Abstract
Background
Over the past two years, SARS-CoV-2 has frequently been documented with various post and para-infectious complications, including cerebrovascular, neuromuscular, and some demyelinating conditions such as acute disseminated encephalomyelitis (ADEM). We report two rare neurological manifestations post-COVID-19 infection; multiple sclerosis (MS) and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD). Further, we reviewed other CNS inflammatory demyelinating diseases (IDDs) associated with SARS-CoV-2, including optic neuritis (ON) and neuromyelitis optica spectrum disorders (NMOSD).
Methods
A descriptive analysis and literature search of Google Scholar and PubMed was conducted by two independent reviewers from December 1st, 2019, to March 30th, 2022, and included all the case studies of MS, MOGAD, NMOSD, and ON associated with COVID-19 infection.
Case presentations
Case 1 (MS) was a 24-year-old female with paresthesia and bilateral weakness one week after COVID-19 symptom onset who showed demyelinating plaques and 12 isolated oligoclonal bands (OCBs). Case 2 (MOGAD) was a 41-year-old male with encephalomyelitis 16 days after COVID-19, who later developed MOG-antibody-associated optic neuritis.
Results
Out of 18 cases, NMOSD was the most common post-COVID manifestation (7, 39%), followed by MOGAD (5, 28%), MS (4, 22%), and isolated ON (2, 11%). The median duration between the onset of COVID-19 symptom onset and neurological symptoms was 14 days. 61% of these were male, with a mean age of 35 years. IVMP was the treatment of choice, and nearly all patients made a full recovery, with zero fatalities.
Conclusions
Although these neurological sequelae are few, physicians must be cognizant of their underlying pathophysiology and associated clinical and neuro-diagnostic findings when treating COVID-19 patients with atypical presentations.
Keywords: COVID-19, SARS-CoV-2, CNS IDD, Virus-induced demyelination, MS, MOGAD, NMOSD, Optic neuritis;
Abbreviations: COVID-19, Coronavirus Disease 2019; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; NIH, National Institutes of Health; ATS/IDSA, American Thoracic Society and Infectious Disease Society of America; CNS, Central Nervous System; IDD, Inflammatory Demyelinating Disease; MS, Multiple Sclerosis; MOGAD, Myelin Oligodendrocyte Glycoprotein Antibody-associated Disease; NMOSD, Neuromyelitis Optica Spectrum Disorders; ON, Optic Neuritis; AQP4, Aquaporin-4; MOG, Myelin Oligodendrocyte Glycoprotein; Ab, Antibody; Ig, Immunoglobulin; MRI, Magnetic Resonance Imaging; RT-PCR, Reverse Transcription-Polymerase Chain Reaction; CSF, Cerebrospinal Fluid
1. Introduction
Coronavirus disease (COVID-19), caused by the SARS-CoV-2 virus, began as a rampant pandemic in 2020 and remains a severe public health crisis today, affecting millions worldwide (WHO, 2022). Among the reported post-infectious sequelae, the prevalence of neurological manifestations associated with COVID-19 infection is estimated between 35 and 85% and includes various inflammatory and demyelinating disorders (Chou et al., 2021; Nolen et al., 2022; Karsidag et al., 2021). Viral organisms often display neurotropic and invasive properties, such that an infection can lead to acute and long-term neurologic complications (Desforges et al., 2019; Kim et al., 2017). Central nervous system (CNS) injury in these circumstances can result either from an autoimmune response towards the virus or directly due to inoculation of the CNS; this is most true of respiratory viruses, including coronaviruses (CoV), with ample evidence of their presence in the brain or cerebrospinal fluid (CSF) (Karsidag et al., 2021; Desforges et al., 2019; Wu et al., 2020).
Coronaviruses (CoV) are a group of enveloped RNA viruses that are responsible for previous outbreaks (SARS-CoV and MERS-CoV) and have reportedly led to diseases like encephalitis, ischemic stroke, and Guillain-Barre syndrome (GBS) (Kim et al., 2017). One significant histopathologic finding in these was the demolition of the myelin sheath covering nerve fibers (Kim et al., 2017). SARS-CoV-2, with similar genetic and clinical characteristics to its predecessors, has also been discovered with demyelination in autopsy reports of COVID-19 patients with coexisting neurological illnesses (Nolen et al., 2022). Disorders such as acute demyelinating encephalomyelitis (ADEM), transverse myelitis (TM), and others have been described as post and para-infectious complications (Al-Ramadan et al., 2021; Moreno-Escobar et al., 2021; Lahiri and Ardila, 2020). Immunological dysfunction and elevated cytokine levels implicated in the pathogenesis of COVID-19 infection have been broadly investigated as a trigger for many of these, suggesting that SARS-CoV-2 may contribute to the induction or exacerbation of long-term neuropathologies (Ismail and Salama, 2022).
Here, we report a series of two patients with radiologically typical and rarely documented post-COVID demyelinating presentations: multiple sclerosis (MS) and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD). Further, we conducted a quantitative analysis of other CNS inflammatory demyelinating diseases (IDDs) associated with COVID-19 infection, including neuromyelitis optica spectrum disorder (NMOSD) and optic neuritis, and have discussed the pathophysiologic mechanisms leading to these complications. Using these case descriptions, we summarize the primary clinical and imaging features and highlight the relevance of CNS-targeting antibodies in the diagnostic process. Our aim through this review is to familiarize and guide clinicians in their approach towards these rare yet burgeoning and potentially disabling post COVID-19 neurological manifestations.
2. Methods
2.1. Literature search
A retrospective chart review of Google Scholar and PubMed was conducted by two independent researchers from December 1st, 2019, to March 30th, 2022. Data was requested using keywords: (Multiple Sclerosis OR MS) AND (Coronavirus Disease 2019 OR COVID-19 OR SARS-CoV-2); (Myelin oligodendrocyte glycoprotein antibody-associated disease OR MOG-IgG mediated disease OR MOG-IgG+ disease OR MOGAD) AND (Coronavirus Disease 2019 OR COVID-19 OR SARS-CoV-2); (Neuromyelitis Optica OR Neuromyelitis Optica Spectrum Disorders OR NMO OR NMOSD) AND (Coronavirus Disease 2019 OR COVID-19 OR SARS-CoV-2); and (Optic Neuritis OR ON) AND (Coronavirus Disease 2019 OR COVID-19 OR SARS-CoV-2). Results were carefully verified to avoid duplicates or overlapping publications.
2.2. Inclusion and exclusion criteria
All related case studies published in English which reported MS, MOGAD, NMOSD, and ON diagnosis following COVID-19 infection were included. Our exclusion criteria were as follows: (a) other CNS or peripheral nervous system (PNS) demyelinating diseases, (b) other types of coronavirus (SARS-CoV/MERS-CoV) infections, (c) articles written in any language other than English, and (d) review articles.
2.3. Data acquisition
From the qualified papers, we extracted information for each patient as available: publication date, country, age/gender, comorbidities, duration between COVID-19 symptoms and neurological symptom onset, clinical presentation, CSF findings, serum aquaporin-4 (AQP4)/myelin oligodendrocyte glycoprotein (MOG) antibody status, imaging findings, as well as treatment and outcomes.
3. Case studies
3.1. Case 1: relapsing-remitting multiple sclerosis (RRMS)
A 24-year-old previously healthy female presented to our clinic on February 14th, 2022, for a second opinion regarding a recent magnetic resonance image (MRI) brain finding suggestive of MS. On September 29th, 2021, she tested positive for COVID-19; within a week, she developed numbness and new-onset weakness, starting in her left leg, which ascended bilaterally. According to the NIH guidelines and ATS/IDSA severity index, this patient was categorized as a mild form of COVID-19 infection (Metlay et al., 2019) (Diagnosis and Treatment of Adults with Community-acquired Pneumonia, 2022). Over the next few months, the weakness in her lower extremities progressed as she struggled with stairs and incline walkways. Electromyography (EMG) performed at an outside neurology facility was normal. Ultimately, she required a cane for ambulation and presented to us with an MRI brain scan (without contrast) that revealed a posterior right frontal and subcortical white matter lesion with subtle periventricular signal changes.
The patient was admitted for further investigations, including an MRI of the brain, cervical, and thoracic spine, which all demonstrated demyelinating plaques (Fig. 1 ). Lumbar puncture (LP) showed elevated CSF protein levels of 52 mg/dL and 12 isolated oligoclonal bands (OCBs) in the CSF. Serum AQP4 and MOG-IgG antibodies were negative. A comprehensive autoimmune and infectious panel was unremarkable, including antinuclear antibodies (ANA), neutrophil cytoplasmic antibodies (ANCA), ENA screen SS-A/Ro and SS-B/La antibodies, anti-Smith (Sm) antibodies, RNP antibodies, anti-Scl-70 antibodies, anti-double-stranded DNA (dsDNA) antibodies, anti-chromatin antibodies, anti-centromere antibodies, and antimitochondrial antibodies. Vitamin B12 and folate levels were within normal limits. The diagnosis of RRMS was confirmed, and the patient received IVMP for five days (tolerated without complications), after which she showed partial improvement in motor function. On her follow-up visit, she displayed steady progress in gait and motor strength; she is now scheduled to start a disease-modifying therapy (DMT).
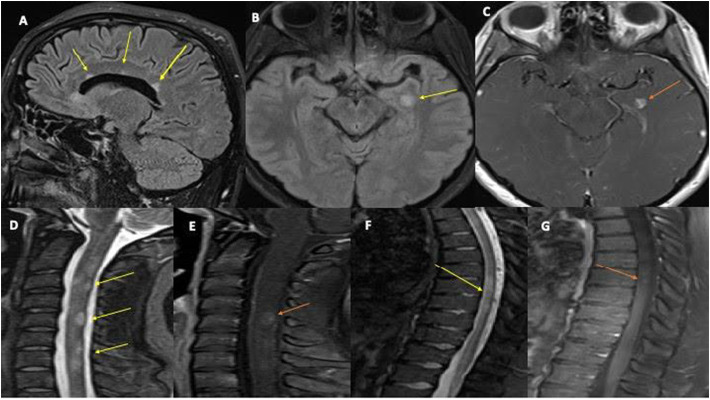
Fig. 1.
MRI Brain sagittal FLAIR images 1(A) showed ovoid periventricular white matter lesion (yellow arrows) and left temporal lesion and corresponding enhancement on axial images 1(B) (yellow arrow) & 1(C) (orange arrow). MRI sagittal STIR images 1(D) of the cervical spine reveal patchy multiple short segment hyperintensity with prominent cord lesion at C2-C3, C4-C5, C5-C6 (yellow arrow), and 1(E) post-contrast sagittal image showed abnormal enhancement at C4-C5 (orange arrow). MRI sagittal STIR image (1F) of the thoracic spine showed intramedullary mid-thoracic cord lesion at T6-T8 (yellow arrow) with corresponding abnormal enhancement at T6 (orange arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Case 2: myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD)
A 41-year-old male with a history of hypertension tested positive for SARS-CoV-2 in early November 2020 and was categorized with mild COVID-19 infection (as per the NIH guidelines and the ATS/IDSA severity index) (Metlay et al., 2019; Diagnosis and Treatment of Adults with Community-acquired Pneumonia, 2022). However, 16 days after the diagnosis, he experienced confusion, shivering, paresthesia, gait instability, and urinary retention. He was then admitted and underwent an MRI of the spine and brain with and without (w/wo) contrast, which displayed abnormal T2-hyperintensity from C2-C4 without post-contrast enhancement, suggestive of longitudinally extensive transverse myelitis (LETM) and post-infectious transverse myelitis (Fig. 2 ). Subsequent CSF analysis revealed 45/uL nucleated cells (90% lymphocytes), 116 mg/dL protein, 37 mg/dL glucose, zero OCBs, and an IgG index of 0.48; serum NMO/anti-AQP4 antibodies were negative. On day 6 of admission, a repeat brain MRI indicated a new T2-FLAIR lesion of the left corona radiata and right parietal subcortical white matter. CSF was negative for viral PCR, and antibiotics were discontinued; yet, the patient remained confused and lethargic. IVMP 1 g/day was administered for five days, following which his mental status improved, and a diagnosis of post-infectious COVID-19 encephalomyelitis was made. The patient was stable and discharged 22 days after admission, without deficits.
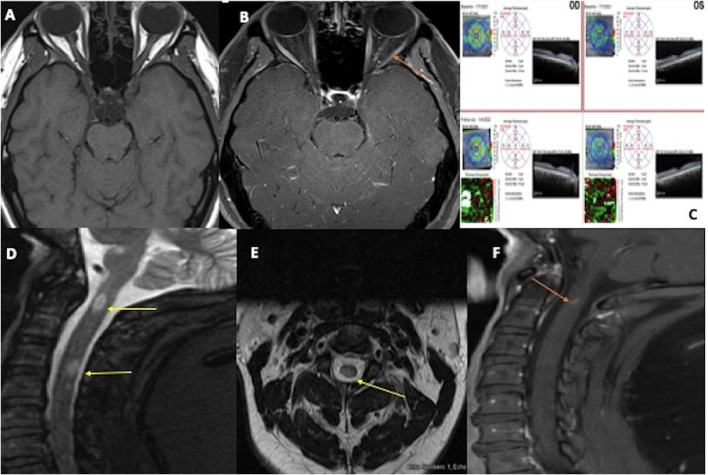
Fig. 2.
MRI orbit Axial T1- weighted pre-contrast 2(A) and post-contrast 2(B) reveals abnormal enhancement of left optic nerve prechiasmatic (intracanalicular); (orange arrow). Fig. 2(C) Optical Coherence Test (OCT) showing baseline and follow-up retinal nerve fiber layer thickness with thinned ganglion cell layer and overall stable rest of the retinal layer on the left compared to the right. MRI sagittal STIR images 2(D) of the cervical spine reveal patchy short segment hyperintensity with prominent cord lesion at C2, C4-C5 (yellow arrow); 2(E) shows axial cut with cord signal alteration at C4-C5 (yellow arrow), and 2(F) post-contrast sagittal image showed abnormal enhancement at C2 (orange arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Six months later, he returned with a mild left-sided headache and ipsilateral retro-orbital pain with visual blurriness occurring over one week. Ophthalmologic examination showed impaired left (20/70) and right (20/25) visual acuity, along with left afferent pupillary defect (APD). Brain and orbital MRI w/wo contrast showed pre-chiasmatic (intracanalicular) enhancement of the left optic nerve, suggestive of optic neuritis (Fig. 2). The patients concerning encephalopathy, optic neuritis, and spinal and brain lesions raised concern for CNS inflammatory post-COVID 19 sequelae. MOG-IgG antibody levels were sent out and tested commercially at the Mayo Clinic via live-cell fluorescent-activated cell sorting assay, which was positive in the serum (1:1000), suggestive of post-COVID MOGAD syndrome. MRI of the brain also showed a decreased conspicuity of the previously visualized white matter lesions. During this admission, he was restarted on IVMP (1 g/day for three days) and was also scheduled to receive five cycles of PLEX therapy. The patient stated significant improvement in his visual acuity and was eventually discharged with a prescription for oral prednisone. At the follow-up visit with neurology, he reported that his vision had nearly returned to baseline. He also no longer experiences any pain and is currently stable since his last admission. Per our recommendations and infectious disease clearance, the patient will continue monthly IVIG therapy for suspected post-COVID MOGAD optic neuritis.
4. Results
We obtained the data of 18 individuals (from a total of 18 case reports) with specific CNS demyelinating manifestations associated with COVID-19 infection (Table S1) (Zhou et al., 2020; Pinto et al., 2020; Domingues et al., 2020; Zoghi et al., 2020; Palao et al., 2020; Corrêa et al., 2021; de Ruijter et al., 2020; Sawalha et al., 2020; Batum et al., 2020; Yavari et al., 2020; Moore et al., 2021; Peters et al., 2021; Žorić et al., 2021; Kogure et al., 2021; Sardar et al., 2021; Ghosh et al., 2020; Barone et al., 2021; Azab et al., 2021). Most of these were male (n = 11) [61%], with a mean age (range) of 35 (15–63) years and a median duration of 14 days between the onset of COVID-19 symptoms onset and neurological symptoms (three cases did not outline the specific interval) (Zhou et al., 2020; Batum et al., 2020; Yavari et al., 2020). Comorbidities were present in one-third of the patients (n = 6) [33%], with diabetes mellitus (DM) type 2 being the most common (n = 2) and non-febrile seizures being the only pre-existing neurologic condition seen in a single patient (Peters et al., 2021); (data unavailable for two cases) (Domingues et al., 2020) (Batum et al., 2020). Out of the 18 cases, seven (39%) were diagnosed with neuromyelitis optica spectrum disorder (NMOSD), five (28%) with MOG-antibody disease (MOGAD), four (22%) with multiple sclerosis (MS)/MS phenotypes, and two (11%) with isolated optic neuritis (ON).
NMOSD (n = 7) was commonly seen with signs of myelitis (n = 4), such as motor (peripheral weakness), sensory (paresthesia/dysesthesia), autonomic (urinary retention), and hypo/areflexia, along with intractable vomiting (n = 2), hiccoughs (n = 1), or other features (n = 1) indicative of APS (area postrema syndrome); optic neuritis was noticed in two cases (one unilateral and one bilateral), with unilateral blindness seen in the third. Among these, one with seronegative (AQP4 Ab-) NMOSD was simultaneously diagnosed with idiopathic intracranial hypertension (IIH) (Sardar et al., 2021), while another was associated with acute myositis and autoimmune thyroiditis (Barone et al., 2021). Interestingly, AQP4 Ab+ encephalomyeloradiculitis (n = 1) (Corrêa et al., 2021) and seronegative encephalomyelitis (with possible APS) (n = 1) (Zoghi et al., 2020) were also noted as hyperintense contrast-enhancing lesions on T2-weighted and FLAIR imaging in areas typical of NMOSD: the anterior fornix, subfornical organ, corticospinal tract, and corpus callosum. Others displayed characteristic MRI findings such as LETM in the cervico-thoracic spine (n = 4) or optic nerve hyperintensity with contrast optic/peri optic enhancement (n = 3).
Among the patients with MOGAD (n = 5), the most frequent manifestations were MOG-associated ON (n = 3), myelitis (n = 1), encephalitis (n = 1), and CNS inflammatory vasculopathy (n = 1). Symptoms included headache (n = 2), ocular pain (n = 2), visual impairment (n = 2), cognitive changes (n = 1), and limb weakness/incoordination (n = 1). Orbital MRI of MOG IgG-mediated ON (n = 3) revealed bilateral and uniform enhancement of optic nerve sheaths (without overt involvement of the chiasm) (n = 2) or was completely normal with only microangiopathic/cortical reductive signs on MRI brain (Žorić et al., 2021). Myelitis (n = 1) appeared as patchy T2-hyperintensities in the lower cervical/upper thoracic regions with mild central thickening and enhancement. Other findings included diffuse cortical T2-FLAIR hyperintensity (with leptomeningeal enhancement) in encephalitis (n = 1) and bilateral T2-hyperintensity in the centrum semiovale in CNS vasculopathy (n = 1).
Two out of the three patients (67%) with MS were female who presented with visual changes, such as diplopia (n = 2), blurred vision (n = 2), and ipsilateral internuclear ophthalmoplegia (INO) [n = 1]. CSF OCBs were positive for only two of these (Palao et al., 2020) (Moore et al., 2021) (five and unspecified OCBs, respectively), with a lumbar puncture (LP) not performed in the third (Yavari et al., 2020). Radiologic findings showed distinctive multiple (n = 2) or single (n = 1) T2-hyperintense lesions, most commonly periventricular (n = 2) in location, with active plaques on MRI of the brain and unilateral enhancing optic nerve lesion on orbital MRI (n = 1). Clinically isolated syndrome (CIS) (n = 1) was reported in a female patient as unilateral paresthesia with a single non-enhancing cervical spine lesion, normal MRI brain, negative OCBs, and concomitant CSF RT-PCR positive for SARS-CoV-2 (Domingues et al., 2020).
Isolated ON was either unilateral (n = 1) or bilateral (n = 1) in presentation. Both cases experienced complete visual loss, wherein one with seropositive MOG-IgG antibodies (Sawalha et al., 2020) attained total recovery only in the left eye; CSF analysis (including OCBs) and serum AQP4/MOG-IgG antibodies were unavailable for the second patient, who eventually gained partial improvement in acuity and colour depth perception (Azab et al., 2021). Imaging results included enhancing and hyperintense swelling of the optic nerves (intraorbital segments) on orbital MRI (n = 2) and mild optic disc swelling (n = 1) on OCT.
The treatment of choice was intravenous methylprednisolone (IVMP) (n = 16); other options included plasmapheresis (PLEX) (n = 5), intravenous immunoglobulin (IVIG) (n = 3), IV antibiotics (n = 3), antivirals (n = 2), and immunosuppressants, such as rituximab (n = 2), azathioprine (n = 1), and interferon (IFN) beta-1a (n = 1). Overall, outcomes were favorable, with nearly all patients achieving a complete recovery and zero fatalities.
5. Discussion
Virus-induced demyelination is not a novel concept and has been observed in numerous human and animal studies (Soldan and Jacobson, 2016; Das, 2010; Johnson and Institute of Medicine (US) Forum on Microbial Threats, 2004). For example, the Epstein-Barr virus (EBV), a highly B-cell tropic virus, has been overwhelmingly implicated in the pathogenesis of MS (Bar-Or et al., 2020) (Guan et al., 2019) (Schirinzi et al., 2021). In fact, it has proven a direct role in MS by interacting with genetic and environmental factors and increasing the susceptibility and severity of attacks; this association highlights the role of viral infection in neurological damage and explains the application of B-cell depleting therapies in MS (Bar-Or et al., 2020) (Guan et al., 2019). Similarly, COVID-19 is a potential risk factor for demyelination in the peripheral and central nervous systems (Schirinzi et al., 2021; Shabani, 2021). Along with MS and MOGAD, as seen in our patients, we documented all existing cases of NMOSD and optic neuritis associated with the SARS-CoV-2 infection (Table S1). Other disorders such as TM, ADEM, acute inflammatory demyelinating polyneuropathy (AIDP), and acute necrotizing encephalopathy have also been described in literature as post and para-infectious CNS complications (Nolen et al., 2022; Al-Ramadan et al., 2021; Moreno-Escobar et al., 2021; Lahiri and Ardila, 2020). However, due to their extensive reporting, we decided to focus our research on these uncommon CNS inflammatory demyelinating diseases (IDD) associated with post COVID-19 infection.
SARS-CoV-2 can invade the CNS either through immune-mediated processes or direct invasion (Karsidag et al., 2021; Kim et al., 2017). Infection results from hematogenous or neuronal retrograde dissemination via disruption of the nasal epithelial barrier or cribriform plate (Ismail and Salama, 2022). Autoptic evidence of monocyte, macrophage, and T-lymphocyte infiltration into the neurovascular walls of COVID-19 patients, suggests that SARS-CoV-2 and pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) can cross the blood-brain barrier (BBB), leading to increased glial activation and the death of neurons and oligodendrocytes (Shabani, 2021; Mohammadi et al., 2020). Other mechanisms include antibody production against myelin via molecular mimicry, activation of toll-like receptors (TLRs), and the affinity for angiotensin-converting enzyme 2 (ACE2) receptors, which can all cause CNS involvement and consequently lead to myelin destruction (Ismail and Salama, 2022; Mohammadi et al., 2020). CSF evaluation can be a mild indicator of this; for example, increased CSF protein has been noted in most COVID-19 patients with neurological complications, along with occasional lymphocytic-predominant pleocytosis (Tandon et al., 2021). However, SARS-CoV-2 itself has been challenging to detect in the CSF of these patients. None of the 30 patients in the study by Neumann et al. had a positive CSF RT-PCR; in another review, only 12% of the tested cases were positive for CSF antibodies towards SARS-CoV-2 with evidence of intrathecal synthesis (Neumann et al., 2020; Lewis et al., 2021).
MS is a classic example of an autoimmune CNS disease characterized by chronic inflammation and demyelination (Thompson et al., 2018a). Cytokine storm, seen in response to SARS-CoV-2 infection, aids in this immune-mediated process by creating a milieu of chemokines and T-cell subsets, such as Th17 (IL-17), Th1 (IL-2), and Treg (IL-10) (Espíndola et al., 2021; Wu and Yang, 2020; Satheesh et al., 2021); most of these are known to induce attacks in patients with MS and amplify the autoimmune status in genetically-predisposed individuals (Schirinzi et al., 2021; Satheesh et al., 2021; Kunkl et al., 2020; Boucher et al., 2007; Kebir et al., 2007). Clinical features primarily involve visual changes (such as diplopia, nystagmus, and INO), along with fatigue and sensory disturbances like paresthesia (Ghasemi et al., 2017; Hauser and Goodin, 2014). These correlate well with our review, wherein symptoms occurred during the recovery phase or within a few weeks of the onset of COVID-19 (Table S1). Similarly, MRI findings diagnostic of MS were also seen, including multiple hypersignal plaques, optic nerve inflammation, and demyelinating lesions (Table S1) (Thompson et al., 2018b). OCBs and elevated proteins in the CSF are reliable markers of MS; however, only two patients reported positive OCBs, with one being unspecified in number (Palao et al., 2020; Moore et al., 2021). In contrast, CSF analysis in our patient (Case 1) revealed 12 OCBs, much higher than those in previous studies (Table S1). Moreover, this case presented typical clinical and imaging findings, including periventricular demyelinating plaques on MRI, meeting the criteria for dissemination in space and time for MS (Thompson et al., 2018b). It is essential to recognize that the pathogenic sequences leading to MS in our patient may have already begun prior to COVID-19 disease (through genetic or environmental influences), in which case, SARS-CoV-2 may have acted as a precipitating factor rather than being a direct cause of MS (Olsson et al., 2017).
Another demyelinating disorder that can be triggered by COVID-19 and targets the optic nerves, brain, and spinal cord is MOG-IgG-associated disease (MOGAD). Myelin Oligodendrocyte Glycoprotein (MOG) is a cell adhesion molecule that belongs to the immunoglobulin (Ig) superfamily and is located on the surface of CNS myelin and oligodendrocytes (Brunner et al., 1989). It maintains the stability of oligodendrocytes, and antibodies against MOG (MOG-IgG) have emerged as a reliable serological biomarker for a subset of CNS IDD, the most common of which is optic neuritis (ON) (Cobo-Calvo et al., 2018; Mariotto et al., 2017; Johns and Bernard, 1999). Likewise, MOG-mediated ON was the most frequent manifestation of MOGAD in our study; others included myelitis, encephalitis, and CNS vasculopathy (Table S1). Based on these results, it would be beneficial to consider MOG-IgG testing in COVID-19 patients with new-onset neurologic symptoms, potentially compatible with MOG-IgG–mediated disease. Here, we reported a case of post-COVID MOGAD (Case 2) with a typical presentation of ON six months after the initial infection, whose earlier findings were suggestive of post-infectious COVID-19 encephalomyelitis (when MOG-IgG titers were not tested). Eventually, he showed an excellent response and achieved a near-complete resolution of symptoms with steroids and IVIG therapy.
On the contrary, neuromyelitis optica (NMO) is an IDD associated with antibodies against aquaporin-4 (AQP4), a protein on the water transport channel membrane of astrocytes (Lennon et al., 2005). It has historically occurred as a para-infectious complication of multiple bacterial and viral infections (including mycobacterium pneumonia, VZV, and HSV) and coexisted with other autoimmune disorders (such as myasthenia gravis), as confirmed in one of our cases (Barone et al., 2021) (Sellner et al., 2010; Park et al., 2013; Machado et al., 2015; Nakamura et al., 2017; Flanagan et al., 2016). The term NMO spectrum disorders (NMOSD) covers the entire clinical spectrum of this distinct disorder, which was defined and stratified based on AQP4-antibody (AQP4-Ab) serology status by the 2015 International Panel for NMO Diagnosis (IPND); among the cardinal features were ON, TM, area postrema syndrome (APS), and brain or brainstem involvement (Wingerchuk et al., 2015). Furthermore, although pathogenic AQP4-Abs are unique to NMO (Lennon et al., 2005), roughly 10–33% of all NMOSD cases are seronegative for AQP4-Ab, out of which nearly half carry serum MOG-Abs (Hamid et al., 2017a) (Hamid et al., 2017b) (Hyun et al., 2016). Indeed, these reflect the clinical vignettes of our patients wherein a slightly higher proportion of AQP4-Ab-negative cases were found (43%), with one of these (33%) being MOG-IgG-positive in a young male with acute bilateral ON (de Ruijter et al., 2020), a feature seen more commonly with MOG-Ab-positive NMOSD (Table S1) (Sato et al., 2014) (Kitley et al., 2014).
6. Conclusion
CNS IDDs, such as MS and MOGAD, are a heterogeneous group of disorders, many of which are associated with viral infections, including COVID-19. All cases discussed here displayed characteristic radiographic and clinical features, including both of our original patients (Cases 1 and 2). Since the detection of SARS-CoV-2 in the CSF has been limited, clinicians may rely on these attributes, along with specific antibody titers, as the early identification of these distinct entities is imperative in informing their respective treatment. Therefore, while these neurological sequelae are few, physicians must be cognizant of their underlying pathophysiology and neuro-diagnostic findings, as identified in this paper, when treating COVID-19 patients with atypical presentations. Moreover, given our evolving understanding of viral-induced demyelination and post-acute COVID-19 immunopathology, we may consider serologic SARS-CoV-2 IgG screening in a patient with a history of a recent viral illness and new-onset manifestations consistent with these CNS IDDs, in the near future.
Disclosures
Parissa Feizi - Reports no disclosure.
Kanika Sharma - Reports no disclosure.
Shreya R Pasham - Reports no disclosure.
Lalit Nirwan - Reports no disclosure.
Joe Joseph - Reports no disclosure.
Shruti Jaiswal - Reports no disclosure.
Shitiz Sriwastava - Reports no disclosure.
Author contribution statement
Conceptualization: Shitiz Sriwastava.;Data curation: Parissa Feizi, Kanika Sharma,Shreya R Pasham,Lalit Nirwan, Joe Joseph, Shruti Jaiswal, Shitiz Sriwastava
Formal analysis:Shitiz Sriwastava;Funding acquisiton:NA;Investogation:Shitiz Sriwastava; Methodology:Kanika Sharma,Shreya R Pasham,Shruti Jaiswal;Project administration:Shitiz Sriwastava;Resources:Shitiz Sriwastava;Software:Shitiz Sriwastava;Supervision:Shitiz Sriwastava;Validation:Parissa Feizi,Shitiz Sriwastava;Visualization:Shitiz Sriwastava;Wrtiting-original drfat:Parissa Feizi, Kanika Sharma,Shreya R Pasham,Lalit Nirwan, Joe Joseph, Shruti Jaiswal, Shitiz Sriwastava;Writing-review editing:Shitiz Sriwastava
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Acknowledgments
West Virginia Clinical and Translational Science Institute, Morgantown, WV; SS supported in part by WVCTSI via award under 5U54GM104942-05.
Code availability
Not applicable.
Ethics approval and consent to participate
Institutional Review Board at West Virginia University authorized the publication of case report, under IRB protocol number: 2004958561.
Informed consent
Informed consent was obtained from patients and as this study was conducted under approval of West Virginia University IRB; IRB protocol number: 2004958561.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jneuroim.2022.577939.
Appendix A. Supplementary data
Summary of case reports of COVID-19-associated CNS demyelinating disorders (NMOSD, MOGAD, MS, and ON)
Data availability
Data will be made available on request.
References
- Al-Ramadan A., Rabab’h O., Shah J., Gharaibeh A. Acute and post-acute neurological complications of COVID-19. Neurol. Int. 2021 Mar 9;13(1):102–119. doi: 10.3390/neurolint13010010. PMID: 33803475; PMCID: PMC8006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab M.A., Hasaneen S.F., Hanifa H., Azzam A.Y. Optic neuritis post-COVID-19 infection. A case report with meta-analysis. Interdiscip. Neurosurg. 2021 Dec;26:101320. doi: 10.1016/j.inat.2021.101320. Epub 2021 Jul 22. PMID: 34312592; PMCID: PMC8295047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone S., Rapisarda L., Manzo L., Mechelli A., Pascarella A., Bruno P., Pasquale M., Trimboli M., Valentino P., Gambardella A. A case of neuromyelitis optica spectrum disorder (NMOSD) and acute myositis following SARS-CoV-2 infection. J. Neurol. Sci. 2021 Oct;429:119862. doi: 10.1016/j.jns.2021.119862. Epub 2021 Oct 8. PMCID: PMC8498504. [DOI] [Google Scholar]
- Bar-Or A., Pender M.P., Khanna R., Steinman L., Hartung H.P., Maniar T., Croze E., Aftab B.T., Giovannoni G., Joshi M.A. Epstein-Barr virus in multiple sclerosis: theory and emerging immunotherapies. Trends Mol. Med. 2020 Mar;26(3):296–310. doi: 10.1016/j.molmed.2019.11.003. Epub 2019 Dec 17. Erratum in: Trends Mol Med. 2021 Apr;27(4):410-411. PMID: 31862243; PMCID: PMC7106557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batum M., Kisabay Ak A., Mavioğlu H. Covid-19 infection-induced neuromyelitis optica: a case report. Int. J. Neurosci. 2020 Dec;30:1–7. doi: 10.1080/00207454.2020.1860036. Epub ahead of print. PMID: 33280477. [DOI] [PubMed] [Google Scholar]
- Boucher A., Desforges M., Duquette P., Talbot P.J. Long-term human coronavirus-myelin cross-reactive T-cell clones derived from multiple sclerosis patients. Clin. Immunol. 2007 Jun;123(3):258–267. doi: 10.1016/j.clim.2007.02.002. Epub 2007 Apr 19. PMID: 17448727; PMCID: PMC7106099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner C., Lassmann H., Waehneldt T.V., Matthieu J.M., Linington C. Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase in the CNS of adult rats. J. Neurochem. 1989 Jan;52(1):296–304. doi: 10.1111/j.1471-4159.1989.tb10930.x. (PMID: 2462020) [DOI] [PubMed] [Google Scholar]
- Chou S.H., Beghi E., Helbok R., Moro E., Sampson J., Altamirano V., Mainali S., Bassetti C., Suarez J.I., McNett M., GCS-NeuroCOVID Consortium and ENERGY Consortium Global incidence of neurological manifestations among patients hospitalized with COVID-19-a report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw. Open. 2021 May 3;4(5) doi: 10.1001/jamanetworkopen.2021.12131. PMID: 33974053; PMCID: PMC8114143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo-Calvo A., Ruiz A., Maillart E., Audoin B., Zephir H., Bourre B., Ciron J., Collongues N., Brassat D., Cotton F., Papeix C., Durand-Dubief F., Laplaud D., Deschamps R., Cohen M., Biotti D., Ayrignac X., Tilikete C., Thouvenot E., Brochet B., Dulau C., Moreau T., Tourbah A., Lebranchu P., Michel L., Lebrun-Frenay C., Montcuquet A., Mathey G., Debouverie M., Pelletier J., Labauge P., Derache N., Coustans M., Rollot F., De Seze J., Vukusic S., Marignier R., OFSEP and NOMADMUS Study Group Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018 May 22;90(21):e1858–e1869. doi: 10.1212/WNL.0000000000005560. (Epub 2018 Apr 25. PMID: 29695592) [DOI] [PubMed] [Google Scholar]
- Corrêa D.G., de Souza Lima F.C., da Cruz Bezerra D., Coutinho A.C. Júnior, Hygino da Cruz LC Júnior. COVID-19 associated with encephalomyeloradiculitis and positive anti-aquaporin-4 antibodies: cause or coincidence? Mult. Scler. 2021 May;27(6):973–976. doi: 10.1177/1352458520949988. Epub 2020 Sep 10. PMID: 32909895. [DOI] [PubMed] [Google Scholar]
- Das Sarma J. A mechanism of virus-induced demyelination. Interdiscip. Perspect. Infect. Dis. 2010;2010:109239. doi: 10.1155/2010/109239. Epub 2010 Jun 21. PMID: 20652053; PMCID: PMC2905936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Le Coupanec A., Dubeau P., Bourgouin A., Lajoie L., Dubé M., Talbot P.J. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019 Dec 20;12(1):14. doi: 10.3390/v12010014. PMID: 31861926; PMCID: PMC7020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnosis and Treatment of Adults with Community-acquired Pneumonia . 2022. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Accessed [May 10, 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues R.B., Mendes-Correa M.C., de Moura Leite F.B.V., Sabino E.C., Salarini D.Z., Claro I., Santos D.W., de Jesus J.G., Ferreira N.E., Romano C.M., Soares C.A.S. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J. Neurol. 2020 Nov;267(11):3154–3156. doi: 10.1007/s00415-020-09996-w. Epub 2020 Jun 20. PMID: 32564153; PMCID: PMC7305694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espíndola O.M., Gomes Y.C.P., Brandão C.O., Torres R.C., Siqueira M., Soares C.N., Lima M.A.S.D., Leite A.C.C.B., Venturotti C.O., Carvalho A.J.C., Torezani G., Araujo A.Q.C., Silva M.T.T. Inflammatory cytokine patterns associated with neurological diseases in coronavirus disease 2019. Ann. Neurol. 2021 May;89(5):1041–1045. doi: 10.1002/ana.26041. Epub 2021 Feb 24. PMID: 33547819; PMCID: PMC8014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan E.P., Cabre P., Weinshenker B.G., Sauver J.S., Jacobson D.J., Majed M., Lennon V.A., Lucchinetti C.F., McKeon A., Matiello M., Kale N., Wingerchuk D.M., Mandrekar J., Sagen J.A., Fryer J.P., Robinson A.B., Pittock S.J. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann. Neurol. 2016 May;79(5):775–783. doi: 10.1002/ana.24617. Epub 2016 Apr 4. PMID: 26891082; PMCID: PMC4988933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi N., Razavi S., Nikzad E. Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. 2017 Apr-Jun;19(1):1–10. doi: 10.22074/cellj.2016.4867. Epub 2016 Dec 21. PMID: 28367411; PMCID: PMC5241505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., De K., Roy D., Mandal A., Biswas S., Biswas S., Sengupta S., Naga D., Ghosh M., Benito-León J. A case of area postrema variant of neuromyelitis optica spectrum disorder following SARS-CoV-2 infection. J. Neuroimmunol. 2020 Nov 11;350:577439. doi: 10.1016/j.jneuroim.2020.577439. Epub ahead of print. PMID: 33333471; PMCID: PMC7657006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Jakimovski D., Ramanathan M., Weinstock-Guttman B., Zivadinov R. The role of Epstein-Barr virus in multiple sclerosis: from molecular pathophysiology to in vivo imaging. Neural Regen. Res. 2019 Mar;14(3):373–386. doi: 10.4103/1673-5374.245462. PMID: 30539801; PMCID: PMC6334604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid S.H.M., Whittam D., Mutch K., Linaker S., Solomon T., Das K., Bhojak M., Jacob A. What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J. Neurol. 2017 Oct;264(10):2088–2094. doi: 10.1007/s00415-017-8596-7. Epub 2017 Aug 24. PMID: 28840314; PMCID: PMC5617862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid S.H., Elsone L., Mutch K., Solomon T., Jacob A. The impact of 2015 neuromyelitis optica spectrum disorders criteria on diagnostic rates. Mult. Scler. 2017 Fe;23(2):228–233. doi: 10.1177/1352458516663853. Epub 2016 Sep 28. PMID: 27553618. [DOI] [PubMed] [Google Scholar]
- Hauser S.L., Goodin D.S. In: Harrison’s Principles of Internal Medicine. Kasper D., Fauci A., Hauser S., Longo D., Jameson J., Loscalzo J., editors. vol. 19e. McGraw Hill; 2014. Multiple sclerosis and other demyelinating diseases.https://accessmedicine.mhmedical.com/content.aspx?bookid=1130§ionid=79756278 Accessed May 18, 2022. [Google Scholar]
- Hyun J.W., Jeong I.H., Joung A., Kim S.H., Kim H.J. Evaluation of the 2015 diagnostic criteria for neuromyelitis optica spectrum disorder. Neurology. 2016 May 10;86(19):1772–1779. doi: 10.1212/WNL.0000000000002655. Epub 2016 Apr 13. PMID: 27164713. [DOI] [PubMed] [Google Scholar]
- Ismail I.I., Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J. Neuroimmunol. 2022 Jan 15;362:577765. doi: 10.1016/j.jneuroim.2021.577765. Epub 2021 Nov 9. PMID: 34839149; PMCID: PMC8577051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns T.G., Bernard C.C.A. The structure and function of myelin Oligodendrocyte glycoprotein. J. Neurochem. 1999;72:1–9. doi: 10.1046/j.1471-4159.1999.0720001.x. [DOI] [PubMed] [Google Scholar]
- Johnson R.T. In: The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary. Institute of Medicine (US) Forum on Microbial Threats, Knobler S.L., O’Connor S., Lemon S.M., et al., editors. National Academies Press (US); Washington (DC): 2004. Demyelinating diseases.https://www.ncbi.nlm.nih.gov/books/NBK83700/ Available from: [PubMed] [Google Scholar]
- Karsidag S., Sahin S., Ates M.F., Cinar N., Kendirli S. Demyelinating disease of the central nervous system concurrent with COVID-19. Cureus. 2021 Aug 19;13(8) doi: 10.7759/cureus.17297. PMID: 34552833; PMCID: PMC8449512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H., Kreymborg K., Ifergan I., Dodelet-Devillers A., Cayrol R., Bernard M., Giuliani F., Arbour N., Becher B., Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007 Oct;13(10):1173–1175. doi: 10.1038/nm1651. Epub 2007 Sep 9. PMID: 17828272; PMCID: PMC5114125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., Ahn J.Y., Kim M.K., Choi J.P. Neurological complications during treatment of Middle East respiratory syndrome. J. Clin. Neurol. 2017 Jul;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. PMID: 28748673; PMCID: PMC5532318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitley J., Waters P., Woodhall M., Leite M.I., Murchison A., George J., Küker W., Chandratre S., Vincent A., Palace J. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol. 2014 Mar;71(3):276–283. doi: 10.1001/jamaneurol.2013.5857. (PMID: 24425068) [DOI] [PubMed] [Google Scholar]
- Kogure C., Kikushima W., Fukuda Y., Hasebe Y., Takahashi T., Shibuya T., Sakurada Y., Kashiwagi K. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: a case report. Medicine (Baltimore) 2021 May 14;100(19) doi: 10.1097/MD.0000000000025865. PMID: 34106635; PMCID: PMC8133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkl M., Frascolla S., Amormino C., Volpe E., Tuosto L. T helper cells: the modulators of inflammation in multiple sclerosis. Cells. 2020 Feb 19;9(2):482. doi: 10.3390/cells9020482. PMID: 32093011; PMCID: PMC7072830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri D., Ardila A. COVID-19 pandemic: a neurological perspective. Cureus. 2020 Apr 29;12(4) doi: 10.7759/cureus.7889. PMID: 32489743; PMCID: PMC7255551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V.A., Kryzer T.J., Pittock S.J., Verkman A.S., Hinson S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005 Aug 15;202(4):473–477. doi: 10.1084/jem.20050304. Epub 2005 Aug 8. PMID: 16087714; PMCID: PMC2212860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A., Frontera J., Placantonakis D.G., Lighter J., Galetta S., Balcer L., Melmed K.R. Cerebrospinal fluid in COVID-19: A systematic review of the literature. J. Neurol. Sci. 2021 Feb 15;421:117316. doi: 10.1016/j.jns.2021.117316. Epub 2021 Jan 10. PMID: 33561753; PMCID: PMC7833669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C., Amorim J., Rocha J., Pereira J., Lourenço E., Pinho J. Neuromyelitis optica spectrum disorder and varicella-zoster infection. J. Neurol. Sci. 2015 Nov 15;358(1–2):520–521. doi: 10.1016/j.jns.2015.09.374. Epub 2015 Oct 4. PMID: 26440423. [DOI] [PubMed] [Google Scholar]
- Mariotto S., Ferrari S., Monaco S., Benedetti M.D., Schanda K., Alberti D., Farinazzo A., Capra R., Mancinelli C., De Rossi N., Bombardi R., Zuliani L., Zoccarato M., Tanel R., Bonora A., Turatti M., Calabrese M., Polo A., Pavone A., Grazian L., Sechi G., Sechi E., Urso D., Delogu R., Janes F., Deotto L., Cadaldini M., Bianchi M.R., Cantalupo G., Reindl M., Gajofatto A. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J. Neurol. 2017 Dec;264(12):2420–2430. doi: 10.1007/s00415-017-8635-4. Epub 2017 Oct 23. PMID: 29063242; PMCID: PMC5688213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., Cooley L.A., Dean N.C., Fine M.J., Flanders S.A., Griffin M.R., Metersky M.L., Musher D.M., Restrepo M.I., Whitney C.G. Diagnosis and treatment of adults with community-acquired pneumonia. an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019 Oct 1;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. PMID: 31573350; PMCID: PMC6812437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S., Moosaie F., Aarabi M.H. Understanding the immunologic characteristics of neurologic manifestations of SARS-CoV-2 and potential immunological mechanisms. Mol. Neurobiol. 2020 Dec;57(12):5263–5275. doi: 10.1007/s12035-020-02094-y. Epub 2020 Sep 1. PMID: 32869183; PMCID: PMC7458880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L., Ghannam M., Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. 2021 Mar;22:100299. doi: 10.1016/j.ensci.2020.100299. Epub 2020 Dec 4. PMID: 33313429; PMCID: PMC7717878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Escobar M.C., Kataria S., Khan E., Subedi R., Tandon M., Peshwe K., Kramer J., Niaze F., Sriwastava S. Acute transverse myelitis with Dysautonomia following SARS-CoV-2 infection: a case report and review of literature. J. Neuroimmunol. 2021 Apr 15;353:577523. doi: 10.1016/j.jneuroim.2021.577523. Epub 2021 Feb 20. PMID: 33640717; PMCID: PMC7895682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Iwasaki Y., Takahashi T., Kaneko K., Nakashima I., Kunieda T., Kaneko S., Kusaka H. A case of MOG antibody-positive bilateral optic neuritis and meningoganglionitis following a genital herpes simplex virus infection. Mult. Scler. Relat. Disord. 2017 Oct;17:148–150. doi: 10.1016/j.msard.2017.07.023. Epub 2017 Jul 27. PMID: 29055448. [DOI] [PubMed] [Google Scholar]
- Neumann B., Schmidbauer M.L., Dimitriadis K., Otto S., Knier B., Niesen W.D., Hosp J.A., Günther A., Lindemann S., Nagy G., Steinberg T., Linker R.A., Hemmer B., Bösel J., PANDEMIC and the IGNITE study groups Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J. Neurol. Sci. 2020 Nov 15;418:117090. doi: 10.1016/j.jns.2020.117090. Epub 2020 Aug 11. PMID: 32805440; PMCID: PMC7417278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen L.T., Mukerji S.S., Mejia N.I. Post-acute neurological consequences of COVID-19: an unequal burden. Nat. Med. 2022 Jan;28(1):20–23. doi: 10.1038/s41591-021-01647-5. (PMID: 35039657) [DOI] [PubMed] [Google Scholar]
- Olsson T., Barcellos L.F., Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017 Jan;13(1):25–36. doi: 10.1038/nrneurol.2016.187. Epub 2016 Dec 9. PMID: 27934854. [DOI] [PubMed] [Google Scholar]
- Palao M., Fernández-Díaz E., Gracia-Gil J., Romero-Sánchez C.M., Díaz-Maroto I., Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2020 Oct;45:102377. doi: 10.1016/j.msard.2020.102377. Epub 2020 Jul 7. PMID: 32698095; PMCID: PMC7340057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.S., Hwang S.J., Shin J.H., Kim D.S. A recurrent longitudinally extensive transverse myelitis with Aquaporin-4(AQP4) antibody after herpes zoster. J. Neurol. Sci. 2013 Nov 15;334(1–2):69–71. doi: 10.1016/j.jns.2013.07.2510. Epub 2013 Jul 31. PMID: 23953947. [DOI] [PubMed] [Google Scholar]
- Peters J., Alhasan S., Vogels C.B.F., Grubaugh N.D., Farhadian S., Longbrake E.E. MOG-associated encephalitis following SARS-COV-2 infection. Mult. Scler. Relat. Disord. 2021 May;50:102857. doi: 10.1016/j.msard.2021.102857. Epub 2021 Feb 23. PMID: 33647592; PMCID: PMC7900751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A.A., Carroll L.S., Nar V., Varatharaj A., Galea I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol. Neuroimmunol. Neuroinflamm. 2020 Jun 10;7(5) doi: 10.1212/NXI.0000000000000813. PMID: 32522768; PMCID: PMC7309522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruijter N.S., Kramer G., Gons R.A.R., Hengstman G.J.D. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: a case report. Mult. Scler. Relat. Disord. 2020 Nov;46:102474. doi: 10.1016/j.msard.2020.102474. Epub 2020 Sep 1. PMID: 32892062; PMCID: PMC7462544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar S., Safan A., Okar L., Sadik N., Adeli G. The diagnostic dilemma of bilateral optic neuritis and idiopathic intracranial hypertension coexistence in a patient with recent COVID-19 infection. Clin. Case Rep. 2021 Jun 10;9(6) doi: 10.1002/ccr3.4347. PMID: 34136250; PMCID: PMC8190579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satheesh N.J., Salloum-Asfar S., Abdulla S.A. The potential role of COVID-19 in the pathogenesis of multiple sclerosis-a preliminary report. Viruses. 2021 Oct 17;13(10):2091. doi: 10.3390/v13102091. PMID: 34696521; PMCID: PMC8540806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato D.K., Callegaro D., Lana-Peixoto M.A., Waters P.J., de Haidar Jorge F.M., Takahashi T., Nakashima I., Apostolos-Pereira S.L., Talim N., Simm R.F., Lino A.M., Misu T., Leite M.I., Aoki M., Fujihara K. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014 Feb 11;82(6):474–481. doi: 10.1212/WNL.0000000000000101. Epub 2014 Jan 10. PMID: 24415568; PMCID: PMC3937859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawalha K., Adeodokun S., Kamoga G.R. COVID-19-induced acute bilateral optic neuritis. J. Investig. Med. High Impact Case Rep. 2020 Jan-Dec;8 doi: 10.1177/2324709620976018. 2324709620976018. PMID: 33238757; PMCID: PMC7705770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirinzi T., Landi D., Liguori C. COVID-19: dealing with a potential risk factor for chronic neurological disorders. J. Neurol. 2021 Apr;268(4):1171–1178. doi: 10.1007/s00415-020-10131-y. Epub 2020 Aug 27. PMID: 32852580; PMCID: PMC7450256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellner J., Hemmer B., Mühlau M. The clinical spectrum and immunobiology of parainfectious neuromyelitis optica (Devic) syndromes. J. Autoimmun. 2010 Jun;34(4):371–379. doi: 10.1016/j.jaut.2009.09.013. Epub 2009 Oct 22. PMID: 19853412. [DOI] [PubMed] [Google Scholar]
- Shabani Z. Demyelination as a result of an immune response in patients with COVID-19. Acta Neurol. Belg. 2021 Aug;121(4):859–866. doi: 10.1007/s13760-021-01691-5. Epub 2021 May 2. PMID: 33934300; PMCID: PMC8088756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan S.S., Jacobson S. Virus-induced demyelination: the case for virus(es) in multiple sclerosis. Neurotrop. Viral Infect. 2016 Apr 8:175–220. doi: 10.1007/978-3-319-33189-8_6. PMCID: PMC7122906. [DOI] [Google Scholar]
- Tandon M., Kataria S., Patel J., Mehta T.R., Daimee M., Patel V., Prasad A., Chowdhary A.A., Jaiswal S., Sriwastava S. A comprehensive systematic review of CSF analysis that defines neurological manifestations of COVID-19. Int. J. Infect. Dis. 2021 Mar;104:390–397. doi: 10.1016/j.ijid.2021.01.002. Epub 2021 Jan 9. PMID: 33434662; PMCID: PMC7837002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.J., Baranzini S.E., Geurts J., Hemmer B., Ciccarelli O. Multiple sclerosis. Lancet. 2018 Apr 21;391(10130):1622–1636. doi: 10.1016/S0140-6736(18)30481-1. Epub 2018 Mar 23. PMID: 29576504. [DOI] [PubMed] [Google Scholar]
- Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., Fujihara K., Galetta S.L., Hartung H.P., Kappos L., Lublin F.D., Marrie R.A., Miller A.E., Miller D.H., Montalban X., Mowry E.M., Sorensen P.S., Tintoré M., Traboulsee A.L., Trojano M., Uitdehaag B.M.J., Vukusic S., Waubant E., Weinshenker B.G., Reingold S.C., Cohen J.A. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018 Fe;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. Epub 2017 Dec 21. PMID: 29275977. [DOI] [PubMed] [Google Scholar]
- WHO Coronavirus (COVID-19) Dashboard. 2022. https://covid19.who.int/
- Wingerchuk D.M., Banwell B., Bennett J.L., Cabre P., Carroll W., Chitnis T., de Seze J., Fujihara K., Greenberg B., Jacob A., Jarius S., Lana-Peixoto M., Levy M., Simon J.H., Tenembaum S., Traboulsee A.L., Waters P., Wellik K.E., Weinshenker B.G., International Panel for NMO Diagnosis International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015 Jul 14;85(2):177–189. doi: 10.1212/WNL.0000000000001729. Epub 2015 Jun 19. PMID: 26092914; PMCID: PMC4515040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020 Jun;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. Epub 2020 Mar 11. PMID: 32205092; PMCID: PMC7156211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavari F., Raji S., Moradi F., Saeidi M. Demyelinating changes alike to multiple sclerosis: a case report of rare manifestations of COVID-19. Case Rep. Neurol. Med. 2020 Dec 28;2020:6682251. doi: 10.1155/2020/6682251. PMID: 33425411; PMCID: PMC7774298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Jones-Lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R. Myelin Oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J. Neuroophthalmol. 2020 Sep;40(3):398–402. doi: 10.1097/WNO.0000000000001049. PMID: 32604245; PMCID: PMC7382408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi A., Ramezani M., Roozbeh M., Darazam I.A., Sahraian M.A. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult. Scler. Relat. Disord. 2020 Sep;44:102324. doi: 10.1016/j.msard.2020.102324. Epub 2020 Jun 24. PMID: 32615528; PMCID: PMC7311915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žorić L., Rajović-Mrkić I., Čolak E., Mirić D., Kisić B. Optic neuritis in a patient with seropositive myelin oligodendrocyte glycoprotein antibody during the post-COVID-19 period. Int. Med. Case Rep. J. 2021 May 25;14:349–355. doi: 10.2147/IMCRJ.S315103. PMID: 34079389; PMCID: PMC8165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of case reports of COVID-19-associated CNS demyelinating disorders (NMOSD, MOGAD, MS, and ON)
Data Availability Statement
Data will be made available on request.