Abstract
Context
Adrenocorticotropin (ACTH) loading is used to increase the success rate of adrenal vein sampling (AVS).
Objective
We aimed to determine the effect of intraprocedural cortisol measurement (ICM) on ACTH-stimulated AVS (AS-AVS) owing to a lack of reliable data on this topic.
Methods
This multicenter, retrospective, observational study took place in 28 tertiary centers in Japan. Among 4057 patients enrolled, 2396 received both basal AVS (B-AVS) and AS-AVS and were divided into 2 groups according to whether ICM was used. The effect of ICM on AS-AVS was measured.
Results
In patients who underwent both AVS procedures, the ICM group had significantly higher success rates for both B-AVS and AS-AVS than the non-ICM group did. However, the probability of failure of AS-AVS after a successful B-AVS and the probability of success of AS-AVS after a failed B-AVS were not significantly different in the 2 groups. For subtype diagnosis, propensity-score matching revealed no significant difference between the 2 groups, and the discrepancy rate between B-AVS and AS-AVS for subtype diagnosis was also not significantly different.
Conclusion
ICM significantly increased the success rate of B-AVS and AS-AVS in protocols in which both AVS procedures were performed and had no effect on subtype diagnosis. However, in protocols in which both AVS procedures were performed, the results suggest ICM may not be necessary when performing AS-AVS if ICM is used only when B-AVS is performed. Our study suggests that ICM during AVS plays an important role and should be recommended.
Keywords: primary aldosteronism, adrenal vein sampling, intraprocedural cortisol measurement
Primary aldosteronism (PA) is the most common cause of secondary endocrine hypertension [1]. Patients with PA are at a higher risk of developing cardiovascular disease than those with essential hypertension [2]. Therefore, it is essential to determine a treatment strategy that depends on the PA subtype. Adrenal vein sampling (AVS) is recommended for patients with PA when surgical treatment is considered [3]. As recommended, unilateral PA confirmed by AVS should be optimally treated with laparoscopic adrenalectomy [4]. Some researchers have used less invasive methods to diagnose PA subtypes, including clinical features [5] and predictive machine-learning models [6]. However, AVS is essential for detecting aldosterone hypersecretion sites. The use of 11C-metomidate has been reported as a diagnostic method for localization, but its sensitivity and specificity remain inadequate [7]. Therefore, AVS, which assesses the hypersecretion of aldosterone from the left and right adrenal glands, has been considered the gold standard for PA subtyping [1].
The success rate of AVS varies across facilities [4, 8, 9], and improving the success rate in patients with PA is challenging. In AVS, catheters are selectively inserted into both adrenal veins, and adrenal venous blood is collected. The main reason for AVS failure is the unsuccessful insertion of a catheter into the adrenal vein, either because the vein is too narrow or because of anatomical variants [4]. Successful catheterization for AVS is determined by an increase in cortisol levels in the blood between the inferior vena cava and adrenal vein [8]. Although multiple criteria have been developed, the higher the adrenal vein-to-inferior vena cava cortisol ratio, the higher the reliability. It generally takes several hours or more to measure cortisol concentrations, and the success or failure of AVS can be determined only after the procedure is completed.
One method of increasing the success rate of AVS is to develop an institution-specific protocol [10] with the involvement of specialists. Furthermore, a recent meta-analysis reported that intraoperative computed tomography is effective, especially in cases in which AVS is considered difficult or in centers with low surgeon success rates [11]. Another method used in major centers worldwide and recommended in Japan is the use of adrenocorticotropin (ACTH) loading; the advantage of ACTH loading is that it increases the concentration gradient between the adrenal vein and the inferior vena cava, which is used to determine the success or failure of AVS. However, it has been reported that ACTH loading may affect subtype diagnosis [12-14]. In the same patients, a divergence in AVS subtype diagnosis before and after ACTH loading was shown, especially with ACTH-simulated AVS, resulting in a greater proportion of patients being diagnosed with bilateral PA. Thus, ACTH loading increases the success rate of AVS, but the clinical effect remains controversial.
Intraprocedural cortisol measurement (ICM) has recently been increasingly reported as a method to improve the success rate of AVS. Real-time measurement of cortisol levels in adrenal venous blood can determine the success or failure of AVS, and there have been multiple reports of improved success rates primarily due to rapid ICM during AVS [15-20]. In particular, recent reports have shown that using rapid cortisol measurement during AVS can reduce radiation exposure [21, 22] and that ICM is useful, especially in facilities with low AVS success rates. Furthermore, a point-of-care testing (POCT) device that measures cortisol levels has been developed in Japan, and its usefulness has been reported [19]. Thus, ICM during AVS is becoming more widespread, especially in specialized facilities. However, no large-scale multicenter studies have evaluated the effect of ICM on ACTH-stimulated (AS)-AVS. In addition, it is necessary to evaluate whether ICM has the same potential to influence disease type diagnosis as ACTH loading. Therefore, we evaluated the effect of ICM on PA practice using data from the registry of the Japan Rare/Intractable Adrenal Diseases Study (JRAS), a multicenter, retrospective, observational study conducted in Japan.
Materials and Methods
Study Design
This was a nationwide, multicenter, observational study. Eleven institutions with known ICM use and duration during AVS and 17 control centers with no history of ICM participated in this study.
Adrenal Vein Sampling
AVS is usually performed at baseline and repeated with ACTH loading. However, some facilities use only one method. Furthermore, there are 3 techniques for ACTH loading: intravenous bolus, continuous infusion, and intravenous bolus plus continuous infusion. A successful selective catheterization depends on the adrenal vein-to-inferior vena cava cortisol ratio (ie, a selectivity index [SI] of > 2 for basal AVS [B-AVS] and > 5 for AS-AVS). Successful AVS was defined as successful insertion into the bilateral adrenal veins. The lateralization ratio (LR) was defined as the ipsilateral adrenal vein aldosterone-to-cortisol ratio divided by the contralateral aldosterone-to-cortisol ratio. Unilateral aldosterone overproduction was confirmed by an LR greater than 2 on B-AVS and an LR greater than 4 on AS-AVS.
Intraprocedural Cortisol Measurement
This study used data from PA patients from 28 centers; in 11 of these centers, AVS with ICM was performed. Any of the 4 ICM measuring instruments was used in each institution. The electrochemiluminescence immunoassay (ECLIA) method using the COBAS system and a kit from Roche Diagnostics KK was the most used method, with a measurement time of 18 minutes; it was used in 6 centers. The next most commonly used method was the immunochromatographic assay based on gold nanoparticles (GN-ICA), using the Quick Cortisol Kit from Trust Medical Co Ltd (Kasai) [20, 23]. This method allowed the quantitative measurement of plasma cortisol concentrations within 6 minutes and the semiquantitative measurement within 5 minutes without laboratory technical skills; this was used in 4 institutions. Chemiluminescent enzyme immunoassay (CLEIA) using Accuraseed (FUJIFILM Wako Pure Chemical Corp) [24] was used in 2 institutions; the reaction and processing time was 6 minutes using this method. A competitive enzyme immunoassay (competitive EIA) (Tosoh Corp) was used in 1 institution; 10 minutes were required for the antigen–antibody reaction and processing time using this method. Multiple types of equipment were used at different time points during the study in one institution. Meanwhile, different types of equipment were used simultaneously in another institution.
Patients
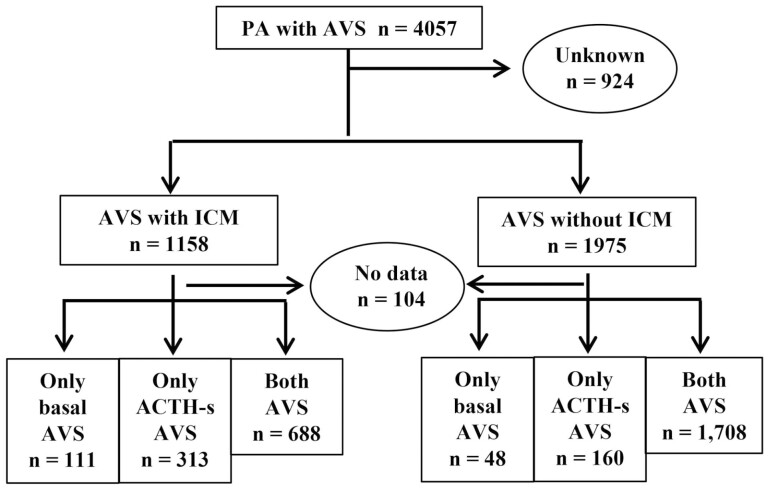
The patients enrolled in this study (n = 4057) underwent AVS for PA subtyping (Fig. 1). The patients were enrolled at each institution between January 2006 and December 2018. Patients with an aldosterone-to-renin ratio (ARR) of 200 or greater and 1 or more positive functional confirmation tests were considered as having PA. Patients aged 20 to 90 years were included in this study. Of these enrolled patients, 924 were first excluded because their ICM status was unknown. Moreover, according to the presence or absence of ICM, 1158 patients in the group with ICM and 1975 patients in that without ICM were enrolled; these patients were further divided into 3 subgroups depending on the AVS method. The first group received only B-AVS, the second received only AS-AVS, and the third received both B-AVS and AS-AVS.
Figure 1.
Number of adrenal vein sampling (AVS) enrollments divided into 2 groups according to intraprocedural cortisol measurement (ICM) status.
This study was conducted according to the guidelines for clinical studies published by the Ministry of Health, Labour, and Welfare, Japan. The study adhered to the principles of the Declaration of Helsinki. The study was approved by the central ethics committee of Kyoto University (R1868-4) and was conducted with permission from the director or ethics committee of each institution.
Statistical Analyses
The study data are expressed as mean ± SEM. The chi-square test was used to test for differences in proportions. The Mann-Whitney U test was used to compare 2 independent groups, and the Wilcoxon signed rank test was used to compare 2 related samples. Propensity-score matching using logistic regression was used to calculate the predictive probability for each patient and to form pairs of patients with similar values to separate the data into 2 groups for analysis. All statistical analyses were performed using Prism 8 (GraphPad Software Inc) or IBM SPSS Statistics for Windows, version 22 (SPSS Inc). Statistical significance was set at P less than .05.
Results
Clinical Characteristics and Outcomes of Primary Aldosteronism Patients Who Underwent Adrenocorticotropin-stimulated Adrenal Vein Sampling
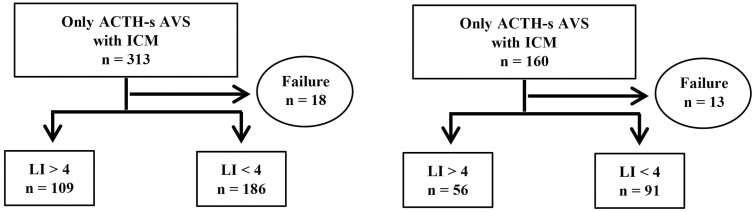
The characteristics of patients enrolled in AS-AVS only are shown in Table 1. A total of 473 patients were classified into 2 groups according to the use of ICM during AVS; concerning the ACTH-loading method, intravenous bolus and intravenous bolus plus continuous infusion were performed in 175 and 136 patients in the ICM group, respectively. In the non-ICM group, the number of patients who received the intravenous bolus, continuous infusion, and intravenous bolus plus continuous infusion were 12, 24, and 74, respectively. Clinically, there were no significant differences between the 2 groups regarding age, systolic and diastolic blood pressure, serum potassium concentration, potassium replacement therapy, plasma renin activity, plasma aldosterone concentration (PAC), and ARR. The ICM group had a significantly higher proportion of males and a significantly higher body mass index (BMI) than the non-ICM group did; regarding AVS results, SI was significantly higher in the non-ICM group than in the ICM group for both right and left adrenal veins. However, the success rate of AVS was higher in the ICM group than in the non-ICM group but not significantly so (94% vs 92%; P = .33) (Fig. 2); subtype diagnosis by LI in AVS was not significantly different between the 2 groups (37% vs 38%; P = .84); complications during AVS were not different between the 2 groups (P = .16).
Table 1.
Clinical characteristics and outcomes of the intraprocedural cortisol measurement (ICM) and non-ICM groups who underwent only adrenocorticotropin-stimulated adrenal vein sampling
| Only ACTH-s AVS with ICM | Only ACTH-s AVS without ICM | ||
|---|---|---|---|
| No. of patients | 313 | 160 | |
| ACTH-loading method, n (intravenous bolus/continuous infusion/intravenous bolus plus continuous infusion/unknown) | 0/175/136/2 | 18/37/105/0 | |
| Age, mean (range), y | 52 (25-81) | 54 (27-74) | P = .05 |
| Sex, men/women | 154/159 | 59/101 | P = .01 |
| Body mass index | 25 ± 4 | 24 ± 4 | P = .01 |
| Systolic blood pressure, mm Hg | 142 ± 19 | 143 ± 19 | P = .35 |
| Diastolic blood pressure, mm Hg | 89 ± 13 | 87 ± 14 | P = .30 |
| Serum potassium, mEq/L | 3.6 ± 0.5 | 3.7 ± 0.5 | P = .13 |
| Plasma renin activity, ng/mL/h | 0.7 ± 6.3 | 0.4 ± 0.5 | P = .19 |
| Plasma aldosterone concentration, pg/mL | 171 ± 121 | 166 ± 122 | P = .65 |
| Aldosterone-to-renin ratio | 1193 ± 1325 | 1152 ± 1324 | P = .41 |
| Selectivity index of right adrenal vein | 29 ± 17 | 38 ± 23 | P < .01 |
| Selectivity index of left adrenal vein | 29 ± 16 | 36 ± 19 | P < .01 |
| Potassium replacement therapy, n (yes/no) | 71/236 | 48/110 | P = .09 |
| Complication during AVS, n (yes/no) | 3/234 | 6/153 | P = .16 |
Abbreviations: ACTH, adrenocorticotropin; ACTH-s, ACTH-stimulated; AVS, adrenal vein sampling; ICM, intraprocedural cortisol measurement.
Figure 2.
Number of successful adrenal vein sampling (AVS) and subtype diagnosis among patients who received only adrenocorticotropin (ACTH)-stimulated AVS, classified by whether intraprocedural cortisol measurement (ICM) was performed.
Clinical Characteristics and Outcomes of Primary Aldosteronism Patients Who Underwent Basal and Adrenocorticotropin-stimulated Adrenal Vein Sampling
The characteristics of the patients who underwent both B-AVS and AS-AVS are shown in Table 2. A total of 2396 patients were classified into 2 groups according to the use of ICM during AVS. Concerning the ACTH-loading method, 473, 6, and 207 patients in the ICM group received intravenous bolus, continuous infusion, and intravenous bolus plus continuous infusion, respectively. However, in the group that did not receive ICM, 1250, 16, and 372 patients were allocated to intravenous bolus, continuous infusion, and intravenous bolus plus continuous infusion, respectively. Clinically, there were no significant differences in age, sex, systolic blood pressure, diastolic blood pressure, or plasma renin activity between the 2 groups. Serum potassium levels were significantly lower in the non-ICM group than in the ICM group, as well as the percentage receiving potassium replacement therapy, PAC, and ARR. Regarding AVS results, SI in both the right adrenal vein and left adrenal vein was significantly higher in both B-AVS and AS-AVS in the ICM group than in the non-ICM group. Complications during AVS did not differ between the 2 groups.
Table 2.
Clinical characteristics and outcomes of the intraprocedural cortisol measurement (ICM) and non-ICM groups who underwent both basal and adrenocorticotropin-stimulated adrenal vein sampling
| Both AVS with ICM | Both AVS without ICM | ||
|---|---|---|---|
| No. of patients | 688 | 1708 | |
| ACTH loading method, n (intravenous bolus/continuous infusion/intravenous bolus plus continuous infusion/unknown) | 473/6/207/2 | 1250/16/372/70 | |
| Age, mean (range), y | 53 (20-77) | 52 (21-84) | P = .14 |
| Sex, men/women | 344/344 | 823/885 | P = .44 |
| Body mass index | 25 ± 4 | 25 ± 4 | P = .23 |
| Systolic blood pressure, mm Hg | 142 ± 19 | 140 ± 17 | P = .07 |
| Diastolic blood pressure, mm Hg | 87 ± 14 | 86 ± 13 | P = .32 |
| Serum potassium, mEq/L | 3.8 ± 0.5 | 3.7 ± 0.5 | P < .01 |
| Plasma renin activity, ng/mL/h | 0.4 ± 0.3 | 0.4 ± 0.3 | P = .82 |
| Plasma aldosterone concentration, pg/mL | 218 ± 338 | 261 ± 234 | P < .01 |
| Aldosterone-to-renin ratio | 851 ± 1638 | 1155 ± 1625 | P < .01 |
| Selectivity index of right adrenal vein | 22 ± 78 | 15 ± 31 | P < .01 |
| Selectivity index of left adrenal vein | 20 ± 32 | 19 ± 32 | P = .01 |
| Selectivity index of right adrenal vein | 48 ± 35 | 44 ± 30 | P < .01 |
| Selectivity index of left adrenal vein | 44 ± 24 | 38 ± 24 | P < .01 |
| Potassium replacement therapy, n (yes/no) | 93/557 | 462/1184 | P < .01 |
| Complication during AVS, n (yes/no) | 14/669 | 46/1585 | P = .32 |
Abbreviations: ACTH, adrenocorticotropin; AVS, adrenal vein sampling; ICM, intraprocedural cortisol measurement.
Effect of Intraprocedural Cortisol Measurement on the Success Rate of Insertion into the Adrenal Vein During Consecutive Basal Adrenal Vein Sampling (AVS) and Adrenocorticotropin-stimulated AVS
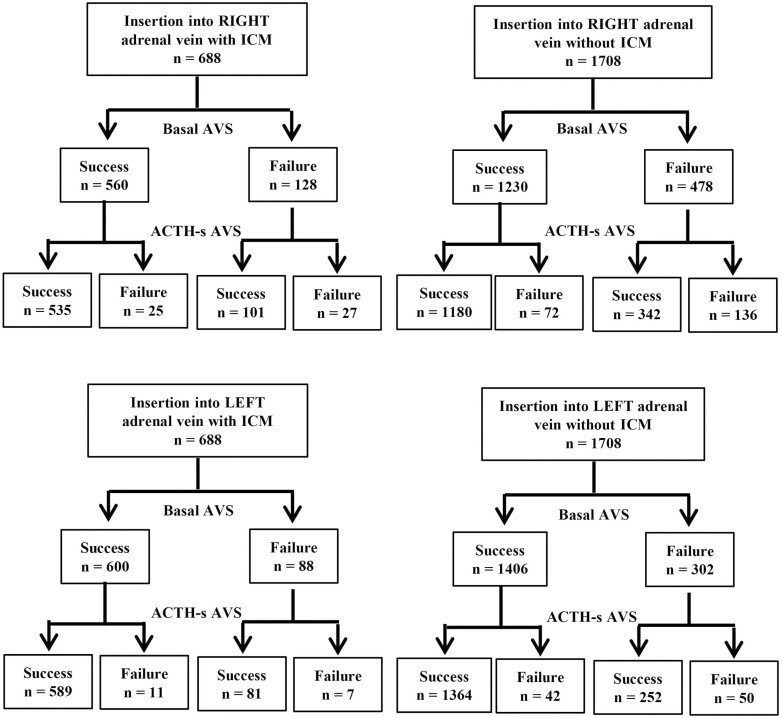
For B-AVS, the success rate of insertion into the right adrenal vein was significantly higher with ICM (566/688, 81%) than without ICM (1230/1708, 72%) (P < .01), and for AS-AVS, the success rate of insertion into the right adrenal vein was significantly higher with ICM (636/688, 92%) than without ICM (1522/1708, 89%) (P = .01) (Fig. 3). There was no significant difference in the failure rate of AS-AVS after successful insertion of basal AVS with or without ICM (4% vs 4%; P = .70). Similarly, there was no significant difference in the success rate of AS-AVS after a failed B-AVS, regardless of ICM (79% vs 72%; P = .12).
Figure 3.
Number of successful insertions into the left and right adrenal veins in patients who underwent basal adrenal vein sampling (AVS) and adrenocorticotropin (ACTH)-stimulated AVS in the 2 groups classified based on intraprocedural cortisol measurement (ICM) use.
The results for insertion into the left adrenal vein were similar to those for the right adrenal vein. The success rate of insertion into the left adrenal vein was significantly higher when ICM was used for both B-AVS (600/688, 87% vs 1406/1708, 82%; P < .01) and AS-AVS (670/688, 97% vs 1616/1708, 95%; P < .01) than when ICM was not used (see Fig. 3). However, the failure rate of AS-AVS in patients with successful insertion with B-AVS (2% vs 3%; P = .17) and the success rate of ACTH-stimulated AVS in patients with failed insertion with basal AVS (92% vs 83%; P = .06) did not differ between the 2 groups.
Effect of Intraprocedural Cortisol Measurement on Adrenal Vein Sampling (AVS) Success Rate and Subtype Diagnosis in Patients Receiving Both Basal AVS and Adrenocorticotropin-stimulated AVS
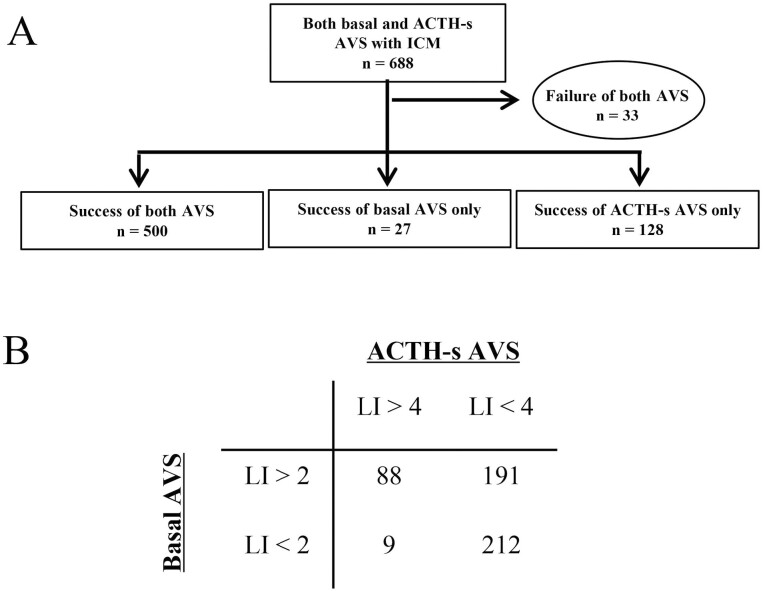
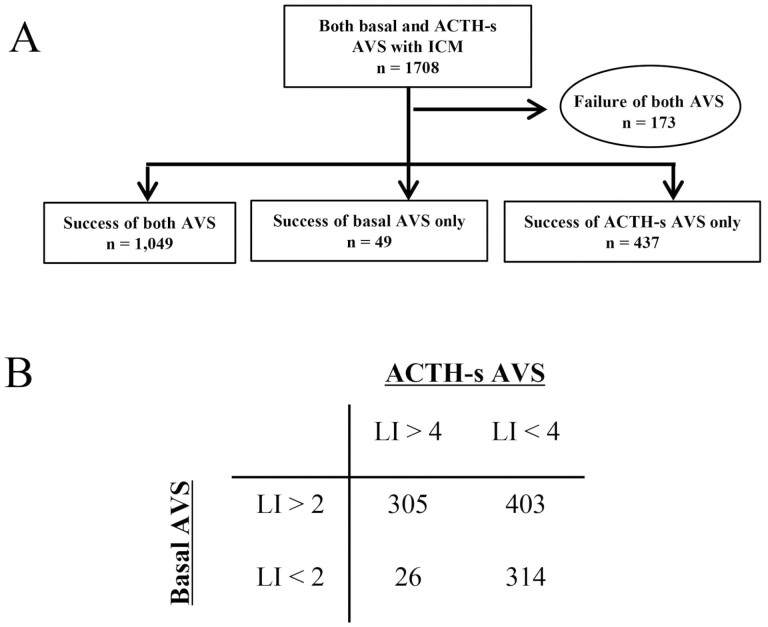
In patients who underwent both B-AVS and AS-AVS, the percentage of cases in which both B-AVS and AS-AVS were successfully inserted into both the left and right adrenal veins was significantly higher in the ICM group than in the non-ICM group (500/688, 73% vs 1049/1708, 61%; P < .01) (Figs. 4A and 5A).
Figure 4.
Number of A, cases in which both adrenal vein samplings (AVS) were successful and B, subtype diagnosis in cases in which intraprocedural cortisol measurement (ICM) was performed and both basal AVS and adrenocorticotropin (ACTH)-stimulated AVS were performed.
Figure 5.
Number of A, successful adrenal vein sampling (AVS) cases and B, subtype diagnosis in cases in which both basal AVS and adrenocorticotropin (ACTH)-stimulated AVS were performed without intraprocedural cortisol measurement (ICM) (one case excluded because of insufficient data in B).
In terms of AVS subtype diagnosis, the non-ICM group was significantly more frequently diagnosed with unilateral PA than the ICM group in both B-AVS (56% vs 68%; P < .01) and AS-AVS (19% vs 32%; P < .01) (Figs. 4B and 5B). However, the proportion of cases with so-called subtype diagnosis discordance, in which the subtype diagnosis of B-AVS and AS-AVS did not match, was similar in the 2 groups (40% vs 41%; P = .74) (see Figs. 4B and 5B)
Effect of Intraprocedural Cortisol Measurement on Adrenal Vein Sampling (AVS) Success Rate and Subtype Diagnosis after Propensity Matching in Patients Who Underwent Both Basal and Adrenocorticotropin-stimulated AVS
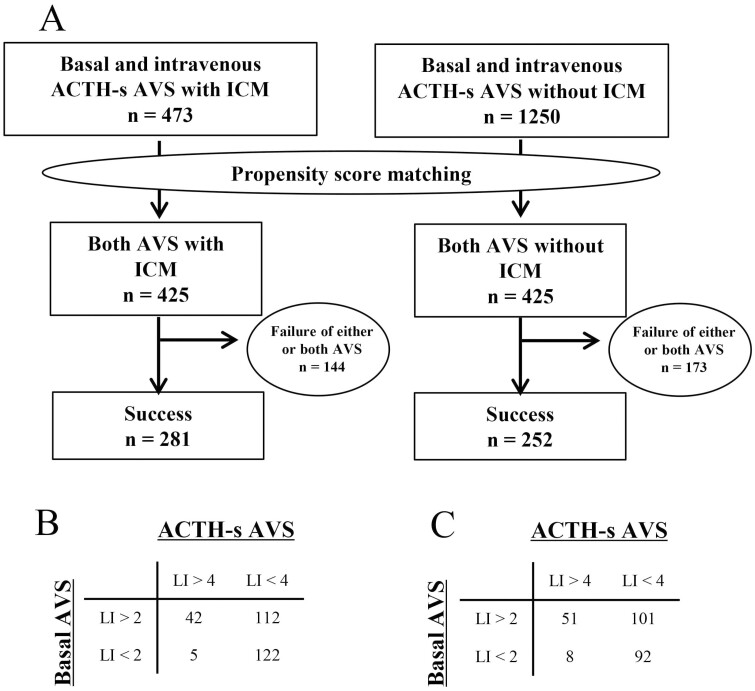
We first limited the ACTH-loading method to the most common intravenous administration and employed age, sex, BMI, presence of hypokalemia, presence of adrenal tumor, tumor diameter, systolic blood pressure, diastolic blood pressure, serum potassium concentration, presence of potassium replacement therapy, PAC, ARR, and antihypertensive medication as variables. Propensity-score matching was performed on 473 patients in the ICM group and 1250 patients in the non-ICM group who underwent B-AVS and AS-AVS with intravenous administration (Fig. 6A), and 425 patients in each group were selected. After propensity-score matching, the ratio of successful insertion into both right and left adrenal veins was significantly higher in the ICM group than in the non-ICM group for both AVS (Fig. 6A). Regarding subtype diagnosis by AVS, there was no significant difference in the proportion of patients diagnosed with unilateral PA in both B-AVS (55% vs 60%; P = .22) and AS-AVS (17% vs 23%; P = .06). There was also no significant difference in the proportion of cases with a discrepancy in subtype diagnosis between B-AVS and AS-AVS.
Figure 6.
A, Propensity-score matching for cases undergoing adrenocorticotropin (ACTH)-stimulated adrenal vein sampling (AVS) with basal AVS and ACTH intravenous administration indicates the number of successes for both AVS procedures after case selection. In addition, B shows the subtype diagnosis in the group that performed intraprocedural cortisol measurement (ICM) and C shows for subtype diagnosis in the group that did not use ICM.
Comparison of the Effect of Different Intraprocedural Cortisol Measurements on Adrenal Vein Sampling
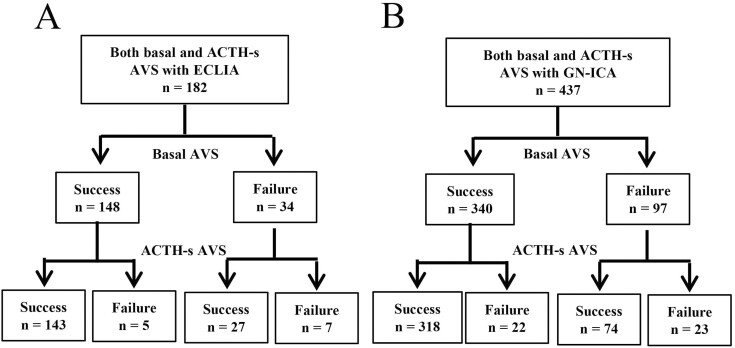
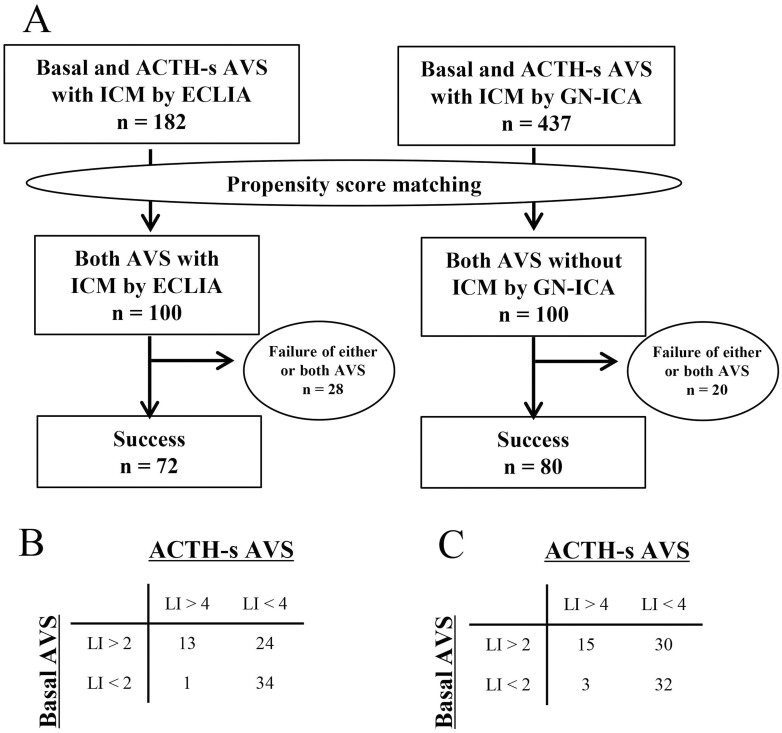
The 2 most commonly used ICM procedures in the cases included in this study were ECLIA and GN-ICA, with ECLIA used at 5 centers and GN-ICA at 4 centers. To compare ICA, 182 cases had both AVS performed at ECLIA and 182 cases had both AVS performed at GN-ICA, with no significant difference between the 2 groups for B-AVS (81% vs 78%; P = .39) and AS-AVS (93% vs 90%; P = .22) (Fig. 7). To evaluate the effect on subtype diagnosis, propensity-score matching was performed using age, sex, BMI, presence of hypokalemia, presence of adrenal tumor, tumor diameter, systolic blood pressure, diastolic blood pressure, serum potassium concentration, presence of potassium replacement therapy, PAC, ARR, antihypertensive medication, and ACTH administration method during AVS as variables, and 100 individuals each from the 2 groups were selected. A comparison performed after propensity-score matching showed no significant difference between the 2 groups in both AVS success rates (72% vs 80%; P = .25) (Fig. 8A) and the diagnostic rate of unilateral PA in B-AVS (51% vs 56%; P = .63) and AS-AVS (19% vs 23%; P = .69), respectively (Fig. 8B and 8C).
Figure 7.
Number of successful adrenal vein sampling (AVS) cases in which basal AVS and adrenocorticotropin (ACTH)-stimulated AVS were performed when using A, electrochemiluminescence immunoassay (ECLIA) and B, immunochromatographic assay based on gold nanoparticles (GN-ICA), respectively.
Figure 8.
Comparison of adrenal vein sampling (AVS) success rate and subtype diagnosis between cases using electrochemiluminescence immunoassay (ECLIA) and immunochromatographic assay based on gold nanoparticles (GN-ICA) after propensity-score matching. A, is the number of successful cases of both AVS after case selection. Also shown in B is for subtype diagnosis in the ECLIA group and C shows the subtype diagnosis in the GN-ICA group.
Discussion
Cortisol and aldosterone concentrations are measured in AVS. The success of selective catheter insertion into the adrenal vein is confirmed by the fact that the cortisol concentration obtained from the adrenal vein is higher than that in the IVC blood; SI is commonly used as an index to determine this, but different cutoff values have been reported [25-29]. Specifically, the SI for B-AVS ranges from 1.1 to 3.0, and the SI for AS-AVS ranges from 2.0 to 5.0 [30]. In the Adrenal Vein Sampling International Study, which involved representative sites globally, the most used SIs were 2.0 for B-AVS and 3.0 or 5.0 for AS-AVS. In the Endocrine Society Clinical Practice Guidelines [4], the SI is 2.0 and 5.0 for protocols without ACTH stimulation and for protocols with ACTH stimulation, respectively; these criteria were adopted in this study. These SI values are also recommended in the Japanese PA guidelines in 2021.
AVS is the gold standard method for subtyping PA; however, the main challenge is its low success rate. Moreover, in this study, the success rate for the right adrenal vein was evidently lower than that of the left adrenal vein. It has been reported that even if catheter insertion into the unilateral adrenal vein is unsuccessful, the aldosterone-to-cortisol ratio of the adrenal vein to the inferior vena cava in which catheter insertion was successful can be used for localization. However, this method requires further study. The main reason for ICM during AVS is to increase the success rate of AVS [20]. During AVS, it is possible to measure cortisol levels in the adrenal veins and inferior vena cava and predict the success or failure of catheter insertion into the adrenal vein using ICM. If the catheter insertion is inappropriate, then the catheter position can be adjusted and repeat blood sampling can be performed. Even if the catheter is inserted correctly into the adrenal vein, it may be dislodged during blood collection, especially during AVS. In addition, multiple adrenal veins are anatomical abnormalities. In such situations, the use of an ICM can provide a quick solution. The selection of samples with appropriate SIs can be performed by the quantitative or semiquantitative assessment of cortisol levels. In this multicenter, retrospective study, the SIs of the left and right adrenal veins for B-AVS were significantly higher in the ICM group than in the non-ICM group. In addition, the success rates of B-AVS and AS-AVS were significantly higher in the ICM group than in the non-ICM group. ACTH loading is the most common method to increase the success rate of AVS [31]. In this study, there was no significant difference in the success rate of AVS between the ICM and non-ICM groups when only AS-AVS was performed. SI was significantly higher in the non-ICM group than in the ICM group. However, this comparison may have been influenced by differences in the ACTH-loading methods between the 2 groups. In both AVS protocols, many patients had successful insertion after ACTH loading, even when insertion into each adrenal vein failed during B-AVS. There was no significant difference in the percentage of patients who went from failure in the B-AVS to success in the AS-AVS in the right (79% vs 72%; P = .12) and left (92% vs 83%; P = .06) adrenal veins, with or without the use of ICM. Therefore, ACTH loading is a powerful method for improving the success rate of AVS.
The main purpose of AVS lies in PA diagnosis and identification of the site of aldosterone overproduction. However, there have been mixed reports on the effect of ACTH loading on disease type diagnosis. In particular, its usefulness for localization diagnosis is unclear, and there have been several reports of discrepancies in the final diagnosis before and after ACTH stimulation [12-14]. Moreover, in the present study, a discrepancy in disease type diagnosis between B-AVS and AS-AVS was evident; concerning the effect of ICM on disease type diagnosis, no significant difference in disease type diagnosis was found when AS-AVS was performed. However, in protocols in which both AVS procedures were performed, a significantly higher percentage of patients in the non-ICM group were diagnosed with unilateral PA in both B-AVS and AS-AVS than in the ICM group. Furthermore, because there were significant differences in clinical characteristics between the 2 groups, we performed a propensity-score matching limited to patients who received the most common intravenous administration of ACTH and found no difference in disease type diagnosis. These results indicate that ICM is unlikely to influence disease type diagnosis. In PA practice, facilities that do not perform ICM may recommend AVS for more typical PA cases, such as those with lower serum potassium levels and higher ARR than those with ICM.
ICM may not be effective in AVS in some situations. First, PA is often associated with Cushing syndrome that is caused by autonomous cortisol production [32, 33]. When aldosterone-producing adenomas produce cortisol, cortisol production in the contralateral adrenal gland is reduced, and this might affect SI. As such, determining the success of adrenal vein insertion using cortisol can be difficult. Therefore, the presence of Cushing syndrome must be assessed before AVS [34]. Second, ICM is labor-intensive, expensive, and requires specialist technicians to operate some of the measuring instruments. However, POCT devices for ICM with nanotechnology have been developed in 2016. The entire assay system for the Quick Cortisol Kit from Trust Medical Co Ltd is both compact and portable; hence, it can be used in any facility [20]. A previous report showed that cortisol measurement using a POCT device during AVS improved cannulation success rates and reduced radiation exposure [23]. Given that POCT equipment does not entail advanced techniques, its demand will likely increase in the future.
In recent years, there have been many studies reporting that cortisol is an inadequate indicator of insertion into the adrenal vein. Substances that may be more useful as indicators than cortisol including 11-deoxycortisol [35], androstenedione [36, 37], 17α-hydroxyprogesterone [36], adrenocortical androgens [38, 39], and metanephrine [37, 40, 41] have been reported. These substances may be particularly useful in cases with high cortisol fluctuations due to stress or other factors, or in cases with aldosterone-producing tumors that may co-produce cortisol. The utility of liquid chromatography–tandem mass spectrometry in evaluating profiles has also been reported [42, 43]. In Japan, many of the aforementioned hormones are difficult to measure in one’s own facility, and rapid measurement may still be difficult. However, if further evidence reveals a hormone more useful as an indicator of AVS than cortisol, the establishment of a rapid measurement device may lead to further breakthroughs in AVS.
This is the first multicenter study to evaluate the efficacy of ICM for AS-AVS, assessing the success rate of ICM not only in B-AVS but also in AS-AVS, its effect on disease type diagnosis, and AVS complications. The success rate of AVS was similar to the findings of previous reports, not only for B-AVS but also for AS-AVS. However, there was no significant difference in the success rate of AS-AVS with or without ICM after determining the success or failure of B-AVS in protocols that performed both AVS. Furthermore, when only AS-AVS was performed, there was no significant difference in success rates with or without the use of ICM. These results suggest that it is not always necessary to use ICM during AS-AVS if the protocol is to perform both AVS or only AS-AVS. This result is important because there are many different methods of ICM, and some methods require multiple additional physicians or technicians, or even costs, when performing AVS. In addition, previous reports suggest that ICM directly confirms the success or failure of insertion into the adrenal vein, thus reducing the need for intraoperative contrast to confirm the catheter position and decreasing the amount of radiation exposure. On the other hand, there was concern that the results of ICM might lead to repeated insertion into the adrenal vein and an increase in intraoperative complications, but such results were not observed in this study. These results suggest that ICM is a very useful technique for performing AVS, and its use should be considered according to each institution’s success rate and method of implementation.
This study is not without limitations. First, this was a retrospective and observational study. Second, the information in the registry did not allow us to assess the time required for AVS, which is an important challenge with the use of ICM or the number of repeated insertions into the adrenal vein based on the results of ICM. Finally, there is no consensus protocol for ICM. A randomized trial is currently underway in Europe to evaluate the efficacy of ICM [44] and is expected to provide further insight into the effect of ICM on AVS. Further prospective studies are needed to assess the benefits and effect of ICM on examination time and complications.
In conclusion, the present multicenter JRAS study showed that ICM improves the success rate of B-AVS and AS-AVS; furthermore, ICM, unlike ACTH loading, has no effect on subtype diagnosis. Notably, the use of ICM does not increase intraoperative AVS complications. Our study also compared the efficacy between ICMs, but no significant differences were found between the 2 types of ICMs. However, depending on the protocol, it is not always necessary to use ICM, especially for AS-AVS, and it is desirable to decide based on the actual situation at each facility. These results suggest that ICM during AVS plays an important role and should be recommended.
Acknowledgments
This research was published by the JPAS/JRAS Study Group. We would like to thank Editage (www.editage.com) for the English language editing.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- ARR
aldosterone-to-renin ratio
- AS-AVS
adrenocorticotropin-stimulated adrenal vein sampling
- AVS
adrenal vein sampling
- B-AVS
basal adrenal vein sampling
- BMI
body mass index
- ECLIA
electrochemiluminescence immunoassay
- GN-ICA
immunochromatographic assay based on gold nanoparticles
- ICM
intraprocedural cortisol measurement
- LR
lateralization ratio
- JRAS
Japan Rare/Intractable Adrenal Diseases Study
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- POCT
point-of-care testing
- SI
selectivity index
Contributor Information
Mitsuhiro Kometani, Department of Health Promotion and Medicine of the Future, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa 920-8641, Japan.
Takashi Yoneda, Email: endocrin@med.kanazawa-u.ac.jp, Department of Health Promotion and Medicine of the Future, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa 920-8641, Japan.
Shigehiro Karashima, Department of Health Promotion and Medicine of the Future, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa 920-8641, Japan.
Yoshiyu Takeda, Department of Health Promotion and Medicine of the Future, Kanazawa University Graduate School of Medicine, Kanazawa, Ishikawa 920-8641, Japan; Department of Internal Medicine, Asanogawa General Hospital, Kanazawa, Ishikawa 910-8621, Japan.
Mika Tsuiki, Department of Endocrinology and Metabolism, National Hospital Organization Kyoto Medical Center, Kyoto 612-8555, Japan.
Akihiro Yasoda, Department of Endocrinology and Metabolism, National Hospital Organization Kyoto Medical Center, Kyoto 612-8555, Japan.
Isao Kurihara, Department of Endocrinology, Metabolism, and Nephrology, Keio University School of Medicine, Tokyo 160-8582, Japan.
Norio Wada, Department of Diabetes and Endocrinology, Sapporo City General Hospital, Sapporo 060-8604, Japan.
Takuyuki Katabami, Division of Metabolism and Endocrinology, Department of Internal Medicine, St. Marianna University School of Medicine, Yokohama City Seibu Hospital, Yokohama 241-0811, Japan.
Masakatsu Sone, Division of Metabolism and Endocrinology, Department of Internal Medicine, St. Marianna University School of Medicine, Kawasaki, Kanagawa 216-8511, Japan.
Takamasa Ichijo, Department of Diabetes and Endocrinology, Saiseikai Yokohamashi Tobu Hospital, Yokohama 230-8765, Japan.
Kouichi Tamura, Department of Medical Science and Cardiorenal Medicine, Yokohama City University Graduate School of Medicine, Yokohama 236-0004, Japan; Division of Nephrology and Hypertension, Yokohama City University Medical Center, Yokohama 232-0024, Japan.
Yoshihiro Ogawa, Department of Medicine and Bioregulatory Science, Kyushu University, Fukuoka 812-8582, Japan.
Hiroki Kobayashi, Division of Nephrology, Hypertension, and Endocrinology, Nihon University School of Medicine, Tokyo 173-8610, Japan.
Shintaro Okamura, Department of Endocrinology, Tenri Hospital, Tenri 632-0015, Japan.
Nobuya Inagaki, Department of Diabetes, Endocrinology, and Nutrition, Kyoto University, Kyoto 606-8501, Japan.
Junji Kawashima, Department of Metabolic Medicine, Faculty of Life Science, Kumamoto University, Kumamoto 860-8556, Japan.
Megumi Fujita, Division of Nephrology and Endocrinology, University of Tokyo, Tokyo 113-0033, Japan.
Kenji Oki, Department of Molecular and Internal Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima 734-8553, Japan.
Yuichi Matsuda, Department of Cardiology, Sanda City Hospital, Sanda 669-1321, Japan.
Akiyo Tanabe, Division of Endocrinology, National Center for Global Health and Medicine, Tokyo 162-8655, Japan.
Mitsuhide Naruse, Endocrine Center, Ijinkai Takeda General Hospital, Kyoto 601-1495, Japan.
Financial Support
This study was conducted as part of the Japan Primary Aldosteronism Study (JPAS) and Japan Rare/Intractable Adrenal Diseases Study (JRAS) and was supported by the Japan Agency for Medical Research and Development (AMED) (grant Nos. JP17ek0109122 and JP20ek0109352) and the National Center for Global Health and Medicine, Japan (Nos. 27-1402 and 30-1008).
Disclosures
The authors have nothing to disclose.
Data Availability
Availability restrictions apply to some or all of the data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. On request, the corresponding author will provide details of the restrictions and conditions under which access to some data may be provided.
References
- 1. Takeda Y, Karashima S, Yoneda T. Primary aldosteronism, diagnosis and treatment in Japan. Rev Endocr Metab Disord. 2011;12(1):21-25. doi: 10.1007/s11154-011-9164-6 [DOI] [PubMed] [Google Scholar]
- 2. Ohno Y, Sone M, Inagaki N, et al. ; Nagahama Study; JPAS Study Group. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: a multicenter study in Japan. Hypertension. 2018;71(3):530-537. doi: 10.1161/HYPERTENSIONAHA.117.10263 [DOI] [PubMed] [Google Scholar]
- 3. Aono D, Kometani M, Karashima S, et al. Primary aldosteronism subtype discordance between computed tomography and adrenal venous sampling. Hypertens Res. 2019;42(12):1942-1950. doi: 10.1038/s41440-019-0310-y [DOI] [PubMed] [Google Scholar]
- 4. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 5. Umakoshi H, Tsuiki M, Takeda Y, et al. ; JPAS Study Group. Significance of computed tomography and serum potassium in predicting subtype diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103(3):900-908. doi: 10.1210/jc.2017-01774 [DOI] [PubMed] [Google Scholar]
- 6. Burrello J, Burrello A, Pieroni J, et al. Development and validation of prediction models for subtype diagnosis of patients with primary aldosteronism. J Clin Endocrinol Metab. 2020;105(10):dgaa379. [DOI] [PubMed] [Google Scholar]
- 7. Burton TJ, Mackenzie IS, Balan K, et al. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J Clin Endocrinol Metab. 2012;97(1):100-109. doi: 10.1210/jc.2011-1537 [DOI] [PubMed] [Google Scholar]
- 8. Vonend O, Ockenfels N, Gao X, et al. ; German Conn’s Registry. Adrenal venous sampling: evaluation of the German Conn’s registry. Hypertension. 2011;57(5):990-995. doi: 10.1161/HYPERTENSIONAHA.110.168484 [DOI] [PubMed] [Google Scholar]
- 9. Fingeret AL, Lee JA. Adrenal venous sampling in primary hyperaldosteronism. Curr Surg Rep. 2014;2(1):38. [Google Scholar]
- 10. Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol (Oxf). 2009;70(1):14-17. doi: 10.1111/j.1365-2265.2008.03450.x [DOI] [PubMed] [Google Scholar]
- 11. Hafezi-Nejad N, Gullotti DM, Bailey CR, Lessne ML, Holly BP. Does intraprocedural CT improve the success rate of adrenal venous sampling? A systematic review and meta-analysis of data from 809 patients. Cardiovasc Intervent Radiol. 2022;45(1):29-40. doi: 10.1007/s00270-021-02954-7 [DOI] [PubMed] [Google Scholar]
- 12. Wolley MJ, Ahmed AH, Gordon RD, Stowasser M. Does ACTH improve the diagnostic performance of adrenal vein sampling for subtyping primary aldosteronism? Clin Endocrinol (Oxf). 2016;85(5):703-709. doi: 10.1111/cen.13110 [DOI] [PubMed] [Google Scholar]
- 13. El Ghorayeb N, Mazzuco TL, Bourdeau I, et al. Basal and post-ACTH aldosterone and its ratios are useful during adrenal vein sampling in primary aldosteronism. J Clin Endocrinol Metab. 2016;101(4):1826-1835. doi: 10.1210/jc.2015-3915 [DOI] [PubMed] [Google Scholar]
- 14. Kometani M, Yoneda T, Aono D, et al. Impact of aldosterone-producing cell clusters on diagnostic discrepancies in primary aldosteronism. Oncotarget. 2018;9(40):26007-26018. doi: 10.18632/oncotarget.25418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woods JJ, Sampson ML, Ruddel ME, Remaley AT. Rapid intraoperative cortisol assay: design and utility for localizing adrenal tumors by venous sampling. Clin Biochem. 2000;33(6):501-503. doi: 10.1016/s0009-9120(00)00141-7 [DOI] [PubMed] [Google Scholar]
- 16. Mengozzi G, Rossato D, Bertello C, et al. Rapid cortisol assay during adrenal vein sampling in patients with primary aldosteronism. Clin Chem. 2007;53(11):1968-1971. doi: 10.1373/clinchem.2007.092080 [DOI] [PubMed] [Google Scholar]
- 17. Auchus RJ, Michaelis C, Wians FH Jr, et al. Rapid cortisol assays improve the success rate of adrenal vein sampling for primary aldosteronism. Ann Surg. 2009;249(2):318-321. doi: 10.1097/SLA.0b013e3181961d77 [DOI] [PubMed] [Google Scholar]
- 18. Reardon MA, Angle JF, Abi-Jaoudeh N, et al. Intraprocedural cortisol levels in the evaluation of proper catheter placement in adrenal venous sampling. J Vasc Interv Radiol. 2011;22(11):1575-1580. doi: 10.1016/j.jvir.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 19. Rossi E, Regolisti G, Perazzoli F, et al. Intraprocedural cortisol measurement increases adrenal vein sampling success rate in primary aldosteronism. Am J Hypertens. 2011;24(12):1280-1285. doi: 10.1038/ajh.2011.148 [DOI] [PubMed] [Google Scholar]
- 20. Yoneda T, Karashima S, Kometani M, et al. Impact of new quick gold nanoparticle-based cortisol assay during adrenal vein sampling for primary aldosteronism. J Clin Endocrinol Metab. 2016;101(6):2554-2561. doi: 10.1210/jc.2016-1011 [DOI] [PubMed] [Google Scholar]
- 21. Augustin AM, Dalla Torre G, Fuss CT, Fassnacht M, Bley TA, Kickuth R. Reduction of radiation exposure in adrenal vein sampling: impact of the rapid cortisol assay. Rofo. 2021;193(12):1392-1402. doi: 10.1055/a-1535-2566 [DOI] [PubMed] [Google Scholar]
- 22. Liu Z, He M, Song X, et al. Computed tomography image fusion, coaxial guidewire technique, fast intraprocedural cortisol testing technique improves success rate and decreases radiation exposure, procedure time, and contrast use for adrenal vein sampling. J Hypertens. 2021;39(9):1918-1925. doi: 10.1097/HJH.0000000000002852 [DOI] [PubMed] [Google Scholar]
- 23. Page MM, Taranto M, Ramsay D, et al. Improved technical success and radiation safety of adrenal vein sampling using rapid, semi-quantitative point-of-care cortisol measurement. Ann Clin Biochem. 2018;55(5):588-592. Doi: 10.1177/0004563218760352 [DOI] [PubMed] [Google Scholar]
- 24. Morimoto R, Ono Y, Tezuka Y, et al. Rapid screening of primary aldosteronism by a novel chemiluminescent immunoassay. Hypertension. 2017;70(2):334-341. doi: 10.1161/HYPERTENSIONAHA.117.09078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toniato A, Bernante P, Rossi GP, Pelizzo MR. The role of adrenal venous sampling in the surgical management of primary aldosteronism. World J Surg. 2006;30(4):624-627. doi: 10.1007/s00268-005-0482-2 [DOI] [PubMed] [Google Scholar]
- 26. Mulatero P, Bertello C, Rossato D, et al. Roles of clinical criteria, computed tomography scan, and adrenal vein sampling in differential diagnosis of primary aldosteronism subtypes. J Clin Endocrinol Metab. 2008;93(4):1366-1371. doi: 10.1210/jc.2007-2055 [DOI] [PubMed] [Google Scholar]
- 27. Stowasser M, Gordon RD. Primary aldosteronism—careful investigation is essential and rewarding. Mol Cell Endocrinol. 2004;217(1–2):33-39. doi: 10.1016/j.mce.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 28. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA.. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136(6):1227-1235. doi: 10.1016/j.surg.2004.06.051 [DOI] [PubMed] [Google Scholar]
- 29. Satoh F, Abe T, Tanemoto M, et al. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res. 2007;30(11):1083-1095. doi: 10.1291/hypres.30.1083 [DOI] [PubMed] [Google Scholar]
- 30. Kempers MJE, Lenders JWM, van Outheusden L, et al. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151(5):329-337. doi: 10.7326/0003-4819-151-5-200909010-00007 [DOI] [PubMed] [Google Scholar]
- 31. Rossi GP, Pitter G, Bernante P, Motta R, Feltrin G, Miotto D. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J Hypertens. 2008;26(5):989-997. doi: 10.1097/HJH.0b013e3282f9e66a [DOI] [PubMed] [Google Scholar]
- 32. Hiraishi K, Yoshimoto T, Tsuchiya K, et al. Clinicopathological features of primary aldosteronism associated with subclinical Cushing’s syndrome. Endocr J. 2011;58(7):543-551. doi: 10.1507/endocrj.k10e-402 [DOI] [PubMed] [Google Scholar]
- 33. Ohno Y, Sone M, Inagaki N, et al. ; JPAS/JRAS Study Group. Latent autonomous cortisol secretion from apparently nonfunctioning adrenal tumor in nonlateralized hyperaldosteronism. J Clin Endocrinol Metab. 2019;104(10):4382-4389. doi: 10.1210/jc.2018-02790 [DOI] [PubMed] [Google Scholar]
- 34. Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151-160. doi: 10.1161/HYPERTENSIONAHA.113.02097 [DOI] [PubMed] [Google Scholar]
- 35. Nilubol N, Soldin SJ, Patel D, et al. 11-Deoxycortisol may be superior to cortisol in confirming a successful adrenal vein catheterization without cosyntropin: a pilot study. Int J Endocr Oncol. 2017;4(2):75-83. doi: 10.2217/ije-2016-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ceolotto G, Antonelli G, Maiolino G, et al. Androstenedione and 17-α-hydroxyprogesterone are better indicators of adrenal vein sampling selectivity than cortisol. Hypertension. 2017;70(2):342-346. doi: 10.1161/HYPERTENSIONAHA.117.09415 [DOI] [PubMed] [Google Scholar]
- 37. Ceolotto G, Antonelli G, Caroccia B, et al. Comparison of cortisol, androstenedione and metanephrines to assess selectivity and lateralization of adrenal vein sampling in primary aldosteronism. J Clin Med. 2021;10(20):4755. doi: 10.3390/jcm10204755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang W, Zhu K, Li H, et al. The value of adrenal androgens for correcting cortisol lateralization in adrenal venous sampling in patients with normal cortisol secretion. Int J Endocrinol. 2019;2019:2860810. doi: 10.1155/2019/2860810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li H, Zhang X, Shen S, et al. Adrenal androgen measurement for assessing the selectivity of adrenal venous sampling in primary aldosteronism. Steroids. 2018;134:16-21. doi: 10.1016/j.steroids.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 40. Dream S, Park S, Yen TW, et al. Utility of epinephrine levels in determining adrenal vein cannulation during adrenal venous sampling for primary aldosteronism. Endocr Pract. 2022;28(3):276-281. doi: 10.1016/j.eprac.2021.09.009 [DOI] [PubMed] [Google Scholar]
- 41. Dekkers T, Deinum J, Schultzekool LJ, et al. Plasma metanephrine for assessing the selectivity of adrenal venous sampling. Hypertension. 2013;62(6):1152-1157. doi: 10.1161/HYPERTENSIONAHA.113.01601 [DOI] [PubMed] [Google Scholar]
- 42. Eisenhofer G, Dekkers T, Peitzsch M, et al. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin Chem. 2016;62(3):514-524. doi: 10.1373/clinchem.2015.251199 [DOI] [PubMed] [Google Scholar]
- 43. Peitzsch M, Dekkers T, Haase M, et al. An LC-MS/MS method for steroid profiling during adrenal venous sampling for investigation of primary aldosteronism. J Steroid Biochem Mol Biol. 2015;145:75-84. doi: 10.1016/j.jsbmb.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 44. Cesari M, Ceolotto G, Rossitto G, Maiolino G, Seccia TM, Rossi GP. The intra-procedural cortisol assay during adrenal vein sampling: rationale and design of a randomized study (I-Padua). High Blood Press Cardiovasc Prev. 2017;24(2):167-170. doi: 10.1007/s40292-017-0192-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability restrictions apply to some or all of the data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. On request, the corresponding author will provide details of the restrictions and conditions under which access to some data may be provided.