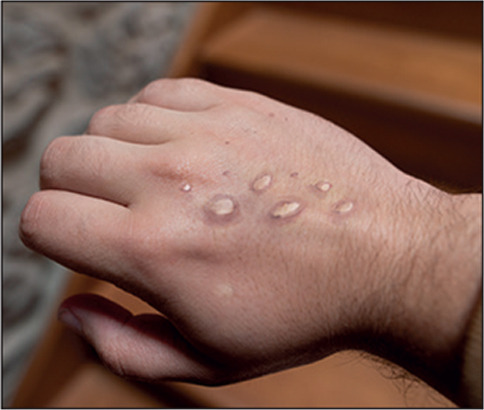
As of July 21, 2022, WHO has reported 15 734 laboratory-confirmed monkeypox infections, including children, in 75 countries across five continents.1 The unprecedented widespread geographical distribution of this poxvirus shows the risk for a potential public health emergency of international concern. These laboratory-confirmed monkeypox infections are more than double the total number of cases than in the previous situation report published 2 weeks earlier on July 9, 2022, emphasising the sustained transmission of the monkeypox virus. However, these reported figures are likely to be an underestimation of the actual number of infections due to inadequate clinical recognition of monkeypox virus infection and the long incubation period of the virus (5–21 days). Current estimates of disease burden reflect the situation from previous weeks, and the actual number of infected individuals could exceed 30 000.
The potential for sustained human-to-human transmission of the monkeypox virus was previously believed to be low. The re-emergence in 2022 suggests improved transmissibility, possibly enabled by an improved viral adaptation to the human host and promoted by the increased exposure of an immunologically naive population to orthopoxviruses. In the coming months, with increased travel by a population previously constrained by COVID-19 restrictions and forthcoming events that include substantial gatherings of people, the current monkeypox virus outbreak could rapidly become uncontrolled, especially as its clinical presentation appears to be more subtle than previous descriptions.1 Although clinical outcomes seem to be favourable, with only three deaths reported in Africa,1 the current outbreak has mostly affected healthy adults at low risk of complications. However, with increasing case numbers, vulnerable populations, such as people who are immunocompromised, children, and pregnant women, could become infected. Epidemiological data from previous monkeypox outbreaks in Africa suggest that the secondary attack rate could be as high as 12·3% among smallpox-unvaccinated household contacts of individuals with monkeypox, particularly affecting children younger than 15 years.2
Compared with healthy adults, complications are more frequent in children and people who are immunocompromised, with an increased risk of bacterial superinfection, sepsis, keratitis, respiratory complications due to pharyngeal abscess and pneumonia, or encephalitis.2, 3 Previous monkeypox outbreaks have reported increased mortality and hospitalisation rates in children, even in high-income countries, such as the USA, in which the only two severe presentations during the 2003 outbreak were observed in the paediatric population.4
Although information regarding the effects of monkeypox infection in pregnant women is scarce, vertical transmission of monkeypox has been associated with fetal demise and congenital infection.5 By analogy with smallpox infection, the disease is expected to be more severe in pregnant women than in healthy individuals who are not pregnant, particularly during their third trimester.6
All available smallpox vaccines offer good protection against monkeypox infection and can be used for pre-exposure or postexposure prophylaxis. A reduced rate of secondary attack is observed in previously vaccinated household contacts of individuals with monkeypox.2 In Canada and the USA, the Modified vaccinia Ankara vaccine (MVA-BN; Bavarian Nordic, Denmark) is licensed for both smallpox and monkeypox prevention among adults. In Europe, where MVA-BN is known as IMVANEX, the vaccine has only received authorisation for use against smallpox. This third-generation vaccine offers a better safety profile than previous versions of the vaccine due to a non-replicating agent that restricts the risk of dissemination and contagiousness of the vaccine agent, which is particularly useful for people who are immunocompromised. Vaccination will be crucial in future control of monkeypox outbreaks and potentially other emerging or re-emerging orthopoxviruses.
Unfortunately, as seen during the COVID-19 pandemic, vaccine acceptance remains challenging. It is therefore urgent for health systems to prepare and educate communities with straightforward, simple, and factual information, as doing so will be crucial to protect pregnant women, children, and other individuals who are at risk of infection. Unlike the COVID-19 pandemic, for which novel vaccines against SARS-CoV-2 had to be developed, data regarding the safety and efficacy of smallpox vaccines have been available for decades, including data in vulnerable populations. Several randomised controlled trials have shown the safety of MVA-BN among patients with HIV with or without AIDS.7 Furthermore, although scarce, data regarding this non-replicating vaccine in pregnant women are reassuring; animal studies have also shown no increased risk of fetal malformations.8 A modified version of MVA-BN was safely used as a vector encoding Ebola proteins in a randomised controlled trial of children aged 1–17 years, thus emphasising its potential future use in this population.9
How the current monkeypox outbreak will evolve and the extent of its effects on public health are still unclear. Nevertheless, how can the unabated emergence and re-emergence of monkeypox be explained after more than 40 years of effective vaccinal control of orthopoxvirus infections? Anticipatory guidance is required, and public health efforts focused on protecting vulnerable populations, especially people who are immunocompromised, pregnant women, and children, should be widely implemented.
© 2022 MarioGuti/iStock
We declare no competing interests.
References
- 1.PAHO. WHO . Pan American Health Organization, World Health Organization; Washington, DC: 2022. Monkeypox situation report.https://www.paho.org/en/documents/weekly-situation-report-monkeypox-multi-country-outbreak-response-region-americas-22-july#:~:text=As%20of%2021%20July%202022,in%20the%20Western%20Pacific%20Region [Google Scholar]
- 2.Jezek Z, Marennikova SS, Mutumbo M, Nakano JH, Paluku KM, Szczeniowski M. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis. 1986;154:551–555. doi: 10.1093/infdis/154.4.551. [DOI] [PubMed] [Google Scholar]
- 3.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:561–564. [PubMed] [Google Scholar]
- 5.Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824–828. doi: 10.1093/infdis/jix260. [DOI] [PubMed] [Google Scholar]
- 6.Nishiura H. Smallpox during pregnancy and maternal outcomes. Emerg Infect Dis. 2006;12:1119–1121. doi: 10.3201/eid1207.051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overton ET, Lawrence SJ, Stapleton JT, et al. A randomized phase II trial to compare safety and immunogenicity of the MVA-BN smallpox vaccine at various doses in adults with a history of AIDS. Vaccine. 2020;38:2600–2607. doi: 10.1016/j.vaccine.2020.01.058. [DOI] [PubMed] [Google Scholar]
- 8.Bavarian Nordic Full prescribing information. 2021. https://www.fda.gov/media/131078/download
- 9.Afolabi MO, Ishola D, Manno D, et al. Safety and immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in children in Sierra Leone: a randomised, double-blind, controlled trial. Lancet Infect Dis. 2022;22:110–122. doi: 10.1016/S1473-3099(21)00128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


