Abstract
Two acid-inducible genes, aniC and aciK, that require anaerobiosis and tyrosine for expression were identified as orf326a encoding a potential amino acid/polyamine antiporter and hyaB encoding hydrogenase I, respectively. Cyclic AMP (cAMP) receptor protein, cAMP, and TyrR, regulator of aromatic amino acid metabolism, were strong positive regulators of both genes.
Salmonella typhimurium undergoes extensive molecular and physiological changes following both subtle and dramatic alterations in environmental pH (5, 12). Changes that occur in response to acid pH shifts include an adaptation to acid stress called the acid tolerance response (ATR), which helps protect the organism from potentially lethal acid environments (13). The ATR involves the induction of a series of polypeptides called acid shock proteins (ASPs), some of which are presumed to protect the cell from acid stress (4, 19, 22). While the ASPs discussed above were found by using two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis, other acid-inducible genes have been identified by using gene fusion technology. Many of these acid pH-inducible genes require medium components for induction in addition to low pH (14). Although these genes have no known role in acid tolerance, they are presumed to contribute in some way to survival in low-pH environments. Our estimates indicate that over 100 genes are transcriptionally sensitive to pH. In spite of the large number of genes that respond to environmental pH, the transcriptional mechanisms by which acidic pH alters gene expression are poorly understood.
The best-characterized acid-inducible gene systems are the lysine and arginine decarboxylases of Escherichia coli. The cadBA operon, encoding lysine decarboxylase (cadA) and a lysine/cadaverine antiporter (cadB), is controlled by acid pH through a membrane sensor protein called CadC (25). The arginine decarboxylase system (adi) is more complex than the cad operon in that adi requires anaerobiosis, acid, and a combination of amino acids for maximal induction (31). A positive regulator has been identified in E. coli (adiY), but an antiporter that would exchange arginine for its decarboxylation product, agmatine, has not (32).
Previously, lacZ gene fusion techniques were used to identify acid-inducible genes in S. typhimurium, or regulators of those genes, that may be involved in acid tolerance (11, 14). Most of the genes found were of unknown function. One regulatory locus, atrE (also called oxrG), had a clear effect on inducible acid tolerance. This regulator controlled the expression of three acid-inducible loci called aniC, aniI, and aciK that also required an anaerobic environment and tyrosine for induction. The ani designation reflected the original identification of these genes as being anaerobiosis induced, while the aci designation was based on an initial screen for acid induction. None of these genes were characterized further at the time. Since atrE participated in the acid tolerance response, the known targets of this regulator were identified and their regulation by tyrosine and pH was more clearly defined. The strains used throughout this study are listed in Table 1.
TABLE 1.
Bacterial strains
| Strain | Genotype | Source or reference |
|---|---|---|
| S. typhimurium | ||
| SF430 (TT15239) | putA1019::MudQ | K. Sanderson |
| SF431 (TT15240) | putA1019::MudP | K. Sanderson |
| SF434 (TT15245) | pyrF2690::MudQ | K. Sanderson |
| SF435 (TT15246) | pyrF2690::MudP | K. Sanderson |
| SF436 (TT15625) | tre-152::MudQ | K. Sanderson |
| SF437 (TT15245) | tre-152::MudP | K. Sanderson |
| SF463 (TT10423) | LT2/pNK972 Tn10 transposase | K. Sanderson |
| SF530 (UK1) | Wild type | R. Curtiss III (9) |
| JF2690 | UK1 rpoSΩpRR10(Ap) | 22 |
| JF3306 | UK1 aniC1052::MudJ | 14 |
| JF3307 | UK1 aciK(hyaB)2013::MudJ | 14 |
| JF3308 | UK1 atrE406::Tn10 | 14 |
| JF3330 | UK1 aniC1052::MudJ atrE406::Tn10 | This paper |
| JF3331 | UK1 aciK(hyaB)2013::MudJ atrE406::Tn10 | This paper |
| JF3335 | UK1 rpoS::Ap atrE406::Tn10 | This paper |
| JF3338 | UK1 aniC1052::MudJ rpoSΩpRR10(Ap) | This paper |
| JF3339 | UK1 rpoSΩpRR10(Ap) aciK(hyaB)2013::MudJ | This paper |
| JF3343 | LT2 aniC1::Tn10 | This paper |
| JF3351 | Δ(aniC-adiA) | This paper |
| JF3496 | UK1 aniC1052::MudJ tyrR411::Tn10dTc (55% to sapD::MudJ) | This paper |
| JF3586 | UK1 aniC1052::MudJ crp-773::Tn10 | This paper |
| JF3587 | UK1 hyaB(aniK)1052::MudJ crp-773::Tn10 | This paper |
| JF3602 | UK1 aniC1052::MudJ atrE406::Tn10/pJC100 tyrR+ (Apr) | This paper |
| JF3603 | UK1 hyaB(aciK)1052::MudJ atrE::Tn10/pJC100 tyrR+ (Apr) | This paper |
| JF3618 | UK1 aniC1052::MudJ tyrR411::Tn10dTc/pJC100 tyrR+ (Apr) | This paper |
| JF3633 | UK1 tyrR411::Tn10dTc | This paper |
| JF3634 | UK1 aniC1052::MudJ fnr-2::Tn10 | This paper |
| JF3635 | UK1 hyaB(aniK)1052::MudJ fnr-2::Tn10 | This paper |
| JF4053 | UK1 hyaB(aniK)1052::MudJ tyrR411::Tn10dTc | This paper |
| E. coli | ||
| EF346 | pKPF242 (aniC-MuL junction) | This paper |
| EF348 | pKPF243 [aciK (hyaB)-MuL junction] | This paper |
| EF422 | pKPF276 (tyrR-Tn10 junction) | This paper |
| EK112 (XL1-Blue) | recA1 lac mutant endA1 gyrA96 thi hsdR17 supE44 relA1/F′ proAB+lacIqlacZΔ(M15) | Stratagene, Inc. |
Identification of aniC and aciK.
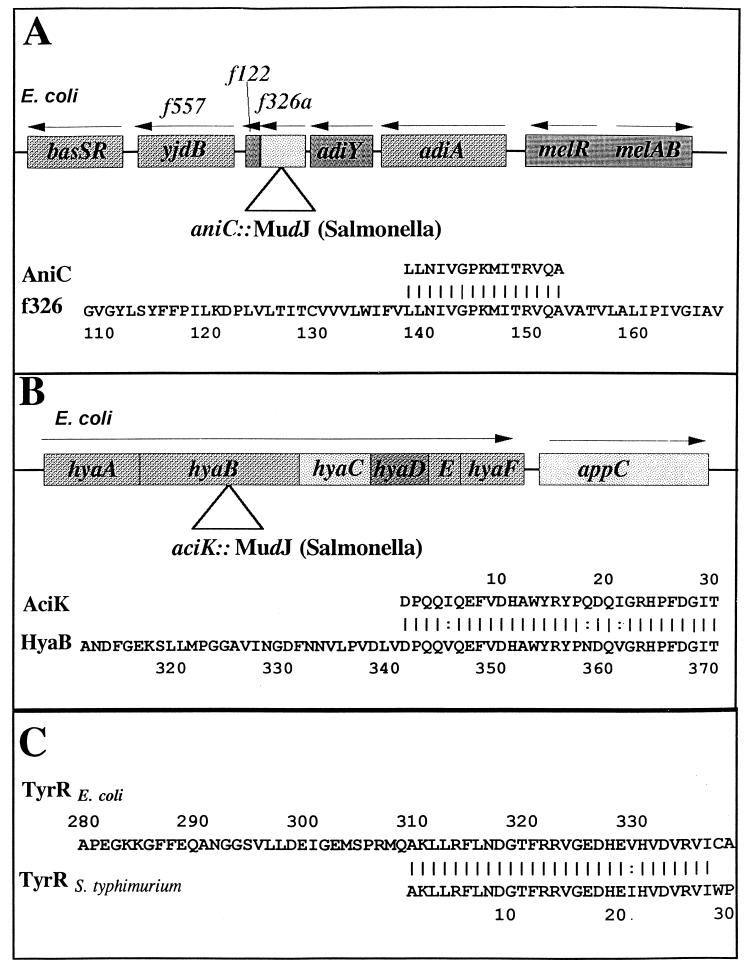
Transcriptional fusions of aniC and aciK with the reporter gene lacZ were originally created via MudJ insertions. The left ends of MudJ junctions were cloned from chromosomal digests by first identifying the sizes of SalI restriction fragments containing the kanamycin resistance gene via Southern blot hybridization with a kanamycin gene probe. Fragments of the appropriate size were excised and extracted from an agarose gel and ligated to SalI-digested pBluescript SK+ vector (Stratagene, La Jolla, Calif.). The ligated mixtures were transformed via CaCl2 into XL1-Blue (EK112), with selection for resistance to ampicillin. Sequencing of the junction sites was performed by using an oligonucleotide specific to the left end of Mu (Oligo 47; 5′CCAATGTCCTCCCGGTTTTT). The results of homology searches using the predicted translation product of aniC indicated that the gene from S. typhimurium is homologous to orf326a in the adi region of E. coli (100% identity over 15 amino acids), with the insertion having occurred after codon 137, based on the E. coli sequence (Fig. 1A). This identity was also consistent with respect to map position as both the E. coli and S. typhimurium genes map to similar locations (93 centisomes).
FIG. 1.
Locations of MudJ insertions and homologies. Locations of MudJ insertions in the S. typhimurium homologs of E. coli aniC (orf326a) (A), hyaB (B), and tyrR (C). The upper diagrams in panels A and B illustrate gene organization in E. coli and the approximate site of the MudJ insertion in S. typhimurium as determined by the sequence analysis shown at the bottom of each panel.
Combining E. coli orf326a with the downstream open reading frame, orf122 (Fig. 1A), would produce a protein with striking homology to a specific class of amino acid antiporters such as CadB (lysine/cadaverine antiporter) and PotE (ornithine/putrescine antiporter) that exchange extracellular amino acids for their intracellular decarboxylation products. It should be noted that it is not clear whether the “split gene” found in the E. coli sequence is real or the result of a sequencing error. Inducible amino acid decarboxylase systems typically include an amino acid decarboxylase enzyme and an amino acid/polyamine antiporter. In acidic environments, protons leaking into the cell across the cell membrane will be consumed by the amino acid decarboxylation reaction (e.g., lysine decarboxylase to form cadaverine). However, to efficiently consume protons there must be a means to rapidly transport additional substrate (e.g., lysine) into the cell. The inducible lysine and ornithine decarboxylase systems include antiporter systems that exchange intracellular product (e.g., cadaverine) for extracellular substrate (e.g., lysine). The result of this exchange is the gradual alkalinization of the medium as more protons leak into the cell and are consumed. Mutants lacking either the decarboxylase or the antiporter will not alkalinize the media. As is the case in E. coli, the aniC (orf326a)::MudJ insertion in Salmonella maps near adiA. This is evident from mutant JF3351, which contains a deletion extending from an aniC::Tn10 insertion (JF3343) that also results in complete loss of arginine decarboxylase activity (data not shown). The natural supposition based upon the proximity of aniC to adiA in E. coli was that AniC may be the antiporter for the arginine decarboxylation product (agmatine). However, the aniC::MudJ mutant of S. typhimurium exhibited normal arginine decarboxylase activity as assayed by Moeller decarboxylase media (data not shown). It remains possible that AniC is an arginine/agmatine antiporter but that multiple antiporters exist such that eliminating one would not eliminate the ability to alkalinize media.
Homology analysis of the aciK-Mu junction indicated that aciK is homologous to hyaB, the second gene in the six-gene hya operon encoding hydrogenase I in E. coli (87% identity over 30 amino acids [23]). The insertion occurred after codon 340 based on the E. coli sequence (Fig. 1B). Based upon the map position of hya in E. coli (22 centisomes), one would predict that this gene should reside near 25 centisomes in S. typhimurium, yet previous reports indicated that it mapped between 33 and 36 min (37 to 40 centisomes [14]). To resolve this apparent discrepancy, the location of aciK (hyaB) was confirmed by Southern hybridization to be between 37 and 40 centisomes on the S. typhimurium linkage map. A biotin-labeled 320-bp aciK fragment prepared from pKPF243 hybridized to DNA from MuP22 lysates prepared from SF434 and SF436 (carrying DNA between 37 to 40 centisomes) but failed to hybridize to MuP22 lysates from SF430 or SF431 (carrying DNA from 22 to 28 centisomes) (data not shown). These results indicated that hya is located at a position in S. typhimurium different from that in E. coli and probably lies within the major chromosomal inversion that distinguishes these two organisms (27). The role of hydrogenase 1 (the product of the hya operon) in cellular physiology is not known, other than its suspected use for dihydrogen oxidation (28).
Regulation of aciK (hyaB) and aniC.
Previous results indicated that aciK (hyaB) and aniC are best expressed under anaerobic, acidic conditions in complex media (1, 2). The reason these genes were poorly expressed in minimal media proved to be that they had a requirement for tyrosine as a coinducer (14). Since one of these genes is the S. typhimurium homolog of hyaB, we decided to examine the expression of both genes under conditions shown previously to affect hya-lac expression in E. coli. In these studies, β-galactosidase was measured according to the method of Miller (24) with cells grown to mid-log phase prior to assay. In E. coli, hya is repressed by nitrate and induced by formate under anaerobic conditions (7). The results of studies with S. typhimurium are shown in Table 2. The data reaffirmed that maximum induction of aniC and hyaB requires anaerobiosis, acid pH, and tyrosine. As reported previously, nitrate stimulated expression of these genes at neutral to alkaline pH (2), contrary to what has been reported for E. coli hya (7). However, under optimally inducing acidic conditions (i.e., pH 5.8, anaerobic with tyrosine), nitrate clearly reduced expression of both genes. Nitrate repression was more dramatic for hyaB (24-fold) than for aniC (2-fold), indicating a major difference in the regulation of these genes. Repression of hyaB by nitrate was consistent with what has been reported for hyaB in E. coli, although tyrosine was not used in that study and the pH conditions were not clearly defined. Formate was then tested for its ability to induce aniC and hyaB in S. typhimurium. As noted above, growth with formate increased expression of hya in E. coli. However, when tested with S. typhimurium, formate did not increase expression of either aniC or hyaB (aciK), contrary to results with E. coli hya (3). Formate had no effect on S. typhimurium hyaB expression, even under anaerobic conditions with tyrosine (Table 2). The reason for this difference is unclear; however, we have moved hyaB::MudJ into several clinical strains of S. enterica and found similar results. Consequently, the phenotype is consistent among the salmonella strains tested.
TABLE 2.
Regulation of aniC and aciK (hyaB) by pH, anaerobiosis, tyrosine, nitrate, and formate
| Strain | Genotype | β-Galactosidase activity (Miller units)a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Aerobic, pH 5.8, tyrosineb | Anaerobic
|
||||||||
| pH 7.7, tyrosine
|
pH 5.8
|
||||||||
| No tyrosine
|
Tyrosine
|
||||||||
| No KNO3 | KNO3c | No formate | Formated | No addition | Formate | KNO3 | |||
| JF3306 | aniC::MudJ | 0 | 6 | 21 | 0 | 0 | 333 | 350 | 172 |
| JF3307 | hyaB::MudJ | 0 | 0 | 17 | 0 | 0 | 318 | 331 | 13 |
Cells were grown in Vogel and Bonner E minimal medium supplemented with 0.4% glucose (EG media) (35). Anaerobiosis was achieved by paraffin oil overlay.
Tyrosine was added at 100 μM.
Potassium nitrate (KNO3) was added at 0.5%.
Sodium formate was added at 0.1%.
Previous results with E. coli hya-lac fusions also indicated that induction in log phase requires the alternate sigma factor ςS (3). The effects of an rpoSΩpRR10(Ap) mutation on aniC and hya expression in S. typhimurium are shown in Table 3. In addition, the effect of the previously identified (14) positive regulator of these genes, atrE, is also shown. The results clearly indicated that both aniC and hyaB are under positive control by atrE. In addition, both genes were in some manner negatively, not positively, regulated by RpoS. RpoS control was modest, 2-fold for aniC and 1.5-fold for hyaB (aciK), suggesting that the effect could be indirect. Nevertheless, these results were once again the opposite of those reported for the E. coli hya operon, where RpoS was required for maximal activity.
TABLE 3.
Regulation of aniC-lacZ and aciK (hyaB)-lacZ by rpoS, crp, atrE, and tyrR
| Strain | Genotype | β-Galactosidase activity (Miller units)
|
|||
|---|---|---|---|---|---|
| pH 7.7a
|
pH 5.8
|
||||
| − Tyrosine | + Tyrosine | − Tyrosine | + Tyrosine | ||
| JF3306 | aniC::MudJ | 0 | 3 | 2 | 358 |
| JF3330 | aniC::MudJ atrE::Tn10 | 1 | 2 | 3 | 13 |
| JF3496 | aniC::MudJ tyrR::Tn10dTc | 2 | 2 | 1 | 0 |
| JF3338 | aniC::MudJ rpoSΩpRR10(Ap) | 0 | 5 | 1 | 738 |
| JF3307 | hyaB::MudJ | 0 | 0 | 0 | 318 |
| JF4053 | hyaB::MudJ tyrR::Tn10dTc | 0 | 0 | 0 | 0 |
| JF3331 | hyaB::MudJ atrE::Tn10 | 0 | 0 | 0 | 3 |
| JF3339 | hyaB::MudJ rpoSΩpRR10(Ap) | 0 | 0 | 0 | 476 |
| JF3586 | aniC::MudJ crp::Tn10 | 0 | 0 | 0 | 0 |
| JF3587 | hyaB::MudJ crp::Tn10 | 0 | 0 | 0 | 0 |
| JF3591 | aniC::MudJ cya::Tn10 | 0 | 0 | 0 | 6 |
| JF3582 | hyaB (aciK)::MudJ cya::Tn10 | 0 | 0 | 0 | 0 |
| JF3602 | aniC::MudJ atrE::Tn10/pJC100 (tyrR) | 0 | 0 | 0 | 13 |
| JF3603 | hyaB (aciK)::MudJ atrE::Tn10 | 0 | 0 | 0 | 0 |
| JF3618 | aniC::MudJ tyrR::Tn10dTc/pJC100 | 0 | 6 | 95 | 697 |
| JF3634 | aniC::MudJ fnr::Tn10 | 0 | 0 | 0 | 422 |
| JF3635 | hyaB (aciK)::MudJ fnr::Tn10 | 0 | 0 | 0 | 248 |
Cells were grown anaerobically in EG medium at pH 7.7 or pH 5.8 with or without tyrosine (100 μM).
We have previously observed that cyclic AMP (cAMP) receptor protein (CRP) and cAMP affected the expression of other acid pH-regulated genes (11). Consequently, the effects of crp and cya mutations on the expression of these genes were tested. Both aniC and hyaB exhibited an absolute requirement for the presence of CRP and cAMP for expression (Table 3). This also contrasts with the situation for the E. coli hya operon, which did not require CRP for induction (7). Again, several strains of salmonellae were tested for this phenomenon, with similar results. Consistent with the situation for E. coli hya (7), anaerobic control of S. typhimurium hyaB did not require Fnr (Table 3) or Arc (data not shown) (18, 33, 34). The reason for the different hyaB regulatory features noted when comparing E. coli and S. typhimurium is not apparent. Since hyaB is located at different map positions in the two organisms, it may be that the evolutionary process of moving the gene also altered its regulation.
The regulator of aromatic amino acid metabolism, TyrR, controls the tyrosine requirement for aniC and hyaB expression.
A newly identified gene involved in the regulation of this system was identified following random Tn10dTc transposition into an aniC::MudJ strain. Tn10dTc insertions were generated by first introducing pNK972, containing the Tn10 transposase gene, into the strain targeted for Tn10dTc transposition. Tn10dTc does not contain the transposase gene. The strain containing pNK972 was then transduced (20) with P22 HT phage propagated on SF463 (TT10423). SF463 contains a Tn10dTc insertion on an F factor. Because there is no homology between the F factor and the recipient chromosome, tetracycline-resistant transductants arise only through transposition. Transposon mutagenesis was performed on MacConkey lactose medium and screened under aerobic and anaerobic conditions (GasPak Systems; Becton Dickinson). Tcr transductants were screened anaerobically on MacConkey lactose medium. Of approximately 10,000 insertion mutants screened, one produced a white colony under anaerobic conditions (JF3496). The insertion eliminated expression of both aniC (JF3496) and hyaB (JF4053; Table 3). Mapping of the Tn10 insertion was accomplished by using the Mud-P22 prophage system (6), which placed the insertion near sapD located at 33 centisomes (16, 17).
Identification of this regulatory gene as tyrR was made through sequencing the Tn10dTc insertion site by a minicircle technique. The Tn10dTc insertion with flanking DNA was identified from a SalI digest from JF3496 via Southern hybridization with a biotin-labeled tet gene. SalI does not cleave within the Tn10dTc transposon. The DNA was excised and extracted from an agarose gel, diluted 1:20, and ligated. An oligonucleotide homologous to the inverted repeats at the ends of Tn10 was used to PCR amplify DNA flanking Tn10 (Oligo 51; 5′GACAAGATGTGGATCCACCTTAAC). A 700-bp fragment was cloned into the pCRII TA cloning vector (Invitrogen, San Diego, Calif.) and used as a probe against SalI-digested chromosomal DNA from JF3496 and SF530 (tyrR+). The hybridization pattern indicated that the 700-bp fragment overlapped the insertion. The DNA sequence was then determined from the T7 primer site on pCRII. The results shown in Fig. 1C indicated that this gene is the S. typhimurium homolog of tyrR (90% identity over 30 amino acids), a regulator of aromatic amino acid biosynthesis and transport (15, 26).
The effects of the tyrR::Tn10dTc insertion on gene expression are shown in Table 3. Confirmation that the insertion is in tyrR was obtained by showing that the function of the S. typhimurium tyrR::Tn10dTc insertion could be complemented with a plasmid carrying E. coli tyrR+ (Table 3, JF3618). The plasmid pJC100 was kindly provided by R. Somerville and does not express any E. coli genes other than tyrR (30). No previous report has suggested a role for TyrR in the regulation of any acid pH- or anaerobically controlled gene. Based upon these results, it is reasonable to predict that TyrR bound to tyrosine acts as a positive regulator of gene expression in this complex regulatory system.
An important feature of regulatory regions controlled by TyrR is the presence of one or more 18-bp TyrR boxes (TGTAAAN6TTTACA [26]). A scan of the E. coli sequences revealed potential TyrR boxes upstream of hyaA, the first gene in the hya operon, and upstream of adiY, the gene immediately upstream of orf326a (the aniC homolog). These observations are consistent with a role for TyrR in the regulation of these genes, although no evidence that TyrR or tyrosine controls these genes in E. coli has been presented. Because aniC (encoding a potential amino acid antiporter) is located close to adiA (encoding arginine decarboxylase), we questioned whether tyrR might control adiA expression. However, tests in Moeller decarboxylase medium indicated that tyrR::Tn10dTc did not affect arginine decarboxylase activity (data not shown).
Conclusions.
We have identified two acid- and anaerobiosis-regulated genes in S. typhimurium as encoding hydrogenase I (hyaB, formerly aciK) and a potential amino acid antiporter (aniC). Regulatory factors required for their expression were also revealed. In addition to a previously identified gene designated atrE, we have now identified TyrR and CRP as essential regulators. TyrR, normally considered a regulator of aromatic amino acid biosynthesis and transport, plays an important role in regulating the expression of both genes and explains the requirement for tyrosine in their induction. TyrR, in E. coli, is a 53-kDa protein that represses the expression of aroFGHLM, tyrB, tyrR, and aroP (8). It activates mtr (a tryptophan-specific transport system) and can repress (in the presence of phenylalanine) or activate (in the presence of tyrosine) the tyrosine transport gene tyrP (26). Much is known about the function of TyrR in E. coli, but potential involvement in controlling low-pH- and anaerobiosis-inducible genes was not suspected. The function of the TyrR regulon under acidic and anaerobic conditions is a mystery. Experiments designed to reveal an anaerobic- or acid-medium phenotype related to tyrosine have so far failed. Tyrosine does not appear to provide an anaerobic growth advantage to wild-type versus tyrR mutant strains of S. typhimurium (data not shown).
A third essential positive regulator of these genes was determined to be the CRP which has previously been implicated in the pH control of another low-pH-regulated gene, aniG (now identified as exu [24a]). How pH controls the expression of these genes is unknown, but there are several possibilities. First, pH may affect DNA topology in the region of the target genes through alteration in DNA supercoiling (21). This has been suggested for several environmentally regulated genes (10). The alteration in DNA topology would influence the ability of TyrR and CRP to bind to their respective target DNA sequences. A second possibility is that there is another as yet unidentified pH sensor (possibly AtrE) that transmits a signal to the target genes. Finally, either TyrR or CRP might sense alterations in internal pH. Although one would not expect large differences in internal pH at external pH values between 6 and 8, the differences may be more significant under anaerobic conditions and in the presence of organic acid fermentation end products.
Many of the acid pH-induced genes identified thus far require coinducers, such as tyrosine, for expression (11, 14, 19, 25, 29). The mechanisms used to integrate acid pH and coregulator signals are not fully elucidated but clearly vary depending upon the system. It is apparent that S. typhimurium possesses specific genetic systems that sense and respond to encounters with acidic pH. Which pH response systems become engaged depends in large measure on the chemical composition of the environment. While the adaptive advantage of some of these systems (cadBA) is apparent (e.g., acid tolerance), the benefits of others (aniC and hyaB) remain enigmatic.
Acknowledgments
We thank R. Somerville, K. Sanderson, and R. Curtiss III for their generous gifts of various strains and plasmids. Various discussions with M. Moreno and M. Spector were especially helpful and are gratefully acknowledged.
This work was supported by an award (GM48017) from the National Institutes of Health.
REFERENCES
- 1.Aliabadi Z, Park Y K, Slonczewski S L, Foster J W. Novel regulatory loci controlling oxygen and pH-regulated gene expression in Salmonella typhimurium. J Bacteriol. 1988;170:842–851. doi: 10.1128/jb.170.2.842-851.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliabadi Z, Warren F, Mya S, Foster J W. Oxygen-regulated stimulons of Salmonella typhimurium identified by Mud(Aplac) operon fusions. J Bacteriol. 1986;165:780–786. doi: 10.1128/jb.165.3.780-786.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atlung T, Kundsen K, Heerfordt L, Brondsted L. Effects of ςs and the transcriptional activator AppY on induction of the Escherichia coli hya and cbdAB-appA operons in response to carbon and phosphate starvation. J Bacteriol. 1997;179:2141–2146. doi: 10.1128/jb.179.7.2141-2146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearson B L, Wilson L, Foster J W. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J Bacteriol. 1998;180:2409–2417. doi: 10.1128/jb.180.9.2409-2417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bearson S, Bearson B, Foster J W. Acid stress responses in enterobacteria. FEMS Microbiol Lett. 1997;147:173–180. doi: 10.1111/j.1574-6968.1997.tb10238.x. [DOI] [PubMed] [Google Scholar]
- 6.Benson N R, Goldman B S. Rapid mapping in Salmonella typhimurium with Mud-P22 prophages. J Bacteriol. 1992;174:1673–1681. doi: 10.1128/jb.174.5.1673-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brondsted L, Atlung T. Anaerobic regulation of the hydrogenase 1 (hya) operon of Escherichia coli. J Bacteriol. 1994;176:5423–5428. doi: 10.1128/jb.176.17.5423-5428.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui J, Ni L, Somerville R L. ATPase activity of TyrR, a transcriptional regulatory protein for sigma70 RNA polymerase. J Biol Chem. 1993;268:13023–13025. [PubMed] [Google Scholar]
- 9.Curtiss R, III, Porter S B, Munson M, Tinge S A, Hassan J O, Gentry-Weeks C, Kelly S M. Nonrecombinant and recombinant avirulent Salmonella vaccines for poultry. In: Blankenship L C, Bailey J H S, Cox N A, Stern N J, Meinersmann R J, editors. Colonization control of human bacterial enteropathogens in poultry. New York, N.Y: Academic Press; 1981. pp. 169–198. [Google Scholar]
- 10.Dorman C J. DNA topology and the global control of bacterial gene expression: implications for the regulation of virulence gene expression. Microbiology. 1995;141:1271–1280. doi: 10.1099/13500872-141-6-1271. [DOI] [PubMed] [Google Scholar]
- 11.Foster J, Aliabadi Z. pH-regulated gene expression in Salmonella: genetic analysis of aniG and cloning of the earA regulator. Mol Microbiol. 1989;3:1605–1615. doi: 10.1111/j.1365-2958.1989.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 12.Foster J W. Low pH adaptation and the acid tolerance response of Salmonella typhimurium. Crit Rev Microbiol. 1995;21:215–237. doi: 10.3109/10408419509113541. [DOI] [PubMed] [Google Scholar]
- 13.Foster J W, Hall H K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990;172:771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster J W, Park Y K, Bang I S, Karem K, Betts H, Hall H K, Shaw E. Regulatory circuits involved with pH-regulated gene expression in Salmonella typhimurium. Microbiology. 1994;140:341–352. doi: 10.1099/13500872-140-2-341. [DOI] [PubMed] [Google Scholar]
- 15.Gollub E G, Liu K P, Sprinson D B. tyrR, a regulatory gene of tyrosine biosynthesis in Salmonella typhimurium. J Bacteriol. 1973;115:1094–1102. doi: 10.1128/jb.115.3.1094-1102.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groisman E A, Ochman H. How to become a pathogen. Trends Microbiol. 1994;2:289–293. doi: 10.1016/0966-842x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 17.Groisman E A, Parra-Lopez C, Salcedo M, Lipps C J. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11939–11943. doi: 10.1073/pnas.89.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunsalus R, Park S. Aerobic-anaerobic gene regulation in Escherichia coli: control by the ArcAB and Fnr regulons. Res Microbiol. 1994;145:437–450. doi: 10.1016/0923-2508(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 19.Hall H K, Foster J W. The role of Fur in the acid tolerance response of Salmonella typhimurium is physiologically and genetically separable from its role in iron acquistion. J Bacteriol. 1996;178:5683–5691. doi: 10.1128/jb.178.19.5683-5691.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holley E A, Foster J W. Bacteriophage P22 as a vector for Mu mutagenesis in Salmonella typhimurium: isolation of nad-lac and pnc-lac gene fusions. J Bacteriol. 1982;152:959–962. doi: 10.1128/jb.152.2.959-962.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karem K, Foster J W. The influence of DNA topology on the environmental regulation of a pH-regulated locus in Salmonella typhimurium. Mol Microbiol. 1993;10:75–86. doi: 10.1111/j.1365-2958.1993.tb00905.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee I S, Lin J, Hall H K, Bearson B, Foster J W. The stationary-phase sigma factor ςs(RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium. Mol Microbiol. 1995;17:155–167. doi: 10.1111/j.1365-2958.1995.mmi_17010155.x. [DOI] [PubMed] [Google Scholar]
- 23.Menon N K, Robbins J, Peck H D J, Chatelus C Y, Choi E-S, Przybyla A E. Cloning and sequencing of a putative Escherichia coli [NiFe] hydrogenase-1 operon containing six open reading frames. J Bacteriol. 1990;172:1969–1977. doi: 10.1128/jb.172.4.1969-1977.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J H. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 24a.Moreno, M., and J. W. Foster. Unpublished data.
- 25.Neeley M N, Dell C L, Olsen E R. Roles of LysP and CadC in mediating the lysine requirement for acid induction of the Escherichia coli cad operon. J Bacteriol. 1994;176:3278–3285. doi: 10.1128/jb.176.11.3278-3285.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pittard A, Davidson B. TyrR protein of Escherichia coli and its role as repressor and activator. Mol Microbiol. 1991;5:1585–1592. doi: 10.1111/j.1365-2958.1991.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 27.Sanderson K E, Roth J R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988;52:485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawers R G, Jamieson D J, Higgins C F, Boxer D H. Characterization and physiological roles of membrane-bound hydrogenase isoenzymes from Salmonella typhimurium. J Bacteriol. 1986;168:398–404. doi: 10.1128/jb.168.1.398-404.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlensog V, Bock A. Identification and sequence analysis of the gene encoding the transcriptional activator of the formate hydrogenlyase system of Escherichia coli. Mol Microbiol. 1990;4:1319–1326. doi: 10.1111/j.1365-2958.1990.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 30.Somerville R L, Shieh T-L N, Hagewood B, Cui J. Gene expression from multicopy T7 promoter vectors proceeds at single copy rates in the absence of T7 RNA polymerase. Biochem Biophys Res Commun. 1991;181:1056–1062. doi: 10.1016/0006-291x(91)92044-k. [DOI] [PubMed] [Google Scholar]
- 31.Stim K P, Bennett G N. Nucleotide sequence of the adi gene, which encodes the biodegradative acid-induced arginine decarboxylase of Escherichia coli. J Bacteriol. 1993;175:1221–1234. doi: 10.1128/jb.175.5.1221-1234.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stim-Herndon K P, Flores T M, Bennett G N. Molecular characterization of adiY, a regulatory gene which affects expression of the biodegradative acid-induced arginine decarboxylase (adiA) of Escherichia coli. Microbiology. 1996;142:1311–1320. doi: 10.1099/13500872-142-5-1311. [DOI] [PubMed] [Google Scholar]
- 33.Unden G, Schirawski J. The oxygen-responsive transcriptional regulator FNR of Escherichia coli: the search for signals and reactions. Mol Microbiol. 1997;25:205–210. doi: 10.1046/j.1365-2958.1997.4731841.x. [DOI] [PubMed] [Google Scholar]
- 34.Unden G, Trageser M. Oxygen regulated gene expression in Escherichia coli: control of anaerobic respiration by the FNR protein. Antonie Leeuwenhoek. 1991;59:65–76. doi: 10.1007/BF00445650. [DOI] [PubMed] [Google Scholar]
- 35.Vogel H J, Bonner D M. Acetylornithase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]