Abstract
Purpose
Amikacin liposome inhalation suspension (ALIS), which efficiently allows amikacin to reach the pulmonary periphery for effect while minimising systemic adverse effects, was recently approved for treating Mycobacterium avium complex (MAC) infections. The international Phase 3 open-label clinical trials showed promising results, contributing to sputum culture conversion, but few studies have examined the efficacy and adverse effects of ALIS using real-world data. We identified the clinical outcome and adverse effects of ALIS in the early phase of treatment, for more effective and safe use in clinical practice.
Patients and Methods
The study population consisted of patients with MAC lung disease (MAC-LD), introduced to ALIS therapy after July 2021 at Keio University Hospital due to poor response to multidrug therapy. The sputum smear/culture results, symptoms, adverse effects, and the serum amikacin concentrations of the early phase of ALIS inhalation therapy were examined.
Results
A total of 11 patients (9 women; median age 64.6 years) were included in this study. The median disease duration of MAC-LD was 13.7 years, and all patients exhibited a positive culture at the beginning of ALIS inhalation. Three of the six patients (50.0%) who were initially sputum-smear-positive were confirmed to have become sputum-smear-negative within one month, including one culture conversion. ALIS inhalation therapy caused some adverse effects in nine patients (81.8%); however, no serious systemic adverse effects were observed. The most common adverse effect was hoarseness (72.7%), which mostly occurred around 1 week after initiation. The medians of peak serum amikacin concentrations were 1.4 and 2.3 μg/mL for the first and third inhalations, respectively. Trough serum concentrations just before the third inhalation were <1.2 μg/mL in all patients.
Conclusion
ALIS therapy might be a treatment option for patients with refractory MAC infection with long disease duration and a poor response to guideline-based therapy.
Keywords: Mycobacterium avium complex, MAC, nontuberculous mycobacteria, NTM, amikacin liposome inhalation suspension, ALIS
Introduction
The prevalence of lung disease caused by nontuberculous mycobacteria (NTM) has been increasing worldwide in recent years.1–3 There are over 200 different species of NTM, and the Mycobacterial avium complex (MAC) accounts for the highest percentage of these species in most regions of the world.4 The annual prevalence of NTM infections is estimated to be 24.9 cases/100,000 population (2016) in Japan, which is higher than that of tuberculosis.5 MAC lung disease (MAC-LD) accounts for approximately 90% of these cases.6,7 Currently, a 3-drug combination therapy including macrolide is recommended for patients with macrolide-susceptible MAC-LD.8 However, intolerance and adverse effects resulting from treatment with multiple antimicrobial agents for MAC-LD are major challenges in long-term management.9,10 The annual mortality rate of this disease is estimated to be 1–2% owing to the increase in refractory and long-course cases, and the recent extension in life expectancy.7
In 2007, the American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) recommended parenteral aminoglycoside therapy as an initial treatment for cavitary MAC disease and for cases previously treated with a standard multidrug regimen.11 Hence, aminoglycoside antibiotics, including amikacin (AMK) and streptomycin (SM), have been intravenously or intramuscularly administered for refractory cases.12,13 Such systemic administration sometimes causes renal, auditory, and vestibular toxicity;14 moreover, the low penetration of these antibiotics into the lung, owing to their chemical nature, is problematic.15 Several researchers, including our group, have studied inhalation therapy using a commercially available compressor nebuliser to improve the efficacy, safety, and feasibility of using injectable AMK sulfate solution, with promising results.16–18
Amikacin liposome inhalation suspension (ALIS) was developed to achieve high concentrations of AMK in peripheral lung tissue and alveolar macrophages while minimising systemic exposure. ALIS contributed to the primary endpoint of improving the sputum culture conversion rate at up to 6 months in the international phase 3 open-label trial (CONVERT).19 In response, the ATS/European Respiratory Society (ERS)/European Society of Clinical Microbiology and Infectious Diseases (ESCMID)/IDSA Clinical Practice Guideline published in 2020 recommended the addition of ALIS to the treatment regimen for patients with MAC-LD who have failed to recover after at least 6 months of guideline-based therapy (GBT).8 Some studies outside of clinical trials examined the efficacy and adverse effects of ALIS, but have been limited to surveillance of symptoms.20 Moreover, in vitro studies have shown that liposomal AMK is readily taken up by macrophages, but details on the pharmacokinetics (PK)/pharmacodynamics (PD) in real-world clinical settings are unknown. Since it was approved in Japan in March 2021, we administered ALIS to 11 patients with MAC disease who had an inadequate response to previous therapy. The aim of this study was to accumulate knowledge on the effects, PK, and adverse effects of ALIS in the early stages after its introduction, to achieve more effective and safer treatment.
Materials and Methods
Study Population and Data Collection
The study population consisted of patients with MAC-LD showing an inadequate response to prior treatment with multi-drug combination therapy at Keio University Hospital. MAC-LD was diagnosed based on the ATS/ERS/ESCMID/IDSA guidelines published in 2020.8 The medical records of 11 patients who commenced ALIS treatment after July 2021 were retrospectively reviewed and analysed. We obtained informed consent from all these patients. The Research Ethics Committee of Keio University Hospital reviewed and approved this study (20110267).
ALIS Inhalation
A 590 mg dose of ALIS (Arikayce; Insmed Inc., Bridgewater, NJ, USA) was administered once daily through inhalation using the dedicated eFlow® rapid nebuliser system (Lamira; PARI Pharma GmbH Co., Ltd., Munich, Germany), according to the procedure specified by the manufacturer. Patients were hospitalised for approximately 5 days to receive training on the inhalation technique from the medical staff. After discharge from the hospital, inhalation was continued at a fixed time every day in principle, but the frequency of inhalation was reduced based on adverse events.
Microbiological Examinations
Sputum specimens were cultured either in mycobacteria growth indicator tubes (Becton, Dickinson and Company, Sparks, MD, USA) or on egg-based solid media (Kyokuto Pharmaceutical Industrial Co., Ltd., Tokyo, Japan), as previously described.21 All the isolates were identified as Mycobacterium tuberculosis or each NTM species using the AccuProbe culture identification test (Gen-Probe Inc., San Diego, CA, USA) and real-time polymerase chain reaction (Cobas Amplicor; Roche Diagnostics, Indianapolis, IN, USA). The minimum inhibitory concentration (MIC) of clarithromycin (CLA) and AMK were determined using BrothMIC NTM with 7H9 Middlebrook liquid media (Kyokuto Pharmaceutical Industrial Co., Ltd.) formulated using the Clinical and Laboratory Standards Institute’s (CLSI) standard 24A. An MIC ≥ 32 μg/mL was defined as CLA resistance.22 In all cases, smear test results of all sputum submitted within one month of initiation and the first culture results after one month were recorded. Sputum cultures were performed for up to 8 weeks in order to determine results.
Clinical Symptoms
The presence or absence of clinical symptoms, including cough, sputum production, dyspnea, hemoptysis, and fever, were evaluated before and after ALIS inhalation therapy, based on medical records.
Adverse Events
Patients were monitored for subjective symptoms after ALIS inhalation. Ototoxicity and vestibular toxicity were assessed by an otorhinolaryngologist before therapy for all patients. Assessment by an otorhinolaryngologist was performed 1 month after ALIS introduction and at the time when patients complained of symptoms. When it was deemed difficult to continue treatment due to adverse events, ALIS was temporarily discontinued and then considered for resumption at an appropriate time. The serum AMK concentrations were evaluated immediately before and 1 h after the first and third ALIS inhalations.
Statistical Analysis
Summary statistics for quantitative markers were expressed as medians and interquartile ranges. Differences were analysed using McNemar’s non-parametric test for paired proportions.
Results
Patient Characteristics
Eleven patients meeting the ATS/ERS/ESCMID/IDSA guideline’s diagnostic criteria for MAC-LD were administered ALIS through inhalation. The patient characteristics are shown in Table 1. The majority of patients (81.8%) were women, and the median age at the initiation of ALIS inhalation was 64.6 years (interquartile range: 60.0–75.1 years). The median time from diagnosis to the initiation of ALIS was 13.7 years (interquartile range: 6.3–15.8 years). All 11 patients had a positive culture at the beginning of ALIS inhalation, with ten (90.9%) with an M. avium infection and one (9.1%) with a Mycobacterium intracellulare infection. Four patients (36.4%) had at least one cavitary lesion in the lungs. The distribution of MAC types based on HRCT images of patients was 72.7% nodular bronchiectatic (NB) type and 27.3% NB + fibrocavitary (FC) type. All patients were taking at least two GBT drugs at baseline, primarily macrolide (81.8%), ethambutol (90.9%), and rifamycin (72.7%).
Table 1.
Patient Background Data (n=11)
| Backgrounds | Median [Interquartile Range] or Number (%) |
|---|---|
| Age, years | 64.6 [60.0–75.1] |
| Sex, Male/Female | 2 (18.2)/9 (81.8) |
| Time from diagnosis until the initiation of treatment, years | 13.7 [6.3–15.8] |
| Weight, kg | 49.4 [44.7–51.5] |
| BMI, kg/m2 | 19.8 [16.4–22.4] |
| Smoking history | 2 (18.2) |
| Medical history | |
| Bronchial asthma | 2 (18.2) |
| Malignancy | 2 (18.2) |
| Diabetes mellitus | 1 (9.1) |
| Mycobacterium species | |
| M. avium | 10 (90.9) |
| M. intracellulare | 1 (9.1) |
| Presence of cavitary lung lesions | 4 (36.4) |
| Radiological pattern | |
| NB/FC/NB+FC/unclassified | 8 (72.7)/0 (0)/3 (27.3)/0 (0) |
| Number of GBT drugs in regimen (at baseline) | |
| 0/1/2/3/4 or more | 0 (0)/0 (0)/5 (45.5)/4 (36.4)/2 (18.2) |
| Drug class (at baseline) | |
| Macrolide | 9 (81.8) |
| Ethambutol | 10 (90.9) |
| Rifamycin | 8 (72.7) |
| Fluoroquinolone | 3 (27.3) |
| Others | 0 (0) |
| Generic name of drug (at baseline) | |
| Clarithromycin | 3 (27.3) |
| Azithromycin | 3 (27.3) |
| Erythromycin | 3 (27.3) |
| Ethambutol | 10 (90.9) |
| Rifampicin | 8 (72.7) |
| Sitafloxacin | 3 (27.3) |
Abbreviations: FC, fibrocavitary type; GBT, guideline-based therapy; NB, nodular bronchiectatic type.
Case Data Summaries
Table 2 summarises the case data for the 11 patients with MAC-LD who received ALIS inhalation therapy. Susceptibility testing showed that the majority of patients (54.5%) had CLA-resistant isolates; all but one of the patients were not using CLA at the time of ALIS initiation. All patients had been priorly treated with at least three different antimicrobial agents. In particular, CLA and ethambutol (EB) were included in the pre-treatment of all patients; rifampicin was also used, except for one patient (case 4). Four patients (cases #5, #6, #8, and #9) had priorly received AMK intravenous drip or inhalation treatment. For two of them (cases #5 and #8), the MIC of AMK was >16 μg/mL. One patient (case #8) underwent surgical resection of MAC lesions. The median duration of total treatment before ALIS introduction was 9.9 years (interquartile range: 6.3–15.8 years). All patients was sputum-culture-positive at the beginning of ALIS inhalation, but three patients (cases #3, #4, and #6) were culture negative at 1 month; two of them (cases #4 and #6) were culture positive again at subsequent follow-up, while the remaining one (cases #3) achieved culture conversion.
Table 2.
Clinical Characteristics of Patients with Mycobacterium avium Complex Receiving ALIS
| Case | Sex | Age | Species | MIC of CLA (μg/mL) | MIC of AMK (μg/mL) | Radiological Pattern | Prior Treatment | Total Treatment Duration Before ALIS (Year) | Drugs at ALIS Initiation | Sputum Culture Results Before ALIS Initiation | Sputum Culture Results 1 Month After ALIS Initiation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | F | 56 | M.avium | >32 | 4 | NB | CLA, EB, RFP, RFB, EM, STFX | 4.5 | EB, RFP | + | + |
| #2 | F | 69 | M.avium | 4 | 1 | NB+FC | CLA, EB, RFP, KM | 19.8 | CLA, EB, RFP | + | + |
| #3 | M | 76 | M.avium | >32 | 16 | NB | CLA, EB, RFP, EM | 13.7 | EB, RFP, EM | + | – |
| #4 | F | 69 | M.avium | 0.5 | 2 | NB | CLA, EB, AZM | 3.9 | EB, AZM | + | – |
| #5 | F | 63 | M.avium | >32 | >16 | NB+FC | CLA, EB, RFP, AMK div, AMK inh, STFX, LVFX | 15.8 | EB, RFP, STFX | + | + |
| #6 | F | 75 | M.avium | 0.5 | 16 | NB | CLA, EB, RFP, RFB, AZM, AMK div, AMK inh | 9.9 | EB, AZM | + | – |
| #7 | F | 79 | M.avium | >32 | 4 | NB | CLA, EB, RFP | 13.9 | CLA, RFP | + | + |
| #8 | F | 64 | M.avium | >32 | >16 | NB | CLA, EB, RFP, EM, SM, AMK div, AMK inh, STFX, SPFX, CPFX, ope | 21.3 | EB, RFP, EM, STFX | + | + |
| #9 | M | 61 | M.avium | >32 | 8 | NB | CLA, EB, RFP, EM, AMK div, STFX | 7.3 | EB, RFP, EM, STFX | + | + |
| #10 | F | 60 | M.avium | 2 | 8 | NB+FC | CLA, EB, RFP, AZM, SM, CZM, LVFX | 8.4 | CLA, EB, RFP | + | + |
| #11 | F | 56 | M.intracellulare | 0.25 | 4 | NB | CLA, EB, RFP, AZM | 6.3 | EB, AZM | + | + |
Note: Sputum culture “+” denotes positive and “-” denotes negative.
Abbreviations: ALIS, amikacin liposome inhalation suspension; MIC, minimum inhibitory concentration; M, male; F, female; M.avium, Mycobacterium avium; M.intracellulare, Mycobacterium intracellulare; div, intravenous drip; inh, inhalation (non-liposomal); ope, operation; CLA, clarithromycin; EB, ethambutol; RFP, rifampicin; RFB, rifabutin; EM, erythromycin; STFX, sitafloxacin; KM, kanamycin; AZM, azithromycin; AMK, amikacin; LVFX, levofloxacin; SM, streptomycin; SPFX, sparfloxacin; CPFX, ciprofloxacin; CZM, clofazimine; NB, nodular bronchiectatic type; FC, fibrocavitary type.
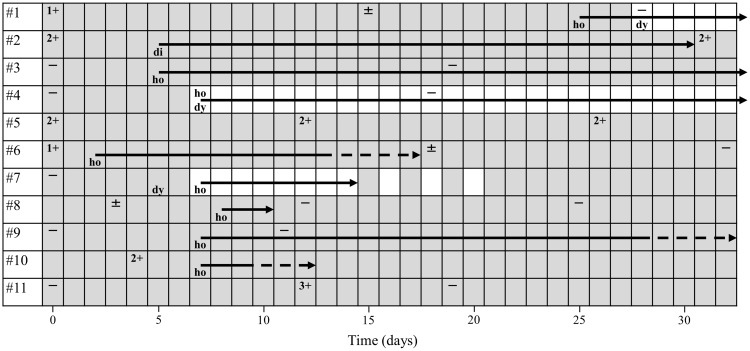
Figure 1 shows the sputum smear results over time and the duration of adverse effects in each case up to 1 month after the introduction of ALIS therapy. Of the six patients (cases #1, #2, #5, #6, #8, and #10) with positive sputum smears at the start of the study, three (cases #1, #6, and #8) achieved negative sputum smear within 1 month. On the other hand, three patients (cases #2, #5, and #10) did not show a decrease in the amount of bacteria; their smear results were all 2+, indicating relatively high bacterial levels. Five patients (cases #3, #4, #7, #9, and #11) were smear-negative at the initiation and none were positive at the 1-month follow-up.
Figure 1.
Sputum smear results over time and duration of adverse effects in each case. Halftone pattern indicates the period during ALIS treatment. Smear results are shown as (-) to (3+) depending on the amount of bacteria based on the bacterial collection method. The end point of the solid arrow indicates the day when the side effect disappeared, and the end point of the dotted arrow indicates a state of general improvement.
Eight patients (72.7%) completed ALIS inhalation during the first month of treatment, whereas three (cases #1, #4, and #7) discontinued or reduced their dose due to adverse effects. Case #7 discontinued inhalation for about 1 week due to hoarseness from day 7, and then resumed every other day for 1 week. After confirming that hoarseness had improved, daily inhalation was performed from day 21 onwards. After the period shown in Figure 1, a similar treatment schedule was followed for case #1, whose hoarseness appeared relatively late. Case #4 had persistent hoarseness for several months, during which time inhalation could not be resumed.
Adverse Events After ALIS Inhalation
Adverse event profiling related to ALIS is shown in Table 3. Nine patients (81.8%) had some adverse effects due to ALIS inhalation therapy; however, there were no severe systemic adverse events. The most common adverse effect was hoarseness (72.7%), and some patients were found to have laryngitis (eg, case #1). Hoarseness appeared around 1 week after initiation in most cases, but there was considerable variation in duration. Physicians were instructed to continue or reduce inhalation according to the severity and tolerability of the symptoms, both of which eventually improved. Other notable symptoms were oral discomfort (45.5%) and dysphonia (45.5%), with pharyngolaryngeal symptoms being the most common. Notably, adverse events related to hypersensitivity pneumonitis appeared in two patients (cases #8 and #9) more than 50 days after ALIS initiation. There was no appearance of auditory toxicity, presumably due to ALIS treatment, and no specific complaints of hearing difficulty.
Table 3.
Adverse Event Profiling of ALIS (n=11)
| Adverse Event | n (%) |
|---|---|
| Uncomfortable feeling in the oral cavity | 5 (45.5) |
| Hoarseness | 8 (72.7) |
| Dysphonia | 5 (45.5) |
| Hypersensitivity pneumonitis | 2 (18.2) |
| Hemoptysis | 1 (9.1) |
| Digestive symptom | 1 (9.1) |
| Vertigo | 1 (9.1) |
| Renal toxicity | 0 (0) |
| Auditory toxicity | 0 (0) |
Abbreviation: ALIS, amikacin liposome inhalation suspension.
Clinical Symptoms Before and After ALIS Inhalation
Table 4 presents the percentages of patients who had clinical symptoms, including coughing, sputum, dyspnea, hemoptysis, and fever, before and after ALIS inhalation therapy. Cough and sputum decreased in two patients and blood sputum decreased in one. Conversely, there were no symptoms that lead to an increase in the number of patients complaining.
Table 4.
Clinical Symptoms Before and After ALIS (Number [%])
| Clinical Symptom | Before Treatment (n=11) | After Treatment (n=11) | P valuea |
|---|---|---|---|
| Cough | 9 (81.8) | 7 (63.6) | 0.1573 |
| Sputum | 10 (90.9) | 8 (72.7) | 0.1573 |
| Dyspnea | 3 (27.3) | 3 (27.3) | 0.5637 |
| Hemoptysis | 3 (27.3) | 2 (18.2) | 1.0000 |
| Fever | 1 (9.1) | 1 (9.1) | 1.0000 |
Note: aMcNemar’s test.
Abbreviation: ALIS, amikacin liposome inhalation suspension.
Serum AMK Concentrations
The peak serum concentrations, measured 1 h after the start of the first ALIS inhalation, were <2.4 μg/mL in all 11 patients (Table 5A). Furthermore, the result of the measurement immediately before initiation of the third ALIS inhalation, regarded as the trough serum concentration, was <1.2 μg/mL for all patients (Table 5B). However, the peak serum concentration after the third inhalation sparsely ranged from <0.8 to >4.0 μg/mL (Table 5C). The third peak concentration was higher than the first for all but two patients (cases #3 and #11). In particular, for case #11, there was no difference in peak and trough concentrations for both the first and third inhalations, suggesting a problem with the inhalation technique.
Table 5.
Serum Amikacin Concentrations (n=11)
| Serum Amikacin Concentrations (μg/mL) | n (%) |
|---|---|
| (A) Serum amikacin concentrations 1 h after the start of the first ALIS inhalation (peak concentration) | |
| < 0.8 | 0 (0) |
| 0.8 to <1.6 | 8 (72.7) |
| 1.6 to <2.4 | 3 (27.3) |
| 2.4 to <3.2 | 0 (0) |
| 3.2 to <4.0 | 0 (0) |
| ≥ 4.0 | 0 (0) |
| (B) Serum amikacin concentrations just before the start of the third ALIS inhalation (trough concentrations) | |
| < 0.8 | 9 (81.8) |
| 0.8 to <1.2 | 2 (18.2) |
| ≥ 1.2 | 0 (0) |
| (C) Serum amikacin concentrations 1 h after the start of the third ALIS inhalation (peak concentration) | |
| < 0.8 | 1 (9.1) |
| 0.8 to <1.6 | 1 (9.1) |
| 1.6 to <2.4 | 4 (36.4) |
| 2.4 to <3.2 | 2 (18.2) |
| 3.2 to <4.0 | 2 (18.2) |
| ≥ 4.0 | 1 (9.1) |
Abbreviation: ALIS, amikacin liposome inhalation suspension.
Discussion
We aimed to identify clinical events that occur in the first month after initiation of ALIS inhalation therapy. This study is a valuable case series reporting on treatment efficacy and adverse effects, with a particular focus on the early phase of ALIS introduction. Only one report on real-world data, not clinical trials, of ALIS have been reported;20 the present study is also significant because it is the first to reveal the PK of ALIS in real clinical practice. Eleven patients started ALIS inhalation during their hospitalisation and attended the hospital every 1–2 weeks for a while after discharge. Close follow-up provided an accurate picture of inhalation compliance, treatment efficacy, adverse effects, and serum AMK concentration. A particularly important result was the finding that a certain number of patients exhibited a decrease in the number of bacteria within the first month of initiation, and that many complained of hoarseness within about a week.
Incidentally, the CONVERT study (INS-212) was a randomised, open-label, international Phase III clinical trial designed to evaluate the effect of ALIS on culture conversion in patients with refractory lung disease due to MAC.19 This trial was conducted at more than 125 sites in 18 countries. A comparison of the present study and CONVERT shows that the median patient age in both is about the same, at approximately 65 years. On the other hand, the patients analysed in our study exhibited several factors that made them more difficult to treat compared to those in the CONVERT study, eg, low BMI (19.8 vs 21.3 kg/m2), long disease duration (13.7 vs 4.6 years), and high CLA resistance rates (54.5% vs 22.9%). This was presumably because many patients were referred to our hospital after no improvement upon GBT at other hospitals.
Eight patients (72.7%) completed ALIS inhalation during the first month of treatment without interruption. The remaining three cases discontinued after consultation with a physician, indicating good inhalation compliance. Three of the six patients (50.0%) with positive sputum smears at the initiation of ALIS treatment were confirmed to be sputum-smear-negative within 1 month. In the CONVERT study, the sputum culture conversion rate at 6 months of ALIS inhalation was 29.0%, of which about half was achieved within 1 month of initiation. These results suggest that some patients may benefit from treatment as early as 1 month after initiation. There were two patients with MIC of AMK >16 μg/mL, both of whom had previously received therapy with intravenous or inhaled AMK. It has been reported that these prior treatments can lead to the acquisition of AMK resistance.23 However, CLSI recently set the decision breakpoint for the use of ALIS for MAC treatment at 128 μg/mL or higher.24 It is not clear whether the aforementioned two cases had resistant strains or not, but there was at least no increase in the amount of bacteria in the early-phase of ALIS treatment.
We observed no serious systemic adverse effects within 1 month in all patients who inhaled ALIS. On the other hand, localised adverse effects, such as disorders of the pharyngolarynx in particular, appeared in most patients (72.7%). Many patients complained of hoarseness or dysphonia within a week; however, such symptoms may also be present several weeks after initiation, as we have previously reported (case #1).25 If laryngitis is confirmed by an otolaryngologist, consideration should be given to reducing the frequency of ALIS inhalation. In such cases, resuming intermittent bi-daily dosing after primary interruption is a viable option.20,25 These adverse effects were reversible, and patients were often able to safely resume treatment after a temporary ALIS dose reduction. Laryngitis can also be prevented by gargling with warm water or glycerine post-dosing, rinsing the mouth after completion of nebuliser treatment, and changing the time of ALIS inhalation to the evening.20 Although ALIS-induced hypersensitivity pneumonitis after induction has also been reported,26 the two cases we experienced had a later onset and were not in the early phases of induction. In addition, it occurred at a rate of 3.1% in the CONVERT study, a higher frequency than in the GBT alone group. Non-airway adverse effects are infrequent but occur at a constant rate. Diarrhoea, nausea, and fatigue seem to be the most common among them. ALIS can cause respiratory and gastrointestinal adverse effects, most of which appear in the first month of treatment. Nevertheless, considering the aforementioned therapeutic effects, it is advisable to continue inhalation during this period. It is important for ALIS prescribers to closely follow symptoms, oxygen saturation, X-rays, and not hesitate to consult an otolaryngologist if necessary. Serum Krebs von den Lungen-6 (KL-6) level is also considered a useful biomarker related to disease progression and treatment response of MAC-LD or reflect the clinical activity of drug-induced pneumonia.27,28 Furthermore, it would be effective to share patient information in advance among physicians, nurses, and pharmacists in order to explain possible adverse effects and how to deal with them. The indication for ALIS may expand to other diseases in the future, because there are reports of its efficacy in cystic fibrosis patients with chronic Pseudomonas aeruginosa infection;29 clinical trials have been proposed for the treatment of Mycobacterium abscessus infections using ALIS.30 In this context, this report summarising events that occur early in treatment is valuable.
When inhaled using the dedicated eFlowⓇ nebuliser system, ALIS is efficiently distributed to the alveoli in the peripheral lungs, and liposome technology facilitates its uptake by macrophages, the major infected cells.31 More specifically, 73.9% of the ALIS aerosol particles produced by eFlowⓇ are in the 1–5 μm particle size range, which is considered optimal for delivery to the alveolar region.32,33 Liposomal AMK exhibits better uptake into macrophages than free AMK because of its non-polarity, and kills more intracellular mycobacteria under in vitro conditions.34,35 In the CONVERT study, the maximum serum concentration (Cmax) and area under the curve from 0 to 24 h (AUC24) of AMK were similar on day 1 and at steady state, indicating little or no serum accumulation of AMK upon ALIS inhalation. The medians of the first and third peak concentrations in the present study were 1.4 μg/mL (interquartile range: 1.2–1.6 μg/mL) and 2.3 μg/mL (interquartile range: 1.7–3.2 μg/mL), respectively, with a difference. This may have been because the patients were still unfamiliar with the inhalation technique. In any case, these levels are much lower than the serum AMK level following intravenous therapy,15 and lead to a lower incidence of systemic adverse events such as hearing loss and renal function abnormalities.
This study had several limitations. First, the treatment efficacy was not determined based on sputum culture, which is the golden standard because of the short observation period. It may not be accurate to consider improvement or exacerbation with sputum smear results. However, the CONVERT study has shown that ALIS can produce sputum conversion for as long as several months. Second, differences in blood levels and therapeutic effects of ALIS due to patient mastery of the inhalation technique cannot be ignored. To minimise this factor as much as possible, cognitive function and hand dexterity of the patients were checked by a multidisciplinary medical team before admission, and close follow-up was conducted in the outpatient clinic after discharge.
Conclusion
ALIS may reduce the amount of MAC bacteria in sputum within a month, but adverse effects such as hoarseness often appear within a few weeks. Therefore, it is important to not interrupt treatment completely but employ a multifaceted approach through multidisciplinary collaboration in the early phase of induction.
Acknowledgments
We thank Yuko Tomaki (Keio University, Tokyo, Japan) for assistance with data collection.
Funding Statement
This study was funded by AMED (21fk0108621h0001), JSPS Grant-in-Aid for JSPS fellows (21J21344), JSPS Grant-in-Aid for Young Scientists (21K15667), and JAID Clinical Research Promotion Grant, and JST PRESTO (JPMJPR21R7).
Abbreviations
ALIS, amikacin liposome inhalation suspension; AMK, amikacin; AZM, azithromycin; CLA, clarithromycin; CPFX, ciprofloxacin; CZM, clofazimine; EB, ethambutol; EM, erythromycin; FC, fibrocavitary type; GBT, guideline-based therapy; KL-6, Krebs von den Lungen-6; KM, kanamycin; LVFX, levofloxacin; MAC, Mycobacterium avium complex; MIC, minimum inhibitory concentration; NB, nodular bronchiectatic type; NTM, nontuberculous mycobacterial; PD, pharmacodynamics; PK, pharmacokinetics; RFB, rifabutin; RFP, rifampicin; SM, streptomycin; SPFX, sparfloxacin; STFX, sitafloxacin.
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by the ethics committees of Keio University School of Medicine (20110267) and related research institutions. All adult patients provided written informed consent to participate in this study.
Consent for Publication
The patients provided written informed consent for publication of individual clinical details and all the accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182(7):970–976. doi: 10.1164/rccm.201002-0310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881–886. doi: 10.1164/rccm.201111-2016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn K, Kim YK, Hwang GY, Cho H, Uh Y. Continued upward trend in non-tuberculous mycobacteria isolation over 13 years in a tertiary care hospital in Korea. Yonsei Med J. 2021;62(10):903–910. doi: 10.3349/ymj.2021.62.10.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36(1):13–34. doi: 10.1016/j.ccm.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schildkraut JA, Gallagher J, Morimoto K, et al. Epidemiology of nontuberculous mycobacterial pulmonary disease in Europe and Japan by Delphi estimation. Respir Med. 2020;173:106164. doi: 10.1016/j.rmed.2020.106164 [DOI] [PubMed] [Google Scholar]
- 6.Namkoong H, Kurashima A, Morimoto K, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect Dis. 2016;22(6):1116–1117. doi: 10.3201/eid2206.151086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morimoto K, Iwai K, Uchimura K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc. 2014;11(1):1–8. doi: 10.1513/AnnalsATS.201303-067OC [DOI] [PubMed] [Google Scholar]
- 8.Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71(4):905–913. doi: 10.1093/cid/ciaa1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace RJ Jr., Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996;153(6 Pt 1):1766–1772. doi: 10.1164/ajrccm.153.6.8665032 [DOI] [PubMed] [Google Scholar]
- 10.Miwa S, Shirai M, Toyoshima M, et al. Efficacy of clarithromycin and ethambutol for Mycobacterium avium complex pulmonary disease. A preliminary study. Ann Am Thorac Soc. 2014;11(1):23–29. doi: 10.1513/AnnalsATS.201308-266OC [DOI] [PubMed] [Google Scholar]
- 11.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 12.Ellender CM, Law DB, Thomson RM, Eather GW. Safety of IV amikacin in the treatment of pulmonary non-tuberculous mycobacterial disease. Respirology. 2016;21(2):357–362. doi: 10.1111/resp.12676 [DOI] [PubMed] [Google Scholar]
- 13.Kim OH, Kwon BS, Han M, et al. Association between duration of aminoglycoside treatment and outcome of cavitary mycobacterium avium complex lung disease. Clin Infect Dis. 2019;68(11):1870–1876. doi: 10.1093/cid/ciy804 [DOI] [PubMed] [Google Scholar]
- 14.Peloquin CA, Berning SE, Nitta AT, et al. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis. 2004;38(11):1538–1544. doi: 10.1086/420742 [DOI] [PubMed] [Google Scholar]
- 15.van Ingen J, Egelund EF, Levin A, et al. The pharmacokinetics and pharmacodynamics of pulmonary Mycobacterium avium complex disease treatment. Am J Respir Crit Care Med. 2012;186(6):559–565. doi: 10.1164/rccm.201204-0682OC [DOI] [PubMed] [Google Scholar]
- 16.Jhun BW, Yang B, Moon SM, et al. Amikacin inhalation as salvage therapy for refractory nontuberculous mycobacterial lung disease. Antimicrob Agents Chemother. 2018;62(7). doi: 10.1128/AAC.00011-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagi K, Ishii M, Namkoong H, et al. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect Dis. 2017;17(1):558. doi: 10.1186/s12879-017-2665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivier KN, Shaw PA, Glaser TS, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc. 2014;11(1):30–35. doi: 10.1513/AnnalsATS.201307-231OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith DE, Eagle G, Thomson R, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198(12):1559–1569. doi: 10.1164/rccm.201807-1318OC [DOI] [PubMed] [Google Scholar]
- 20.Swenson C, Lapinel NC, Ali J. Clinical management of respiratory adverse events associated with amikacin liposome inhalation suspension: results from a patient survey. Open Forum Infect Dis. 2020;7(4):ofaa079. doi: 10.1093/ofid/ofaa079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asakura T, Funatsu Y, Ishii M, et al. Health-related quality of life is inversely correlated with C-reactive protein and age in Mycobacterium avium complex lung disease: a cross-sectional analysis of 235 patients. Respir Res. 2015;16:145. doi: 10.1186/s12931-015-0304-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inagaki T, Yagi T, Ichikawa K, et al. Evaluation of a rapid detection method of clarithromycin resistance genes in Mycobacterium avium complex isolates. J Antimicrob Chemother. 2011;66(4):722–729. doi: 10.1093/jac/dkq536 [DOI] [PubMed] [Google Scholar]
- 23.Brown-Elliott BA, Iakhiaeva E, Griffith DE, et al. In vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates. J Clin Microbiol. 2013;51(10):3389–3394. doi: 10.1128/JCM.01612-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia Spp., and Other Aerobic Actinomycetes. CLSI Supplement M62. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 25.Morita A, Namkoong H, Hosoya M, Hasegawa N. Laryngitis after inhalation of liposomal amikacin. Clin Case Rep. 2022;10(2):e05350. doi: 10.1002/ccr3.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidogawa M, Yamasaki K, Nemoto K, Yatera K. Liposomal amikacin inhalation suspension-induced pneumonitis. Intern Med. 2022. doi: 10.2169/internalmedicine.8796-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asakura T, Kimizuka Y, Nishimura T, et al. Serum Krebs von den Lungen-6 level in the disease progression and treatment of Mycobacterium avium complex lung disease. Respirology. 2021;26(1):112–119. doi: 10.1111/resp.13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi H, Yokoyama A, Yasuhara Y, et al. Circulating KL-6 levels in patients with drug induced pneumonitis. Thorax. 2003;58(10):872–875. doi: 10.1136/thorax.58.10.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilton D, Fajac I, Pressler T, et al. Long-term amikacin liposome inhalation suspension in cystic fibrosis patients with chronic P. aeruginosa infection. J Cyst Fibros. 2021;20(6):1010–1017. doi: 10.1016/j.jcf.2021.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henriette Zweijpfenning SM, Chiron R, Essink S, et al. Safety and outcomes of amikacin liposome inhalation suspension for mycobacterium abscessus pulmonary disease: a NTM-NET. Chest. 2022;162:76–81. doi: 10.1016/j.chest.2022.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park HY, Jeong BH, Chon HR, Jeon K, Daley CL, Koh WJ. Lung function decline according to clinical course in nontuberculous mycobacterial lung disease. Chest. 2016;150(6):1222–1232. doi: 10.1016/j.chest.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 32.Wenzler E, Fraidenburg DR, Scardina T, Danziger LH. Inhaled antibiotics for gram-negative respiratory infections. Clin Microbiol Rev. 2016;29(3):581–632. doi: 10.1128/CMR.00101-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Zhang Y, Wurtz W, et al. Characterization of nebulized liposomal amikacin (Arikace) as a function of droplet size. J Aerosol Med Pulm Drug Deliv. 2008;21(3):245–254. doi: 10.1089/jamp.2008.0686 [DOI] [PubMed] [Google Scholar]
- 34.Meers P, Neville M, Malinin V, et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008;61(4):859–868. doi: 10.1093/jac/dkn059 [DOI] [PubMed] [Google Scholar]
- 35.Rose SJ, Neville ME, Gupta R, Bermudez LE. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS One. 2014;9(9):e108703. doi: 10.1371/journal.pone.0108703 [DOI] [PMC free article] [PubMed] [Google Scholar]