Abstract
Background
Studies have shown that COVID-19 vaccination is effective at preventing infection and death in older populations. However, whether vaccination effectiveness is reduced in patients with frailty is unclear. We aimed to compare vaccine effectiveness against hospitalisation and death after COVID-19 during the surge of the delta (B.1.617.2) variant of SARS-CoV-2 according to patients' frailty status.
Methods
In this retrospective cohort study, we used data derived from the US Veterans Health Administration (VHA) facilities and the US Department of Veterans Affairs (VA) COVID-19 Shared Data Resource, which contains information from the VA National Surveillance Tool, death certificates, and National Cemetery Administration. We included veterans aged 19 years or older who tested positive for SARS-CoV-2 using RT-PCR or antigen tests between July 25 and Sept 30, 2021, with no record of a previous positive test. Deaths were identified through VHA facilities, death certificates, and National Cemetery Administration data available from VA databases. We also retrieved data including sociodemographic characteristics, medical conditions diagnosed at baseline, frailty score, and vaccination information. The primary outcomes were COVID-19-associated hospitalisations and all-cause deaths at 30 days from testing positive for SARS-CoV-2. The odds ratio (OR) for COVID-19-associated hospitalisation and hazard ratio (HR) for death of vaccinated patients compared with the unvaccinated patients were estimated according to frailty categories of robust, pre-frail, or frail. Vaccine effectiveness was estimated as 1 minus the OR for COVID-19-associated hospitalisation, and 1 minus the HR for death.
Findings
We identified 57 784 veterans (mean age 57·5 years [SD 16·7], 50 642 [87·6%] males, and 40 743 [70·5%] White people), of whom 28 497 (49·3%) were categorised as robust, 16 737 (29·0%) as pre-frail, and 12 550 (21·7%) as frail. There were 2577 all-cause deaths (676 [26·2%] in the vaccinated group and 1901 [73·8%] in the unvaccinated group), and 7857 COVID-19-associated hospitalisations (2749 [35·0%] in the vaccinated group and 5108 [65·0%] in the unvaccinated group) within 30 days of a positive SARS-CoV-2 test. Vaccine effectiveness against COVID-19-associated hospitalisation within 30 days of a positive SARS-CoV-2 test was 65% (95% CI 61–69) in the robust group, 54% (48–58) in the pre-frail group, and 36% (30–42) in the frail group. By 30 days of a positive SARS-CoV-2 test, the vaccine effectiveness for all-cause death was 79% (95% CI 74–84) in the robust group, 79% (75–83) in the pre-frail group, and 68% (63–71) in the frail group.
Interpretation
Compared with non-frail patients (pre-frail and robust), those with frailty had lower levels of vaccination protection against COVID-19-associated hospitalisation and all-cause death. Future studies investigating COVID-19 vaccine effectiveness should incorporate frailty assessments and actively recruit older adults with frailty.
Funding
Miami VA Healthcare System Geriatric Research Education and Clinical Center.
Introduction
Older adults have been disproportionately affected by the COVID-19 pandemic. Studies have documented higher risk of morbidity, hospital admission, admission to care homes, and death among the older population than in younger people with COVID-19.1 The delta (B.1.617.2) variant of SARS-CoV-2 became dominant in the USA between June and August, 2021, and was characterised by even higher transmissibility, more severe clinical presentation, and higher mortality in unvaccinated individuals than the preceding alpha (B.1.1.7) variant.2 Older adults stand to benefit the most from interventions aimed at preventing infection or mitigating the effects of COVID-19. Studies have shown that COVID-19 vaccination is effective at preventing infection, symptomatic disease, severe complications, and death in older populations, but their responses to vaccines are lower than those of younger adults.3 Post-clinical trial data show age-related reduced immunogenicity and a waning of vaccine protection over time.4 However, there is evidence of a heterogeneity of responses to vaccination in the older population. Clinical trials investigating the efficacy of COVID-19 vaccines have included older adults, but these studies have mostly excluded those with multimorbidity, disability, and frailty.5 These older populations are at high risk of poor outcomes and are, therefore, the most in need of vaccine protection. However, few studies have investigated vaccine effectiveness in these populations.
Research in context.
Evidence before this study
We searched PubMed, Embase, Cochrane Library, Google Scholar, and Web of Science for research articles published between March 1, 2020, and March 1, 2022, using the search terms and keywords [“COVID”] OR [“SARS-CoV-2,”] OR [“novel human coronavirus”] OR [“coronavirus disease 2019”] AND [“vaccines, vaccination, inoculation, immunization”] AND [“frail, frailty”] AND [“mortality, mortalities”] OR [“death”] OR [“survivor, survival”] OR [“hospital, hospitalization, hospitalizations”]. No language restrictions were applied. Reference lists from identified articles were also analysed for additional studies. We included articles that evaluated the effects of mRNA COVID-19 vaccines on hospitalisation and all-cause death according to frailty status. Evidence shows that COVID-19 vaccination is effective at preventing severe complications and all-cause death in older populations; however, their responses to vaccines are lower than those of younger adults. Furthermore, current research reveals a heterogeneity in the responses to vaccination in older adults, although the reasons for this are not well understood. The existing evidence suggests that chronological age might not be an optimal criterion when evaluating the effectiveness of COVID-19 vaccines. Frailty, a state of reduced reserves and increased vulnerability to stressors resulting from multisystemic dysfunction, can be considered a surrogate marker of biological age. Frailty is common in older populations and is responsible for many negative clinical outcomes. It is associated with age-related immune dysregulation characterised by low-grade chronic inflammation and immunosenescence, which are factors that might contribute to decreased vaccine protection. Although vaccine effectiveness has not been well studied in relation to frailty or underlying health status, some studies investigating the efficacy or effectiveness of vaccines in older adults with frailty have shown mixed results.
Added value of this study
Our study showed that COVID-19 vaccine effectiveness against COVID-19-associated hospitalisation and all-cause death varied by frailty status. So far, this is the first retrospective cohort study evaluating the effects of mRNA vaccines on COVID-19-associated hospitalisation or all-cause death after documented SARS-CoV-2 infection according to frailty status. The results of this study are important to build our understanding of frailty as an influential factor in the variability of responses to vaccination in older populations.
Implications of all the available evidence
Although individuals with frailty benefit from COVID-19 mRNA vaccination, the degree of protection against COVID-19-associated hospitalisation and all-cause death seems to be lower than in people without frailty. The evidence from this study suggests that future research design, development, and evaluation of COVID-19 vaccine effectiveness should consider baseline frailty assessments. The findings of reduced vaccine protection in patients with frailty underscore the need to optimise vaccine effectiveness for these patients, with the goal of preventing severe SARS-CoV-2-related outcomes. Future large cohort studies and randomised controlled trials that consider frailty status are essential to gain more information about the effects of COVID-19 vaccination in older adults according to frailty status.
Frailty is characterised by individuals' vulnerability to various stressors resulting from multisystemic dysfunction. Frailty is a common condition in the older population that often coexists and noticeably overlaps with multimorbidity or disability.6 However, there is growing consensus that frailty is a distinctly defined clinical syndrome with several postulated biological mechanisms.7, 8 COVID-19 is a key stressor, and evidence suggests that it has contributed to worse clinical outcomes in older people with frailty than in those without. Moreover, frailty might hinder favourable responses to various effective clinical interventions against COVID-19, including vaccination, which might further compromise clinical outcomes in older people.5 Two mechanisms could lead to the decline in vaccine responsiveness in older individuals with frailty: immunosenescence, which refers to the age-related dysregulation in humoral and cellular immunity, and inflammaging, which describes low-grade chronic inflammation.9 Mounting research suggests that inflammaging and immunosenescence do not invariably occur in all older adults and might account for the variability in vaccine responses seen in individuals with frailty.10, 11 Supporting clinical evidence reveals that frailty might negatively affect individuals' responses to other vaccines, such as the influenza vaccine.12 More research examining the effects of frailty on COVID-19 vaccination responsiveness in patients with previous SARS-CoV-2 infection is needed. Frailty in older adults might be an important factor to consider when designing, developing, and evaluating COVID-19 vaccines.13
The US veteran population is older and predominantly male, and has a high prevalence of chronic medical and mental health multimorbidity, substance abuse, obesity, disability, and frailty,14 all of which are known risk factors for worse outcomes after SARS-CoV-2 infection. Although COVID-19 mRNA vaccines were effective at reducing death in veterans during the delta variant wave, protection was lower for veterans older than 65 years than those aged 65 years or younger, and veterans' frailty status was not assessed.15 As one in three veterans older than 65 years have frailty,16 the determination of frailty status might identify a group at higher risk of both worse clinical outcomes and lower vaccine responsiveness. Our primary aim was to compare vaccine effectiveness against COVID‑19-associated hospitalisation and all-cause death after SARS-CoV-2 infection according to patients' frailty status. Our hypothesis was that vaccine effectiveness against COVID-19-associated hospitalisation and all-cause death during the delta variant wave will be lower in patients with frailty than in robust and pre-frailty groups.
Methods
Study design and population
This retrospective cohort study was done at the Veterans Health Administration (VHA), the largest integrated health-care system in the USA. Data were extracted from the US Department of Veterans Affairs (VA) COVID-19 Shared Data Resource (which was created by the VHA in 2020 to facilitate research into SARS-CoV-217), the VA National Surveillance Tool (NST; which is the authoritative data source for positive and negative COVID-19 cases in the veteran population),18 and the VA Corporate Data Warehouse. We included US veterans aged 19 years or older who tested positive for SARS-CoV-2 on RT-PCR or antigen tests between July 25 and Sept 30, 2021, a period during which the prevalence of COVID-19 resulting from the delta variant exceeded 96% in the USA,19 and had no record of a previous positive test. We retrieved the following data from the VA COVID-19 Shared Data Resource: sociodemographic characteristics, medical conditions diagnosed at baseline (diabetes, hypertension, cardiovascular disease, kidney disease, and chronic obstructive pulmonary disease), COVID-19-related symptoms, and vaccine type and administration date. Data on COVID-19-associated hospitalisations were obtained from the VA COVID-19 Shared Data Resource. Deaths were identified through VHA facilities, death certificates, and National Cemetery Administration data available from VA databases. Previous VA studies have demonstrated high agreement between dates of death recorded in the VA Corporate Data Warehouse database and dates of death recorded in external sources that feed into the VHA Vital Status File.20
Comorbid conditions were captured using International Classification of Diseases 10th revision (ICD-10) codes from the NST outpatient and inpatient data domains. We excluded veterans who received vaccines other than US mRNA vaccines (mRNA-1273, Moderna, and BNT162b2, Pfizer–BioNTech); those missing information regarding vaccine type; those who were partially vaccinated at the time of infection; and those who were admitted to hospital before testing positive for SARS-CoV-2, because these patients were not admitted to hospital for COVID-19. This study was approved by the Institutional Review Board at the Miami VHA and was exempt from the requirement for informed consent because study data were anonymised and de-identified in the VA COVID-19 Shared Data Resource.
Frailty
We categorised frailty using the VA Frailty Index (VA-FI). For each patient, a VA-FI score was computed on the date of testing positive for SARS-CoV-2. The 31-item VA-FI15 was developed based on the deficit accumulation conceptual framework that assumes that frailty is the result of multiple physical, functional, psychological, and social variables.21 Unlike the frailty phenotype, the deficit accumulation approach does not rely on predetermined variables and might be more suitable to our veteran population because it focuses on multimorbidity, cognitive impairment, and disability.22 The VA-FI has been validated in more than 2 million veterans and has been shown to be associated with mortality.23 The VA-FI consists of 31 deficits categorised into five major groups: morbidity, function, sensory loss, cognition and mood, and others (appendix p 2). Each individual deficit was coded with values between 0 and 1, where 0 represented the absence of the deficit and 1 represented its presence. The VA-FI was calculated by adding the values for all deficits and dividing by the total number (ie, 31) of health-care deficits considered for each individual.24 Total index scores were categorised as robust (0 to <0·1), pre-frail (≥0·1 to <0·21), and frail (≥0·21) on the basis of previously published cutoff points.15
Vaccination status
We defined fully vaccinated at the time of infection as at least 7 days from receipt of the second dose of the BNT162b2 vaccine, and at least 14 days from receipt of the second dose of mRNA-1273. These definitions are consistent with the phase 3 clinical trials of the respective vaccines.25 We defined partial vaccination when at least 2 days had passed since receiving the last of two doses of vaccine but not meeting full vaccination criteria.
COVID-19-related symptoms
Symptoms in the 30 days before the date of confirmed SARS-CoV-2 infection that were consistent with potential COVID-19 included abdominal pain, chills, cough, diarrhoea, dyspnoea, fatigue, fever, headache, loss of smell, loss of taste, myalgia, nausea, rhinorrhoea, and sore throat. These were first derived using ICD-10 codes and recorded vital signs and then natural language-processing tools for those symptoms that were not captured by ICD-10 codes.
Statistical analysis
The primary outcomes were COVID-19-associated hospitalisations and all-cause death within 30 days after testing positive for SARS-CoV-2 infection.
Continuous variables are presented as means with SDs and medians with IQRs; categorical variables are presented as numbers with proportions. A Cox proportional-hazards model was used to estimate the hazard ratios (HRs) of death at 30 days from testing positive for SARS-CoV-2 among vaccinated compared with unvaccinated patients, controlling for covariates that are known risk factors for severe infection: age (as a continuous variable), BMI, race, ethnicity, sex, smoking status, being an active patient in the VHA system in the past 12 months, rurality, and treatment with monoclonal antibodies. Multiple imputation by chained equations was used to impute missing values of BMI using the MICE package in R. When information on race, ethnicity, and smoking status was not available, we reported the data as “unknown”. The proportional-hazards assumption was assessed and verified by testing the correlation between the Schoenfeld residuals and survival time. A log–log plot was generated to show the proportional hazards of the robust, pre-frail, and frail groups (appendix p 9). As the proportional-hazards assumption does not hold for COVID-19-associated hospitalisation (p<0·001), we used logistic regression to estimate the odds ratio (OR) of COVID-19-associated hospitalisation within 30 days from testing positive for SARS-CoV-2, controlling for the same covariates as in the Cox model. Vaccine effectiveness was defined as 1 minus the HR for all-cause death and 1 minus the OR for COVID-19-associated hospitalisation. The 95% CI for vaccine effectiveness was calculated as 1 minus the lower end of the 95% CI of the HR or OR to 1 minus the upper end of the 95% CI of the HR or OR. We stratified the analysis according to frailty status (robust, pre-frail, and frail groups). Vaccine effectiveness was calculated according to frailty status for the two mRNA vaccines (mRNA-1273 and BNT162b2) combined and also separately for each vaccine. Since the date of COVID-19 vaccination was not available for unvaccinated participants, we used the date of testing as the index date for both vaccinated and unvaccinated individuals. The time to event (all-cause death) was calculated from the date of a documented positive SARS-CoV-2 test. As patients with frailty were more likely to be vaccinated earlier based on vaccination policies, we did an additional analysis to de-confound the effects from time of vaccination and frailty status by comparing the OR for COVID-19-associated hospitalisation and HR for all-cause death at 30 days for patients vaccinated less than 4 months, 4–6 months, and more than 6 months before infection with unvaccinated patients, according to frailty status. We created a variable indicating vaccination status and time from vaccination to a positive SARS-CoV-2 test: not vaccinated, less than 4 months from vaccination, 4–6 months from vaccination, and longer than 6 months from vaccination. We report adjusted HRs for all-cause death and adjusted ORs for COVID-19-associated hospitalisation at less than 4 months, 4–6 months, and longer than 6 months from vaccination compared with the unvaccinated patients, adjusting for the covariates. We tested whether an interaction between frailty and age existed by adding an interaction term of frailty status and age (as a binary variable indicting whether a patient is 65 years or older) to the model. We report adjusted ORs for COVID-19-associated hospitalisation and adjusted HRs for death in individuals younger than 65 years and those 65 years or older according to frailty status. We also used the same Cox model and logistic regression model to compare the effectiveness of mRNA-1273 versus BNT162b2, adjusting for the covariates and frailty status. In addition, we calculated ORs and HRs for vaccination stratified by age group (<65 years and ≥65 years) and sex. All the statistical analyses were done with R (version 4.0.5).
Role of the funding resource
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
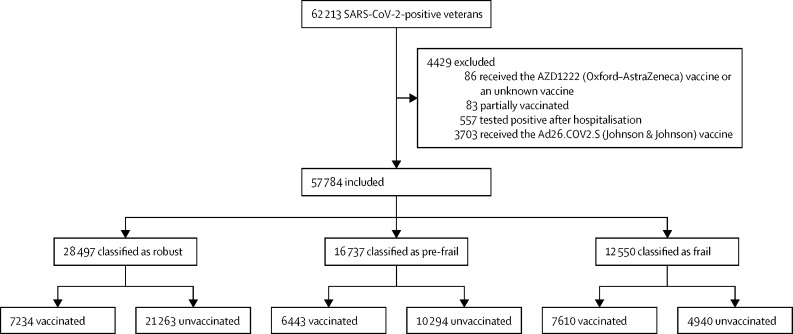
Between July 25 and Sept 30, 2021, 62 213 SARS-CoV-2-positive veterans were identified. 57 784 patients (mean age 57·5 years [SD 16·7], 50 642 [87·6%] males, and 40 743 [70·5%] White people) were included in the analysis (figure ). The final cohort consisted of 28 497 (49·3%) patients in the robust group, 16 737 (29·0%) in the pre-frail group, and 12 550 (21·7%) in the frail group. Baseline characteristics of patients according to frailty status are shown in table 1 . Patients with frailty were older and more likely to have the common chronic comorbidities diabetes, hypertension, chronic obstructive pulmonary disease, cardiovascular disease, and kidney disease than patients in the robust group. 4528 (36·1%) of 12 550 patients in the frail group and 12 950 (45·4%) of 28 497 patients in the robust group had asymptomatic COVID-19. Compared with robust patients, those with frailty were more likely to have abdominal pain and dyspnoea as COVID-19-presenting symptoms. Patients in the frail group were more likely to be fully vaccinated (7610 [60·6%]) than patients in the pre-frail group (6443 [38·5%]) and robust group (7234 [25·4%]).
Figure.
Study profile
Table 1.
Baseline characteristics according to frailty status
| Total (n=57 784) | Robust group (n=28 497) | Pre-frail group (n=16 737) | Frail group (n=12 550) | ||
|---|---|---|---|---|---|
| Age, years | |||||
| Mean (SD) | 57·5 (16·7) | 50·1 (15·8) | 60·8 (14·9) | 69·8 (11·5) | |
| Median (IQR) | 58 (43–72) | 49 (37–62) | 63 (50–72) | 72 (63–76) | |
| Age groups | |||||
| <65 years | 34 955 (60·5%) | 22 419 (78·7%) | 9078 (54·2%) | 3458 (27·6%) | |
| ≥65 years | 22 829 (39·5%) | 6078 (21·3%) | 7659 (45·8%) | 9092 (72·4%) | |
| Sex | |||||
| Male | 50 642 (87·6%) | 24 470 (85·9%) | 14 625 (87·4%) | 11 547 (92·0%) | |
| Female | 7142 (12·4%) | 4027 (14·1%) | 2112 (12·6%) | 1003 (8·0%) | |
| Race | |||||
| White | 40 743 (70·5%) | 19 322 (67·8%) | 12 008 (71·7%) | 9413 (75·0%) | |
| Black | 10 867 (18·8%) | 5408 (19·0%) | 3201 (19·1%) | 2258 (18·0%) | |
| Asian | 480 (0·8%) | 316 (1·1%) | 111 (0·7%) | 53 (0·4%) | |
| American Indian or Alaska Native | 495 (0·9%) | 263 (0·9%) | 147 (0·9%) | 85 (0·7%) | |
| Native Hawaiian or other Pacific Islander | 584 (1·0%) | 310 (1·1%) | 159 (0·9%) | 115 (0·9%) | |
| Unknown | 4615 (8·0%) | 2878 (10·1%) | 1111 (6·6%) | 626 (5·0%) | |
| Ethnicity | |||||
| Hispanic | 4870 (8·4%) | 2781 (9·8%) | 1346 (8·0%) | 743 (5·9%) | |
| Not Hispanic | 49 457 (85·6%) | 23 190 (81·4%) | 14 808 (88·5%) | 11 459 (91·3%) | |
| Unknown | 3457 (6·0%) | 2526 (8·9%) | 583 (3·5%) | 348 (2·8%) | |
| Comorbidities | |||||
| Diabetes | 16 261 (28·1%) | 2771 (9·7%) | 5773 (34·5%) | 7717 (61·5%) | |
| Hypertension | 30 838 (53·4%) | 8339 (29·3%) | 11 277 (67·4%) | 11 222 (89·4%) | |
| Chronic obstructive pulmonary disease | 7659 (13·3%) | 1207 (4·2%) | 2255 (13·5%) | 4197 (33·4%) | |
| Cardiovascular disease | 15 856 (27·4%) | 2524 (8·9%) | 4988 (29·8%) | 8344 (66·5%) | |
| Kidney disease | 10 890 (18·8%) | 1983 (7·0%) | 3087 (18·4%) | 5820 (46·4%) | |
| BMI, kg/m2 | |||||
| ≤20 | 1395 (2·4%) | 509 (1·8%) | 395 (2·4%) | 491 (3·9%) | |
| >20 to ≤30 | 27 355 (47·3%) | 13 829 (48·5%) | 7690 (45·9%) | 5836 (46·5%) | |
| >30 to ≤40 | 23 623 (40·9%) | 11 337 (39·8%) | 7210 (43·1%) | 5076 (40·4%) | |
| >40 | 5411 (9·4%) | 2822 (9·9%) | 1442 (8·6%) | 1147 (9·1%) | |
| Smoking | |||||
| Current | 8707 (15·1%) | 4350 (15·3%) | 2597 (15·5%) | 1760 (14·0%) | |
| Former smoker | 21 753 (37·6%) | 8837 (31·0%) | 6825 (40·8%) | 6091 (48·5%) | |
| Never | 21 672 (37·5%) | 10 820 (38·0%) | 6581 (39·3%) | 4271 (34·0%) | |
| Unknown | 5652 (9·8%) | 4490 (15·8%) | 734 (4·4%) | 428 (3·4%) | |
| Rurality | |||||
| City town | 5480 (9·5%) | 2450 (8·6%) | 1726 (10·3%) | 1304 (10·4%) | |
| Small town or rural | 4147 (7·2%) | 1769 (6·2%) | 1381 (8·3%) | 997 (7·9%) | |
| Urban | 42 893 (74·2%) | 21 351 (74·9%) | 12 294 (73·5%) | 9248 (73·7%) | |
| Monoclonal antibody treatment | 4241 (7·3%) | 1551 (5·4%) | 1365 (8·2%) | 1325 (10·6%) | |
| COVID-19-related symptoms | |||||
| Abdominal pain | 2076 (3·6%) | 788 (2·8%) | 587 (3·5%) | 701 (5·6%) | |
| Chills | 8792 (15·2%) | 4834 (14·0%) | 2347 (12·8%) | 1611 (1·4%) | |
| Cough | 21 820 (37·8%) | 10 457 (36·7%) | 6296 (37·6%) | 5067 (40·4%) | |
| Diarrhoea | 8317 (14·4%) | 3866 (13·6%) | 2359 (14·1%) | 2092 (16·7%) | |
| Dyspnoea | 12 883 (22·3%) | 5390 (18·9%) | 3639 (21·7%) | 3854 (30·7%) | |
| Fatigue | 12 870 (22·3%) | 6063 (21·3%) | 3522 (21·0%) | 3285 (26·2%) | |
| Fever | 15 830 (27·4%) | 7815 (27·4%) | 4291 (25·6%) | 3724 (29·7%) | |
| Headache | 11 840 (20·5%) | 6394 (22·4%) | 3294 (19·7%) | 2152 (17·1%) | |
| Loss of smell | 936 (1·6%) | 468 (1·6%) | 252 (1·5%) | 216 (1·7%) | |
| Loss of taste | 6221 (10·8%) | 3390 (11·9%) | 1733 (10·4%) | 1098 (8·7%) | |
| Myalgia | 8681 (15·0%) | 4769 (16·7%) | 2334 (13·9%) | 1578 (12·6%) | |
| Nausea | 7672 (13·3%) | 3662 (12·9%) | 2123 (12·7%) | 1887 (15·0%) | |
| Rhinorrhoea | 6979 (12·1%) | 3580 (12·6%) | 1953 (11·7%) | 1446 (11·5%) | |
| Sore throat | 7391 (12·8%) | 3942 (13·8%) | 2068 (12·4%) | 1381 (11·0%) | |
| Asymptomatic | 25 000 (43·3%) | 12 950 (45·4%) | 7522 (44·9%) | 4528 (36·1%) | |
| Number of symptoms | |||||
| Mean (SD) | 2·3 (2·9) | 2·3 (3·0) | 2·2 (2·9) | 2·4 (2·9) | |
| Median (IQR) | 1 (0–4) | 1 (0–4) | 1 (0–4) | 1 (0–4) | |
| Fully vaccinated | 21 287 (36·8%) | 7234 (25·4%) | 6443 (38·5%) | 7610 (60·6%) | |
Data are n (%) unless otherwise stated.
The mean time between the date of vaccination and the date of a positive SARS-CoV-2 test was 4·9 months (SD 1·4) in the robust group, 5·2 months (1·3) in the pre-frail group, and 5·4 months (1·2) in the frail group (p<0·0001; appendix p 3). The time between the date of vaccination and the date of a positive SARS-CoV-2 test was more than 6 months for 1638 (22·6%) of 7234 patients in the robust group, 1788 (27·8%) of 6443 patients in the pre-frail group, and 2564 (33·7%) of 7610 patients in the frail group (appendix p 3). The number of COVID-19-associated hospitalisations and all-cause deaths in vaccinated and unvaccinated groups by age group and frailty status are shown in the appendix (p 4).
Within 30 days of a positive SARS-CoV-2 test, there were 7857 COVID-19-associated hospitalisations (2749 [35·0%] in the vaccinated group and 5108 [65·0%] in the unvaccinated group) and 2577 all-cause deaths (676 [26·2%] in the vaccinated group and 1901 [73·8%] in the unvaccinated group; table 2 ). Vaccination effectiveness against 30-day COVID-19-associated hospitalisation was 51% (95% CI 48–53) and all-cause death was 74% (71–76; table 2). ORs for COVID-19-associated hospitalisation and HRs for all-cause death for all the covariates included in the models are in the appendix (p 5).
Table 2.
Vaccination effectiveness against COVID-19-associated hospitalisation and all-cause death within 30 days after a positive SARS-CoV-2 test according to frailty status
|
All patients (n=57 784) |
Robust group (n=28 497) |
Pre-frail group (n=16 737) |
Frail group (n=12 550) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Adjusted OR* or HR* (95% CI) | Vaccine effectiveness (95% CI) | Events | Adjusted OR* or HR* (95% CI) | Vaccine effectiveness (95% CI) | Events | Adjusted OR* or HR* (95% CI) | Vaccine effectiveness (95% CI) | Events | Adjusted OR* or HR* (95% CI) | Vaccine effectiveness (95% CI) | |
| COVID-19-associated hospitalisation | ||||||||||||
| Unvaccinated | 5108 (14·0%) | 1 (ref) | 1 (ref) | 2230 (10·5%) | 1 (ref) | 1 (ref) | 1488 (14·5%) | 1 (ref) | 1 (ref) | 1390 (28·1%) | 1 (ref) | 1 (ref) |
| Vaccinated | 2749 (12·9%) | OR 0·49 (0·47–0·52) | 51% (48–53) | 405 (5·6%) | OR 0·35 (0·31–0·39) | 65% (61–69) | 606 (9·4%) | OR 0·46 (0·42–0·52) | 54% (48–58) | 1738 (22·8%) | OR 0·64 (0·58–0·70) | 36% (30–42) |
| All-cause death | ||||||||||||
| Unvaccinated | 1901 (5·2%) | 1 (ref) | 1 (ref) | 616 (3·0%) | 1 (ref) | 1 (ref) | 616 (6·0%) | 1 (ref) | 1 (ref) | 669 (13·5%) | 1 (ref) | 1 (ref) |
| Vaccinated | 676 (3·2%) | HR 0·27 (0·24–0·29) | 74% (71–76) | 89 (1·2%) | HR 0·21 (0·16–0·26) | 79% (74–84) | 144 (2·2%) | HR 0·21 (0·17–0·25) | 79% (75–83) | 443 (5·8%) | HR 0·32 (0·29–0·37) | 68% (63–71) |
HR=hazard ratio. OR=odds ratio.
Adjusted for age, BMI, race, ethnicity, sex, smoking status, being an active patient in the Veterans Health Administration system in the past 12 months, rurality, and monoclonal antibody treatment.
When comparing the two mRNA vaccines directly, mRNA-1273 was more effective than BNT162b2 (reference) in protection against 30-day COVID-19-associated hospitalisation (OR 0·71, 95% CI 0·65–0·77), but not significantly different against 30-day all-cause death (HR 0·89, 95% CI 0·77–1·04).
By 30 days after a positive SARS-CoV-2 test, 2635 (9·2%) of 28 497 (405 vaccinated and 2230 unvaccinated) patients in the robust group, 2094 (12·5%) of 16 737 (606 vaccinated and 1488 unvaccinated) patients in the pre-frail group, and 3128 (24·9%) of 12 550 (1738 vaccinated and 1390 unvaccinated) patients in the frail group had a COVID-19-associated hospitalisation (table 2). 705 (2·5%; 89 vaccinated and 616 unvaccinated) patients in the robust group, 760 (4·5%; 144 vaccinated and 616 unvaccinated) in the pre-frail group, and 1112 (8·9%; 443 vaccinated and 669 unvaccinated) in the frail group died within 30 days of a positive SARS-CoV-2 test (table 2). Although vaccination provided protection against COVID-19-associated hospitalisation and all-cause death within 30 days after a positive SARS-CoV-2 test for all patients, the protection was lower in the frail group than in the robust and pre-frail groups. By 30 days after a positive SARS-CoV-2 test, vaccination effectiveness against COVID-19-associated hospitalisation was 65% (95% CI 61–69) for the robust group, 54% (48–58) for the pre-frail group, and 36% (30–42) for the frail group, and vaccine effectiveness against all-cause death was 79% (74–84) for the robust group, 79% (75–83) for the pre-frail group, and 68% (63–71) for the frail group. The survival curve shows the lowest probability of survival in the unvaccinated individuals with frailty (appendix p 10). The reduction of vaccine effectiveness for patients with frailty was similar for mRNA-1273 and BNT162b2 vaccines (appendix pp 6–7).
The frail group had lower vaccine effectiveness than the robust and pre-frail groups against COVID-19-associated hospitalisation and all-cause death at 30 days from a positive SARS-CoV-2 test at time intervals from vaccination to a positive SARS-CoV-2 test of less than 4 months, 4–6 months, and longer than 6 months (table 3 ; appendix p 11).
Table 3.
Vaccination effectiveness against COVID-19-associated hospitalisation and all-cause death within 30 days after a positive SARS-CoV-2 test according to frailty status and time from vaccination to a positive SARS-CoV-2 test
|
Robust group |
Pre-frail group |
Frail group |
|||||
|---|---|---|---|---|---|---|---|
| Adjusted OR* or HR* (95% CI) | Vaccine effectiveness (95% CI) | Adjusted OR* or HR* (95% CI) | Vaccine effectiveness (95% CI) | Adjusted OR* or HR* (95% CI) | Vaccine effectiveness (95% CI) | ||
| COVID-19-associated hospitalisation | |||||||
| <4 months from vaccination to a positive SARS-CoV-2 test | |||||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Vaccinated patients | OR 0·35 (0·27–0·44) | 65% (54–73) | OR 0·56 (0·45–0·70) | 44% (30–55) | OR 0·70 (0·59–0·83) | 30% (17–41) | |
| 4–6 months from vaccination to a positive SARS-CoV-2 test | |||||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Vaccinated patients | OR 0·35 (0·30–0·41) | 65% (59–70) | OR 0·42 (0·37–0·49) | 58% (51–63) | OR 0·58 (0·53–0·64) | 42% (36–47) | |
| >6 months from vaccination to a positive SARS-CoV-2 test | |||||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Vaccinated patients | OR 0·36 (0·29–0·44) | 64% (56–71) | OR 0·49 (0·41–0·58) | 51% (42–59) | OR 0·71 (0·63–0·80) | 29% (20–37) | |
| All-cause death | |||||||
| <4 months from vaccination to a positive SARS-CoV-2 test | |||||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Vaccinated patients | HR 0·19 (0·10–0·35) | 81% (65–90) | HR 0·25 (0·16–0·41) | 75% (59–84) | HR 0·37 (0·27–0·50) | 63% (50–73) | |
| 4–6 months from vaccination to a positive SARS-CoV-2 test | |||||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Vaccinated patients | HR 0·19 (0·14–0·26) | 81% (74–86) | HR 0·19 (0·15–0·25) | 81% (75–85) | HR 0·29 (0·25–0·34) | 71% (66–75) | |
| >6 months from vaccination to a positive SARS-CoV-2 test | |||||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| Vaccinated patients | HR 0·25 (0·17–0·35) | 75% (65–83) | HR 0·23 (0·17–0·30) | 77% (70–83) | HR 0·36 (0·31–0·43) | 64% (57–69) | |
HR=hazard ratio. OR=odds ratio.
Adjusted for age, BMI, race, ethnicity, sex, smoking status, being an active patient in the Veterans Health Administration system in the past 12 months, rurality, and monoclonal antibody treatment.
The interaction between frailty and age was significant (the coefficient estimated was −0·34, p<0·0001 for COVID-19-associated hospitalisation; and −0·73, p<0·0001 for all-cause death). The adjusted OR for COVID-19-associated hospitalisation and HR for all-cause death at 30 days for vaccinated versus unvaccinated individuals according to age group (<65 and ≥65 years) and frailty status are shown in table 4 and in the appendix (p 12). There were differences between age groups in the reduction of vaccine effectiveness due to frailty (ie, the difference in effectiveness between the robust group and the frail group). For death, the reduction in effectiveness was 20% (from 90% to 70%) in patients younger than 65 years, and 9% (from 77% to 68%) for those who were 65 years or older (table 4). Similarly, for COVID-19-associated hospitalisation, the reduction was 37% (from 73% to 36%) in patients younger than 65 years and 18% (from 54% to 36%) in older patients (table 4). These results confirmed the interaction effect between age and frailty on vaccine effectiveness: the reduction was more pronounced in younger patients.
Table 4.
Vaccine effectiveness against COVID-19-associated hospitalisation and all-cause death according to frailty status and age group (younger than 65 and 65 years or older)
|
COVID-19-associated hospitalisation |
All-cause death |
|||
|---|---|---|---|---|
| Adjusted OR* (95% CI) | Vaccine effectiveness (95% CI) | Adjusted HR* (95% CI) | Vaccine effectiveness (95% CI) | |
| Age <65 years | ||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Vaccinated patients | 0·40 (0·36–0·44) | 60% (56–64) | 0·17 (0·12–0·24) | 83% (76–88) |
| Age ≥65 years | ||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Vaccinated patients | 0·56 (0·52–0·60) | 44% (40–48) | 0·31 (0·28–0·34) | 69% (66–72) |
| Robust and age <65 years | ||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Vaccinated patients | 0·27 (0·22–0·32) | 73% (68–78) | 0·10 (0·05–0·20) | 90% (80–95) |
| Frail and age <65 years | ||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Vaccinated patients | 0·64 (0·54–0·77) | 36% (23–46) | 0·30 (0·19–0·47) | 70% (53–81) |
| Robust and age ≥65 years | ||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Vaccinated patients | 0·46 (0·39–0·54) | 54% (46–61) | 0·23 (0·18–0·30) | 77% (70–82) |
| Frail and age ≥65 years | ||||
| Unvaccinated patients | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Vaccinated patients | 0·64 (0·58–0·70) | 36% (30–42) | 0·32 (0·29–0·37) | 68% (63–71) |
HR=hazard ratio. OR=odds ratio.
Adjusted for age, BMI, race, ethnicity, sex, smoking status, being an active patient in the Veterans Health Administration system in the past 12 months, rurality, and monoclonal antibody treatment.
Male patients had lower vaccination protection than female patients overall, and vaccination protection declined with age when we further stratified into four age groups (<65 years, 65−74 years, 75−84 years, ≥85 years; appendix p 8).
Discussion
In this study, patients with frailty had the highest rates of vaccination and were more likely to receive a vaccine earlier in the delta variant wave compared with the robust and pre-frail groups. We showed that vaccines were effective against COVID-19-associated hospitalisation and all-cause death in veterans within 30 days of a positive SARS-CoV-2 test. However, and consistent with our hypothesis, vaccine effectiveness varied according to age and frailty status. After adjusting for covariates, vaccine effectiveness against COVID-19-associated hospitalisation and all-cause death at 30 days from testing positive for SARS-CoV-2 was lower in patients who were 65 years or older than in those who were younger than 65 years. Moreover, patients with frailty had lower levels of protection against COVID-19-associated hospitalisation and all-cause death at 30 days after testing positive for SARS-CoV-2 than patients in the robust group. Notably, the reduction in effectiveness in frail, compared with robust, patients was greater in patients younger than 65 years than in those who were 65 years or older. Our results add to the evidence that although frailty is often associated with old age, it is a distinct condition that also occurs in younger individuals. These results suggest that frailty should be an important consideration in the design, development, and testing of efficacy and safety of vaccines.
Consistent with other studies, COVID-19 vaccine effectiveness against hospitalisation and death after SARS-CoV-2 infection was lower in older adults.15 Although overall vaccine effectiveness was high in this population of veterans, results showed a lower response in the frail group, consistent with our hypothesis. To our knowledge, this study represents the first report of an association of frailty with a decline in COVID-19 vaccine effectiveness against hospitalisation and all-cause death regardless of age in patients who have previously tested positive for SARS-CoV-2. The decline in effectiveness does not appear to be related to earlier time of vaccination in older patients with frailty—who are more likely to be vaccinated overall—because the reduction was persistent when the cohort was divided into defined vaccination periods before infection. The estimated CIs were wide, so our study was not powered to evaluate vaccine waning according to frailty status.
Our findings of lower vaccine effectiveness in patients with frailty, combined with the observation that people with frailty are at high risk of poor outcomes, highlight the importance of considering frailty in vaccine design, evaluation, and programme implementation. Potential approaches to optimally tailor vaccine product design to better stimulate immune responses in older and frailer people could include increasing the dose of antigen, optimising use of adjuvants, or using recombinant antigens. All of these approaches have been used in influenza vaccines.26 For example, when the immunogenicity (antibody titres) of a high-dose influenza vaccine was measured as part of a clinical trial, the effects of frailty were not apparent, indicating dose as one avenue to mitigate the impact of frailty on vaccine responses.27 Indeed, consistent with a previous study in a veteran population,18 the mRNA-1273 vaccine, which has a higher active ingredient, was more effective than the BNT162b2 vaccine in protection against COVID-19-associated hospitalisation. The findings of reduced vaccine protection in patients with frailty underscore the need to optimise vaccine effectiveness for these patients and provide support for considering frailty in COVID-19 vaccine development. Future studies that incorporate measurements of humoral and cellular responses to vaccination in individuals according to frailty status might provide additional insights into the mechanisms of frailty and its effects on vaccination. Prospective cohort studies and randomised controlled trials of COVID-19 vaccines that include baseline assessments of frailty status as risk stratification might clarify the role of frailty in protection against death in older adults.
The data on sex differences in immune responses to viral vaccines are mixed.28 Our study showed that COVID-19 vaccine effectiveness was higher in females than males. However, these results should be interpreted with caution due to the lower numbers of female participants included in the analyses. Future studies that include a large number of female participants should explore whether the reduction of vaccine effectiveness associated with frailty differs according to sex.
Strengths of our study are the use of a large-scale, national dataset from a large, integrated health-care system with individual-level demographic and clinical data; inclusion of patients who tested positive for SARS-CoV-2 during the specified period; accurate documentation of vaccine type and date, thereby avoiding the potential confounding from US epidemic phases; and determination of frailty status using a validated model predictive of death in US veterans. Even so, the retrospective cohort study design introduces important limitations. Although the deaths within 30 days after a positive SARS-CoV-2 test are probably mostly related to COVID-19, a small number of deaths not related to COVID-19 might have been included because we did not have a detailed record of the cause of death. Veterans are sometimes admitted to hospitals outside the VHA,29 which might have led to an underestimation of COVID-19-associated hospitalisations. Despite this limitation, the estimated ORs for vaccinated versus unvaccinated patients should not be affected assuming that vaccination status has no effect on whether a patient was admitted to a hospital outside the VHA. We did not consider other factors associated with poor outcomes, such as social determinants of health, symptom duration, and the way that illness might appear in older adults with frailty. Older adults with frailty and COVID-19 might present atypically, thereby delaying diagnosis, which could potentially lead to more deaths despite vaccination.30 Social isolation due to strict physical distancing might have further amplified the problem by reducing contact with relatives and health-care providers who could identify changes in condition. Failing to recognise these symptoms might delay or even preclude testing, potentially underestimating vaccine effectiveness due to an incorrect estimation of the timing of death in relation to the vaccination date or failing to identify the patient's death as occurring in the setting of COVID-19. Our study did not consider the effects of concomitant non-invasive or invasive interventions that, in addition to vaccination, might have improved individuals' survival. Resource allocation issues and more proactive advance care planning might result in the withholding of potentially beneficial treatments for older adults with frailty.31 The predominantly male population in this study might reduce the generalisability of our findings to women, who are often more frail than men. By contrast, the findings of beneficial effects of vaccination on death are remarkable considering that men are more likely to die from COVID-19.
The clinical implications of our study are that, in addition to age and other factors, we need to consider frailty when evaluating COVID-19 vaccines. If confirmed in future studies, the importance of frailty could have far-reaching implications for COVID-19 vaccine development and evaluation. COVID-19 vaccination represents a key therapeutic strategy for patients with frailty because COVID-19 is associated with higher morbidity and death in this population. As with other vaccines, a wide range of interventions could improve COVID-19 vaccine effectiveness in older and frail adults, including higher dosing, use of adjuvants or recombinant antigens, and earlier and more frequent administration of boosters.32 The VHA began administrating booster shots in late September, 2021. Because our cohort included patients who tested positive between July 25 and Sept 30, 2021, the number of patients who got the booster shot before testing positive for SARS-CoV-2 was exceedingly small. Future studies should also investigate vaccine reactogenicity and safety in older adults with frailty. Few studies suggest that older patients with frailty might develop more adverse events associated with vaccination,33 although it is unclear whether these reactions are due to vaccination or to frailty. However, it must be emphasised that if individuals with frailty are excluded from vaccine trials, and if frailty is not explicitly considered in study cohorts, opportunities to identify potential vaccine innovations and to tailor products to generate optimal responses in the context of frailty and older age will be missed. Although the frailty index used in this study was developed in the context of the VHA system, in general when frailty indices conform to prescribed criteria24 their properties are consistent across settings.34 Investigators can operationalise frailty using VA-FI variables—ICD 10 and Current Procedural Terminology codes—which are readily available from electronic health records and administrative databases at most health-care institutions worldwide.
Studies comparing the classification and degree of frailty using different scales have shown that although the results are often consistent, there might be variability depending on the emphasis of the scale,35 so investigation of other commonly used frailty measures in relation to COVID-19 vaccine effectiveness is warranted.
In summary, our study shows that although COVID-19 vaccines are effective against hospitalisation and all-cause death after SARS-CoV-2 infection, the effectiveness is lower in patients with frailty. Future observational and experimental studies investigating COVID-19 vaccine effectiveness should incorporate frailty assessments and actively recruit older adults with frailty.
Data sharing
Data were obtained from the COVID-19 Shared Data Resource maintained by the Department of Veterans Affairs Informatics and Computing Infrastructure (VINCI). The data are available to approved individuals upon request to VINCI after fulfilling specified requirements.
This online publication has been corrected. The corrected version first appeared at thelancet.com/healthy-longevity on September 6, 2022
Declaration of interests
JGR holds a grant from Longeveron and received consulting fees from Pfizer. MKA reports grants from the Canadian Frailty Network, Canadian Institutes of Health Research, Public Health Agency of Canada, Sanofi, Pfizer, Merck, and GlaxoSmithKline, and payments from Pfizer, Sanofi, and Seqirus outside the submitted work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This study was supported by the Miami VA Healthcare System Geriatric Research Education and Clinical Center and was supported using resources and facilities of VINCI, VA HSR RES 13-457. The views expressed are those of the authors and not those of the Geriatric Research Education and Clinical Center or VINCI. This study was not funded by the US National Institutes of Health (NIH), and none of the authors is employed by the NIH or in receipt of an NIH grant.
Contributors
FT, ISH, and JGR contributed to the design of the study. FT extracted the data and did the statistical analysis. FT, ISH, and JGR had full access to and verified the data in the study. JGR and FT wrote the first draft. MKA and ISH critically reviewed and edited the manuscript and did the literature search. All authors had full access to the data and accept responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Rio C, Malani PN, Omer SB. Confronting the delta variant of SARS-CoV-2, summer 2021. JAMA. 2021;326:1001–1002. doi: 10.1001/jama.2021.14811. [DOI] [PubMed] [Google Scholar]
- 3.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373 doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 7.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 8.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mech Ageing Dev. 2012;133:456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Fulop T, Larbi A, Dupuis G, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2018;8 doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrew MK, McElhaney JE. Age and frailty in COVID-19 vaccine development. Lancet. 2021;396:1942–1944. doi: 10.1016/S0140-6736(20)32481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrew MK, Shinde V, Ye L, et al. The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis. 2017;216:405–414. doi: 10.1093/infdis/jix282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrew MK, Schmader KE, Rockwood K, Clarke B, McElhaney JE. Considering frailty in SARS-CoV-2 vaccine development: how geriatricians can assist. Clin Interv Aging. 2021;16:731–738. doi: 10.2147/CIA.S295522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 15.Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AWJS. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375:331–336. doi: 10.1126/science.abm0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among US veterans and its association with mortality, 2002–2012. J Gerontol A Biol Sci Med Sci. 2019;74:1257–1264. doi: 10.1093/gerona/gly232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in US veterans. N Engl J Med. 2022;386:105–115. doi: 10.1056/NEJMoa2115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention COVID Data Tracker. Variant proportions. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 20.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 22.Walston J, Bandeen-Roche K, Buta B, et al. Moving frailty toward clinical practice: NIA Intramural Frailty Science Symposium summary. J Am Geriatr Soc. 2019;67:1559–1564. doi: 10.1111/jgs.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng D, DuMontier C, Yildirim C, et al. Updating and validating the US Veterans Affairs Frailty Index: transitioning from ICD-9 to ICD-10. J Gerontol A Biol Sci Med Sci. 2021;76:1318–1325. doi: 10.1093/gerona/glab071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrew MK, Bowles SK, Pawelec G, et al. Influenza vaccination in older adults: recent innovations and practical applications. Drugs Aging. 2019;36:29–37. doi: 10.1007/s40266-018-0597-4. [DOI] [PubMed] [Google Scholar]
- 27.DiazGranados CA, Dunning AJ, Robertson CA, Talbot HK, Landolfi V, Greenberg DP. Efficacy and immunogenicity of high-dose influenza vaccine in older adults by age, comorbidities, and frailty. Vaccine. 2015;33:4565–4571. doi: 10.1016/j.vaccine.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hynes DM, Koelling K, Stroupe K, et al. Veterans' access to and use of Medicare and Veterans Affairs health care. Med Care. 2007;45:214–223. doi: 10.1097/01.mlr.0000244657.90074.b7. [DOI] [PubMed] [Google Scholar]
- 30.Gan JM, Kho J, Akhunbay-Fudge M, et al. Atypical presentation of COVID-19 in hospitalised older adults. Ir J Med Sci. 2021;190:469–474. doi: 10.1007/s11845-020-02372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maves RC, Downar J, Dichter JR, et al. Triage of scarce critical care resources in COVID-19. An implementation guide for regional allocation: an Expert Panel Report of the Task Force for Mass Critical Care and the American College of Chest Physicians. Chest. 2020;158:212–225. doi: 10.1016/j.chest.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izda V, Jeffries MA, Sawalha AH. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin Immunol. 2021;222 doi: 10.1016/j.clim.2020.108634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torjesen I. Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ. 2021;372:n149. doi: 10.1136/bmj.n149. [DOI] [PubMed] [Google Scholar]
- 34.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were obtained from the COVID-19 Shared Data Resource maintained by the Department of Veterans Affairs Informatics and Computing Infrastructure (VINCI). The data are available to approved individuals upon request to VINCI after fulfilling specified requirements.
This online publication has been corrected. The corrected version first appeared at thelancet.com/healthy-longevity on September 6, 2022