lindholm J, Bjerre P, et al. Frequent occurrence of pituitary apoplexy in patients with non-functioning pituitary adenoma. Clin Endocrinol (Oxf). 2006;64(3):319-322. doi: 10.1111/j.1365-2265.2006.02463.x [DOI] [PubMed] [Google Scholar]
- 14. Puglisi V, Morini E, Biasini F, Vinciguerra L, Lanza G, Bramanti P. Neurological presentation of giant pituitary tumour apoplexy: case report and literature review of a rare but life-threatening condition. J Clin Med. 2022;11(6):1581. doi 10.3390/jcm11061581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bujawansa S, Thondam SK, Steele C, et al. Presentation, management and outcomes in acute pituitary apoplexy: a large single-centre experience from the United Kingdom. Clin Endocrinol (Oxf). 2014;80(3):419-424. doi: 10.1111/cen.12307 [DOI] [PubMed] [Google Scholar]
- 16. Wildemberg LE, Glezer A, Bronstein MD, Gadelha MR. Apoplexy in nonfunctioning pituitary adenomas. Pituitary. 2018;21(2):138-144. doi: 10.1007/s11102-018-0870-x [DOI] [PubMed] [Google Scholar]
- 17. Jho DH, Biller BM, Agarwalla PK, Swearingen B. Pituitary apoplexy: large surgical series with grading system. World Neurosurg. 2014;82(5):781-790. doi: 10.1016/j.wneu.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 18. Sarwar KN, Huda MS, Van de Velde V, et al. The prevalence and natural history of pituitary hemorrhage in prolactinoma. J Clin Endocrinol Metab. 2013;98(6):2362-2367. doi: 10.1210/jc.2013-1249 [DOI] [PubMed] [Google Scholar]
- 19. Kinoshita Y, Tominaga A, Usui S, Arita K, Sugiyama K, Kurisu K. Impact of subclinical haemorrhage on the pituitary gland in patients with pituitary adenomas. Clin Endocrinol (Oxf). 2014;80(5):720-725. doi: 10.1111/cen.12349 [DOI] [PubMed] [Google Scholar]
- 20. Chen L, White WL, Spetzler RF, Xu B. A prospective study of nonfunctioning pituitary adenomas: presentation, management, and clinical outcome. J Neurooncol. 2011;102(1):129-138. doi: 10.1007/s11060-010-0302-x [DOI] [PubMed] [Google Scholar]
- 21. Oldfield EH, Merrill MJ. Apoplexy of pituitary adenomas: the perfect storm. J Neurosurg. 2015;122(6):1444-1449. doi: 10.3171/2014.10.JNS141720 [DOI] [PubMed] [Google Scholar]
- 22. Baker HL Jr. The angiographic delineation of sellar and parasellar masses. Radiology. 1972;104(1):67-78. doi: 10.1148/104.1.67 [DOI] [PubMed] [Google Scholar]
- 23. Gorczyca W, Hardy J. Microadenomas of the human pituitary and their vascularization. Neurosurgery. 1988;22(1 Pt 1):1-6. doi: 10.1227/00006123-198801010-00001 [DOI] [PubMed] [Google Scholar]
- 24. Kruse A, Astrup J, Cold GE, Hansen HH. Pressure and blood flow in pituitary adenomas measured during transsphenoidal surgery. Br J Neurosurg. 1992;6(4):333-341. doi: 10.3109/02688699209023792 [DOI] [PubMed] [Google Scholar]
- 25. Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab. 2000;85(5):1789-1793. doi: 10.1210/jcem.85.5.6611 [DOI] [PubMed] [Google Scholar]
- 26. Zayour DH, Selman WR, Arafah BM. Extreme elevation of intrasellar pressure in patients with pituitary tumor apoplexy: relation to pituitary function. J Clin Endocrinol Metab. 2004;89(11):5649-5654. doi: 10.1210/jc.2004-0884 [DOI] [PubMed] [Google Scholar]
- 27. Cardoso ER, Peterson EW. Pituitary apoplexy: a review. Neurosurgery. 1984;14(3):363-373. doi: 10.1227/00006123-198403000-00021 [DOI] [PubMed] [Google Scholar]
- 28. Di Ieva A, Weckman A, Di Michele J, et al. Microvascular morphometrics of the hypophysis and pituitary tumors: from bench to operating theatre. Microvasc Res. 2013;89:7-14. doi: 10.1016/j.mvr.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 29. Hirano A, Tomiyasu U, Zimmerman HM. The fine structure of blood vessels in chromophobe adenoma. Acta Neuropathol. 1972;22(3):200-207. doi: 10.1007/BF00684523 [DOI] [PubMed] [Google Scholar]
- 30. Schechter J. Ultrastructural changes in the capillary bed of human pituitary tumors. Am J Pathol. 1972;67(1):109-126. [PMC free article] [PubMed] [Google Scholar]
- 31. Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab. 2000;85(3):1159-1162. doi: 10.1210/jcem.85.3.6485 [DOI] [PubMed] [Google Scholar]
- 32. Randall BR, Couldwell WT. Apoplexy in pituitary microadenomas. Acta Neurochir (Wien). 2010;152(10):1737-1740. doi: 10.1007/s00701-010-0706-6 [DOI] [PubMed] [Google Scholar]
- 33. Capatina C, Inder W, Karavitaki N, Wass JA. Management of endocrine disease: pituitary tumour apoplexy. Eur J Endocrinol. 2015;172(5):R179-R190. doi: 10.1530/EJE-14-0794 [DOI] [PubMed] [Google Scholar]
- 34. Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA. Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol (Oxf). 1999;51(2):181-188. doi: 10.1046/j.1365-2265.1999.00754.x [DOI] [PubMed] [Google Scholar]
- 35. Mou C, Han T, Zhao H, Wang S, Qu Y. Clinical features and immunohistochemical changes of pituitary apoplexy. J Clin Neurosci. 2009;16(1):64-68. doi: 10.1016/j.jocn.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Qian Y, Qiao Y, et al. Risk factors for the incidence of apoplexy in pituitary adenoma: a single-center study from southwestern China. Chin Neurosurg J. 2020;6:20. doi: 10.1186/s41016-020-00202-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Möller-Goede DL, Brändle M, Landau K, Bernays RL, Schmid C. Pituitary apoplexy: re-evaluation of risk factors for bleeding into pituitary adenomas and impact on outcome. Eur J Endocrinol. 2011;164(1):37-43. doi: 10.1530/EJE-10-0651 [DOI] [PubMed] [Google Scholar]
- 38. Kato Y, Ogawa Y, Tominaga T. Treatment and therapeutic strategies for pituitary apoplexy in pregnancy: a case series. J Med Case Rep. 2021;15(1):289. doi: 10.1186/s13256-021-02892-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grand’Maison S, Weber F, Bédard MJ, Mahone M, Godbout A. Pituitary apoplexy in pregnancy: a case series and literature review. Obstet Med. 2015;8(4):177-183. doi: 10.1177/1753495X15598917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martinez-Perez R, Kortz MW, Carroll BW, et al. Coronavirus disease 2019 and pituitary apoplexy: a single-center case series and review of the literature. World Neurosurg. 2021;152:e678-e687. doi: 10.1016/j.wneu.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taneja C, Fazeli PK, Gardner PA, Wang EW, Snyderman CH, Mahmud H. Rapidly progressive pituitary apoplexy in a patient with COVID-19 disease treated with endoscopic endonasal surgery. J Neurol Surg Rep. 2022;83(1):e8-e12. doi: 10.1055/s-0041-1742104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liew SY, Seese R, Shames A, Majumdar K. Apoplexy in a previously undiagnosed pituitary macroadenoma in the setting of recent COVID-19 infection. BMJ Case Rep. 2021;14(7):e243607. doi: 10.1136/bcr-2021-243607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Piñar-Gutiérrez A, Remón-Ruiz P, Soto-Moreno A. Case report: pituitary apoplexy after COVID-19 vaccination. Med Clin (Barc). 2022;158(10):498-499. doi: 10.1016/j.medcli.2021.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roncati L, Manenti A. Pituitary apoplexy following adenoviral vector-based COVID-19 vaccination. Brain Hemorrhages. Published online April 19, 2022. doi: 10.1016/j.hest.2022.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mungmunpuntipantip R, Wiwanitkit V. Pituitary apoplexy and COVID-19 vaccination. Med Clin (Barc). 2022;159(2):e11. doi: 10.1016/j.medcli.2021.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frara S, Allora A, Castellino L, di Filippo L, Loli P, Giustina A. COVID-19 and the pituitary. Pituitary. 2021;24(3):465-481. doi: 10.1007/s11102-021-01148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suri H, Dougherty C. Presentation and management of headache in pituitary apoplexy. Curr Pain Headache Rep. 2019;23(9):61. doi: 10.1007/s11916-019-0798-5 [DOI] [PubMed] [Google Scholar]
- 48. Singh TD, Valizadeh N, Meyer FB, Atkinson JL, Erickson D, Rabinstein AA. Management and outcomes of pituitary apoplexy. J Neurosurg. 2015;122(6):1450-1457. doi: 10.3171/2014.10.JNS141204 [DOI] [PubMed] [Google Scholar]
- 49. Dubuisson AS, Beckers A, Stevenaert A. Classical pituitary tumour apoplexy: clinical features, management and outcomes in a series of 24 patients. Clin Neurol Neurosurg. 2007;109(1):63-70. doi: 10.1016/j.clineuro.2006.01.006 [DOI] [PubMed] [Google Scholar]
- 50. Tumyan G, Mantha Y, Gill R, Feldman M. Acute sterile meningitis as a primary manifestation of pituitary apoplexy. AACE Clin Case Rep. 2021;7(2):117-120. doi: 10.1016/j.aace.2020.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sibal L, Ball SG, Connolly V, et al. Pituitary apoplexy: a review of clinical presentation, management and outcome in 45 cases. Pituitary. 2004;7(3):157-163. doi: 10.1007/s11102-005-1050-3 [DOI] [PubMed] [Google Scholar]
- 52. Johnston PC, Hamrahian AH, Weil RJ, Kennedy L. Pituitary tumor apoplexy. J Clin Neurosci. 2015;22(6):939-944. doi: 10.1016/j.jocn.2014.11.023 [DOI] [PubMed] [Google Scholar]
- 53. Pangal DJ, Chesney K, Memel Z, et al. Pituitary apoplexy case series: outcomes after endoscopic endonasal transsphenoidal surgery at a single tertiary center. World Neurosurg. 2020;137:e366-e372. doi: 10.1016/j.wneu.2020.01.204 [DOI] [PubMed] [Google Scholar]
- 54. Goyal P, Utz M, Gupta N, et al. Clinical and imaging features of pituitary apoplexy and role of imaging in differentiation of clinical mimics. Quant Imag Med Surg. 2018;8(2):219-231. doi: 10.21037/qims.2018.03.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Waqar M, McCreary R, Kearney T, Karabatsou K, Gnanalingham KK. Sphenoid sinus mucosal thickening in the acute phase of pituitary apoplexy. Pituitary. 2017;20(4):441-449. doi: 10.1007/s11102-017-0804-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888-3921. doi: 10.1210/jc.2016-2118 [DOI] [PubMed] [Google Scholar]
- 57. Guijt MC, Zamanipoor Najafabadi AH, Notting IC, et al. Towards a pituitary apoplexy classification based on clinical presentation and patient journey. Endocrine. 2022;76(1):132-141. doi: 10.1007/s12020-022-02983-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Budohoski KP, Khawari S, Cavalli A, et al. Long-term oncological outcomes after haemorrhagic apoplexy in pituitary adenoma managed operatively and non-operatively. Acta Neurochir (Wien). 2022;164(4):1115-1123. doi: 10.1007/s00701-022-05119-8 [DOI] [PubMed] [Google Scholar]
- 59. Cabuk B, Kaya NS, Polat C, et al. Outcome in pituitary apoplexy patients, stratified by delay between symptom appearance and surgery: a single center retrospective analysis. Clin Neurol Neurosurg. 2021;210:106991. doi: 10.1016/j.clineuro.2021.106991 [DOI] [PubMed] [Google Scholar]
- 60. Cavalli A, Martin A, Connolly DJ, Mirza S, Sinha S. Pituitary apoplexy: how to define safe boundaries of conservative management? Early and long-term outcomes from a single UK tertiary neurosurgical unit. Br J Neurosurg. 2021;35(3):334-340. doi: 10.1080/02688697.2020.1812523 [DOI] [PubMed] [Google Scholar]
- 61. Marx C, Rabilloud M, Borson Chazot F, Tilikete C, Jouanneau E, Raverot G. A key role for conservative treatment in the management of pituitary apoplexy. Endocrine. 2021;71(1):168-177. doi: 10.1007/s12020-020-02499-8 [DOI] [PubMed] [Google Scholar]
- 62. Nakhleh A, Assaliya Naffa M, Sviri G, Shehadeh N, Hochberg I. Outcomes of pituitary apoplexy: a comparison of microadenomas and macroadenomas. Pituitary. 2021;24(4):492-498. doi: 10.1007/s11102-020-01124-1 [DOI] [PubMed] [Google Scholar]
- 63. Shepard MJ, Snyder MH, Soldozy S, Ampie LL, Morales-Valero SF, Jane JA. Radiological and clinical outcomes of pituitary apoplexy: comparison of conservative management versus early surgical intervention. J Neurosurg. 2021;135(5):1310–1318. doi: 10.3171/2020.9.JNS202899 [DOI] [PubMed] [Google Scholar]
- 64. Zhu Q, Liang Y, Fan Z, et al. Ischemic infarction of pituitary apoplexy: a retrospective study of 46 cases from a single tertiary center. Front Neurosci. 2021;15:808111. doi: 10.3389/fnins.2021.808111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lammert A, Walter MS, Giordano FA, et al. Neuro-endocrine recovery after pituitary apoplexy: prolactin as a predictive factor. Exp Clin Endocrinol Diabetes. 2020;128(5):283-289. doi: 10.1055/a-0640-2915 [DOI] [PubMed] [Google Scholar]
- 66. Almeida JP, Sanchez MM, Karekezi C, et al. Pituitary apoplexy: results of surgical and conservative management clinical series and review of the literature. World Neurosurg. 2019;130:e988-e999. doi: 10.1016/j.wneu.2019.07.055 [DOI] [PubMed] [Google Scholar]
- 67. Kim YH, Cho YH, Hong SH, et al. Postoperative neurologic outcome in patients with pituitary apoplexy after transsphenoidal surgery. World Neurosurg. 2018;111:e18-e23. doi: 10.1016/j.wneu.2017.11.124 [DOI] [PubMed] [Google Scholar]
- 68. Rutkowski MJ, Kunwar S, Blevins L, Aghi MK. Surgical intervention for pituitary apoplexy: an analysis of functional outcomes. J Neurosurg. 2018;129(2):417-424. doi: 10.3171/2017.2.JNS1784 [DOI] [PubMed] [Google Scholar]
- 69. Abbara A, Clarke S, Eng PC, et al. Clinical and biochemical characteristics of patients presenting with pituitary apoplexy. Endocr Connect. 2018;7(10):1058-1066. doi: 10.1530/EC-18-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teixeira JC, Lavrador J, Simão D, Miguéns J. Pituitary apoplexy: should endoscopic surgery be the gold standard? World Neurosurg. 2018;111:e495-e499. doi: 10.1016/j.wneu.2017.12.103 [DOI] [PubMed] [Google Scholar]
- 71. Giritharan S, Gnanalingham K, Kearney T. Pituitary apoplexy—bespoke patient management allows good clinical outcome. Clin Endocrinol (Oxf). 2016;85(3):415-422. doi: 10.1111/cen.13075 [DOI] [PubMed] [Google Scholar]
- 72. Leyer C, Castinetti F, Morange I, et al. A conservative management is preferable in milder forms of pituitary tumor apoplexy. J Endocrinol Invest. 2011;34(7):502-509. doi: 10.3275/7241 [DOI] [PubMed] [Google Scholar]
- 73. Goshtasbi K, Abiri A, Sahyouni R, et al. Visual and endocrine recovery following conservative and surgical treatment of pituitary apoplexy: a meta-analysis. World Neurosurg. 2019;132:33-40. doi: 10.1016/j.wneu.2019.08.115 [DOI] [PubMed] [Google Scholar]
- 74. Tu M, Lu Q, Zhu P, Zheng W. Surgical versus non-surgical treatment for pituitary apoplexy: a systematic review and meta-analysis. J Neurol Sci. 2016;370:258-262. doi: 10.1016/j.jns.2016.09.047 [DOI] [PubMed] [Google Scholar]
- 75. Zaidi HA, Cote DJ, Burke WT, et al. Time course of symptomatic recovery after endoscopic transsphenoidal surgery for pituitary adenoma apoplexy in the modern era. World Neurosurg. 2016;96:434-439. doi: 10.1016/j.wneu.2016.09.052 [DOI] [PubMed] [Google Scholar]
- 76. Gondim JA, de Albuquerque LAF, Almeida JP, et al. Endoscopic endonasal surgery for treatment of pituitary apoplexy: 16 years of experience in a specialized pituitary center. World Neurosurg. 2017;108:137-142. doi: 10.1016/j.wneu.2017.08.131 [DOI] [PubMed] [Google Scholar]
- 77. Kelly PD, Fernando SJ, Malenke JA, Chandra RK, Turner JH, Chambless LB. The effect of timing of surgery in pituitary apoplexy on continuously valued visual acuity. J Neurol Surg B Skull Base. 2021;82(Suppl 3):e70-e78. doi: 10.1055/s-0040-1701217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Turgut M, Ozsunar Y, Başak S, Güney E, Kir E, Meteoğlu I. Pituitary apoplexy: an overview of 186 cases published during the last century. Acta Neurochir (Wien). 2010;152(5):749-761. doi: 10.1007/s00701-009-0595-8 [DOI] [PubMed] [Google Scholar]
- 79. Sahyouni R, Goshtasbi K, Choi E, et al. Vision outcomes in early versus late surgical intervention of pituitary apoplexy: meta-analysis. World Neurosurg. 2019;127:52-57. doi: 10.1016/j.wneu.2019.03.133 [DOI] [PubMed] [Google Scholar]
- 80. Maccagnan P, Macedo CL, Kayath MJ, Nogueira RG, Abucham J. Conservative management of pituitary apoplexy: a prospective study. J Clin Endocrinol Metab. 1995;80(7):2190-2197. doi: 10.1210/jcem.80.7.7608278 [DOI] [PubMed] [Google Scholar]
- 81. Seo Y, Kim YH, Dho YS, et al. The outcomes of pituitary apoplexy with conservative treatment: experiences at a single institution. World Neurosurg. 2018;115:e703-e710. doi: 10.1016/j.wneu.2018.04.139 [DOI] [PubMed] [Google Scholar]
- 82. Gruber A, Clayton J, Kumar S, Robertson I, Howlett TA, Mansell P. Pituitary apoplexy: retrospective review of 30 patients—is surgical intervention always necessary? Br J Neurosurg. 2006;20(6):379-385. doi: 10.1080/02688690601046678 [DOI] [PubMed] [Google Scholar]
- 83. Pal A, Capatina C, Tenreiro AP, et al. Pituitary apoplexy in non-functioning pituitary adenomas: long term follow up is important because of significant numbers of tumour recurrences. Clin Endocrinol (Oxf). 2011;75(4):501-504. doi: 10.1111/j.1365-2265.2011.04068.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the present study.