Abstract
Type III protein secretion systems of nitrogen-fixing rhizobia deliver effector proteins into leguminous host cells to promote or inhibit the nodule symbiosis. However, mechanisms underlying effector-triggered inhibition of nodulation remain largely unknown. Nodulation outer protein T (NopT) of Sinorhizobium sp. NGR234 is an effector protease related to the Pseudomonas effector Avirulence protein Pseudomonas phaseolicola B (AvrPphB). Here, we constructed NGR234 mutants producing different NopT variants and found that protease activity of NopT negatively affects nodulation of smooth crotalaria (Crotalaria pallida). NopT variants lacking residues required for autocleavage and subsequent lipidation showed reduced symbiotic effects and were not targeted to the plasma membrane. We further noticed that Sinorhizobium fredii strains possess a mutated nopT gene. Sinorhizobium fredii USDA257 expressing nopT of NGR234 induced considerably fewer nodules in soybean (Glycine max) cv. Nenfeng 15 but not in other cultivars. Effector perception was further examined in NopT-expressing leaves of Arabidopsis (Arabidopsis thaliana) and found to be dependent on the protein kinase Arabidopsis AvrPphB Susceptible 1 (AtPBS1) and the associated resistance protein Arabidopsis Resistance to Pseudomonas syringae 5 (AtRPS5). Experiments with Nicotiana benthamiana plants indicated that the soybean homolog GmPBS1-1 associated with AtRPS5 can perceive NopT. Further analysis showed that NopT cleaves AtPBS1 and GmPBS1-1 and thus can activate these target proteins. Insertion of a DKM motif at the cleavage site of GmPBS1-1 resulted in increased proteolysis. Nodulation tests with soybeans expressing an autoactive GmPBS1-1 variant indicated that activation of a GmPBS1-1-mediated resistance pathway impairs nodule formation in cv. Nenfeng 15. Our findings suggest that legumes face an evolutionary dilemma of either developing effector-triggered immunity against pathogenic bacteria or establishing symbiosis with suboptimally adapted rhizobia producing pathogen-like effectors.
An effector protease of a nitrogen-fixing bacterium impairs root nodule formation of certain legume host plants and targets a soybean protein kinase known to be involved in plant immunity.
Introduction
Legumes enter a mutualistic nodule symbiosis with nitrogen-fixing bacteria, commonly referred to as rhizobia. Bacterial root infection and nodule development depend on signals from both partners, among them host flavonoids secreted into the rhizosphere and rhizobial lipo-chitooligosaccharides, so-called Nod factors (Perret et al., 2000; Roy et al., 2020). Similar to pathogenic bacteria, rhizobia have evolved strategies to evade or suppress the immune system of plants (Cao et al., 2017; Berrabah et al., 2019). For example, many rhizobial strains, including the Sinorhizobium (Ensifer) model strains NGR234, USDA257, and HH103, use their bacterial type III protein secretion system (T3SS) to translocate effector proteins into host cells (Staehelin and Krishnan, 2015; López-Baena et al., 2016). Depending on the host plant genotype, rhizobial effectors (nodulation outer proteins; Nops) may affect symbiosis differently. Symbiosis-promoting effects of rhizobial effectors on plants are generally assumed to be due to suppression of pattern recognition receptor-triggered immunity (PTI). On the other hand, effector recognition within certain legume cells may cause inhibition of nodule development by resistance mechanisms mediating effector-triggered immunity (ETI) against invading microbes (Staehelin and Krishnan, 2015; López-Baena et al., 2016; Miwa and Okazaki, 2017).
Plant proteins interacting with rhizobial effectors are largely unknown. Soybean (Glycine max) genes encoding proteins possibly interacting with effectors of Sinorhizobium fredii strain HH103 have been obtained by quantitative trait locus analysis but were not further characterized (Wang et al., 2018, 2020; Liu et al., 2021). Biochemical studies showed that the NopL effector of Sinorhizobium sp. NGR234 interacts with mitogen-activated protein kinases and becomes subsequently phosphorylated (Zhang et al., 2011; Ge et al., 2016). NopM of strain NGR234 is a bacterial E3 ubiquitin ligase effector that facilitates the covalent conjugation of ubiquitin from ubiquitin-loaded E2 enzymes to a given protein substrate, including NopM itself (Xin et al., 2012; Xu et al., 2018). The NopD effector of Bradyrhizobium sp. XS1150 can process small ubiquitin-related modifier (SUMO) proteins and cleave SUMO-conjugated proteins (Xiang et al., 2020). NopP of Bradyrhizobium diazoefficiens (B. japonicum) USDA110 is an effector that directly interacts with the soybean resistance (R) protein GmNNL1 (G.max Nodule Number Locus 1), resulting in genotype-specific inhibition of root hair infection (Zhang et al., 2021). Other rhizobial strains producing specific NopP variants can also trigger nodulation blockage in soybeans, a process found to be dependent on allelic polymorphism of the Rj2/Rfg1 R-gene. Attempts failed to identify a protein heterocomplex in this gene-for-gene relationship, however (Yang et al., 2010; Sugawara et al., 2018; Rehman et al., 2019). Using a yeast two-hybrid screen, NopT of Mesorhizobium amphore CCNWGS0123 was recently found to physically interact with two Robinia pseudoacacia proteins of unknown symbiotic function (ATP-citrate synthase alpha chain protein 2 and a hypersensitive-induced response protein) (Luo et al., 2020).
NopT effector proteins of rhizobia have been reported to be cysteine proteases with potential autocleavage activity, which depends on a conserved catalytic triad (cysteine, histidine, and aspartic acid residues) (Dai et al., 2008; Dowen et al., 2009; Kambara et al., 2009; Fotiadis et al., 2012). NopT proteins show sequence similarities to the effector protease Avirulence protein Pseudomonas phaseolicola B (AvrPphB) of Pseudomonas syringae pv. phaseolicola (Shao et al., 2002; Zhu et al., 2004). Mutant analysis indicated that nopT of Sinorhizobium sp. NGR234 can affect nodulation either positively (common bean, Phaseolus vulgaris cv. Yudou No. 1; Vogel’s tephrosia, Tephrosia vogelii) or negatively (sunn hemp, Crotalaria juncea) (Dai et al., 2008; Kambara et al., 2009). NopT of NGR234 (Dai et al., 2008; Kambara et al., 2009), as well as NopT1 and NopT2 of B. diazoefficiens USDA110 (Fotiadis et al., 2012), have been biochemically characterized. Autocleaved NopT proteins of these strains expose an N-terminus that is predicted to be myristoylated and palmitoylated in plant cells. Accordingly, NopT of NGR234 expressed in yeast was found to be lipidated. When fused to green fluorescent protein (GFP), NopT was localized to the plasma membrane of Chinese cabbage (Brassica campestris subsp. napus var. pekinensis) (Dowen et al., 2009). Hence, NopT of NGR234, similar to AvrPphB (Nimchuk et al., 2000; Zhu et al., 2004), appears to hijack the plant lipidation machinery to ensure plasma membrane localization.
Translocated AvrPphB can proteolytically cleave various plant target proteins, thereby inhibiting PTI (Zhang et al., 2010). AvrPphB can cleave AvrPphB Susceptible 1 (PBS1) target proteins in various plant species (Shao et al., 2003; Sun et al., 2017; Carter et al., 2019; Helm et al., 2019). PBS1 proteins are intracellular serine threonine kinases associated with PTI in certain plants (Zhang et al., 2010; Albers et al., 2019). When playing a role in ETI, PBS1 proteins are known or hypothesized to be associated with intracellular immune receptors known as nucleotide-binding leucine-rich repeat proteins (NLRs), namely Resistance to P. syringae 5 (RPS5) in Arabidopsis (Arabidopsis thaliana) (Pottinger and Innes, 2020) and AvrPphB Response 1 in barley (Hordeum vulgare subsp. vulgare) (Carter et al., 2019). Cleavage of Arabidopsis PBS1 (AtPBS1) by AvrPphB causes a conformational change that activates AtRPS5 and triggers ETI-related downstream responses (Shao et al., 2003). Hence, AtPBS1 in combination with the resistance protein AtRPS5 functions as an intracellular sensor that recognizes AvrPphB protease activity in Arabidopsis cells. AtRPS5 can also be activated by autoactive AtPBS1 variants that contain multiple alanine residues in the activation loop (DeYoung et al., 2012).
In this study, we employed a Xanthomonas-pepper (Capsicum annuum cv. ECW-10R) translocation system to confirm NopT translocation into plant cells. Nodulation tests showed that nopT negatively affects the symbiosis between NGR234 and smooth crotalaria (Crotalaria pallida) and various NopT variants were tested for their symbiotic effector activity. We also noticed that the nopT gene in S.fredii strains contains a 19-bp deletion and found that NopT of NGR234, when expressed in S. fredii USDA257, almost completely blocks nodulation of soybean cv. Nenfeng 15. We further investigated ETI-like NopT effects in plant cells and found that they depend on AtPBS1/AtRPS5 in Arabidopsis. Experiments with Nicotiana benthamiana showed cell death responses when NopT was co-expressed with AtPBS1 and AtRPS5. AtPBS1 could be replaced by the soybean homolog GmPBS1-1 in these studies. Further analysis revealed that NopT can cleave PBS1 proteins and that a GmPBS1-1 version modified in the activation loop is more susceptible to cleavage. Finally, we demonstrate that an autoactive form of GmPBS1-1 substantially reduces soybean nodulation, indicating that PBS1-dependent downstream resistance mechanisms impair nodule formation.
Results
Analysis of N-terminal NopT residues in a Xanthomonas-pepper translocation system
Using a Pseudomonas-Arabidopsis test system, previous attempts failed to demonstrate NopT translocation into plant cells (Kimbrel et al., 2013). Here, we used a similar Xanthomonas-pepper system that is based on plant symptoms induced by the Xanthomonas effector AvrBs1 with N-terminal amino acid residues of a given effector (Jiang et al., 2009; Chen et al., 2016). A plasmid (pnopT-avrBs1) was constructed to express a NopT-AvrBs1 protein (residues 1–58 of NopT from Sinorhizobium sp. NGR234 fused to residues 59–455 of AvrBs1) in 8004ΔavrBs1, an avrBs1-deficient mutant of X. campestris strain 8004. When bacteria were infiltrated into pepper leaves, leaf chlorosis was observed in the infiltration zone 75 h later. In contrast, the 8004ΔhrpF mutant (deficient in the translocation protein HrpF) carrying pnopT-avrBs1 was not causing these symptoms (Figure 1A). First phenotypic differences between the two strains were observed at 24 h and a picture taken at 36 h is shown in Supplemental Figure S1. Further control experiments of the effector translocation test system are shown in Chen et al. (2016). In conclusion, the obtained results indicated that the N-terminal NopT residues of the expressed fusion protein were indispensable for T3SS-dependent protein translocation into pepper cells.
Figure 1.
Characterization of NopT and variants of Sinorhizobium sp. NGR234. A, Effector translocation test. The photograph shows a pepper leaf 75 h postinfiltration with X. campestris 8004ΔavrBs1 (left) and 8004ΔhrpF (right) both carrying pnopT-avrBs1. Infiltration holes are marked by circles. B, Nodulation test with Sinorhizobium sp. NGR234 and nopT mutants inoculated on C. pallida. Plants were inoculated with Sinorhizobium sp. NGR234 (parent strain), NGRΔnopT, or NGRΩnopT and the number of nodules were determined at 28 days postinoculation. Data indicate means ± se (n = 9). Significant differences among strains are marked with different letters above columns (Student’s t test; *P ≤ 0.05). C, Schematic view of examined NopT variants. D, Secretion of NopT and indicated variants expressed in the NGRΔnopT mutant. The wild-type strain Sinorhizobium sp. NGR234 and NGRΔnopT carrying the empty vector pFAJ1703 (EV) served as controls. Effector protein expression was induced by 10−6 M apigenin. Culture supernatants harvested 40 h later were subjected to Western blot analysis using an antibody against NopT (the lower band likely represents processed NopT). E, Symbiotic effects of NopT and variants on C. pallida. Indicated NopT proteins were expressed in the NGRΔnopT mutant carrying corresponding plasmids. Plants were harvested 28 days postrhizobial inoculation. Data indicate means ± se (n = 8). Significant differences among strains are indicated by different letters (Student’s t test; P ≤ 0.05). F, Subcellular localization of full-length NopT and variants with a C-terminal GFP tag. Agrobacterium tumefaciens-mediated transformation was used to express the proteins in N. benthamiana. Protoplasts were isolated 48 h postagroinfiltration and analyzed by fluorescence microscopy under green fluorescent (GF) and bright field (BF) conditions. Bar = 20 μm.
Effects of NopT and variants on the symbiosis between NGR234 and C. pallida
Nodulation tests with NGR234 and nopT mutants (NGRΔnopT and NGRΩnopT; Dai et al., 2008) showed that the mutants induced more nodules on C. pallida, indicating that NopT has a negative effect on nodule formation of this legume (Figure 1B). This nodulation phenotype allowed us to test the asymbiotic effector activity of NopT variants which cannot be lipidated (NopT3A), lack autocleavage and subsequent lipidation (NopT6A), or are deficient in protease activity (NopTcatA) (Figure 1C). Western blot analysis with NGRΔnopT carrying corresponding plasmids showed that NopT and variants were expressed and secreted by the bacteria (Figure 1D). The results of the nodulation tests showed that the asymbiotic NopT effects were reduced for NopT3A and NopT6A while NopTcatA was completely inactive (Figure 1E). These findings indicated that autocleavage and lipidation likely promoted NopT activity and that protease activity was indispensable for asymbiotic effector activity. Subcellular localization analysis with N. benthamiana protoplasts showed that NopT fused to GFP was targeted to the plasma membrane. In contrast, corresponding NopT variants failed to do so (Figure 1F).
NopT of NGR234 expressed in a S. fredii strain impairs nodulation of soybean cv. Nenfeng 15
Various nopT effector genes are present in a number of Sinorhizobium, Mesorhizobium, and Bradyrhizobium strains. A phylogenetic tree is shown in Figure 2A. In contrast to the nopT sequence of NGR234, other Sinorhizobium (Ensifer) strains possess a 19-bp deletion in nopT, resulting in a frameshift mutation (Figure 2B). Accordingly, a small 9.9-kDa protein (first 52 residues identical to those of NopT from NGR234) is predicted to be synthesized. Western blot analysis provided clues for the presence of such a truncated protein in the culture supernatant of S. fredii USDA257. A second Western blot band indicated that a secondary start methionine could initiate synthesis of an additional secreted NopT form of USDA257, presumably a 16.4-kDa protein with the N-terminal residues MEAL (Supplemental Figure S2). An amino acid sequence alignment with NopT of NGR234 shows that residues required for lipidation and protease activity (catalytic cysteine) are absent in both secreted NopT forms (Supplemental Figure S3). Furthermore, Western blot analysis of intracellular proteins of USDA257 provided clues for synthesis of a third 22.5-kDa NopT form that could not be detected in the culture supernatant (Supplemental Figure S2).
Figure 2.
Polymorphism of nopT in different rhizobial strains. A, Phylogenetic analysis based on nucleotide sequences from selected strains aligned to the nopT coding sequence of Sinorhizobium sp. NGR234 (accession numbers CP029453, CP029233, CP023069, CP003565, HE616895, U00090, SRXN01000053, JADYVM010000023, NWTC01000041, WP_011084934, WP_040582237.1, WP_011084854, and SPP97927.1). Bootstrap values are indicated next to branches. The sequence of AvrPphB from P. syringae pv. phaseolicola (accession number Q52430) was included for comparison. B, Sequence variations in different Sinorhizobium (Ensifer) strains. In addition to NGR234, full-length nopT genes were identified in two other Sinorhizobium (Ensifer) strains (group I). Soybean nodulating Sinorhizobium (Ensifer) strains from China (group II) possess a 19-bp deletion (corresponding to nucleotides 158–176 of the nopT coding sequence in NGR234). The corresponding sequence of S. fredii PCH1 shows poor alignment and a putative nopT start codon is predicted to be downstream of the shown sequence (group III). Conserved nucleotides are highlighted in blue. Nucleotides identical to the nopT sequence of NGR234 are shown in red. Lp, Lablab purpureus; Cc, Cajanus cajan; Gm, G. max; Pv, Phaseolus vulgaris; PG, Papua New Guinea; IN, India; and CN, China.
Unlike NGR234, strain USDA257 can induce nitrogen-fixing nodules on many soybean accessions. The observed 19-bp deletion in nopT of USDA257 suggests that a nopT gene without deletion in an ancestral S. fredii genome could negatively affect soybean nodulation. Such hypothetical full-length nopT is nearly identical to nopT of NGR234 (Supplemental Figure S4). We, therefore, expressed NopT and variants of NGR234 in strain USDA257. Secretion of the expressed protein was confirmed by Western blot analysis (Figure 3A). Nodulation tests with various soybean accessions showed that nopT of NGR234 expressed in USDA257 negatively affected nodulation of cv. Nenfeng 15 (Figure 3B). Further tests with this accession indicated that NopT3A, NopT6A, and NopTcatA caused significantly weaker effects than nonmodified NopT (Figure 3C). The NGRΔnopT mutant failed to induce nodules on Nenfeng 15, indicating that additional genes in NGR234 are causing symbiotic incompatibility with soybeans (Supplemental Figure S5).
Figure 3.
Sinorhizobium fredii USDA257 expressing NopT from NGR234 shows symbiotic incompatibility with soybean cv. Nenfeng 15. A, Secretion of NopT and indicated variants of Sinorhizobium sp. NGR234 expressed in S. fredii USDA257. USDA257 carrying the EV pFAJ1703 was also analyzed. Effector protein expression was induced by addition of 10−6 M genistein. Culture supernatants harvested 40 h later were subjected to Western blot analysis using an antibody against NopT. B, Nodule formation of different soybean cultivars inoculated with S. fredii USDA257 secreting NopT of NGR234. USDA257 carrying the EV was used as a control. Plants were harvested at 28 days postinoculation. A significant NopT effect was found for cv. Nenfeng 15 (Student’s t test; P ≤ 0.05). C, Results of a similar nodulation test with soybean cv. Nenfeng 15 and USDA257 secreting indicated variants derived from NopT of NGR234. USDA257 secreting nonmodified NopT and USDA257 carrying the EV served as controls. Data in B and C indicate means ± se (n = 10). Significant differences among different strains are marked with different letters (Student’s t test; P ≤ 0.05).
NopT-triggered responses in plant cells depend on PBS1 proteins
NopT of NGR234 expressed in Arabidopsis plants caused chlorotic and necrotic symptoms in previous experiments (Dai et al., 2008). Such effects are reminiscent of the AtPBS1/AtRPS5-mediated ETI induced by the Pseudomonas effector AvrPphB (Shao et al., 2003; Pottinger and Innes, 2020). We, therefore, transiently expressed full-length NopT of NGR234 in leaves of the Arabidopsis mutants pbs1-2 (Warren et al., 1999) and rps5-2 (Warren et al., 1998). Agrobacterium-mediated NopT expression resulted in rapid leaf chlorosis in wild-type plants whereas no visible effects were observed for the pbs1-2 and rps5-2 mutants. These observations indicated that the AtPBS1/AtRPS5 effector recognition system was indispensable for NopT-triggered responses in Arabidopsis (Figure 4A).
Figure 4.
NopT-trigged effects in Arabidopsis and N. benthamiana plants depend on PBS1 proteins and AtRPS5. A, Effects of NopT expressed in Arabidopsis leaves (Col-0 wild-type; pbs1-2 and rps5-2 mutants) 4 days postinfiltration with A. tumefaciens carrying pCAMBIA2301 containing full-length nopT of NGR234 or the EV. NopT-triggered leaf chlorosis was only observed in wild-type plants. Agroinfiltration holes are marked by circles. B, Reconstitution of the AtPBS1/AtRPS5 pathway in N. benthamiana. Agrobacterium-mediated transformation was used to co-express constructs encoding NopT (lacking residues 1–48) or variants, AtPBS1, and AtRPS5. The EV pTA7002 was used as a control. Protein expression was induced by 50 µM DEX (42 h postagroinfiltration) and leaves were photographed 48 h later. The pictures in the lower panel were taken with a UV camera to illustrate formation of necrotic tissue. C, Replacement of AtPBS1 by GmPBS1-1 showed similar effects when NopT proteins and AtRPS5 were co-expressed.
To substantiate these findings, we reconstituted the AtPBS1/AtRPS5 resistance pathway in N. benthamiana leaves using Agrobacterium strains carrying a plasmid containing a dexamethasone (DEX) inducible promoter (Ade et al., 2007). As expected, chlorosis and UV signals associated with necrosis were observed when NopT (processed form lacking residues 1–48) was co-expressed with AtPBS1/AtRPS5. The NopT6A and NopTcatA variants were also active in combination with AtPBS1/AtRPS5; however, effects of NopTcatA appeared to be weaker than those induced by NopT (Figure 4B). Expression of two proteins (AtPBS1/AtRPS5, AtPBS1/NopT, and NopT/AtRPS5) or NopT alone showed no obvious effects in these experiments (Supplemental Figure S6).
Next, we asked whether GmPBS1-1, the prototype of the three similar soybean PBS1 proteins (Helm et al., 2019), is also active in the N. benthamiana expression system. mRNA sequencing indicated that the amino acid sequences of the three soybean PBS1 genes are identical in the cultivars Nenfeng 15, Beidou 35, and Williams 82. Expression of NopT (processed form) with GmPBS1-1/AtRPS5 in N. benthamiana resulted in chlorosis and necrosis-associated UV signals, indicating that GmPBS1-1 could functionally replace AtPBS1. Likewise, NopT6A and NopTcatA were active in combination with GmPBS1-1/AtRPS5 under the used test conditions (Figure 4C). However, unlike the AtPBS1/AtRPS5 combination, the NopT-induced cell death responses in GmPBS1-1/AtRPS5 expressing tissue appeared to be weaker and were not seen in all examined leaves. No cell death responses were observed for expression of two proteins (GmPBS1-1/AtRPS5, GmPBS1-1/NopT; Supplemental Figure S6). AvrPphB (processed form lacking residues 2–62) was causing chlorosis and cell death when GmPBS1-1/AtRPS5 were co-expressed (Supplemental Figure S7). In conclusion, the findings of these experiments indicated that NopT and AvrPphB both could activate AtPBS1 or GmPBS1-1 to trigger AtRPS5-mediated responses in N. benthamiana.
NopT cleaves AtPBS1 and GmPBS1-1
Activation of PBS1 proteins by AvrPphB was found to be dependent on proteolytic cleavage of the activation loop in PBS1 proteins (Shao et al., 2003; Helm et al., 2019; Pottinger and Innes, 2020). We, therefore, expected that NopT possesses a similar proteolytic activity. When expressed in N. benthamiana, processed form of NopT with HA tag (NopT-HA), processed form of AvrPphB with HA tag (AvrPphB-HA), as well as a FLAG-tagged AtPBS1 and GmPBS1-1 could be detected by Western blot analysis. When analyzed 7 h after induction of gene expression by DEX, co-expression of AvrPphB-HA with either AtPBS1-FLAG or GmPBS1-1-FLAG resulted in cleavage of the PBS1 proteins as reported previously (Shao et al., 2003; Helm et al., 2019; Pottinger and Innes, 2020). Under these conditions, no cleavage product was observed for AtPBS1-FLAG or GmPBS1-1-FLAG when NopT-HA was co-expressed (Supplemental Figure S8). When analyzed 42 h after DEX treatment, however, the appearance of a cleavage product band indicated that NopT-HA could cleave AtPBS1-FLAG as well as GmPBS1-1-FLAG (Figure 5A).
Figure 5.
NopT cleaves PBS1 proteins of Arabidopsis and soybean. A, NopT-HA was co-expressed with AtPBS1-FLAG or GmPBS1-1-FLAG in N. benthamiana leaves. Protein expression was induced by 50 µM DEX. Proteins were extracted 42 h later and subjected to Western blot analysis using anti-FLAG and anti-NopT antibodies. AtPBS1-FLAG and GmPBS1-1-FLAG are marked by an arrowhead and the FLAG-tagged cleavage products by an arrow. B, Schematic view of the GmPBS1-1DKM and GmPBS1-1PDKM variants used in further experiments with N. benthamiana. C, Western blot analysis (20 h after DEX treatment) showed that NopT-HA and AvrPphB-HA both released a cleavage product (arrow) from FLAG-tagged GmPBS1-1DKM (arrowhead) while GmPBS1-1PDKM was not cleaved. D, Similar experiments were performed for FLAG-tagged GmPBS1-1DKM co-expressed with processed NopT and the variants NopT6A and NopTcatA (Western blot analysis with proteins 22 h after DEX treatment).
NopT and AvrPphB considerably differ in their autocleavage sites. When expressed in Escherichia coli, processed NopT lacks a methionine residue at its N-terminus (Dai et al., 2008; Dowen et al., 2009), indicating that the autocleavage site was most probably at the position PDKM-G. Alternatively, the cleavage site would be PDK-MG and the methionine proteolytically removed by an E. coli protease (Supplemental Figure S9; Dai et al., 2008; Dowen et al., 2009). In contrast, AvrPphB is auto-processed at the LGDK-G amino acid motif of the activation loop (Shao et al., 2003; Carter et al., 2019; Helm et al., 2019). The cleavage site motif in PBS1 proteins contains conserved GDK residues that are identical to the autocleavage site of AvrPphB (Shao et al., 2003; Carter et al., 2019; Helm et al., 2019) but not NopT. We, therefore, wondered whether NopT cleaves a modified GmPBS1-1 more effectively. GmPBS1-1 variants with an activation loop deduced from the autocleavage site of NopT were tested in the N. benthamiana expression system (GmPBS1-1DKM-FLAG and GmPBS1-1PDKM-FLAG; Figure 5B). Interestingly, NopT-HA and AvrPphB-HA both could efficiently cleave GmPBS1-1DKM-FLAG when co-expressed proteins were analyzed 20 h after DEX treatment. Additional experiments confirmed that GmPBS1-1DKM-FLAG, when compared with nonmodified GmPBS1-1-FLAG, is a better substrate for NopT-HA. In contrast, GmPBS1-1PDKM-FLAG was not cleaved by NopT-HA under the used test conditions (Figure 5C). In addition to NopT-HA (processed form), we also analyzed NopT variants (full length forms without tag) for their capacity to cleave GmPBS1-1DKM-FLAG. Only a faint cleavage product was observed when NopT6A was co-expressed with GmPBS1-1DKM-FLAG while the NopTcatA variant was enzymatically inactive as expected (Figure 5D).
Expression of an autoactive PBS1 variant in soybean roots impairs nodule formation
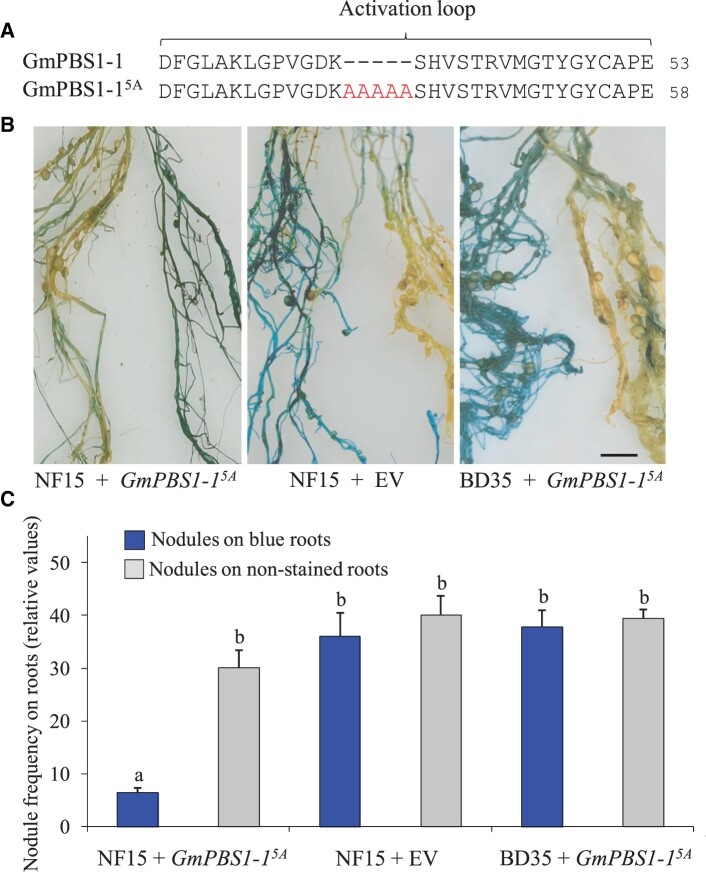
GmPBS1 proteins are targets of the Pseudomonas effector AvrPphB (DeYoung et al., 2012; Helm et al., 2019). GmPBS1 genes are well expressed in roots and nodules of soybean (eFP Browser; http://bar.utoronto.ca/efpsoybean/cgi-bin/efpWeb.cgi). To test whether PBS1-triggered defense responses affect the nodule symbiosis, we constructed a binary vector with a GmPBS1-15A construct under the control of a cauliflower mosaic virus (CaMV) 35S promoter (Figure 6A). The GmPBS1-15A variant contains five alanine residues in the activation loop and therefore represents an autoactive form that can activate ETI in the absence of an effector protease (DeYoung et al., 2012; Helm et al., 2019). The binary vector pCAMBIA1305.1 also contained a β-glucuronidase (GUS) gene expression cassette to visualize formation of transgenic roots. Agrobacterium rhizogenes K599 carrying the constructed plasmid was used to transform soybeans identified to be responsive (cv. Nenfeng 15) and nonresponsive (cv. Beidou 35) to NopT of Sinorhizobium sp. NGR234 (Figure 3B). Plants forming hairy roots were inoculated with S. fredii USDA257 and harvested 28 days later. Transformation efficiency values deduced from GUS stained roots were similar (Supplemental Figure S10). Nodule formation on blue roots of cv. Nenfeng 15 transformed with the plasmid containing GmPBS1-15A DNA was impaired while roots lacking blue coloration (and thus likely nontransgenic) formed much more nodules. Control roots transformed with pCAMBIA1305.1 (containing only the GUS expression cassette) did not show such difference and nodules were equally formed on blue and nonstained roots. Quantitative analysis of nodule frequency data confirmed that transformation with the GmPBS1-15A construct impaired nodule formation of cv. Nenfeng 15. In contrast, nodulation of cv. Beidou 35 was not affected by the GmPBS1-15A construct (Figure 6, B and C). These findings indicate that GmPBS1-15A could induce a PBS1-related resistance pathway in cv. Nenfeng 15 and suggest that such pathway is nonfunctional in cv. Beidou 35.
Figure 6.
Hairy roots of soybean cv. Nenfeng 15 transformed with a GmPBS1-15A construct form fewer nodules. A, Illustration of the GmPBS1-15A variant. B, Photographs of representative Nenfeng 15 roots transformed with the GmPBS1-15A construct or with the EV. Roots of cv. Beidou 35 transformed with the GmPBS1-15A construct were also analyzed. Plants were inoculated with S. fredii USDA257 and harvested 28 days postinoculation. Blue coloration of the roots indicated that GUS was co-transformed. Bar = 0.25 mm. C, Quantification of nodule formation as determined by the frequency of nodules on blue or nonstained roots. Data indicate means ± se (n = 11 for the GmPBS1-15A construct; n = 5 for the EV control). Significantly reduced nodulation was observed on blue roots of cv. Nenfeng 15 transformed with the GmPBS1-15A construct when compared with the other test plants (Student’s t test; P ≤ 0.05). NF15, cv. Nenfeng 15; BD35, cv. Beidou 35; GmPBS1-15A, transformation with pCAMBIA1305.1 containing the GmPBS1-15A expression cassette; and EV, transformation with the EV pCAMBIA1305.1 (containing a GUS expression cassette).
Discussion
Rhizobial NopT proteins are proteases that can affect nodule formation with host legumes depending on the strain-host combination (Dai et al., 2008; Kambara et al., 2009; Teulet et al., 2019; Liu et al., 2021; Songwattana et al., 2021). In this study, we employed a well-established AvrBs1-based Xanthomonas-pepper translocation system (Jiang et al., 2009; Chen et al., 2016) and found that the N-terminal part of NopT was indispensable for T3SS-dependent translocation of the NopT-AvrBs1 protein. Effector translocation is also supported by the finding that processed NopT of NGR234 can be lipidated in eukaryotic cells (Dowen et al., 2009). Furthermore, we found that NopT3A and NopT6A, variants lacking residues required for lipidation, show reduced effector activity in nodulation experiments with C. pallida (NGRΔnopT derivatives) and soybean cv. Nenfeng 15 (USDA257 derivatives). Accordingly, GFP-tagged NopT expressed in N. benthamiana cells was associated with the plasma membrane while NopT3A and NopT6A were not. Likewise, most fluorescence signals of GFP-tagged NopTcatA were not associated with the plasma membrane, suggesting that neither NopTcatA nor plant proteases could process the effector at the autocleavage site. Remarkably, NopTcatA was completely inactive in nodulation experiments with C. pallida but retained weak asymbiotic effector activity in soybean cv. Nenfeng 15. NopTcatA expressed in N. benthamiana also showed certain activity in inducing cell death when co-expressed with GmPBS1-1 (or AtPBS1) and AtRPS5. Hence, NopTcatA could retain weak effector activity, suggesting that binding of NopTcatA to a PBS1 protein was apparently sufficient to cause conformational changes required for activation of downstream responses. In contrast, an enzymatically inactive form of the Pseudomonas effector AvrPphB did not show any visible symptoms in N. benthamiana leaves co-expressing AtPBS1/AtRPS5 (Ade et al., 2007).
This study shows that NopT of NGR234 acts as an asymbiotic effector in C. pallida and soybean cv. Nenfeng 15. In contrast to NGR234, S. fredii strains such as USDA257 and HH103 possess a nopT gene with a frameshift mutation, resulting in synthesis of multiple NopT forms. Nodulation tests carried out recently with a nopT mutant of S. fredii strain HH103 and various soybean accessions suggested that nopT of S. fredii modulates symbiosis either positively or negatively (Liu et al., 2021). Hence, the secreted 9.9-kDa and 16-kDa NopT forms of S. fredii USDA257, although nonlipidated and predicted to be enzymatically inactive (Supplemental Figures S3 and S4), may possess certain symbiotic effector activity. We suggest that NopT forms of S. fredii possess effector activities that are different from full-length NopT of NGR234. During evolution, the mutation causing a 19-bp deletion in nopT of S. fredii strains likely promoted symbiosis with soybeans, perhaps due to lacking interactions with plasma membrane-associated PBS1 proteins or newly acquired interactions with cytoplasmic target proteins that positively affect soybean nodulation. Future work is required to identify such NopT targets in soybean. Candidates for soybean proteins associated with NopT of HH103 have been recently identified by quantitative trait locus analysis (Liu et al., 2021).
Upon cleavage of AtPBS1 by the AvrPphB effector, AtRPS5-dependent ETI responses are induced in Arabidopsis (Shao et al., 2003; Pottinger and Innes, 2020). In this study, we found that transient nopT expression in Arabidopsis causes chlorosis followed by cell death in an AtPBS1/AtRPS5-dependent way. Moreover, we reconstituted the AtPBS1/AtRPS5 pathway in N. benthamiana (Shao et al., 2003) and found that NopT can trigger cell death when NopT, GmPBS1-1, and AtRPS5 were co-expressed. Based on the previously reported AvrPphB/AtPBS1/AtRPS5 protein complex (Shao et al., 2003; Ade et al., 2007; Pottinger and Innes, 2020), we suggest that GmPBS1-1 and AtRPS5 formed a functional heterocomplex that could recognize NopT in N. benthamiana.
Further gene expression experiments with N. benthamiana plants showed that AtPBS1 and GmPBS1-1 are indeed cleaved by NopT of NGR234. Similarly, GmPBS1-2 and GmPBS1-3 are expected to be substrates for NopT because their cleavage site residues are identical to GmPBS1-1. All three GmPBS1 proteins were found to be cleaved by AvrPphB (Helm et al., 2019). Compared with AvrPphB, NopT cleaved AtPBS1 and GmPBS1-1 at lower efficiency in our experiments, whereas the modified GmPBS1-1DKM variant was highly susceptible to NopT cleavage (Figure 5). Thus, NopT apparently shows a preference for substrates containing a DKM cleavage site. In Arabidopsis, several PBS1-like proteins possibly involved in PTI have been identified as substrates for AvrPphB (Zhang et al., 2010). Likewise, cleavage of unknown NopT substrates (not interacting with a resistance protein) are expected to suppress PTI, which may positively affect nodule formation. NopT of M. amphore CCNWGS0123 has an autocleavage site identical to NopT of NGR234, suggesting that both effectors possess similar proteolytic activities. However, we could not identify potential cleavage sites in previously reported NopT-interacting proteins of R. pseudoacacia (Luo et al., 2020).
In the absence of AvrPphB, AtPBS1 with multiple alanine residues in the activation loop can trigger RPS5-dependent resistance in Arabidopsis (DeYoung et al., 2012). Likewise, it was reported that soybean protoplasts show premature cell death when transformed with GmPBS1-1 or GmPBS1-2 variants containing multiple alanine residues in the activation loop (Helm et al., 2019). In our study, expression of autoactive GmPBS1-15A in hairy roots of cv. Nenfeng 15 considerably impaired nodule formation when USDA257 was used for inoculation. These findings suggest that activation of PBS1-dependent ETI in soybean roots can negatively affect nodulation. Our mRNA sequencing results indicated that the coding sequences of GmPBS1-1, GmPBS1-2, and GmPBS1-3 are identical in Nenfeng 15 and Beidou 35 soybeans. We therefore suggest that a PBS1-associated NLR is different in Nenfeng 15 and Beidou 35 soybeans. Protein Blast search results indicated that AtRPS5 and soybean NLRs show only weak sequence similarities. It is worth noting in this context that AtRPS5-related NLR sequences derived from the Nenfeng 15 transcriptome are not completely identical to those from Beidou 35 (Supplemental Table S1). Future work will be required to identify PBS1-interacting NLRs from different soybeans and to study their NopT/PBS1-dependent activation.
In plant–pathogen interactions, effector genes and plant resistance genes rapidly coevolve to avoid ETI and pathogen attack, respectively. Due to this “arms race,” pathogens rapidly change their effector arsenal during evolution (Anderson et al., 2010). Such changes include structural modifications of effectors to escape from recognition by plant resistance proteins or effector-interacting decoy proteins. NopT and other rhizobial effectors such as NopM (Xin et al., 2012; Xu et al., 2018) are structurally and functionally related to effectors of bacterial pathogens. Rhizobia producing such pathogen-like effectors may therefore promote loss of function mutations in resistance genes of host plants, thereby indirectly influencing the “arms race” between legumes and effector-producing pathogens. In our study, the nodulation tests with S. fredii USDA257 expressing NopT of NGR234 indicated that soybeans can be either responsive or nonresponsive to NopT of NGR234. Such genotype-specific differences suggest that NopT-producing rhizobia may function as evolutionary driving force to suppress functions of NLRs associated with PBS1 proteins. In other words, legumes may face an evolutionary dilemma of either developing resistance mechanisms against pathogenic bacteria or establishing symbiosis with rhizobia producing pathogen-like effectors.
Materials and methods
Bacterial strains and plasmids
Characteristics of all strains and plasmids used in this study are shown in Supplemental Table S2 and used primers are listed in Supplemental Table S3. Bacterial growth conditions are described in Supplemental Text S1. Plasmids were mobilized into agrobacteria by electroporation. The AvrBs1-based Xanthomonas-pepper (C.annuum cv. ECW-10R) translocation assay was performed with 8004ΔavrBs1 and 8004ΔhrpF, a mutant of 8004 lacking the translocon gene hrpF (Xu et al., 2008; Jiang et al., 2009). The bacteria were carrying pnopT-avrBs1, a pFAJ1702 (Dombrecht et al., 2001) derivative containing nopT-avrBs1 DNA (encoding residues 1–58 of NopT from Sinorhizobium [Ensifer] sp. NGR234 fused to residues 59–455 of AvrBs1). The wild-type strain 8004 and additional plasmids were included in control experiments (Chen et al., 2016). Sinorhizobium sp. NGR234, NGRΔnopT (Dai et al., 2008), NGRΩnopT (Dai et al., 2008), and S. fredii USDA257 served as rhizobial inoculum strains. Where indicated, the rhizobia were carrying pFAJ1703 (Dombrecht et al., 2001) with inserted nopT sequence (promoter and coding sequence PCR-amplified from Sinorhizobium sp. NGR234). Similar plasmids with DNA encoding NopT variants were prepared by overlap extension PCR (Heckman and Pease, 2007). Mobilization of plasmids into rhizobia was performed by triparental mating using the helper plasmid pRK2013 (Figurski and Helinski, 1979). Agrobacteriumtumefaciens strain GV3101 or EHA105 carrying a given binary vector was used for transient gene expression in N. benthamiana and Arabidopsis. For Arabidopsis transformation, pCAMBIA2301 alone or with a nopT expression cassette (CaMV 35S promoter) were electroporated into A. tumefaciens strain EHA105. For subcellular localization analysis in N. benthamiana cells, nopT fusion constructs with a C-terminal GFP sequence were cloned into pCAMBIA1302 (expression driven by a CaMV 35S promoter). For DEX-inducible gene expression in N. benthamiana, a ligation-free cloning procedure (Applied Biological Materials Inc., Richmond, Canada) was employed to insert nopT, avrPphB, AtPBS1, and GmPBS1-1 constructs into pTA7002 (Aoyama and Chua, 1997). The nopT (encoding NopTΔ1–48) and avrPphB (encoding AvrPphBΔ2–62) constructs contained a C-terminal 2×HA tag. The coding regions of AtPBS1, AtRPS5, and GmPBS1-1 were PCR-cloned from cDNA derived from Arabidopsis and soybean mRNA (the coding sequences of GmPBS1-1 in the cultivars Nenfeng 15 and Beidou 35 were found to be identical to that of Williams 82). Constructs encoding the variants GmPBS1-1PDKM and GmPBS1-1DKM were obtained by overlap extension PCR and then also cloned into pTA7002. All PBS1 constructs inserted into pTA7002 contained a C-terminal 2×FLAG tag sequence. Hairy root transformation was achieved with A. rhizogenes strain K599 carrying pCAMBIA1305.1 (containing a GUS expression cassette) or pCAMBIA1305.1 with a GmPBS1-15A DNA construct (DeYoung et al., 2012; Helm et al., 2019). The GmPBS1-15A DNA fragment was obtained by overlap extension PCR and then cloned into pRT104 (Töpfer et al., 1987). The expression cassette containing a CaMV 35S promoter was then inserted into pCAMBIA1305.1 using a ligation-free cloning procedure. DNA constructs inserted into plasmids were confirmed by sequencing.
Plant material, growth conditions, and nodulation tests
Pepper (C.annuum cv. ECW-10R), thale cress (A.thaliana ecotype Columbia-0 [Col-0] and the mutants pbs1-2 and rps5-2; Warren et al., 1998, 1999), smooth crotalaria (C.pallida; seeds collected at Qingyuan [N23°41′, E113°03′] and Zhuhai [N22°16′, E113°34′]; Guangdong Province, China), N.benthamiana plants, and various soybean (G.max) accessions were used in this study. The soybean cultivars Nenfeng 15 (indeterminate growth type) and Beidou 35 (semi-determinate growth type) have been commonly used in agricultural settings in Northeast China. Pepper, C. pallida and soybean plants were grown in a temperature-controlled greenhouse with a 16-h photoperiod (29°C–31°C for pepper; 25°C–27°C for legumes). Arabidopsis and N. benthamiana plants were grown in a growth room at 22°C–24°C with a 12-h photoperiod. Nodulation tests with C. pallida and soybeans were performed in plastic jar units (one plant per unit) according to a previously described procedure (Staehelin et al., 2006). Details are provided in Supplemental Text S1. Plants were harvested 28 days postrhizobial inoculation.
Effector translocation assay
An AvrBs1-based Xanthomonas-pepper system was employed as described previously (Jiang et al., 2009; Chen et al., 2016). Details are described in Supplemental Text S1.
Analysis of NopT forms in rhizobial culture supernatants
Secreted proteins of rhizobial liquid cultures were concentrated by precipitation with trichloroacetic acid and subjected to Western blot analysis using a previously prepared anti-NopT antibody (Dai et al., 2008). A detailed protocol is provided in Supplemental Text S1.
Transient gene expression in Arabidopsis and N. benthamiana plants
Agrobacterium tumefaciens strain EHA105 carrying pCAMBIA2301 containing nopT or pCAMBIA2301 alone (EV) was used for transformation of leaves of 4–5 weeks Arabidopsis plants according to previously described procedures (Shao et al., 2003). Subcellular localization analysis of NopT proteins was performed with N. benthamiana cells expressing proteins containing a C-terminal GFP tag. Protoplasts were isolated 48 h postagroinfiltration of leaves and analyzed by fluorescence microscopy. Details of the procedure are shown in Supplemental Text S1. Agrobacteriumtumefaciens GV3101 carrying various DEX-inducible constructs was used for gene expression in N. benthamiana as described previously (Ade et al., 2007) with minor modifications. Overnight grown cultures were resuspended in 10 mM MgCl2 (OD600 ∼ 0.62), supplemented with 100 μM acetosyringone and incubated for 3 h at room temperature. Bacterial infiltration was performed with leaves of 3- to 4-week-old N. benthamiana plants. For experiments requiring co-expression of different genes, corresponding bacterial suspensions were mixed at equal ratios. Gene expression was induced 42 h postagroinfiltration by spraying the leaves with 50 μM DEX. Samples were collected at different time points. Where indicated, cell death responses were visualized by photographing the leaves with a UV camera (Syngene G: BOX-HR-E-M imaging system [Syngene; Bangalore, India]) or with a D7100 Nikon camera.
Cleavage of PBS1 proteins by effector proteases
NopT and AvrPphB were co-expressed with PBS1 proteins in N. benthamiana leaves. The expressed proteins contained a HA or FLAG tag. Samples were frozen in liquid nitrogen at different time points after DEX application. Information on protein extraction, SDS-PAGE and Western blot analysis is provided in Supplemental Text S1.
Hairy root transformation
Agrobacterium rhizogenes-mediated hairy root transformation was used to co-express GmPBS1-15A and GUS in Nenfeng 15 and Beidou 35 soybeans. Details of the procedure are shown in Supplemental Text S1. Plants forming hairy roots were transferred to plastic jar units (see above) and inoculated with S. fredii USDA257 2 days later. Nodule formation was analyzed 28 days postinoculation. GUS staining was performed according to a published protocol (D’Haeze et al., 1998). Transformation efficiency was estimated by counting the number of GUS-stained hairy roots per plant and by determining the ratio of GUS-stained (blue) roots to nonstained roots based on a gridline intersection method (Giovannetti and Mosse, 1980).
mRNA sequencing of the cultivars Nenfeng 15 and Beidou 35
Transcriptome sequencing was performed by Biomarker Technologies (Beijing, China) using cv. Williams 82 as reference genome. To identify AtRPS5-related sequences, obtained cDNAs were translated into amino acid sequences and a protein Blast was performed with AtRPS5 as a query sequence. Further details are provided in Supplemental Text S1.
Sequence alignment and phylogenetic analysis
Alignment of nucleotide or protein sequences was performed with Clustal W software (Thompson et al., 1994). Manual postalignment adjustment was performed for the alignment shown in Figure 2B. Phylogenetic analysis of nucleotide sequences related to the nopT sequence of Sinorhizobium sp. NGR234 was conducted in MEGA X (Kumar et al., 2018) using the Maximum-Likelihood method based on the Tamura–Nei model (Tamura and Nei, 1993).
Accession numbers
GmPBS1-1, GmPBS1-2, and GmPBS1-3 sequences of soybean cv. Nenfeng 15 obtained from mRNA sequencing (identical to corresponding sequences in cv. Beidou 35) were submitted to the GenBank database (accession numbers OK338064, OK338065, and OK338066). The accession numbers of AtPBS1 (AT5g13160) and AtRPS5 (At1g12220) are AY078037 and BT010583, respectively. Accession numbers for nopT sequences and avrPphB are shown in Figure 2.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Results of the effector translocation test (36 h after bacterial infiltration).
Supplemental Figure S2. Analysis of NopT forms produced by S. fredii USDA257.
Supplemental Figure S3. Amino acid sequence alignment of proposed NopT forms secreted by S. fredii USDA257 with NopT of Sinorhizobium sp. NGR234.
Supplemental Figure S4. Amino acid sequence alignment of a hypothetical NopT protein of S. fredii USDA257 (in an ancestral genome) with NopT of Sinorhizobium sp. NGR234.
Supplemental Figure S5. NGR234 and NGRΔnopT do not induce nodules on soybean cv. Nenfeng 15.
Supplemental Figure S6. Co-expression of two proteins and expression of NopT alone does not induce visible responses in N. benthamiana leaves.
Supplemental Figure S7. AvrPphB co-expressed with GmPBS1-1/AtRPS5 induces necrosis in N. benthamiana leaves.
Supplemental Figure S8. Western blot analysis of PBS1 proteins co-expressed with AvrPphB-HA and NopT-HA in N. benthamiana (7 h after DEX application).
Supplemental Figure S9. AvrPphB and NopT protein sequences differ at their autocleavage site.
Supplemental Figure S10. GUS-staining results of hairy roots of soybeans transformed with a GmPBS1-15A construct.
Supplemental Table S1. NLR sequences derived from cDNA of cv. Nenfeng 15 showing highest similarity with AtRPS5.
Supplemental Table S2. Strains and plasmids used in this study.
Supplemental Table S3. Primers used in this study.
Supplementary Material
Acknowledgments
We thank Qi Sun, Christian Wagner, and all laboratory members for their help with various aspects of this work. DNA encoding AvrPphB was kindly provided by Jian-Min Zhou (National Institute of Biological Sciences, Beijing, China), X. campestris strains and pepper seeds by Wei Jiang (Guangxi University, Nanning, China), pFAJ1702 and pFAJ1703 by Jan Michiels (Katholieke Universiteit Leuven, Heverlee, Belgium), pTA7002 by Nam-Hai Chua (The Rockefeller University, New York, USA), S. fredii USDA257 by Patrick E. Elia (USDA Agricultural Research Service, Beltsville, USA), soybean seeds by Dawei Xin (Northeast Agricultural University, Harbin, China), Arabidopsis mutants by Roger W. Innes (Indiana University, Bloomington, USA), and N. benthamiana seeds by Jian-Feng Li (Sun Yat-sen University).
Funding
This work was supported by the National Natural Science Foundation of China (grants 32170256 and 31470197), by the Guangdong Natural Science Foundation (Grants 2016A030313299 and 2017B030311005), the Science Foundation of the State Key Laboratory of Biocontrol, and the Guangdong Key Laboratory of Plant Resources.
Conflict of interest statement. None declared.
Contributor Information
Asaf Khan, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, East Campus, 510006 Guangzhou, China.
Syed F Wadood, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, East Campus, 510006 Guangzhou, China.
Min Chen, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, East Campus, 510006 Guangzhou, China.
Yan Wang, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, East Campus, 510006 Guangzhou, China.
Zhi-Ping Xie, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, East Campus, 510006 Guangzhou, China.
Christian Staehelin, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, East Campus, 510006 Guangzhou, China.
A.K., S.F.W., M.C., and Y.W. performed the research. All authors designed the experiments and analyzed the data. C.S. wrote the manuscript with the help of A.K., S.F.W., and Z.-P.X.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Christian Staehelin (cst@mail.sysu.edu.cn).
References
- Ade J, DeYoung BJ, Golstein C, Innes RW (2007) Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Procs Natl Acad Sci USA 104: 2531–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers P, Üstün S, Witzel K, Kraner M, Börnke F (2019) A remorin from Nicotiana benthamiana interacts with the Pseudomonas type-III effector protein HopZ1a and is phosphorylated by the immune-related kinase PBS1. Mol Plant Microbe Interact 32: 1229–1242 [DOI] [PubMed] [Google Scholar]
- Anderson JP, Gleason CA, Foley RC, Thrall PH, Burdon JB, Singh KB (2010) Plants versus pathogens: an evolutionary arms race. Funct Plant Biol 37: 499–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Chua NH (1997) A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11: 605–612 [DOI] [PubMed] [Google Scholar]
- Berrabah F, Ratet P, Gourion B (2019) Legume nodules: massive infection in the absence of defense induction. Mol Plant Microbe Interact 32: 35–44 [DOI] [PubMed] [Google Scholar]
- Cao Y, Halane MK, Gassmann W, Stacey G (2017) The role of plant innate immunity in the legume-rhizobium symbiosis. Annu Rev Plant Biol 68: 535–561 [DOI] [PubMed] [Google Scholar]
- Carter ME, Helm M, Chapman AV, Wan E, Restrepo Sierra AM, Innes RW, Bogdanove AJ, Wise RP (2019) Convergent evolution of effector protease recognition by Arabidopsis and barley. Mol Plant Microbe Interact 32: 550–565 [DOI] [PubMed] [Google Scholar]
- Chen M, Xiang QW, Ge YY, Huang QY, Liang Y, Xu CC, Zhang D, Zhang MX, Zhu PF, Xie ZP, et al. (2016) Use of a Xanthomonas/pepper translocation system for characterization of rhizobial type 3 effectors. InSayyed RZ, Reddy MS, Al-Turki AI, eds, Recent Trends in PGPR Research for Sustainable Crop Productivity. Scientific Publishers, Jodhpurt, India, pp 174–178 [Google Scholar]
- D'Haeze W, Gao M, De Rycke R, Van Montagu M, Engler G, Holsters M (1998) Roles for azorhizobial Nod factors and surface polysaccharides in intercellular invasion and nodule penetration, respectively. Mol Plant Microbe Interact 11: 999–1008 [Google Scholar]
- Dai WJ, Zeng Y, Xie ZP, Staehelin C (2008) Symbiosis-promoting and deleterious effects of NopT, a novel type 3 effector of Rhizobium sp. strain NGR234. J Bacteriol 190: 5101–5110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung BJ, Qi D, Kim SH, Burke TP, Innes RW (2012) Activation of a plant nucleotide binding-leucine rich repeat disease resistance protein by a modified self protein. Cell Microbiol 14: 107–-1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrecht B, Vanderleyden J, Michiels J (2001) Stable RK2-derived cloning vectors for the analysis of gene expression and gene function in gram-negative bacteria. Mol Plant Microbe Interact 14: 426–430 [DOI] [PubMed] [Google Scholar]
- Dowen RH, Engel JL, Shao F, Ecker JR, Dixon JE (2009) A family of bacterial cysteine protease type III effectors utilizes acylation-dependent and-independent strategies to localize to plasma membranes. J Biol Chem 284: 15867–15879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76: 1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis CT, Dimou M, Georgakopoulos DG, Katinakis P, Tampakaki AP (2012) Functional characterization of NopT1 and NopT2, two type III effectors of Bradyrhizobium japonicum. FEMS Microbiol Lett 327: 66–77 [DOI] [PubMed] [Google Scholar]
- Ge YY, Xiang QW, Wagner C, Zhang D, Xie ZP, Staehelin C (2016) The type 3 effector NopL of Sinorhizobium sp. strain NGR234 is a mitogen-activated protein kinase substrate. J Exp Bot 67: 2483–2494 [DOI] [PubMed] [Google Scholar]
- Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84: 489–500 [Google Scholar]
- Heckman KL, Pease LR (2007) Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc 2: 924. [DOI] [PubMed] [Google Scholar]
- Helm M, Qi M, Sarkar S, Yu H, Whitham SA, Innes RW (2019) Engineering a decoy substrate in soybean to enable recognition of the soybean mosaic virus NIa protease. Mol Plant Microbe Interact 32: 760–769 [DOI] [PubMed] [Google Scholar]
- Jiang W, Jiang BL, Xu RQ, Huang JD, Wei HY, Jiang GF, Cen WJ, Liu J, Ge YY, Li GH, et al. (2009) Identification of six type III effector genes with the PIP box in Xanthomonas campestris pv. campestris and five of them contribute individually to full pathogenicity. Mol Plant Microbe Interact 22: 1401–1411 [DOI] [PubMed] [Google Scholar]
- Kambara K, Ardissone S, Kobayashi H, Saad MM, Schumpp O, Broughton WJ, Deakin WJ (2009) Rhizobia utilize pathogen-like effector proteins during symbiosis. Mol Microbiol 71: 92–106 [DOI] [PubMed] [Google Scholar]
- Kimbrel JA, Thomas WJ, Jiang Y, Creason AL, Thireault CA, Sachs JL, Chang JH (2013) Mutualistic co-evolution of type III effector genes in Sinorhizobium fredii and Bradyrhizobium japonicum. PLoS Pathog 9: e1003204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sun M, Chen Q, Xin D, Sun X (2021) Mapping quantitative trait loci related to nodule number in soybean (Glycine max (L.) Merr.) in response to the Sinorhizobium (Ensifer) fredii HH103 NopT type III effector. J Plant Interact 16: 126–135 [Google Scholar]
- López-Baena FJ, Ruiz-Sainz JE, Rodríguez-Carvajal MA, Vinardell JM (2016) Bacterial molecular signals in the Sinorhizobium fredii-soybean symbiosis. Int J Mol Sci 17: 755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Liu D, Jiao S, Liu S, Wang X, Shen X, Wei G (2020) Identification of Robinia pseudoacacia target proteins responsive to Mesorhizobium amphore CCNWGS0123 effector protein NopT. J Exp Bot 71: 7347–7363 [DOI] [PubMed] [Google Scholar]
- Miwa H, Okazaki S (2017) How effectors promote beneficial interactions. Curr Opin Plant Biol 38: 148–154 [DOI] [PubMed] [Google Scholar]
- Nimchuk Z, Marois E, Kjemtrup S, Leister RT, Katagiri F, Dangl JL (2000) Eukaryotic fatty acylation drives plasma membrane targeting and enhances function of several type III effector proteins from Pseudomonas syringae. Cell 101: 353–363 [DOI] [PubMed] [Google Scholar]
- Perret X, Staehelin C, Broughton WJ (2000) Molecular basis of symbiotic promiscuity. Microbiol Mol Biol Rev 64: 180–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottinger SE, Innes RW (2020) RPS5-Mediated disease resistance: fundamental insights and translational applications. Annu Rev Phytopathol 58: 139–160 [DOI] [PubMed] [Google Scholar]
- Rehman HM, Cheung W-L, Wong KS, Xie M, Luk CY, Wong FL, Li MW, Tsai SN, To WT, Chan LY, et al. (2019) High-throughput mass spectrometric analysis of the whole proteome and secretome from Sinorhizobium fredii strains CCBAU25509 and CCBAU45436. Front Microbiol 10: 2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Liu W, Nandety RS, Crook A, Mysore KS, Pislariu CI, Frugoli J, Dickstein R, Udvardi MK (2020) Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 32: 15–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW (2003) Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301: 1230–1233 [DOI] [PubMed] [Google Scholar]
- Shao F, Merritt PM, Bao Z, Innes RW, Dixon JE (2002) A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109: 575–588 [DOI] [PubMed] [Google Scholar]
- Songwattana P, Chaintreuil C, Wongdee J, Teulet A, Mbaye M, Piromyou P, Gully D, Fardoux J, Zoumman AMA, Camuel A, et al. (2021) Identification of type III effectors modulating the symbiotic properties of Bradyrhizobium vignae strain ORS3257 with various Vigna species. Sci Rep 11: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin C, Forsberg LS, D’Haeze W, Gao M-Y, Carlson RW, Xie ZP, Pellock BJ, Jones KM, Walker GC, Streit WR, et al. (2006) Exo-oligosaccharides of Rhizobium sp. strain NGR234 are required for symbiosis with various legumes. J Bacteriol 188: 6168–6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin C, Krishnan HB (2015) Nodulation outer proteins: double-edged swords of symbiotic rhizobia. Biochem J 470: 263–274 [DOI] [PubMed] [Google Scholar]
- Sugawara M, Takahashi S, Umehara Y, Iwano H, Tsurumaru H, Odake H, Suzuki Y, Kondo H, Konno Y, Yamakawa T, et al. (2018) Variation in bradyrhizobial NopP effector determines symbiotic incompatibility with Rj2-soybeans via effector-triggered immunity. Nat Commun 9: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Huang G, Fan F, Wang S, Zhang Y, Han Y, Zou Y, Lu D (2017) Comparative study of Arabidopsis PBS1 and a wheat PBS1 homolog helps understand the mechanism of PBS1 functioning in innate immunity. Sci Rep 7: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10: 512–526 [DOI] [PubMed] [Google Scholar]
- Teulet A, Busset N, Fardoux J, Gully D, Chaintreuil C, Cartieaux F, Jauneau A, Comorge V, Okazaki S, Kaneko T, et al. (2019) The rhizobial type III effector ErnA confers the ability to form nodules in legumes. Proc Natl Acad Sci USA 116: 21758–21768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Töpfer R, Matzeit V, Gronenborn B, Schell J, Steinbiss HH (1987) A set of plant expression vectors for transcriptional and translational fusions. Nucleic Acids Res 15: 5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang J, Liu C, Ma C, Li C, Zhang Y, Qi Z, Zhu R, Shi Y, Zou J, et al. (2018) Identification of soybean genes whose expression is affected by the Ensifer fredii HH103 effector protein NopP. Int J Mol Sci 19: 3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang J, Ma C, Zhou Z, Yang D, Zheng J, Wang Q, Li H, Zhou H, Sun Z, et al. (2020) QTL mapping and data mining to identify genes associated with the Sinorhizobium fredii HH103 T3SS effector NopD in soybean. Front Plant Sci 11: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RF, Henk A, Mowery P, Holub E, Innes RW (1998) A mutation within the leucine-rich repeat domain of the Arabidopsis disease resistance gene RPS5 partially suppresses multiple bacterial and downy mildew resistance genes. Plant Cell 10: 1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RF, Merritt PM, Holub E, Innes RW (1999) Identification of three putative signal transduction genes involved in R gene-specified disease resistance in Arabidopsis. Genetics 152: 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang QW, Bai J, Cai J, Huang QY, Wang Y, Liang Y, Zhong Z, Wagner C, Xie ZP, Staehelin C (2020) NopD of Bradyrhizobium sp. XS1150 possesses SUMO protease activity. Front Microbiol 11: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin DW, Liao S, Xie ZP, Hann DR, Steinle L, Boller T, Staehelin C (2012) Functional analysis of NopM, a novel E3 ubiquitin ligase (NEL) domain effector of Rhizobium sp. strain NGR234. PLoS Pathog 8: e1002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CC, Zhang D, Hann DR, Xie ZP, Staehelin C (2018) Biochemical properties and in planta effects of NopM, a rhizobial E3 ubiquitin ligase. J Biol Chem 293: 15304–15315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RQ, Blanvillain S, Feng JX, Jiang BL, Li XZ, Wei HY, Kroj T, Lauber E, Roby D, Chen B, et al. (2008) AvrACXcc8004, a type III effector with a leucine-rich repeat domain from Xanthomonas campestris pathovar campestris confers avirulence in vascular tissues of Arabidopsis thaliana ecotype Col-0. J Bacteriol 190: 343–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Tang F, Gao M, Krishnan HB, Zhu H (2010) R gene-controlled host specificity in the legume–rhizobia symbiosis. Proc Natl Acad Sci USA 107: 18735–18740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wang M, Sun Y, Zhao P, Liu C, Qing K, Hu X, Zhong Z, Cheng J, Wang H, et al. (2021) Glycine max NNL1 restricts symbiotic compatibility with widely distributed bradyrhizobia via root hair infection. Nat Plants 7: 73–86 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, et al. (2010) Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7: 290–301 [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen XJ, Lu HB, Xie ZP, Staehelin C (2011) Functional analysis of the type 3 effector nodulation outer protein L (NopL) from Rhizobium sp. NGR234: symbiotic effects, phosphorylation, and interference with mitogen-activated protein kinase signaling. J Biol Chem 286: 32178–32187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Shao F, Innes RW, Dixon JE, Xu Z (2004) The crystal structure of Pseudomonas avirulence protein AvrPphB: a papain-like fold with a distinct substrate-binding site. Proc Natl Acad Sci USA 101: 302–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.