Abstract
Objectives:
Owing to advances in antiretroviral therapy (ART), we re-examined minimum ART adherence levels necessary to achieve sustained HIV-1 viral load (VL) suppression among people living with HIV who use drugs (PLHIV-PWUD).
Design and methods:
We used data from ACCESS, a community-recruited prospective cohort of PLHIV-PWUD in Vancouver, Canada. We calculated adherence using the proportion of days of ART dispensed in the year before each VL measurement. We used generalized linear mixed-effects models to identify adherence- and ART regimen-related correlates of VL suppression (<200 copies/mL). We employed probit regression models and generated dose-response curves to estimate the minimum adherence level needed to produce VL suppression in 90% of measures, stratified by regimen and calendar-year.
Results:
Among 837 ART-exposed PLHIV-PWUD recruited between 1996 to 2017, the overall estimated adherence level necessary to achieve 90% VL suppression was 93% (95% CI: 90–96). This differed by regimen: 69% (95% CI: 45–92) for integrase inhibitor (INSTI)-, 96% (95% CI: 92–100) for boosted protease inhibitor (bPI)-, and 98% (95% CI: 91–100) for nonnucleoside reverse transcriptase inhibitor-based regimens. In multivariable analysis, INSTI-based regimens were positively associated with VL suppression (vs. bPIs), while un-boosted PIs and other regimens were negatively associated. We observed a decreasing temporal trend of estimated adherence necessary for 90% VL suppression, dropping to 64% (95% CI: 50–77) during 2016–2017.
Conclusion:
While high levels of ART adherence were necessary to achieve consistent VL suppression, the minimum necessary adherence levels decreased over time. Overall, INSTI-based regimens performed the best, suggesting that they should be preferentially prescribed to PLHIV-PWUD.
Keywords: Adherence, ART, HIV viral load suppression, integrase inhibitor, substance use, people who use drugs
INTRODUCTION
Thanks to advancements in antiretroviral therapy (ART), HIV infection has become a chronic and manageable condition with life expectancy among people living with HIV (PLHIV) approaching that of HIV-negative individuals [1,2]. By maintaining a suppressed plasma HIV-1 RNA viral load (VL), not only is disease progression halted [3] but the risk of HIV transmission is effectively null [4,5]. In order to benefit from the individual- and community-level benefits resulting from ART, current regimens require PLHIV to take ART on a daily basis over the course of their lifetime [3]. Importantly, suboptimal adherence, over the long-term, leads to higher VLs [6,7] and negative outcomes such as higher risks of disease progression [8], drug resistance [9], and HIV transmission [10].
Maintaining optimal levels of adherence to ART remains a major challenge across settings and populations. Many barriers to achieving clinically acceptable adherence levels have been identified, including limited access to and retention in care, treatment toxicity, treatment fatigue, comorbid conditions, and stigma [11–14]. Given these barriers and the advent of newer ART regimens, it is important to characterize what level of adherence may be necessary for optimal individual- and community-level outcomes. This is particularly important among subgroups of PLHIV, including PLHIV who use drugs (PLHIV-PWUD) who are known to be disproportionately affected by barriers to ART adherence [15]: unstable housing (e.g., homelessness, residential eviction), incarceration, food insecurity, and prohibited income generation activities are critical structural barriers to optimal HIV outcomes and likely operate through their impacts on adherence [16–19]. Furthermore, PLHIV-PWUD can be exposed to drug-drug interactions—between unregulated substances, ART, as well as opioid agonist therapy medications in some cases—which can affect the ART adherence level needed for VL suppression among this subpopulation [20].
While previous studies have shown that a near perfect level of ART adherence (i.e., ≥95%) was needed in order to achieve and maintain durable VL suppression [21,22], these studies were conducted during periods when older ART regimens, such as un-boosted protease inhibitors (PIs), were prevalent. More recent analyses have demonstrated that with newer formulations of ART—which have improved potency, pharmacokinetics and safety profiles [23,24]—lower levels of adherence may be sufficient [25–28] to maintain suppression. However, the available evidence for PLHIV-PWUD, particularly with newer ART regimens, is limited [29,30]. We are unaware of any study among people who use unregulated drugs that estimates the minimum ART adherence required for HIV VL suppression using data from the last decade. Thus, this study aimed to calculate the adherence level necessary to achieve VL suppression, across different ART regimens and time periods, among PLHIV-PWUD in Vancouver, Canada, a setting with universal no-cost healthcare including all HIV medications and centralized ART dispensation records.
METHODS
Study design and population
Data for this study was drawn from the AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), an ongoing open prospective cohort of adults living with HIV who use unregulated drugs (other than or in addition to cannabis) in Vancouver, Canada, that recruited its first participants in 1996. Eligibility and study procedures have been described in detail previously [31]. In brief, participants are recruited through community-based methods such as street outreach with a focus in the Downtown Eastside neighbourhood, an area with a long-standing open drug market alongside high levels of marginalization, criminalization, and HIV infection.
After providing written informed consent, at recruitment and every six months thereafter, participants complete an interviewer-administered questionnaire and provide blood and urine samples for further analysis. The questionnaire collects information on socio-demographics, substance use patterns, access to health and social services, and other relevant social/structural exposures. A $40 honorarium is provided to participants after completing each study visit. In addition, at baseline, participants provide their personal health number, a unique and persistent identifier for all residents of British Columbia (BC). This identifier is used to perform confidential data linkages with the British Columbia Centre for Excellence in HIV/AIDS (BC-CfE) Drug Treatment Programme (DTP), which dispenses HIV medications at no cost to all PLHIV in the province of BC. These linkages allow for a complete longitudinal clinical and laboratory profile for each ACCESS participant, including all CD4 cell counts and HIV VL tests, as well as data on all ART dispensations, including dates, quantity, and regimen dispensed. Of note, this linkage includes the results of all CD4 cell count and HIV VL tests conducted by the research study or during clinical care. The University of British Columbia/Providence Healthcare Research Ethics Board approved the ACCESS study.
For the present analysis, we included participants recruited between June 12, 1996, and December 31, 2017, who had ≥1 day(s) of ART dispensation at least 360 days prior to the end of the study period.
Outcome of interest
The primary outcome of interest was VL suppression, defined as a VL measure less than 200 copies/mL. We included all VL measures taken >360 days after the earliest date of ART dispensation. For each VL measure included in the analysis, there were at least 360 days of corresponding ART dispensation data preceding each measure. This was the case even when the same participant had multiple VL measures with overlapping adherence timeframes (i.e., if a person had more than one VL test within a 360-day adherence measurement period).
Antiretroviral adherence levels
As done in a previous study [25], adherence to ART was calculated as the proportion of days covered (PDC). The PDC is a pharmacy claims-based measure of the proportion of days for which medication is available for a person during a specific measurement period [32]. By dividing the number of days of ART coverage during the measurement period by the total length of the measurement period, ART PDC is obtained. Specifically, for each HIV VL test, we calculated the days of ART PDC in the previous 360 days and grouped it into five categories: <50%; 50 to <80%; 80 to <85%; 85 to <90%; 90 to <95%; ≥95%.
Treatment regimens
For each HIV VL measure and using all ART dispensed within the period beginning 360 days prior to the HIV VL measure and ending at the date of the HIV VL measure, we categorized the ART regimen into one of five categories: (1) integrase inhibitor (INSTI)-based; (2) nonnucleoside reverse transcriptase inhibitor (NNRTI)-based; (3) boosted protease inhibitor (bPI)-based; (4) un-boosted PI-based; and (5) all “other” types (i.e., all regimens not categorized as an INSTI-, NNRTI- or PI-based, e.g., INSTI-two-NRTI-PI.) In the event of >1 regimen in that 360-day period, we used the regimen dispensed for >180 days (>50% of the time).
Other measures
We considered various factors that we hypothesized may be associated with VL suppression among PLHIV-PWUD, including sociodemographic characteristics (age, sex, self-reported ancestry), calendar-year of observation (grouped into the following eras: 1996–1999, introduction of combination ART; 2000–2005, steady state of ART use; 2006–2009, second expansion of ART distribution; 2010–2015, aggressive scale-up of ART among key populations as part of local seek, test, treat and retain efforts; and 2016–2017, scale-up of INSTI-based regimens as first-line in HIV treatment guidelines) [33], physician experience, time on ART, and history of injection drug use. Physician experience referred to the number of patients living with HIV that the participant’s prescribing physician had previously enrolled in the province-wide HIV treatment programme as reflected in the DTP data. Time on ART was calculated as the time in years between the earliest date of ART dispensation in the DTP and the date of VL measure. Age, sex, self-reported ancestry, and history of injection drug use were determined via responses to the study questionnaire.
Statistical analysis
First, we examined characteristics of the study sample at baseline, stratified by VL suppression. Next, we examined the association between each explanatory variable and VL suppression, using generalized linear mixed-effects models (GLMM) with a logit-link function. We included random intercepts to account for the clustering of repeated observations from the same participants over time. Both bivariable and multivariable GLMM analyses were performed. Multivariable models included ART regimens, adherence categories, and all other explanatory variables. A complete case analysis was done. Finally, we used probit regression models to estimate the predicted probabilities that were plotted in the dose-response curves to evaluate the minimum ART adherence level (ART PDC) needed to produce VL suppression in 90% of measures (i.e., 90% VL suppression benchmark).
To further investigate the effects of developments in HIV care over time, including increasing availability of more potent ART regimens, we also used probit regression models and dose-response curves to estimate the minimum ART PDC needed to achieve the 90% VL suppression benchmark, stratified by ART regimen and by calendar-time period. In addition, in sub-analyses we explored whether the required levels of ART adherence to reach this benchmark differed by patterns of recent substance use (last six months) based on self-reported data (injection drug use, non-injection drug use only, opioid use, and stimulant use). All statistical analyses were done using SAS software version 9.4 (SAS, Cary, NC), and all p-values are two-sided.
RESULTS
Among the 1,008 PLHIV-PWUD recruited into ACCESS between June 12, 1996, and December 31, 2017, 837 (83.1%) participants had at least ≥1 day(s) of ART dispensation at least 360 days prior to the end of the study period and were included in the present analysis. These participants contributed a total of 38,815 VL measures (median: 41 per participant, quartile 1 to quartile 3 [Q1–Q3]: 24–63). Baseline characteristics of participants, stratified by VL suppression, using the closest VL measure to the participant’s baseline date, are presented in Table 1. The median age at baseline was 39 years old (Q1–Q3: 32.8–45.1), 558 (66.7%) participants were male, 454 (54.2%) self-identified as White, and 377 (45%) as Black, Indigenous, or other people of colour. The majority had a history of injection drug use (779, 93.1%). Over the study period, bPIs were the most frequent ART regimen (21,213, 54.7% of all VL measures), followed by NNRTIs (9,224, 23.8%).
Table 1.
Baseline characteristics of 837 PLHIV-PWUD, stratified by VL suppression (<200 copies/mL), Vancouver, Canada (1996–2017).
| Characteristic | Total, No. (%) (n = 837) | VL suppression, No. (%) |
|
|---|---|---|---|
| Yes (n = 408) | No (n = 429) | ||
|
| |||
| Individual-level | |||
|
| |||
| Age in years, Median (Q1–Q3) | 39 (32.8–45.1) | 42.5 (35.5–47.9) | 36.6 (31.1–42.3) |
| Male | 558 (66.7) | 276 (49.5) | 282 (50.5) |
| White | 454 (54.2) | 221 (48.7) | 233 (51.3) |
| History of injection drug use | 779 (93.1) | 382 (49) | 397 (51) |
|
| |||
| ART-related characteristics | |||
|
| |||
| ART category | |||
| PI (boosted) | 307 (36.7) | 194 (63.2) | 113 (36.8) |
| PI (un-boosted) | 113 (13.5) | 22 (19.5) | 91 (80.5) |
| NNRTI | 274 (32.7) | 165 (60.2) | 109 (39.8) |
| INSTI | 21 (2.5) | 17 (81) | 4 (19) |
| Other | 122 (14.6) | 10 (8.2) | 112 (91.8) |
| ART adherence | |||
| ≥95% | 304 (36.3) | 238 (78.3) | 66 (21.7) |
| 90 to <95% | 36 (4.3) | 25 (69.4) | 11 (30.6) |
| 85 to <90% | 49 (5.9) | 30 (61.2) | 19 (38.8) |
| 80 to <85% | 32 (3.8) | 18 (56.2) | 14 (43.8) |
| 50 to <80% | 138 (16.5) | 55 (39.9) | 83 (60.1) |
| <50% | 278 (33.2) | 42 (15.1) | 236 (84.9) |
| Calendar-year of observation | |||
| 1996–1999 | 193 (23.1) | 13 (6.7) | 180 (93.3) |
| 2000–2005 | 204 (24.4) | 93 (45.6) | 111 (54.4) |
| 2006–2009 | 194 (23.2) | 129 (66.5) | 65 (33.5) |
| 2010–2015 | 228 (27.2) | 158 (69.3) | 70 (30.7) |
| 2016–2017 | 18 (2.2) | 15 (83.3) | 3 (16.7) |
| Physician experience, Median (Q1–Q3) | 71 (26–180) | 89 (40–231.5) | 71 (19–135) |
PLHIV, people living with HIV; PWUD, people who use drugs; VL, viral load; Q1–Q3, quartile 1–quartile 3; ART, antiretroviral therapy; PI, protease inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; INSTI, integrase strand transfer inhibitor. Percentages (%) in the VL suppression (Yes/No) columns were calculated as row percentages (e.g., 276/558 = 49.5%)
Table 2 presents the results of the unadjusted and adjusted analyses of factors associated with VL suppression. As shown, in unadjusted analyses, compared to bPIs, participants who received INSTI-based regimens had increased odds of achieving VL suppression (Odds Ratio [OR] = 1.95, 95% Confidence Interval [CI]: 1.68–2.26). Conversely, participants on NNRTIs (OR = 0.44, 95% CI: 0.40–0.49), un-boosted PIs (OR = 0.14, 95% CI: 0.12–0.16) and other regimens (OR = 0.51, 95% CI: 0.46–0.56) were less likely to achieve VL suppression. In adjusted analyses, INSTI-based regimens (adjusted OR [aOR] = 1.26, 95% CI: 1.07–1.48) remained associated with higher odds of VL suppression, while un-boosted PIs and other ART regimens remained associated with lower odds (aOR = 0.69, 95% CI: 0.58–0.82 and aOR = 0.73, 95% CI: 0.64–0.83, respectively). As also shown in Table 2, compared to ART adherence levels ≥95%, all ART adherence categories under 95% were negatively associated with VL suppression in a dose-response fashion in both unadjusted and adjusted analyses, ranging from aOR = 0.54 (95% CI: 0.47–0.62) for adherence levels 90 to <95% to aOR = 0.09 (95% CI: 0.08–0.10) for adherence levels <50%.
Table 2.
Unadjusted and adjusted GLMM quantifying factors associated with VL suppression (<200 copies/mL) among PLHIV-PWUD, Vancouver, Canada (1996–2017).
| Characteristic | Odds Ratio |
|
|---|---|---|
| Unadjusted (95% CI) |
Adjusted (95% CI) |
|
| Individual-level factors | ||
|
| ||
| Age, per year older | 1.22 (1.22–1.23)* | 1.05 (1.04–1.07)* |
| Male | 1.19 (0.92–1.55) | 0.95 (0.76–1.20) |
| White | 1.14 (0.89–1.45) | 0.90 (0.73–1.11) |
| History of injection drug | 1.00 (0.83–1.21) | 1.00 (0.81–1.25) |
|
| ||
| ART-related characteristics | ||
|
| ||
| ART category | ||
| PI (boosted) | Ref | Ref |
| PI (un-boosted) | 0.14 (0.12–0.16)* | 0.69 (0.58–0.82)* |
| NNRTI | 0.44 (0.40–0.49)* | 1.12 (1.00–1.25) |
| INSTI | 1.95 (1.68–2.26)* | 1.26 (1.07–1.48)* |
| Other | 0.51 (0.46–0.56)* | 0.73 (0.64–0.83)* |
| ART adherence | ||
| ≥95% | Ref | Ref |
| 90 to <95% | 0.52 (0.46–0.58)* | 0.54 (0.47–0.62)* |
| 85 to <90% | 0.51 (0.45–0.57)* | 0.50 (0.44–0.56)* |
| 80 to <85% | 0.32 (0.28–0.36)* | 0.33 (0.29–0.38)* |
| 50 to <80% | 0.20 (0.19–0.22)* | 0.23 (0.21–0.25)* |
| <50% | 0.06 (0.06–0.07)* | 0.09 (0.08–0.10)* |
| Calendar-year of observation | ||
| 1996–1999 | Ref | Ref |
| 2000–2005 | 11.25 (9.13–13.87)* | 7.65 (6.02–9.72)* |
| 2006–2009 | 32.82 (26.52–40.61)* | 12.05 (9.19–15.82)* |
| 2010–2015 | 79.86 (64.51–98.86)* | 15.67 (11.53–21.31)* |
| 2016–2017 | 115.30 (91.78–144.86)* | 15.02 (10.55–21.38)* |
| Time on ART, per 1-year increase | 1.24 (1.23–1.25)* | 1.04 (1.02–1.06)* |
| Physician experience, per 100-patient increase | 1.41 (0.73–2.73) | 0.92 (0.52–1.63) |
PLHIV, people living with HIV; PWUD, people who use drugs; VL, viral load; CI, confidence interval; ART, antiretroviral therapy; PI, protease inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; INSTI, integrase strand transfer inhibitor
p<0.05
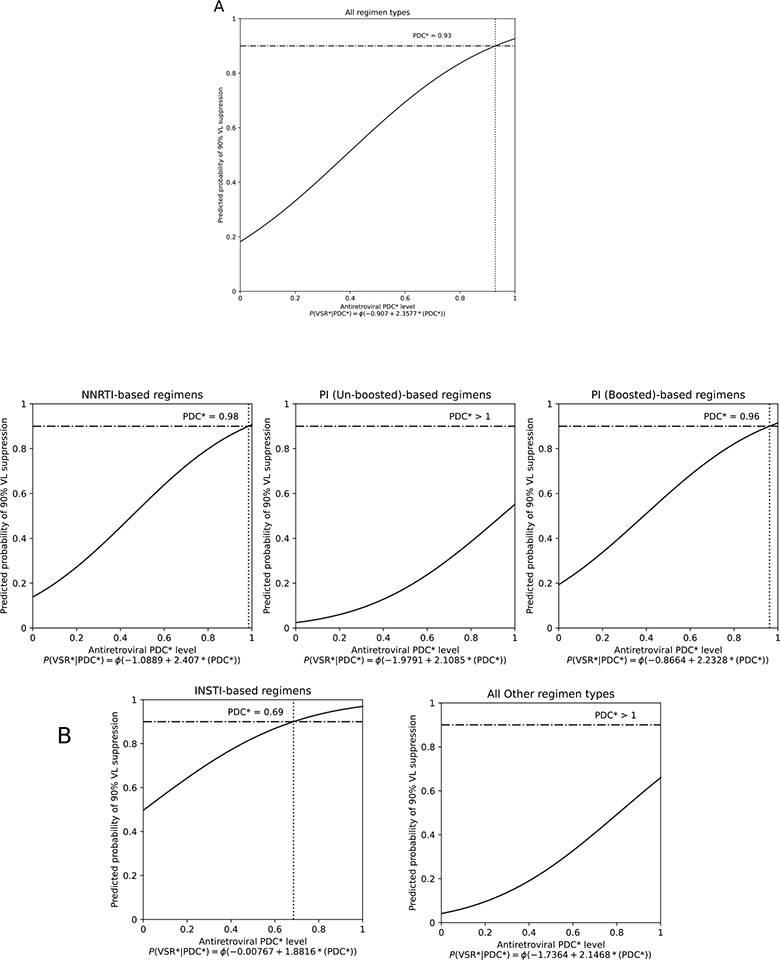
Figure 1 displays dose-response curves that estimate the minimum adherence level needed to achieve VL suppression in 90% of the VL measures, overall, and stratified by ART regimen. As shown, the ART PDC necessary to achieve the 90% VL suppression benchmark was 93% overall (95% CI: 90–96) and varied by ART regimen: 69% (95% CI: 45–92) for INSTI-, 96% (95% CI: 92–100) for bPI-, and 98% (95% CI: 91–100) for NNRTI-based regimens. Un-boosted PIs and “other” ART regimens did not reach the 90% VL suppression benchmark even with 100% adherence levels.
Figure 1. Estimation of the minimum adherence level needed to produce HIV VL suppression in 90% of tests overall (A) and by ART regimen type (B), among 837 PHLIV-PWUD in Vancouver, Canada, 1996–2017.
*PDC, proportion of days covered; INSTI, integrase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor. The horizontal lines represent the 90% benchmark for viral suppression and the dotted vertical lines represent the PDC level at which 90% of HIV VL measures were suppressed (HIV-1 RNA <200 copies/mL).
Compared to the period 1996–1999, more recent time periods were positively associated with VL suppression, ranging from aOR = 7.65 (95% CI: 6.02–9.72) for 2000–2005 to aOR = 15.02 (95% CI: 10.55–21.38) for 2016–2017 (Table 2). A decreasing temporal trend of ART PDC necessary to achieve consistent VL suppression was also observed in the probit models (Figure 2). Specifically, while in the earliest two periods (1996–1999 and 2000–2005) not even perfect levels of adherence were sufficient to achieve the 90% VL suppression benchmark, subsequent periods achieved this goal when participants maintained adherence levels of at least 92% (95% CI: 85–99) between 2006–2009, 76% (95% CI: 69–83) between 2010–2015, and 64% (95% CI: 50–77) between 2016–2017.
Figure 2. Estimation of the minimum adherence level needed to produce HIV VL suppression in 90% of tests by calendar-year of observation, among 837 PHLIV-PWUD in Vancouver, Canada, 1996–2017.
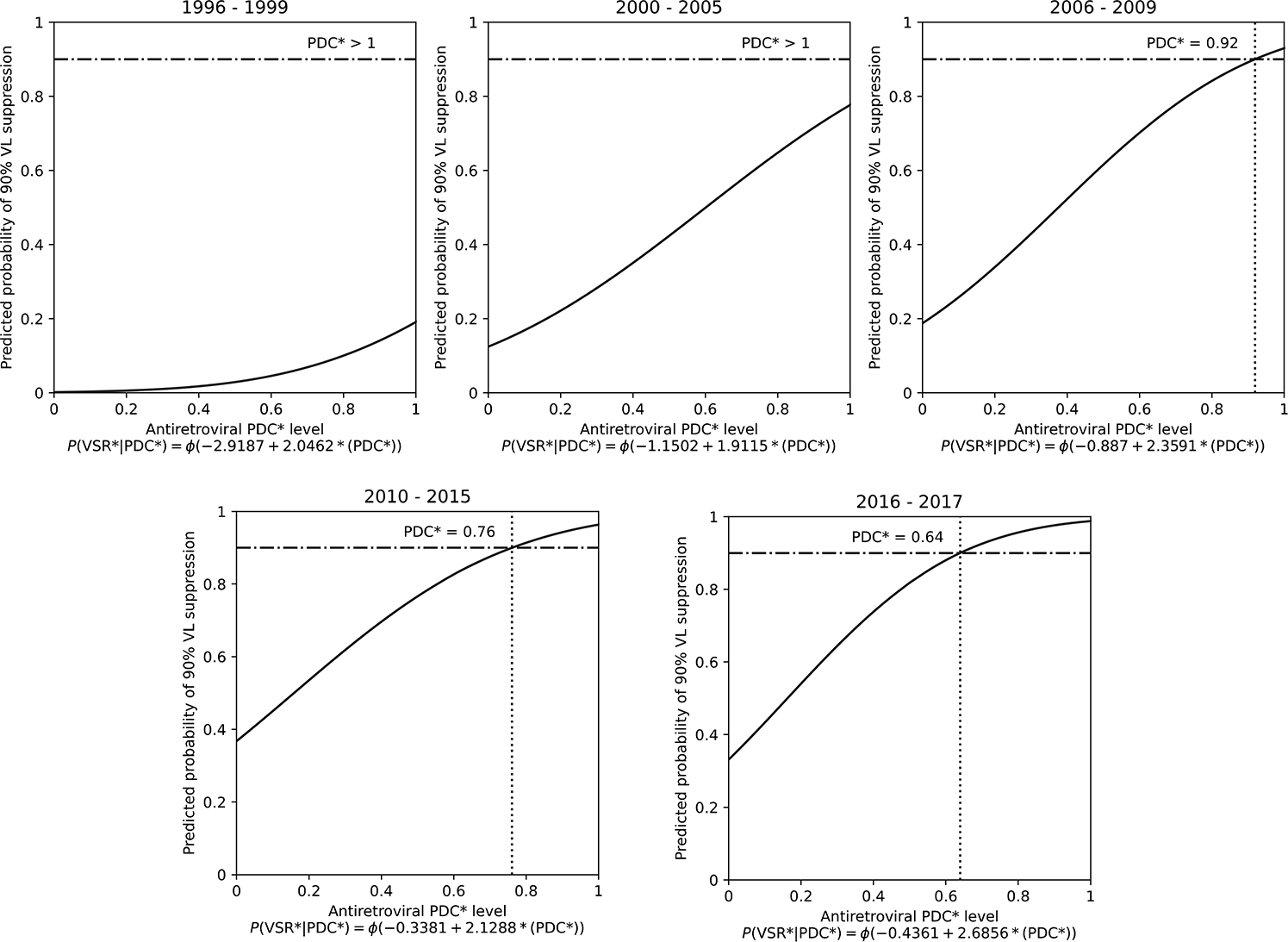
*PDC, proportion of days covered. The horizontal lines represent the 90% benchmark for viral suppression and the dotted vertical lines represent the PDC level at which 90% of HIV VL measures were suppressed (HIV RNA <200 copies/mL).
Our sub-analyses revealed that the minimum adherence needed to reach the 90% VL suppression benchmark was overall similar across substance use pattern sub-groups: 91% (95% CI: 85–96) for people using injection drugs, 90% (95% CI: 83–96) for people only using non-injection drugs, 90% (95% CI: 82–96) for people using opioids, and 87% (95% CI: 81–92) for those using stimulants (Figure 3). Similar to the main analysis, INSTI-based regimens required low ART PDC to achieve the 90% benchmark across all sub-groups (ranging from 65% to 69%), except for people only using non-injection drugs for whom required levels were 92% (Supplemental Figures S1–S4).
Figure 3. Estimation of the minimum adherence level needed to produce HIV VL suppression in 90% of tests by patterns of recent substance use, among 837 PHLIV-PWUD in Vancouver, Canada, 1996–2017.
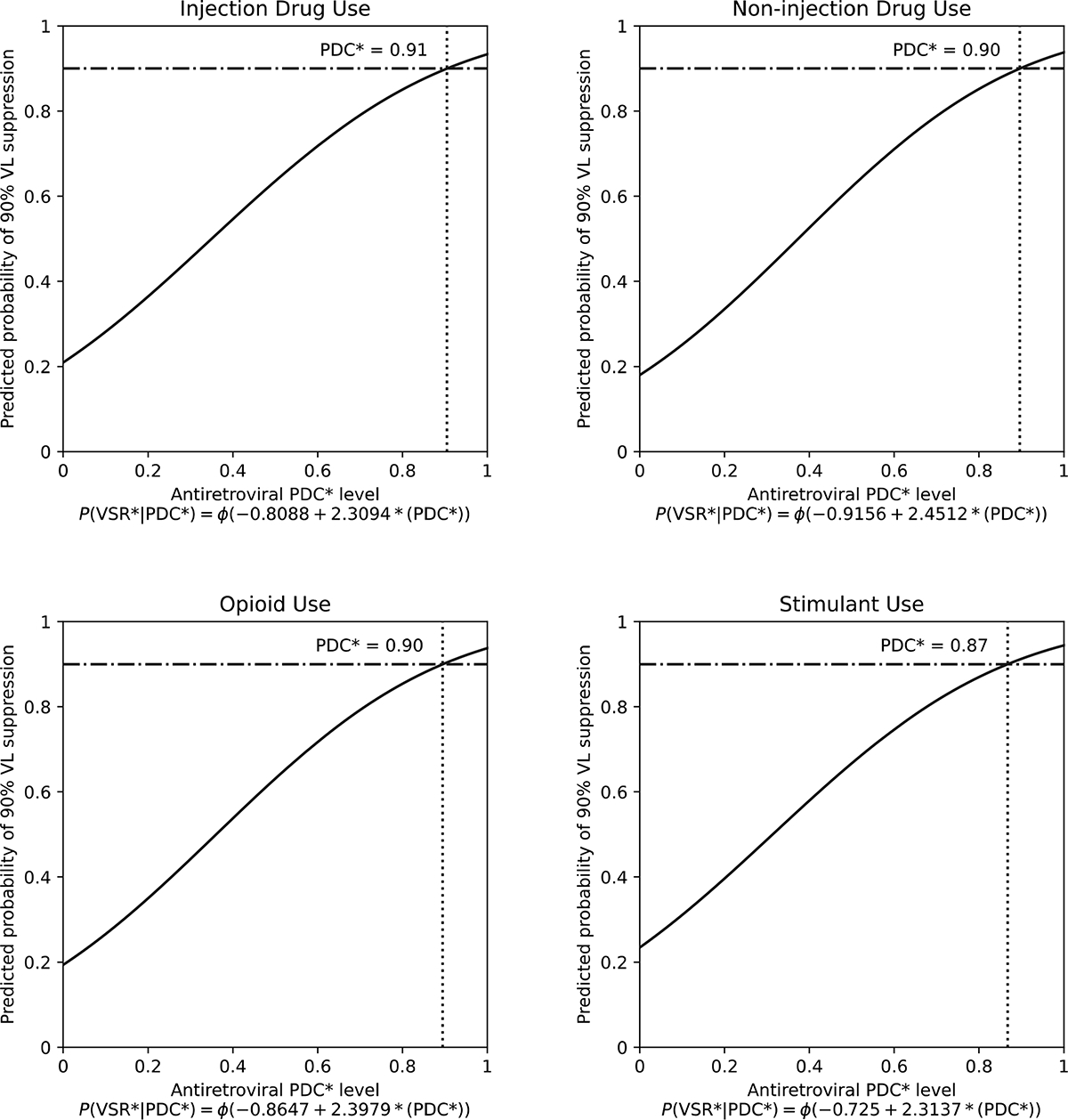
*PDC, proportion of days covered. The horizontal lines represent the 90% benchmark for viral suppression and the dotted vertical lines represent the PDC level at which 90% of HIV VL measures were suppressed (HIV RNA <200 copies/mL).
DISCUSSION
In the present analysis, we observed that, among more than 800 PLHIV-PWUD in Vancouver, Canada, followed from 1996 to 2017, a high level of ART adherence (93% ART PDC overall; 95% CI: 90–96) was necessary to attain VL suppression in 90% of VL measures. The level of adherence necessary to reach this threshold, though, varied by regimen type. INSTI-based regimens needed only 69% (95% CI: 45–92) ART PDC. On the other hand, bPI- and NNRTI-based regimens needed almost perfect levels of adherence (≥95%). Of note, when focusing on the last available period, the estimated level of adherence needed to achieve the 90% VL suppression benchmark significantly decreased from 93 to 64%.
Our analysis found that ART adherence levels lower than 95% were negatively associated with VL suppression and that high adherence levels (>90%) were needed to achieve the 90% VL suppression benchmark. This finding is in line with previous studies conducted among PLHIV-PWUD [29,30] and is also consistent with earlier studies conducted among the general population of PLHIV at the beginning of the introduction of ART, when regimens were less potent and more toxic [21,22]. However, more recent studies conducted among PLHIV showed that adherence levels less than 95% could still be associated with VL suppression [25–28] and that ART adherence levels of around 80% could be sufficient to achieve the 90% VL suppression goal [25]. The differences observed between these and our study could be in part a result of the fact that PLHIV-PWUD make up a unique population that experiences various barriers to ART adherence [12,34,35]. Indeed, previous research has documented the negative impacts of substance use and related disorders on ART adherence, particularly when left untreated [15]. Specifically, socio-structural determinants of health such as homelessness, incarceration, food insecurity, and socioeconomic marginalization are known to substantially impact ART adherence, particularly among marginalized populations [16–19]. Moreover, drug-drug interactions between unregulated substances and ART medications, many of which are metabolized by the same pathways, may also affect the ART adherence level needed for VL suppression among PLHIV-PWUD [20,36].
Another reason that could explain this relatively high overall level of adherence needed to achieve consistent VL suppression observed in the present analysis is the specific assessed period. While we obtained data from 1996 to 2017, the study by Byrd et al. [25] was based on data taken from 2014 to 2016, when newer and more effective regimens were in use. Notably, both GLMM analyses and probit regression models demonstrated that, in our study, more recent calendar-years were associated with a higher likelihood of VL suppression and a lower ART PDC needed to achieve consistent VL suppression, with the last calendar-time period only requiring 64% adherence (95% CI: 50–77) to achieve the 90% VL suppression benchmark. These findings could be partially attributable to the increased use of more potent ART drugs such as INSTIs due to changes in ART guidelines [37].
While Byrd et al. [25] found no statistical difference between INSTI- and PI-based regimens, our study demonstrated that INSTI-based regimens were associated with higher odds of VL suppression. Moreover, consistent with earlier work among PLHIV (not exclusive to PWUD) in recent years [25,26], we also found that adherence levels necessary to attain consistent VL suppression varied by ART regimen type. Specifically, the necessary adherence level to achieve the 90% VL suppression benchmark in participants receiving INSTI-based regimens was the lowest, at 69% (95% CI: 45–92), compared to other regimens, which was consistent across the different patterns of substance use evaluated, with the exception of non-injection drug use. While the high level of ART PDC required to achieve consistent VL suppression observed among people only using non-injection drugs on INSTI-based regimens is surprising, the small sample size in the latter precludes any noteworthy conclusion. These findings are likely a result of INSTIs’ higher potency and genetic barrier and improved pharmacokinetic profiles, which, in turn, may make them more forgiving to missed doses [23,24,38,39]. Furthermore, compared to PIs and NNRTIs, INSTIs are known to have less serious adverse effects and minor drug-drug interactions not only with unregulated substances but also with opioid agonist therapy medications that could decrease ART levels [40,41].
Given the continued and persistent deficits in HIV-related treatment outcomes among PLHIV-PWUD compared to other key populations [42,43], our findings underscore the importance of prioritizing the prescription of newer ART such as INSTI-based regimens among PLHIV-PWUD who face multiple barriers to achieving perfect adherence. Of note, local clinical guidelines still include NNRTIs and bPIs along with INSTIs as first-line therapies based on their cost-effectiveness profiles [44]. Acknowledging the multiple adherence barriers that this population faces, having INSTIs as a preferred first-line treatment may be necessary for PLHIV-PWUD who wish to realize the full benefits of ART. Importantly, while our study found that lower adherence levels may effectively achieve VL suppression among PLHIV-PWUD treated with INSTI-based regimens, sub-optimal adherence may result in low-level ongoing viral replication leading to immune activation and associated increased morbidity and mortality [8]. As such, HIV care providers should continue stressing the importance of working toward the maximum ART adherence possible through comprehensive counselling support and pairing with effective pharmacotherapies where possible. Considering that some patients cannot be perfectly adherent to their medications due to contextual barriers, these results may be helpful when considering those barriers, particularly those affecting PWUD, and the optimal ART regimens for these individuals.
Results from this study should be interpreted considering several limitations. First, we employed a non-random sampling strategy to recruit participants into our cohort and, thus, our findings may not necessarily represent the larger population of PLHIV-PWUD in Vancouver or other jurisdictions. Second, the province of BC offers free HIV care and ART for PLHIV, so cost was not a barrier to adherence for participants in this study, which may be different from other systems without no-cost HIV care. Third, we did not account for ART resistance in the models. The presence of resistance can interfere with VL suppression regardless of ART adherence levels. That said, ART regimens should be chosen taking into consideration resistance patterns. We did account for HIV-physician experience which, in an earlier study, was associated with swifter VL suppression following ART initiation (independent of adherence), probably through better regimen choice and pre-initiation viral genotyping [45]. Fourth, due to limitations on pharmacy refill data, our analysis did not distinguish patterns of adherence (e.g., prolonged versus intermittent periods of non-adherence). Though beyond the scope of the present study, future studies could explore whether such patterns have differential impacts on VL suppression. Finally, the PDC method is a proxy for adherence. The PDC calculates how much time a person has access to medication, not actual pill-taking behaviour (including taking medication according to the prescriber’s instructions or on the prescribed schedule), potentially overestimating adherence. However, using pharmacy dispensation data to calculate adherence has an advantage over other methods (e.g., self-report and pill counts) potentially impacted by social-desirability and recall bias [46]. Further, measures of adherence based on pharmacy dispensation records have been shown to predict VL suppression and survival [47].
In summary, this study found an overall high level of adherence necessary to achieve VL suppression in 90% of the measurements among PLHIV-PWUD in Vancouver, Canada, over a 21-year period. However, encouragingly, when restricting our analysis to observations from the 2016–2017 period, we found that the needed adherence level to achieve the 90% VL suppression benchmark substantially decreased. Among the different ART regimens, INSTIs required a lower level of adherence to maintain consistent VL suppression. These results support current recommendations for including INSTI-based regimens as a first-line treatment in HIV therapeutic guidelines [44], especially among PLHIV-PWUD, and the need for ongoing development and implementation of strategies to improve ART adherence among this subpopulation.
Supplementary Material
ACKNOWLEDGMENTS
This work took place on the traditional and unceded territories of the xʷməθkwəy̓əm (Musqueam), Skwxwú7mesh (Squamish), and sel̓íl̓witulh (Tsleil-Waututh) Nations. We thank the study participants for their contributions to the research, as well as current and past researchers and staff. We also thank Dr. Julio Montaner and the British Columbia Centre for Excellence in HIV/AIDS for facilitating access to Drug Treatment Program data.
This work was supported by the US National Institute on Drug Abuse (NIDA) at the US National Institutes of Health (NIH) (U01-DA021525). MES is supported by a Michael Smith Foundation for Health Research (MSFHR)/St Paul’s Foundation Scholar Award. MJM is supported by NIDA (U01-DA021525) and is the Canopy Growth professor of cannabis science at the University of British Columbia, a position created by unstructured gifts to the University from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. NF is supported by a Philip Owen Professorship in Addiction Medicine at the University of British Columbia, and a Scholar Award from the MSFHR/St. Paul’s Hospital Foundation.
Sources of funding:
This work was supported by the US National Institute on Drug Abuse (NIDA) at the US National Institutes of Health (NIH) (U01-DA021525). MES is supported by a Michael Smith Foundation for Health Research (MSFHR)/St Paul’s Foundation Scholar Award. MJM is supported by NIDA (U01-DA021525). MJM is the Canopy Growth professor of cannabis science at the University of British Columbia, a position created by unstructured gifts to the University from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. NF is supported by a Philip Owen Professorship in Addiction Medicine at the University of British Columbia, and a Scholar Award from the MSFHR/St. Paul’s Hospital Foundation.
Footnotes
CONFLICTS OF INTEREST
The University of British Columbia has received unstructured funding from NG Biomed, Ltd, an applicant to the Canadian federal government, for a license to produce medical cannabis, to support MJM’s research. All other authors report no potential conflicts.
REFERENCES
- 1.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS Lond Engl 2014; 28:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med 2016; 375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA 2016; 316:171–181. [DOI] [PubMed] [Google Scholar]
- 6.Martin M, Del Cacho E, Codina C, Tuset M, De Lazzari E, Mallolas J, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses 2008; 24:1263–1268. [DOI] [PubMed] [Google Scholar]
- 7.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis Off Publ Infect Dis Soc Am 2002; 34:1115–1121. [DOI] [PubMed] [Google Scholar]
- 8.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS Lond Engl 2001; 15:1181–1183. [DOI] [PubMed] [Google Scholar]
- 9.Harrigan PR, Hogg RS, Dong WWY, Yip B, Wynhoven B, Woodward J, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis 2005; 191:339–347. [DOI] [PubMed] [Google Scholar]
- 10.Lingappa JR, Hughes JP, Wang RS, Baeten JM, Celum C, Gray GE, et al. Estimating the Impact of Plasma HIV-1 RNA Reductions on Heterosexual HIV-1 Transmission Risk. PLOS ONE 2010; 5:e12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shubber Z, Mills EJ, Nachega JB, Vreeman R, Freitas M, Bock P, et al. Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis. PLoS Med 2016; 13. doi: 10.1371/journal.pmed.1002183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannheimer S, Hirsch-Moverman Y. What We Know and What We Do Not Know About Factors Associated with and Interventions to Promote Antiretroviral Adherence. Curr Infect Dis Rep 2015; 17:13. [DOI] [PubMed] [Google Scholar]
- 13.Claborn KR, Meier E, Miller MB, Leffingwell TR. A Systematic Review of Treatment Fatigue among HIV-infected Patients Prescribed Antiretroviral Therapy. Psychol Health Med 2015; 20:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi K, Wood E, Kerr T, Dong H, Nguyen P, Puskas CM, et al. Factors associated with optimal pharmacy refill adherence for antiretroviral medications and plasma HIV RNA non-detectability among HIV-positive crack cocaine users: a prospective cohort study. BMC Infect Dis 2016; 16:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Socias ME, Milloy M-J. Substance Use and Adherence to Antiretroviral Therapy: What Is Known and What Is Unknown. Curr Infect Dis Rep 2018; 20:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson LA, Kerr TH, Dobrer S, Puskas CM, Guillemi SA, Montaner JSG, et al. Socioeconomic marginalization and plasma HIV-1 RNA nondetectability among individuals who use illicit drugs in a Canadian setting. AIDS 2015; 29:2487–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milloy M-J, Kerr T, Bangsberg DR, Buxton J, Parashar S, Guillemi S, et al. Homelessness as a structural barrier to effective antiretroviral therapy among HIV-seropositive illicit drug users in a Canadian setting. AIDS Patient Care STDs 2012; 26:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milloy M-J, Montaner JSG, Wood E. Incarceration of People Living with HIV/AIDS: Implications for Treatment-as-Prevention. Curr HIV/AIDS Rep 2014; 11:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer AW, Weiser SD, McCoy SI. Does Food Insecurity Undermine Adherence to Antiretroviral Therapy? A Systematic Review. AIDS Behav 2015; 19:1510–1526. [DOI] [PubMed] [Google Scholar]
- 20.Desai N, Burns L, Gong Y, Zhi K, Kumar A, Summers N, et al. An update on drug-drug interactions between antiretroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol 2020; 16:1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133:21–30. [DOI] [PubMed] [Google Scholar]
- 22.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS Lond Engl 2000; 14:357–366. [DOI] [PubMed] [Google Scholar]
- 23.Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother 2011; 45:372–379. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CA, Robinson L, Tseng A, MacArthur RD. New antiretroviral drugs: a review of the efficacy, safety, pharmacokinetics, and resistance profile of tipranavir, darunavir, etravirine, rilpivirine, maraviroc, and raltegravir. Expert Opin Pharmacother 2009; 10:2445–2466. [DOI] [PubMed] [Google Scholar]
- 25.Byrd KK, Hou JG, Hazen R, Kirkham H, Suzuki S, Clay PG, et al. Antiretroviral Adherence Level Necessary for HIV Viral Suppression Using Real-World Data. J Acquir Immune Defic Syndr 1999 2019; 82:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Sauer B, Zhang Y, Nickman NA, Jamjian C, Stevens V, et al. Adherence and virologic outcomes among treatment-naïve veteran patients with human immunodeficiency virus type 1 infection. Medicine (Baltimore) 2018; 97:e9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to Antiretroviral Therapy and Virologic Failure: A Meta-Analysis. Medicine (Baltimore) 2016; 95:e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon LL, Gharibian D, Chong K, Chun H. Comparison of HIV Virologic Failure Rates Between Patients with Variable Adherence to Three Antiretroviral Regimen Types. AIDS Patient Care STDs 2015; 29:384–388. [DOI] [PubMed] [Google Scholar]
- 29.Viswanathan S, Detels R, Mehta SH, Macatangay BJC, Kirk GD, Jacobson LP. Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). AIDS Behav 2015; 19:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viswanathan S, Justice AC, Alexander GC, Brown TT, Gandhi NR, McNicholl IR, et al. Adherence and HIV RNA Suppression in the Current Era of Highly Active Antiretroviral Therapy. J Acquir Immune Defic Syndr 1999 2015; 69:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’Shaughnessy MV, Montaner JS, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA 1998; 280:547–549. [DOI] [PubMed] [Google Scholar]
- 32.PDC-PH_Summary_2019–12-18.pdf. https://www.pqaalliance.org/assets/docs/PDC-PH_Summary_2019-12-18.pdf (accessed 3 May2021).
- 33.Montaner JSG, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet Lond Engl 2006; 368:531–536. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez A, Barinas J, O’Cleirigh C. Substance Use: Impact on Adherence and HIV Medical Treatment. Curr HIV/AIDS Rep 2011; 8:223. [DOI] [PubMed] [Google Scholar]
- 35.Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdóttir TB, Richter C, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med 2014; 12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antoniou T, Tseng AL-I. Interactions between recreational drugs and antiretroviral agents. Ann Pharmacother 2002; 36:1598–1613. [DOI] [PubMed] [Google Scholar]
- 37.Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JVR, Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet Lond Engl 2009; 374:796–806. [DOI] [PubMed] [Google Scholar]
- 38.Himmel DM, Arnold E. Non-Nucleoside Reverse Transcriptase Inhibitors Join Forces with Integrase Inhibitors to Combat HIV. Pharm Basel Switz 2020; 13. doi: 10.3390/ph13060122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cusato J, Allegra S, Nicolò AD, Calcagno A, D’Avolio A. Precision medicine for HIV: where are we? Pharmacogenomics 2018; 19:145–165. [DOI] [PubMed] [Google Scholar]
- 40.Baecke C, Gyssens IC, Decoutere L, van der Hilst JCH, Messiaen P. Prevalence of drug-drug interactions in the era of HIV integrase inhibitors: a retrospective clinical study. Neth J Med 2017; 75:235–240. [PubMed] [Google Scholar]
- 41.Meemken L, Hanhoff N, Tseng A, Christensen S, Gillessen A. Drug-Drug Interactions With Antiviral Agents in People Who Inject Drugs Requiring Substitution Therapy. Ann Pharmacother 2015; 49:796–807. [DOI] [PubMed] [Google Scholar]
- 42.Aceijas C, Oppenheimer E, Stimson GV, Ashcroft RE, Matic S, Hickman M. Antiretroviral treatment for injecting drug users in developing and transitional countries 1 year before the end of the “Treating 3 million by 2005. Making it happen. The WHO strategy” (“3 by 5”). Addict Abingdon Engl 2006; 101:1246–1253. [DOI] [PubMed] [Google Scholar]
- 43.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet Lond Engl 2010; 376:355–366. [DOI] [PubMed] [Google Scholar]
- 44.2020_06_30-BC-CfE_Adult_ARV_Therapeutic_Guidelines.pdf. http://bccfe.ca/sites/default/files/uploads/Guidelines/2020_06_30-BC-CfE_Adult_ARV_Therapeutic_Guidelines.pdf (accessed 23 Mar2021).
- 45.Sangsari S, Milloy M-J, Ibrahim A, Kerr T, Zhang R, Montaner J, et al. Physician experience and rates of plasma HIV-1 RNA suppression among illicit drug users: an observational study. BMC Infect Dis 2012; 12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whalley Buono E, Vrijens B, Bosworth HB, Liu LZ, Zullig LL, Granger BB. Coming full circle in the measurement of medication adherence: opportunities and implications for health care. Patient Prefer Adherence 2017; 11:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood E, Montaner JSG, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, et al. Adherence to antiretroviral therapy and CD4 T-cell count responses among HIV-infected injection drug users. Antivir Ther 2004; 9:229–235. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.