Abstract
Background:
Few treatments exist for the cognitive symptoms of schizophrenia. Pharmacological agents resulting in glutamate N-methyl-d-aspartate (NMDA) receptor hypofunction, such as MK-801, mimic many of these symptoms and disrupt neural activity. Recent evidence suggests that deep brain stimulation (DBS) of the medial septal nucleus (MSN) can modulate medial prefrontal cortex (mPFC) and hippocampal activity and improve spatial memory.
Objective:
Here, we examine the effects of acute MK-801 administration on oscillatory activity within the septohippocampal circuit and behavior. We also evaluate the potential for MSN stimulation to improve cognitive behavioral measures following MK-801 administration.
Methods:
59 Sprague Dawley male rats received either acute intraperitoneal (IP) saline vehicle injections or MK-801 (0.1 mg/kg). Theta (5–12 Hz), low gamma (30–50 Hz) and high frequency oscillatory (HFO) power were analyzed in the mPFC, MSN, thalamus and hippocampus. Rats underwent MSN theta (7.7 Hz), gamma (100 Hz) or no stimulation during behavioral tasks (Novel object recognition (NOR), elevated plus maze, Barnes maze (BM)).
Results:
Injection of MK-801 resulted in frequency-specific changes in oscillatory activity, decreasing theta while increasing HFO power. Theta, but not gamma, stimulation enhanced the anxiolytic effects of MK-801 on the elevated plus maze. While MK-801 treated rats exhibited spatial memory deficits on the Barnes maze, those that also received MSN theta, but not gamma, stimulation found the escape hole sooner.
Conclusions:
These findings demonstrate that acute MK-801 administration leads to altered neural activity in the septohippocampal circuit and impaired spatial memory. Further, these findings suggest that MSN theta-frequency stimulation improves specific spatial memory deficits and may be a possible treatment for cognitive impairments caused by NMDA hypofunction.
Keywords: MK-801, Hippocampus, Medial septal nucleus, theta, Gamma, Deep brain stimulation, Spatial working memory
1. Introduction
Schizophrenia is a severe neuropsychiatric disorder that affects 1% of the population.(Stepnicki et al., 2018) Current treatments, including antipsychotic drugs, supportive psychotherapy, and vocational and cognitive behavioral therapy, are predominantly effective for controlling positive symptoms (56–81% response rate)(Dunayevich et al., 2006; Haddad and Correll, 2018; Lambert et al., 2010; Leucht et al., 2017) but offer little relief for negative (Elis et al., 2013; Lambert et al., 2010; Lincoln et al., 2012) and cognitive symptoms (<40% response rate). (Buchanan et al., 2017; Carbon and Correll, 2014; Elis et al., 2013) Furthermore, medications often have debilitating side effects, necessitating new treatment options.(Stroup et al., 2000).
Development of novel treatments for schizophrenia is limited by the fact that the pathophysiology underlying the disease is not well understood. In addition to dysregulation of the dopamine and glutamatergic axes, there is evidence of dysfunction in neural networks involved in spatial navigation and memory. The mesolimbic system, which plays a crucial role in cognition and sensorimotor processing,(Green and Arduini, 1954; Vertes, 2005) has been found to be altered in schizophrenia.(Uhlhaas and Singer, 2010) Specifically, the septohippocampal circuit plays a crucial role in multiple cognitive and emotional constructs seen in schizophrenia, including memory, attention, anxiety, and sensorimotor processing.(Green and Arduini, 1954; Menard and Treit, 1996; Tsanov, 2017; Vertes, 2005) This circuit is primarily composed of the medial prefrontal cortex (mPFC), medial septal nucleus (MSN), and hippocampus (Fig. 1).
Fig. 1.
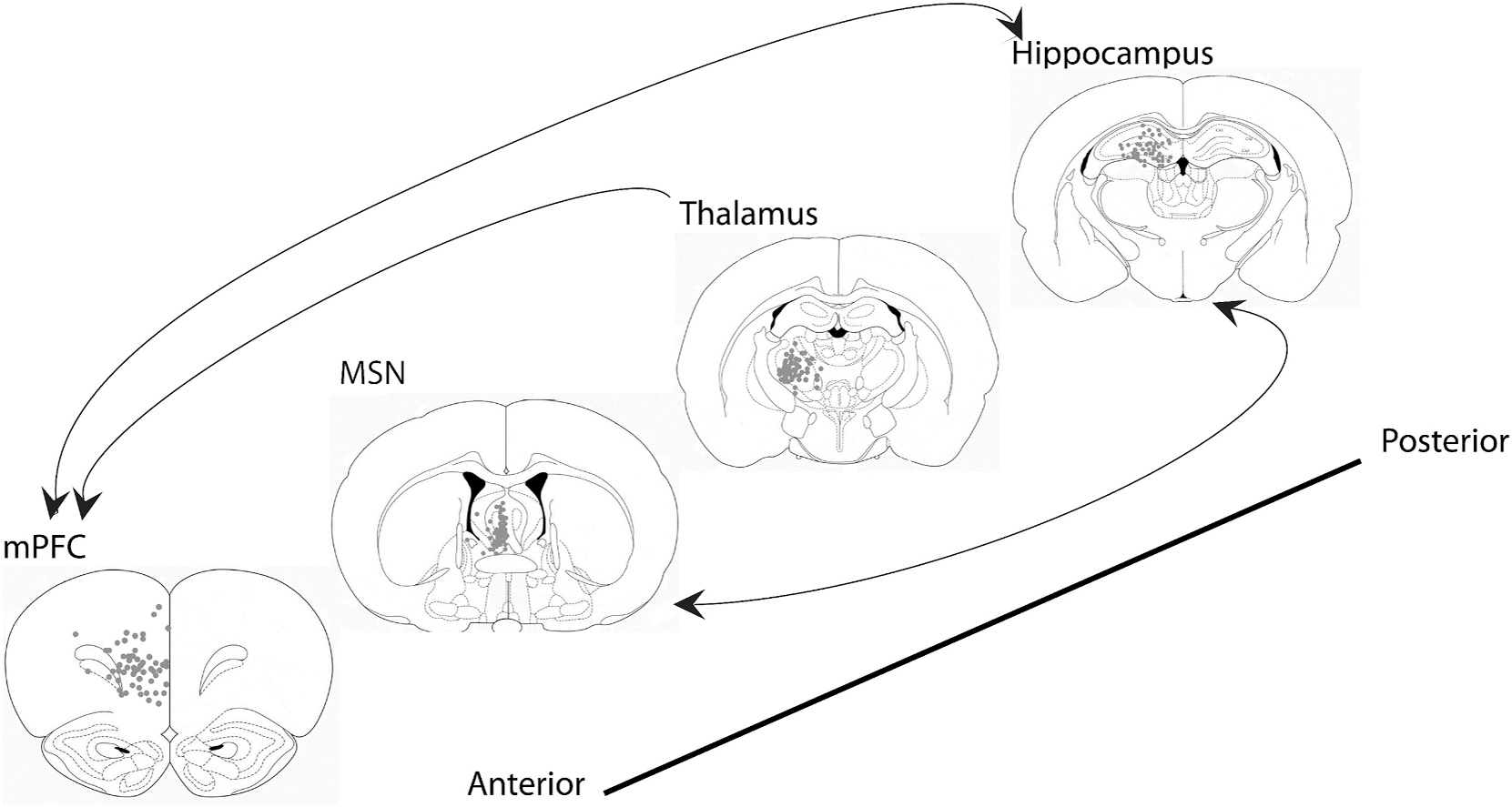
Atlas schematic and electrode localization of the MSN-hippocampal-mPFC-thalamic circuit. Representative coronal sections (from anterior to posterior) of the mPFC, MSN, thalamus and hippocampus demonstrate the location of electrode tips. Arrows demonstrate canonical directionality of neural transmission. Rats with stimulating electrodes not within the MSN or recording electrodes not within the mPFC, thalamus or hippocampus were excluded from the study. (Figures E-H modified from the atlas of “The rat brain in stereotaxic coordinates, 5th Edition” by Paxinos and Watson).
Mounting evidence suggests that glutamate hypofunction may underlie neural network dysfunction associated with schizophrenia.(Amitai and Markou, 2010; Billingslea et al., 2014; Carlen et al., 2012; Cousijn et al., 2015; Finlay et al., 2015; Javitt, 2000; Javitt, 2007; Javitt et al., 2004; Korotkova et al., 2010; Lee et al., 2018; Paine and Carlezon Jr., 2009) Hippocampal NMDA receptors in parvalbumin-positive interneurons have been shown to mediate hippocampal oscillatory synchrony,(Korotkova et al., 2010) facilitating learning and memory. NMDA receptor antagonist drugs, such as MK-801 and ketamine, have been found to both decrease hippocampal theta (5–12 Hz) frequency activity,(Carlen et al., 2012; Cousijn et al., 2015; Korotkova et al., 2010; Lee et al., 2018) and increase high frequency oscillatory activity (HFO, 130–180 Hz) (Olszewski et al., 2013; Ye et al., 2018) leading to deficits in spatial memory, attention, and social interaction.(Carlen et al., 2012; Korotkova et al., 2010).
Given the role that neural network dysfunction plays in schizophrenia spectrum disorders, there has been increasing interest in utilizing neuromodulation strategies to treat such disorders. Deep brain stimulation (DBS) is a form of neuromodulation that applies electrical stimulation to focal regions of the brain to modulate the activity of specific neural networks. It is used to treat diseases with underlying neural network dysfunction, including movement disorders such as Parkinson’s disease, essential tremor, and dystonia, as well as cognitive and psychiatric disorders, such as obsessive-compulsive disorder and epilepsy. Although its use in psychiatric disorders is in its nascent phase, there is evidence from rodent studies that DBS may also be beneficial in improving cognitive symptoms.(Ma and Leung, 2014) Specifically, we are targeting the MSN, which is known to generate and maintain hippocampal theta oscillations.(Buzsaki, 2005; McNaughton et al., 2006) Moreover, we have previously demonstrated that exogenous low frequency MSN theta stimulation can restore hippocampal theta oscillations and improve spatial working memory in disease processes with abnormal hippocampal theta oscillations such as traumatic brain injury and epilepsy.(Lee et al., 2015; Lee et al., 2017).
In this current study, we examined the effects of MK-801 on septohippocampal circuitry oscillations and evaluated the potential of MSN theta stimulation to restore spatial memory deficits in MK-801 treated male rats.
2. Methods
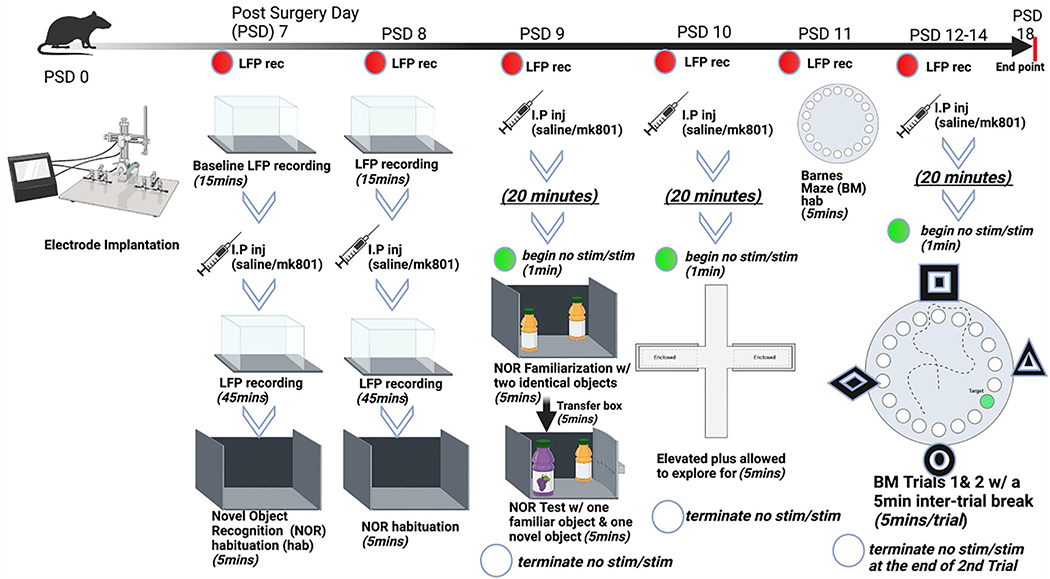
Fifty-nine adult male Sprague-Dawley rats (Charles River Laboratories; Hollister, CA) underwent unilateral implantation of medial prefrontal cortex (mPFC), reticular thalamic, and hippocampal recording electrodes as well as two ipsilateral medial septal nucleus (MSN) stimulating electrodes. Intraperitoneal (IP) injections of either 0.1 mg/kg (+)MK-801 hydrogen maleate (CAS # 77086–22-7, M107, Sigma-Aldrich, St. Louis, MO) (n = 33) or saline of equivalent volume: 0.1–0.3 ml (n = 26) were administered after baseline local field potential (LFP) recordings and twenty minutes prior to each behavioral task. All rats went through all behavioral tasks: (1) novel object recognition (NOR), (2) elevated plus maze, and (3) Barnes maze (BM) (Fig. 2). During behavioral tasks, theta (7.7 Hz), gamma (100 Hz) or no stimulation were administered one minute prior to each task and were terminated at the end of each task. Video-LFP recordings were collected throughout the entirety of every task. Saline-treated rats were divided into three groups: no stimulation (n = 14), theta (7.7 Hz) stimulation (n = 6) or gamma (100 Hz) stimulation (n = 6). MK-801-treated rats were divided into three groups: (1) no stimulation (n = 12), (2) theta stimulation (n = 12) or (3) gamma stimulation (n = 9). Rats were housed under 12-h light/dark cycles with access to standard rat chow and water ad lib. All procedures were approved by the University of Southern California, Institutional Animal Care and Use Committee (IACUC #20946).
Fig. 2.
Experimental Design. This graphical representation demonstrates the timeline of the experiments. PSD: post-surgery day, LFP: local field potential, IP: intraperitoneal.
2.1. Electrode placement
Rats were anesthetized using 4% isoflurane in O2/N2O (1:2) carrier gas and sustained at 2% isoflurane to maintain surgical anesthesia. Under aseptic conditions, a midline incision on the scalp and circular 2 mm diameter craniotomies were performed to implant tungsten electrodes (E363T/2/SPC ELEC 0.008”/.2MM, Plastics One Inc., Roanoke, VA) into the right mPFC (+3.8 mm A), +0.6 mm L, −3.8 mm DV), right MSN (−0.5 mm AP, 1.0 mm L; −6.8 mm DV at 12.8 degrees to avoid the dural sinus), right reticular thalamus (−2.0 mm AP, +2.4 mm L; −6.0 mm DV), and right CA3-CA1 dorsal hippocampal fissure (AP -3.3 mm from bregma, lateral +2.0 mm from midline, depth: −3.6 mm). An additional electrode was implanted into the corpus callosum (+1.5 mm AP; +1.0 mm L; −4.0 mm DV) as a reference electrode. The electrodes were then wired into an electrode interface board (EIB) and secured to the skull with screws and methylmethacrylate. The locations of the mPFC, thalamic, and hippocampal recording electrodes and MSN stimulating electrodes are shown in Fig. 1. Data from electrodes not found to be in target regions were removed from further analysis (n = 3).
2.2. Local field potential recordings
On post-surgery day (PSD) 7, a 15-min baseline local-field potential (LFP) recording was taken prior to the administration of either saline or MK-801. After injection, an additional 45 min of LFP activity was recorded in an observation box (enclosed translucent Plexiglas cage, 25×45×50cm). LFP activity was amplified through a unity gain headstage (HS-16-QC, Neuralnyx) and recorded using the Cheetah Digital Lynx SX Data Acquisition System (Neuralynx, Bozeman, MT). Measures of power spectral density were obtained using Thomson’s multi-taper analysis (pmtm() in Matlab’s Signal Processing Toolbox). To compare power before and after injection, the power spectral density estimate from the post-injection time period was subtracted from the preinjection period and divided by the total of the two periods (Post−Pre)/(Pre + Post) to create a normalized measure between −1 to 1 to compare across animals. These difference measures were then averaged for frequency bands of interest (theta: 5–12 Hz, low gamma: 30–50 Hz, HFO: 130–180) for each rat.
2.3. Medial septal nucleus stimulation paradigm
Electrical stimulation of the MSN was delivered using the following stimulation parameters: 7.7 Hz or 100 Hz frequency, 100 μsec square wave pulse width, and current of 80 μA (STG 4008, Multi Channel Systems, Baden-Württemberg, Germany). During behavioral tasks, stimulation was started one minute prior to each trial or task and terminated at the end of the task.(Lee et al., 2013; Lee et al., 2015).
2.4. Novel object recognition
On post-surgery day (PSD) 9, rats were evaluated on a novel object recognition (NOR) task to test attention and memory.(Antunes and Biala, 2012) The task consists of three phases: habituation (carried out on PSD 7 and 8 after LFP recordings), familiarization (PSD 9), and test phase (PSD 9). All phases were carried out in the same platform (100 cm × 100 cm × 50 cm). On PSD 7 and 8 animals were allowed to habituate under ambient light without any stimulation or any objects present and allowed to explore the test platform for five minutes. On PSD 9, rats were administered an IP injection of either saline or MK-801. Twenty minutes after injection, rats were exposed to theta, gamma or no stimulation starting one minute prior to a task and throughout the task. For the novel object recognition task, rats were evaluated for object exploration with two identical objects (familiarization phase) for five minutes on the platform. During a five-minute inter-phase interval, the rats were returned to their travel box (under continuous stimulation). Rats were then re-evaluated with one of the identical objects replaced with a novel, but similar object (test phase) for 5 five minutes on the platform. Video-LFP recordings were collected throughout all three phases. The time spent exploring the novel object during the test phase was analyzed.
2.5. Elevated plus maze
On PSD 10, the elevated plus maze was utilized to evaluate anxiety. (Feyissa et al., 2017) The maze consists of four arms arranged in a plusshape (110.49 cm × 10.16 cm × 80.01 cm). Two of the arms facing opposite each other were closed with sidewalls, and the other two arms were open. The closed arms are considered a safe, comfortable environment for the rat, whereas the open arms are open and reveal an elevated, anxiety-induced space. On PSD 10, twenty minutes post IP injection of either saline or MK-80, rats either underwent theta, gamma, or no stimulation for one minute prior to the behavior. The stimulation continued during the behavior, where the rat was placed at the center of the maze and subsequently allowed to explore for five minutes. Video-LFP recordings were collected throughout the entire five-minute test phase. The number of entries into the open arms was quantified.
2.6. Barnes maze
On PSDs 12–14 the Barnes Maze (BM) was performed as previously described to evaluate for spatial memory, spatial reference memory (short and long term), and cognitive flexibility.(Barnes, 1979a; Lee et al., 2013; Lee et al., 2015) The Barnes maze consists of a black, circular, acrylonitrile butadiene styrene (ABS) plastic table with a diameter of 122 cm. There were twenty (10 cm in diameter) holes spaced equally around the periphery of the table with a dark escape box (19.69 cm × 11.43 cm × 11.75 cm) under the designated target hole. Black curtains surrounded the table with four distinct visual cues spaced equally on the curtains. On PSD 11, neither IP injection nor stimulation was administered. Each rat was connected to the LFP recording system and allowed five minutes to habituate under ambient lighting to the BM platform without the presence of visual cues, the escape box, or white noise. LFP recordings were collected during the habituation phase. On PSDs 12–14, each test trial was characterized by bright overhead lights, visual cues around the periphery, and continuous white noise. The escape box was placed underneath the target hole, and each rat received either IP saline or MK-801 injection twenty minutes prior to the first trial of each day. A one-minute stimulation period (theta, gamma or no stimulation) preceded the start of the first trial and was continued throughout the intertrial interval and second trial. For each trial, the rat was first placed in an opaque, cylindrical container at the center of the table. After one minute, the container was raised, and the rats were allowed five minutes to find and enter the hole with the escape box. If the rat did not find the escape hole, it was placed inside the escape box at the end of the trial. After each trial, rats were kept in the escape box for one minute prior to being placed back in their travel box. Beginning on PSD 12, each animal was tested twice a day for a total of 3 days. Dependent variables included latency to find the escape box, search strategy pattern, and locomotor speed during each trial. Locomotor speed (distance traveled divided by escape latency) was calculated by determining path length during each Barnes maze trial using TopScan Lite version 2.0 (CleverSys Inc., Reston, Virginia).
Search strategies were categorized as spatial, peripheral, or random. (Lee et al., 2013; Lee et al., 2015) The spatial search strategy was defined as the rodent using direct pathways towards the escape hole, indicating the use of a spatial memory strategy. The peripheral search strategy was defined as circling the edge of the platform in a serial manner prior to finding the escape hole, implying the use of a non-spatial strategy to solve the task. The random search strategy was defined as searching nonconsecutive holes prior to the escape hole without a discernible search pattern.
2.7. Histology
Rats were euthanized 18 days after surgery by anesthesia (Isoflurane) and were transcardially perfused with 100 ml of 0.1 M sodium phosphate buffer saline (PBS, pH −7.4), followed by 50 ml of 4% paraformaldehyde (pH 7.4). Brains were extracted and stored in 4% paraformaldehyde at 4 °C. Serial coronal sections were cut at 100-μm thickness with a vibratome (Leica VT 1200; Leica Biosystems, Buffalo Grove, IL) starting at +3.8 mm Bregma and ending at −4.80 mm Bregma in order to capture all tissue spanning between the mPFC and the caudal aspects of the hippocampus. Sections in the vicinity of electrodes were mounted onto 0.1% gelatin-subbed slides and stained with NeuroTrace 530/615 Red Fluorescent Nissl Stain (N21482, ThermoFisher Scientific, Waltham, MA, USA).
2.8. Statistical analysis
Statistical analyses were performed using Matlab (The MathWorks Inc.) and R (R Core Team, 2021). Standard parametric tests (t-test, ANOVA) were used except when specified otherwise for data that did not meet the assumptions for parametric statistics. Bonferroni-Holm corrections to p-values were used to control for familywise error. A chi-square analysis was used to compare differences in search strategy between the groups on the Barnes maze. For all tests, alpha was set to 0.05 (two-tailed).
3. Results
3.1. ACUTE MK-801 administration alters oscillatory activity in a frequency-dependent manner
To determine the effect of MK-801 on the oscillatory activity in the septo-hippocampal circuit, we compared power in the theta (5–12 Hz), low gamma (30–50 Hz), and HFO (130–180 Hz) bands. Power analysis was conducted on a 5-min period two minutes before the end of each recording since peak behavioral activation occurs within 20–30 min of an MK-801 injection (Parras et al., 2020) and to reduce potential artifacts as rats habituated to the recording box.
To obtain a normalized change in power that could be averaged across rats, the post-injection power was subtracted from the preinjection power. The result was divided by the total power from both periods (Post−Pre)/(Post+Pre), resulting in a value between −1 and 1. Positive values indicate a relative increase in power while negative values indicate a relative decrease.
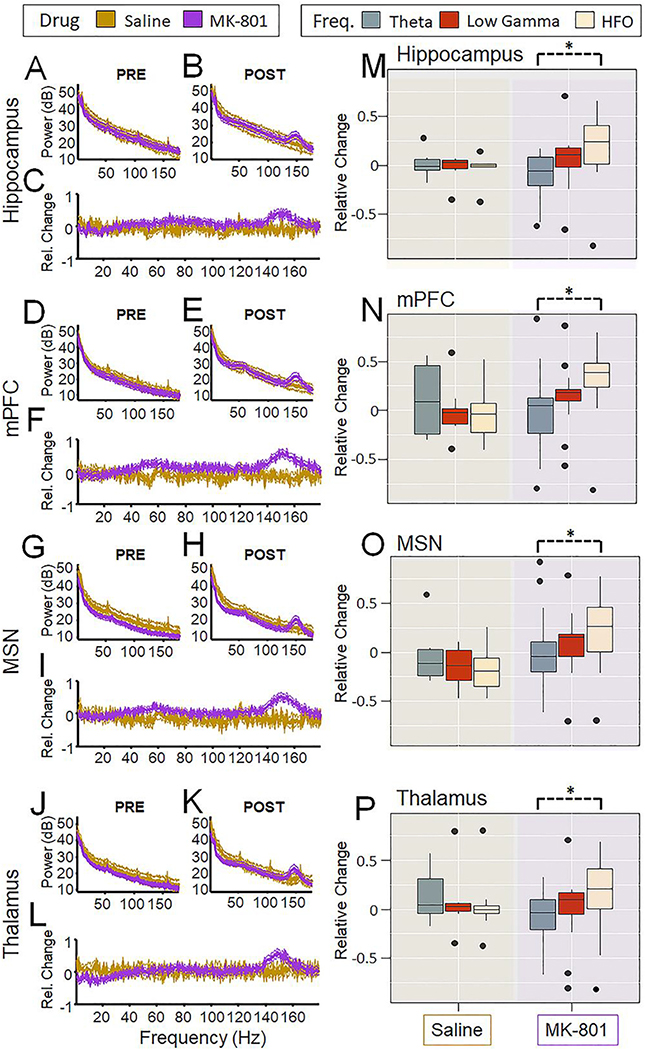
A multi-factorial ANOVA comparing the effects of region, drug, and frequency on power change revealed a significant interaction between the frequency and drug condition (F(1.5,24.7) = 5.84, p = 0.013); there was no main effect of region (F(1.6,26.7) = 0.65, p = 0.504) nor a drug × region × power interaction (F(3.0, 47.3) = 0.30, p = 0.82). Fig. 3 displays relative changes in power by drug and frequency band for each region. This suggests that across all regions examined, MK-801 had different impacts on the power by frequency, relatively decreasing theta power while increasing HFO power.
Fig. 3.
MK-801 has frequency-specific effects across the septo-hippocampal circuit. Power was compared before and after injection in Saline and MK-801 treated animals. Power spectral densities from the hippocampus (A) before injection and (B) after injection for both groups are shown. Lines indicate mean +/− SEM. (C) The relative change was measured by taking the difference in the power spectral density from the post-injection time period from the pre-injection time period and dividing by the total power of the two time periods together, yielding a normalized difference measure between −1 and 1. Lines indicate mean +/− SEM. This was repeated for the mPFC (D—F), the MSN (G-I), and the Thalamus (J-L). (M-P) To determine the effect of drug, frequency and region on normalized power change, a muti-factorial ANOVA was performed with drug as a between-groups variable and frequency and region as within-groups variables. There was a significant drug × frequency interaction condition (F (1.5,24.7) = 5.84, p = 0.013). This is denoted with a dashed line to indicate this effect was across regions as there was no significant main effect of region (F (1.6,26.7) = 0.65, p = 0.504) or drug × frequency × region interaction (F(3.0, 47.3) = 0.30, p = 0.82).
3.2. Novel object recognition is not altered following acute administration of MK-801
To evaluate the role of NMDA receptor antagonism in learning and memory, rats were assessed in the novel object recognition task. ANOVA revealed a main effect of stimulation type (F(2,38) = 3.67, p = 0.035; Fig. 4A), but no main effect of drug (saline vs. MK-801) or significant stimulation × drug interaction (F(2,38) = 2.97, p = 0.063). Post-hoc comparisons revealed that saline-gamma treated rats spent significantly more time with the novel object than did saline-theta (p = 0.046, Bonferroni-holm corrected) or saline- no stimulation rats (p = 0.046). Increased time spent exploring the novel item relative to the familiar item has been used as a metric for object recognition due to preference of rodents for novelty,(Leger et al., 2013) and studies indicate that this behavior is hippocampal-dependent.(Broadbent et al., 2010) This data suggests that gamma stimulation of unimpaired (saline-treated) rats may result in improved object recognition memory.
Fig. 4.
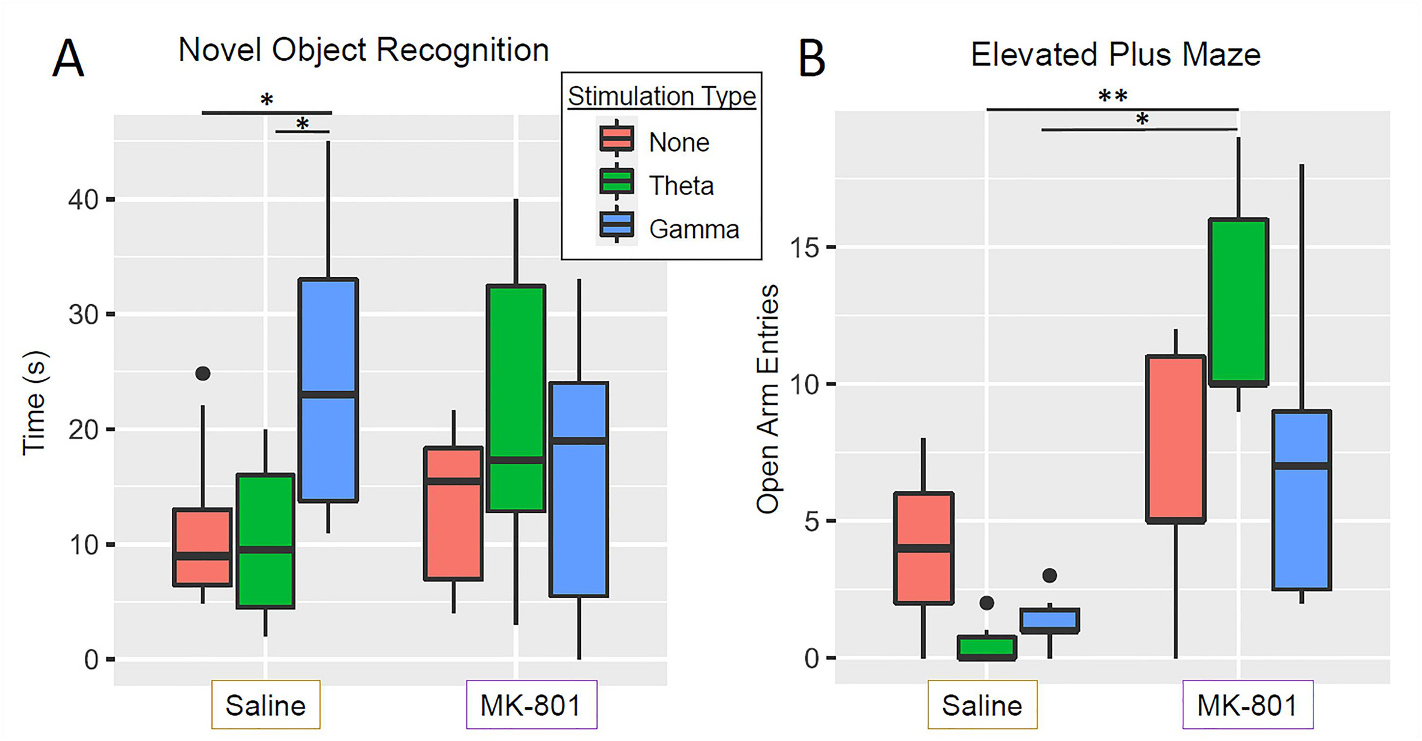
Novel object recognition and elevated plus maze results. (A) ANOVA revealed a significant main effect of stimulation type (F(2,38) = 3.67, p = 0.035) but not of drug (F(1,38) = 0.06, p = 0.807) nor a drug × stimulation type interaction (F(2,38) = 2.97, p = 0.063). Post-hoc pairwise t-tests revealed that saline-gamma stimulated rats spent significantly more time around the novel object than did saline-no stimulation rats (p = 0.046, Bonferroni-Holm corrected) and saline −theta rats (p = 0.046, Bonferroni-Holm corrected). (B) Evaluation of open arm entries on the elevated plus maze using a Kruskel wallis test showed a significant effect of group (X(5) = 19.85, p = 0.001) and post hoc Dunn tests showed a significant difference between MK-801- theta and saline-gamma (p = 0.022, Bonferroni-Holm corrected) and MK801-theta compared to saline-theta (p = 0.002, Bonferroni-Holm corrected). *p < 0.05, **p < 0.01, ***p < 0.001.
3.3. MSN theta stimulation reduces anxiety behaviors
To test the role of NMDA signaling in anxiety-associated behaviors, rats were evaluated on the elevated plus maze, in which a greater number of open-arm entries is considered to indicate lower anxiety. Kruskal-Wallis rank tests between the 6 groups revealed a significant effect of group (χ2 (5) = 19.85, p = 0.001; Fig. 4B), and post-hoc Dunn tests revealed that MK801- treated rats with MSN theta stimulation had significantly more open arm entries than either saline-treated rats with MSN theta stimulation (p = 0.022, Bonferroni-Holm corrected) or saline-treated rats with MSN gamma stimulation (p = 0.002, Bonferroni- Holm corrected). This suggests that theta stimulation may enhance the anxiolytic effects of MK-801.
3.4. Medial septal nucleus stimulation improves spatial memory following MK-801 administration
In order to evaluate the effects of MSN stimulation on MK-801 NMDA receptor antagonism-induced spatial memory impairments, rats were evaluated on the Barnes maze. Rodents exhibiting a shorter escape latency and more direct search strategies over time are associated with having better spatial memory. Rats performed the task over the course of three days and were evaluated on latency to find the escape hole, number of errors, and search strategy employed.
To determine the if there was an effect of day, drug, and stimulation type on the latency in which rats were able to find the escape hole, we performed a mixed ANOVA with 2 between-subjects factors (stimulation type and drug) and one within-subject factor (day) on log-transformed latency times. There was a significant main effect of stimulation type (F2,50) = 4.55, p = 0.015) and of day (F(2,100) = 28.84, p < 0.0001), and a significant stimulation × drug × day interaction (F(4,100) = 3.23, p = 0.016). Post hoc comparisons revealed that for MK-801 treated rats, MSN theta-stimulated rats had significantly shorter latencies to finding the escape hole than either MSN gamma-stimulated rats (p = 0.0008, Bonferroni-Holm corrected) or non-stimulated rats (p = 0.014). There was no significant difference; however, between the MSN gamma stimulated and non-stimulated rats (p = 0.126, Bonferroni-Holm corrected). Within saline treated rats, there was no significant difference between the non-stimulated rats and theta stimulated rats (p = 0.261); however, the gamma stimulated rats took significantly longer to find the escape hole than the non-stimulated rats (p = 0.012). This suggests that MSN gamma stimulation in unimpaired (saline-treated) rats results in worse spatial memory but does not alter impaired rats (MK-801-treated). Interestingly, MSN theta stimulation does not improve spatial memory in unimpaired (saline-treated) rats but improves spatial memory in impaired (MK-801-treated) rats.
As NMDA antagonists are known to affect locomotor speed, we evaluated speed on the Barnes maze. While MK-801 rats ran faster than saline rats (F(1,30) = 14.33, p = 0.0007), there was no significant effect of stimulation type (F(2,30) = 0.47, p = 0.626). Moreover, there was no significant stimulation type by drug interaction (F(2,30) = 0.59, p = 0.556). Because there were no stimulation-related differences in speed in the MK-801 rats, the improved performance of the MK801 theta-treated rats relative to the saline- and gamma- treated rats is not attributable to locomotor speed.
We also evaluated the number of errors made by rats, defined as the number of occurrences during which rats explored an incorrect escape hole. MK-801 rats made significantly more errors than saline treated rats (F(1,36) = 0.49, p = 0.615; Fig. 5C); however, there was no main effect of stimulation type (F(2,36) = 0.49, p = 0.615; Fig. 5C) nor a significant stimulation type × drug interaction (F(2,36) = 2.30, p = 0.115). These findings further suggest that MK-801 disrupts the rat’s ability to find the correct escape hole.
Fig. 5.
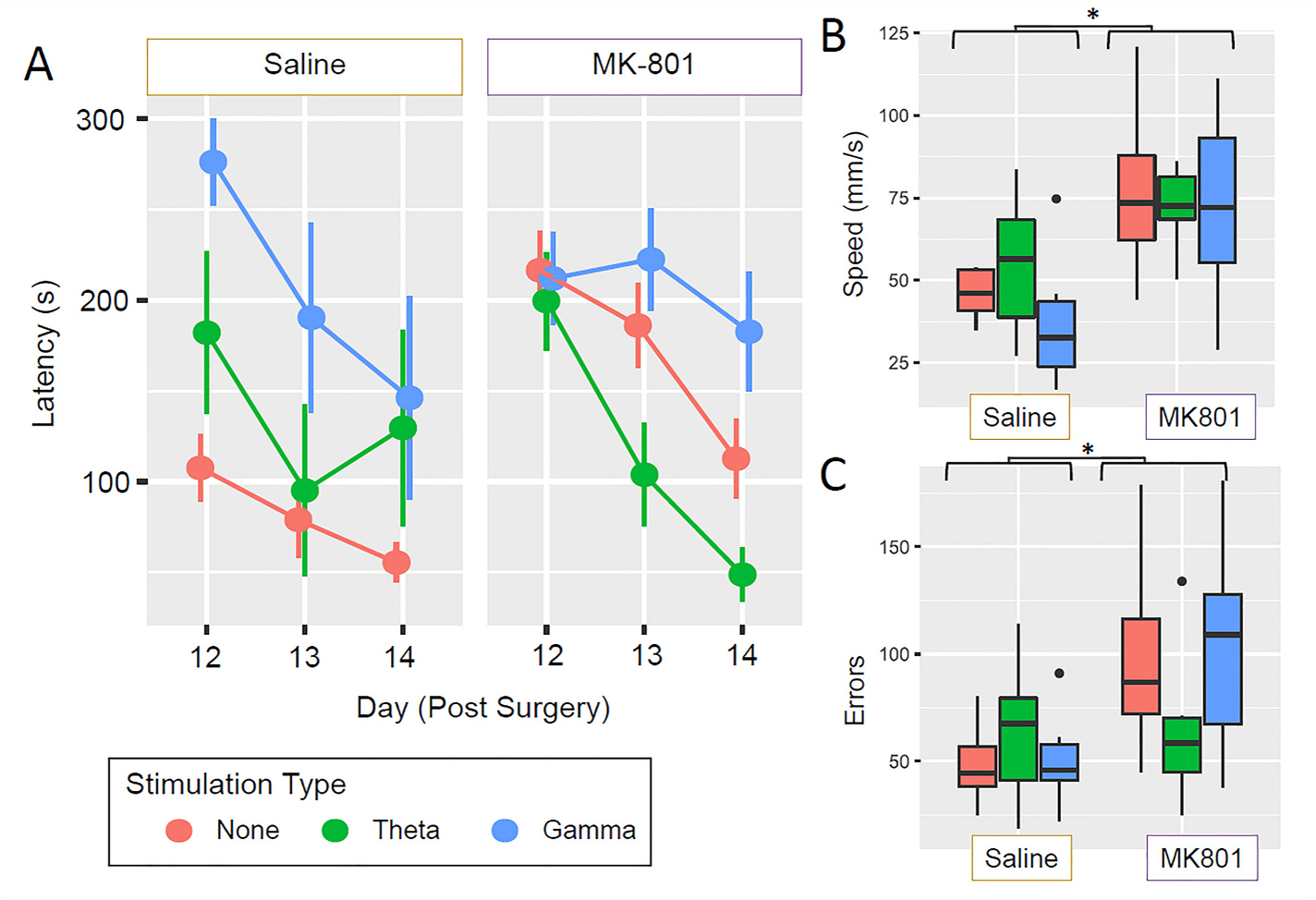
Barnes maze results. (A) There was a significant main effect of stimulation type (F2,50) = 4.55, p = 0.015) and of day (F(2,100) = 28.84, p < 0.0001), and a significant stimulation × drug × day interaction (F(4,100) = 3.23, p = 0.016). Post hoc comparisons revealed that for MK801 treated male rats, theta-stimulated rats had significantly shorter latencies to find the escape hole than both gamma stimulated rats (p = 0.0008, Bonferroni-Holm corrected) and nonstimulated rats (p = 0.014, Bonferroni-Holm corrected). There was no significant difference, however, between gamma stimulated and non-stimulated rats (p = 0.126, Bonferroni-Holm correct) Within saline treated rats, there was no significant difference between non-stimulated rats and theta-stimulated rats (p = 0.261), however, gamma stimulated rats took significantly longer to find the escape hole than nonstimulated rats (p = 0.012, Bonferroni-Holm adjusted). (B) MK-801 treated rats ran significantly faster than saline treated rats (F(1,30) = 14.33, p = 0.0007) but there as no effect of stimulation type (F (2,30) = 0.47, p = 0.626) nor a stimulation × drug interaction (F(2,30) = 0.60, p = 0.556). (C) MK-801-treated rats made a greater number of errors than saline treated rats (F(1,36) = 0.49, p = 0.615), however there was no main effect of stimulation type (F(2,36) = 0.49, p = 0.615) nor a significant stimulation type × drug interaction (F(2,36) = 2.30, p = 0.115). *p < 0.05, **p < 0.01, ***p < 0.001.
In addition to evaluating the latency to find the escape hole, we evaluated search strategies that rats employed to find the escape hole (random, peripheral, direct search strategies) (Barnes, 1979b). A chi-square analysis demonstrated a significant difference in strategy between the saline and MK-801 rats (χ2(2) = 10.23, p = 0.006; Fig. 6). While saline rats mostly used spatial or peripheral strategies (51.2% of trials), MK-801 rats predominantly utilized a random search strategy (73.8% of trials) across each of the six trials. The saline rats utilized the spatial search strategy during 35.2% of the trials, while the MK-801 rats utilized the spatial search strategy in 26.2% of the trials. MSN theta stimulation of the MK-801 rats led to a significant improvement in search strategy, as there was an increased use of the spatial search strategy (37.5% of trials) compared to the non-stimulated MK-801 rats (χ2(2) = 6.963), p = 0.031). Interestingly, MSN stimulation led to a more random search strategy in saline rats relative to non-stimulated saline controls (MSN theta: 58.3% of trials (χ2(2) = 7.230, p = 0.027) and MSN gamma: 80.6% of trials (χ2(2) = 9.568, p = 0.008).
Fig. 6.
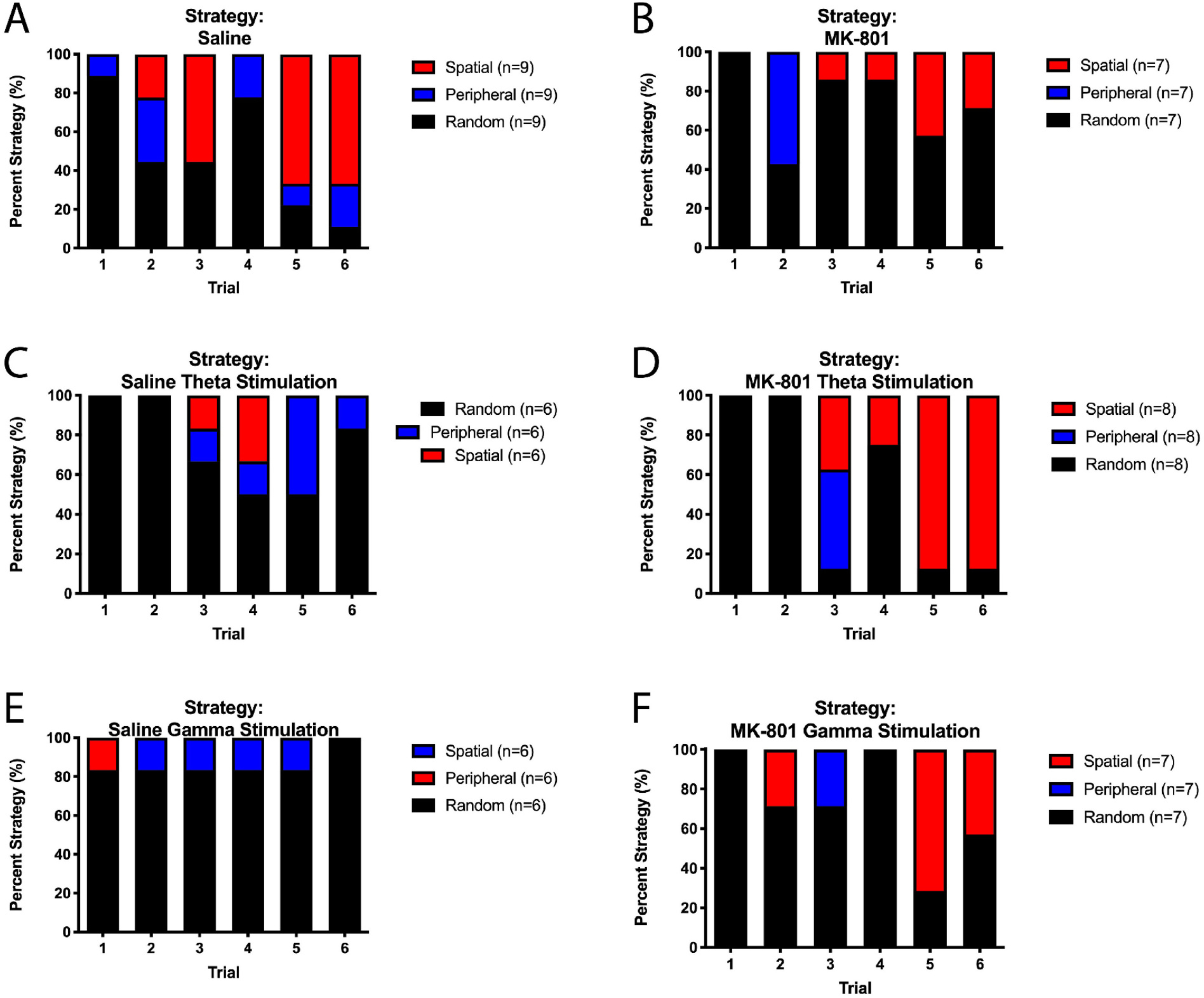
Barnes maze search strategy. There is a significant difference in search strategies between saline control and MK-801 rats (χ2(df = 2) = 10.23, p = 0.006). (A) Saline control rats utilized spatial or peripheral strategies (51.2% of trials), while (B) MK-801 rats utilized these strategies in only 26.2% of trials. (C) In saline inject rats, MSN theta stimulation led to a more random search strategy in non-stimulated saline rats (χ2(df = 2) = 7.2303, p = 0.027). (D) MSN theta stimulation of MK-801 rats led to a significant improvement in search strategy, as there was an increased use of the spatial search strategies (37.5% of trials) compared to nonstimulated MK-801 rats (χ2(df = 2) = 6.963), p = 0.031) and no difference compared to non-stimulated saline rats (p = 0.657). (E) Saline rats with MSN gamma stimulation utilized more random search strategies throughout the Barnes maze compared to saline non-stimulated rats (χ2(df = 2) = 9.568, p = 0.008). (F) Similarly, MK-801-injected rats with MSN gamma stimulation had different search strategies compared to saline controls rats (χ2(df = 2) = 6.638, p = 0.036) and did not differ from non-stimulated MK-801 rats (p = 0.553).
4. Discussion
The current study adds to a growing literature on the effects of NMDA antagonism on neural oscillatory activity and evaluates the effects of MSN deep brain stimulation on behavior with three novel findings: (1) MK-801 resulted in frequency-specific changes to oscillatory activity across four different brain regions (MSN, thalamus, mPFC, hippocampus). Specifically, the change in theta power and HFO power were significantly different in MK-801 treated animals, with theta showing a general decrease while HFO power increased. (2) MSN theta stimulation in the setting of MK-801 reduced anxiety behavior. (3) MSN theta, but not gamma frequency stimulation, administered during the Barnes maze task significantly improved spatial memory.
4.1. MK-801 administration results in frequency-specific changes to oscillatory activity
In the present study we found that MK-801 administration had differing effects on theta and HFO power, resulting in a relative decrease in theta power compared to increase in HFO power. This is in agreement with previous studies that, despite variations in dosage, administration method, and the behavior of rats during recording, studies have consistently illustrated the attenuating effect of NMDA antagonists on mPFC and hippocampal theta power.(Kiss et al., 2013; Kittelberger et al., 2012; Lazarewicz et al., 2010; Leung and Desborough, 1988; Puma et al., 1996; Saunders et al., 2012) and HFO power.(Hudson et al., 2020; Ye et al., 2018).
Of note, our analysis of power change in saline and MK-801 treated animals did not show any region specificity as there was no significant effect of region or region × drug × frequency interaction (Fig. 3). This may indicate that the septo-hippocampal circuit is similarly altered by NMDA antagonism across multiple nodes of the circuit, which may be responsible for wide-spread cognitive deficits; however, this cannot be concluded from the present study as no regions outside the septohippocampal network were evaluated.
4.2. MSN theta stimulation in the setting of MK-801 reduced anxiety behavior
While there is some evidence that NMDA receptor antagonism has anxiolytic effects,(Silote et al., 2020) there is discordance among preclinical studies on its effects on anxiety and fear-related behaviors. In fact, the anxiolytic effects can be altered by dosing, sex differences, species, and/or schedule of administration. Acute administration of MK-801 in this current study did not result in alterations in anxiety on the elevated plus maze (no difference in number of entries into the open arms); however, MK-801 rats with theta, but not gamma, stimulation resulted in anxiolytic behavior compared to saline controls (as represented by the increased number of entries).
The medial prefrontal cortex and ventral hippocampus are key structures in the regulation of anxiety. Previous work demonstrates that increased mPFC hyperactivity has been correlated with anxiety.(Liu et al., 2019) The MSN is integral in modulating both direct projections from the MSN to the mPFC and hippocampus as well as the indirect connections between the mPFC and hippocampus. While this current study did not demonstrate an anxiolytic effect of acute MK-801 alone, MK-801 theta stimulation may have augmented the anxiolytic effects of MK-801 and normalized mPFC oscillatory activities. Interestingly, gamma stimulation did not affect anxiety measures. Further work is necessary to evaluate the potential for MSN stimulation as an adjunct to NMDA receptor antagonism as an anxiolytic. As mPFC dopaminergic and hippocampal glutamatergic release is important in the regulation of anxiety,(Liu et al., 2019; Saitoh et al., 2014) future studies are necessary to understand the effects of stimulation on neurotransmitter release.
4.3. Effects of MK-801 on septohippocampal activity and cognition
The hippocampus is involved in many cognitive functions, including learning and memory. Theta oscillations and synchrony in the septohippocampal circuit have been shown to play a crucial role in these functions. Studies have shown that theta power increases in both the human and rodent hippocampus during spatial memory tasks.(Watrous et al., 2013) In addition, Previous evidence suggests that deficits in hippocampal theta oscillations are associated with difficulty with spatial memory,(Lee et al., 2013; Lee et al., 2015; Lee et al., 2017; McNaughton et al., 2006) Furthermore, MSN lesions, which functions as the generator of the hippocampal theta activity, result in memory impairments similar to those of hippocampal lesions, indicating the MSN’s role in theta generation and cognitive functioning(McNaughton et al., 2006).
Furthermore, existing evidence demonstrates that NMDA antagonism of the septohippocampal circuit impairs cognition. For example, infusion of the NMDA receptor antagonist AP-5 into the MSN directly has been shown to interfere with spatial memory during water maze tasks.(Elvander-Tottie et al., 2006) Furthermore, Cas-mediated ablation of NMDA receptors in parvalbumin positive hippocampal interneurons disrupt spatial and recognition memory.(Korotkova et al., 2010).
As seen in this current study, MK801-induced NMDA receptor antagonism results in an altered distribution of power across frequency bands, as theta was reduced while HFOs were increased in all the brain regions that were studied, including the hippocampus and MSN. This was seen concurrently with impaired spatial recognition in the Barnes maze test in the MK801-treated rats. Therefore, our study is consistent with the previous studies and bridges NMDA antagonism-induced theta reduction and memory impairment.
4.4. Improved performance on the Barnes maze is due to enhanced spatial memory
Data presented here suggests that spatial memory can be enhanced by MSN theta frequency-specific stimulation. Here, we demonstrate that acute administration of MK-801 leads to longer latencies and more errors to find the escape hole in the Barnes maze compared to saline controls (Fig. 5). MSN theta stimulation in MK-801 rats significantly reduced the latency to find the escape hole to levels similar to that of saline controls. This was noticeable on the second and third day of testing, after the rats had the opportunity to learn the location of the hidden escape box. Moreover, while MK-801 rats moved faster than saline rats, there was no difference in speed between MSN theta and gamma stimulation, while gamma-stimulated rats took longer to find the escape hole. Similar to previous data in traumatic brain injury rats with theta deficits,(Lee et al., 2013) MSN theta stimulation did not result in a non-specific, hyper-arousal effect such as speed.
Moreover, the saline vehicle groups utilized the spatial search strategy more frequently than the MK-801 rats and MK-801 rats with MSN theta stimulation used the spatial search strategy more often (37.5%) than non-stimulated MK-801 rats (Fig. 6). Taken together, these data suggest that MSN theta stimulation improves spatial memory in MK-801-treated animals.
Therefore, by demonstrating that MK801 can negatively modulate both theta oscillations and memory and that frequency-specific stimulation of the MSN can reverse this effect, the present study further relates theta to memory. Although the underlying mechanisms are poorly understood, our study further establishes that memory impairment may be mediated by desynchronization of theta oscillations within the septohippocampal circuit, including the MSN-hippocampal pathway mediating spatial memory, and mPFC-hippocampal pathway mediating spatial declarative memory.(Jo et al., 2007; Sapiurka et al., 2016) Interestingly, MK-801 NMDA receptor antagonism did not result in impairments to novel object recognition. This is possibly due to a difference in the circuitry mediating these tasks. Active synchronization of theta rhythms of brain regions involved in spatial memory, such as CA1 and the mPFC, is required to maintain their coordination with respect to each other. Septal stimulation may potentially realign oscillatory patterns and restore successful encoding of spatial memory.(Jones and Wilson, 2005; Kundu et al., 2018).
Another potential mechanism is that disrupting hippocampal theta oscillations may disrupt the ability of spatially selective cells such as place cells or grid cells to encode space. Place cells and are known to fire regularly with respect to theta phase(Skaggs et al., 1996) and inhibiting theta rhythm oscillations results in a loss of spatial periodicity of grid cells, disabling spatial memory encoding and leading to subsequent spatial memory impairment.(Korotkova et al., 2010) MSN theta stimulation may potentially restore the ability of theta to organize place cell and grid cell activity, and thereby improve spatial memory.
4.5. Stimulation does not enhance behavior in saline control rats
As previously described, MSN theta or gamma stimulation does not enhance behavior in saline control rats and may even worsen spatial memory.(Lee et al., 2015) Indeed, saline treated rats with MSN gamma stimulation resulted in longer latencies on the Barnes maze compared to saline vehicle rats without MSN stimulation. Interestingly, saline rats with MSN theta stimulation had similar latencies on the Barnes maze compared to saline control rats. Moreover, saline-treated rats with either MSN theta or MSN gamma stimulation utilized random search strategies more frequently than saline-treated rats without stimulation. While MK-801 rats had increased locomotor speed compared to saline controls, MSN stimulation did not alter the speed of MK-801-treated rats. Taken together, these data suggest that MSN theta stimulation may be a neurorestorative treatment as opposed to one that merely increases overall activity.
4.6. Limitations
Of note, the current study is limited to male rats. Specific differences in behavior have been previously described including that sex hormones have an effect on NMDA receptor antagonism behavioral measures, including ataxia, locomotion and inhibition of stationary behavior. (Feinstein and Kritzer, 2013) Additionally, while behaviors were analyzed after acute MK-801 injections, there were repeated injections of MK-801 throughout the duration of the study, which could potentially result in a chronic NMDA receptor blockade.
4.7. Clinical implications
Deep brain stimulation (DBS) has historically been used to treat movement disorders, such as Parkinson’s disease, essential tremor, and dystonia. Although its use in psychiatric disorders is in its nascent phase, there is evidence in rodents that DBS may also be beneficial in improving psychotic symptoms.(Ma and Leung, 2014) In this study, we have demonstrated frequency-specific oscillatory deficits that were accompanied by behavioral deficits. We also found that MSN theta-frequency specific stimulation improved spatial memory in an acute MK-801 rat model. While the exact mechanisms underlying these stimulation effects is still unknown, these data demonstrate that DBS neuromodulation has the potential to improve hippocampal circuit function and cognitive performance in an acute NMDA receptor hypofunction model, suggesting an exciting potential treatment strategy for cognitive dysfunction in psychiatric disorders characterized by NMDA hypofunction.
Acknowledgements
We would like to thank Martha Abilia Viera Murillo for her assistance on this manuscript.
Keck School of Medicine Dean’s Pilot Funding Program Grant (DJL-PI) NIMH: 1K08MH121757-01A1 (DJL-PI).
Footnotes
Author disclosure statement
No competing financial interests exist.
References
- Amitai N, Markou A, 2010. Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol. Psychiatry 68, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes M, Biala G, 2012. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process 13, 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, 1979a. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol 93, 74–104. [DOI] [PubMed] [Google Scholar]
- Barnes CA, 1979b. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol 93, 74–104. [DOI] [PubMed] [Google Scholar]
- Billingslea EN, et al. , 2014. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology 39, 1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, et al. , 2010. Object recognition memory and the rodent hippocampus. Learn. Mem 17, 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, et al. , 2017. A randomized clinical trial of oxytocin or galantamine for the treatment of negative symptoms and cognitive impairments in people with schizophrenia. J. Clin. Psychopharmacol 37, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, 2005. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15, 827–840. [DOI] [PubMed] [Google Scholar]
- Carbon M, Correll CU, 2014. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr 19 (Suppl. 1), 38–52 quiz 35–7, 53. [DOI] [PubMed] [Google Scholar]
- Carlen M, et al. , 2012. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol. Psychiatry 17, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, et al. , 2015. Modulation of hippocampal theta and hippocampal-prefrontal cortex function by a schizophrenia risk gene. Hum. Brain Mapp 36, 2387–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunayevich E, et al. , 2006. Characteristics of two alternative schizophrenia remission definitions: relationship to clinical and quality of life outcomes. Schizophr. Res 86, 300–308. [DOI] [PubMed] [Google Scholar]
- Elis O, et al. , 2013. Psychosocial treatments for negative symptoms in schizophrenia: current practices and future directions. Clin. Psychol. Rev 33, 914–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvander-Tottie E, et al. , 2006. N-methyl-D-aspartate receptors in the medial septal area have a role in spatial and emotional learning in the rat. Neuroscience 142, 963–978. [DOI] [PubMed] [Google Scholar]
- Feinstein I, Kritzer MF, 2013. Acute N-methyl-D-aspartate receptor hypofunction induced by MK801 evokes sex-specific changes in behaviors observed in open-field testing in adult male and proestrus female rats. Neuroscience 228, 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa DD, et al. , 2017. Individual differences in male rats in a behavioral test battery: a multivariate statistical approach. Front. Behav. Neurosci 11, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM, et al. , 2015. Effects of prefrontal cortex and hippocampal NMDA NR1-subunit deletion on complex cognitive and social behaviors. Brain Res 1600, 70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JD, Arduini AA, 1954. Hippocampal electrical activity in arousal. J. Neurophysiol 17, 533–557. [DOI] [PubMed] [Google Scholar]
- Haddad PM, Correll CU, 2018. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther. Adv. Psychopharmacol 8, 303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson MR, et al. , 2020. NMDA receptors on parvalbumin-positive interneurons and pyramidal neurons both contribute to MK-801 induced gamma oscillatory disturbances: complex relationships with behaviour. Neurobiol. Dis 134, 104625. [DOI] [PubMed] [Google Scholar]
- Javitt DC, 2000. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol. Neurootol 5, 207–215. [DOI] [PubMed] [Google Scholar]
- Javitt DC, 2007. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol 78, 69–108. [DOI] [PubMed] [Google Scholar]
- Javitt DC, et al. , 2004. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycine-site agonists. Neuropsychopharmacology 29, 300–307. [DOI] [PubMed] [Google Scholar]
- Jo YS, et al. , 2007. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J. Neurosci 27, 13567–13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA, 2005. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol 3, e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, et al. , 2013. Rhythmic theta and delta activity of cortical and hippocampal neuronal networks in genetically or pharmacologically induced N-methyl-D-aspartate receptor hypofunction under urethane anesthesia. Neuroscience 237, 255–267. [DOI] [PubMed] [Google Scholar]
- Kittelberger K, et al. , 2012. Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct. Funct 217, 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, et al. , 2010. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68, 557–569. [DOI] [PubMed] [Google Scholar]
- Kundu B, et al. , 2018. Deep brain stimulation for the treatment of disorders of consciousness and cognition in traumatic brain injury patients: a review. Neurosurg. Focus 45, E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M, et al. , 2010. Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin. Neurosci 12, 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz MT, et al. , 2010. Ketamine modulates theta and gamma oscillations. J. Cogn. Neurosci 22, 1452–1464. [DOI] [PubMed] [Google Scholar]
- Lee DJ, et al. , 2013. Medial septal nucleus theta frequency deep brain stimulation improves spatial working memory after traumatic brain injury. J. Neurotrauma 30, 131–139. [DOI] [PubMed] [Google Scholar]
- Lee DJ, et al. , 2015. Septohippocampal neuromodulation improves cognition after traumatic brain injury. J. Neurotrauma 32, 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, et al. , 2017. Stimulation of the medial septum improves performance in spatial learning following pilocarpine-induced status epilepticus. Epilepsy Res 130, 53–63. [DOI] [PubMed] [Google Scholar]
- Lee M, et al. , 2018. Rodent mismatch negativity/theta neuro-oscillatory response as a translational neurophysiological biomarker for N-methyl-D-aspartate receptor-based new treatment development in schizophrenia. Neuropsychopharmacology 43, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, et al. , 2013. Object recognition test in mice. Nat. Protoc 8, 2531–2537. [DOI] [PubMed] [Google Scholar]
- Leucht S, et al. , 2017. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am. J. Psychiatry 174, 927–942. [DOI] [PubMed] [Google Scholar]
- Leung LW, Desborough KA, 1988. APV, an N-methyl-D-aspartate receptor antagonist, blocks the hippocampal theta rhythm in behaving rats. Brain Res 463, 148–152. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, et al. , 2012. Moving from efficacy to effectiveness in cognitive behavioral therapy for psychosis: a randomized clinical practice trial. J. Consult. Clin. Psychol 80, 674–686. [DOI] [PubMed] [Google Scholar]
- Liu L, et al. , 2019. Dopamine D1 receptor in the medial prefrontal cortex mediates anxiety-like behaviors induced by blocking glutamatergic activity of the ventral hippocampus in rats. Brain Res 1704, 59–67. [DOI] [PubMed] [Google Scholar]
- Ma J, Leung LS, 2014. Deep brain stimulation of the medial septum or nucleus accumbens alleviates psychosis-relevant behavior in ketamine-treated rats. Behav. Brain Res 266, 174–182. [DOI] [PubMed] [Google Scholar]
- McNaughton N, et al. , 2006. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus 16, 1102–1110. [DOI] [PubMed] [Google Scholar]
- Menard J, Treit D, 1996. Lateral and medial septal lesions reduce anxiety in the plusmaze and probe-burying tests. Physiol. Behav 60, 845–853. [DOI] [PubMed] [Google Scholar]
- Olszewski M, et al. , 2013. NMDA receptor antagonist-enhanced high frequency oscillations: are they generated broadly or regionally specific? Eur. Neuropsychopharmacol 23, 1795–1805. [DOI] [PubMed] [Google Scholar]
- Paine TA, Carlezon WA Jr., 2009. Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits in rats. Neuropharmacology 56, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras GG, et al. , 2020. The effect of NMDA-R antagonist, MK-801, on neuronal mismatch along the rat auditory thalamocortical pathway. Sci. Rep 10, 12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puma C, et al. , 1996. Intraseptal infusion of selective and competitive glutamate receptor agonist NMDA and antagonist D-2-amino-5-phosphonopentanoic acid spectral implications for the physostigmine-induced hippocampal theta rhythm in urethane-anesthetized rats. Exp. Brain Res 109, 384–392. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2021. R: A language and environment for statistical computing R Foundation for StatisticalComputing, Vienna, Austria. URL http://www.R-project.org/. [Google Scholar]
- Saitoh A, et al. , 2014. Activation of the prelimbic medial prefrontal cortex induces anxiety-like behaviors via N-methyl-D-aspartate receptor-mediated glutamatergic neurotransmission in mice. J. Neurosci. Res 92, 1044–1053. [DOI] [PubMed] [Google Scholar]
- Sapiurka M, et al. , 2016. Distinct roles of hippocampus and medial prefrontal cortex in spatial and nonspatial memory. Hippocampus 26, 1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, et al. , 2012. NMDA antagonists recreate signal-to-noise ratio and timing perturbations present in schizophrenia. Neurobiol. Dis 46, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silote GP, et al. , 2020. Ketamine effects on anxiety and fear-related behaviors: current literature evidence and new findings. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 100, 109878. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, et al. , 1996. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172. [DOI] [PubMed] [Google Scholar]
- Stepnicki P, et al. , 2018. Current concepts and treatments of schizophrenia. Molecules 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup TS, et al. , 2000. Comparative effectiveness of antipsychotic drugs in schizophrenia. Dialogues Clin. Neurosci 2, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, 2017. Speed and oscillations: medial septum integration of attention and navigation. Front. Syst. Neurosci 11, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W, 2010. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci 11, 100–113. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2005. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus 15, 923–935. [DOI] [PubMed] [Google Scholar]
- Watrous AJ, et al. , 2013. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus 23, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, et al. , 2018. Ten-hour exposure to low-dose ketamine enhances corticostriatal cross-frequency coupling and hippocampal broad-band gamma oscillations. Front Neural Circuit 12, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]