Abstract
Pre-exposure prophylaxis (PrEP) decreases human immunodeficiency virus (HIV) acquisition among persons who inject drugs (PWID); however, its uptake has been suboptimal. We explored HIV risk perceptions and PrEP interest among drug detoxification center patients in the context of the ongoing opioid overdose epidemic. We conducted in-depth interviews of patients (n=24) and professional key informants (n=10 physicians, case managers, nurses, and harm reduction educators), and thematic analysis of coded data.
Mean age of participants (patients) was 37 years; 54% identified as male and 67% as White. Although 71% reported injecting drugs and 62% had condomless sex in the past 6 months, participants had mixed HIV risk perceptions, and some viewed PrEP as an undesirable indicator of elevated HIV risk. Nevertheless, many participants viewed drug detoxification as a first step towards embarking on a “healthier lifestyle,” with some narratives identifying opportunities for delivering PrEP information and services in this setting.
Opportunities exist to expand PrEP at drug detoxification centers, but initiatives are needed to educate patients and staff on indications and benefits of this prevention tool. Interventions are also needed to determine the best strategies for implementing PrEP adoption in this setting.
Keywords: human immunodeficiency virus, pre-exposure prophylaxis, persons who inject drugs, drug detoxification center
1.1. INTRODUCTION
In October 2020, the U.S. Centers for Disease Control and Prevention issued an Official Health Advisory regarding clustered human immunodeficiency virus (HIV) outbreaks attributed to injection drug use in at least thirteen diverse areas of the United States since 2015 (Centers for Disease Control and Prevention, 2020). This included one large outbreak involving ≥129 molecularly or epidemiologically linked HIV cases in two cities in Massachusetts between 2015–2018 (Alpren et al, 2019). The combined context of the ongoing opioid epidemic and increasing fentanyl in local drug supplies, threaten efforts to end HIV transmission among persons who inject drugs (PWID) (Alpren et al, 2019).
Pre-exposure prophylaxis (PrEP) is an HIV prevention tool (USPSTF, 2019) that is underutilized among PWID despite studies showing high levels of clinical indication (Earlywine et al, 2020). Existing research documents barriers to PrEP relating to limited awareness of PrEP and low HIV risk perceptions, challenges with healthcare access, medication adherence, and competing health priorities (Allen et al, 2020; Biello et al, 2018; Walters et al, 2020). Research also shows that PWID prefer receiving PrEP information and services outside of traditional healthcare settings (e.g., through syringe service programs and other community-based organizations; Biello et al, 2018).
Drug detoxification centers provide medically-supervised withdrawal for patients with substance use disorders and represent promising venues for delivering HIV prevention services (CSAT, 2006). While patients at detoxification centers may be interested in PrEP (Assoumou et al, 2021a), to our knowledge, no studies outside of the Bangkok Tenofovir Study have conducted more in-depth investigations into attitudes and barriers to uptake in drug detoxification settings (Martin et al, 2016).
2.1. METHODS
Between November 2018 and August 2019, we conducted an IRB-approved qualitative study with patients at a drug and alcohol detoxification center in Boston, MA (Assoumou et al, 2020). Eligible participants were adults (≥18 years of age) who spoke English and were admitted to the center with self-reported drug use. We administered brief questionnaires assessing socio-demographics and sexual and drug-related behaviors that increase HIV transmission risk. Next, informed by the Information-Motivation-Behavioral Skills (IMB) model (Figure 1; Assoumou et al, 2021b), trained research assistants conducted semi-structured interviews exploring HIV risk perceptions and PrEP interest. Participants received $20 gift cards as compensation. During key informant interviews with clinicians from the detoxification center and a neighboring medical center, we explored PrEP uptake barriers and facilitators within the detoxification center and nearby healthcare facilities.
Figure 1.
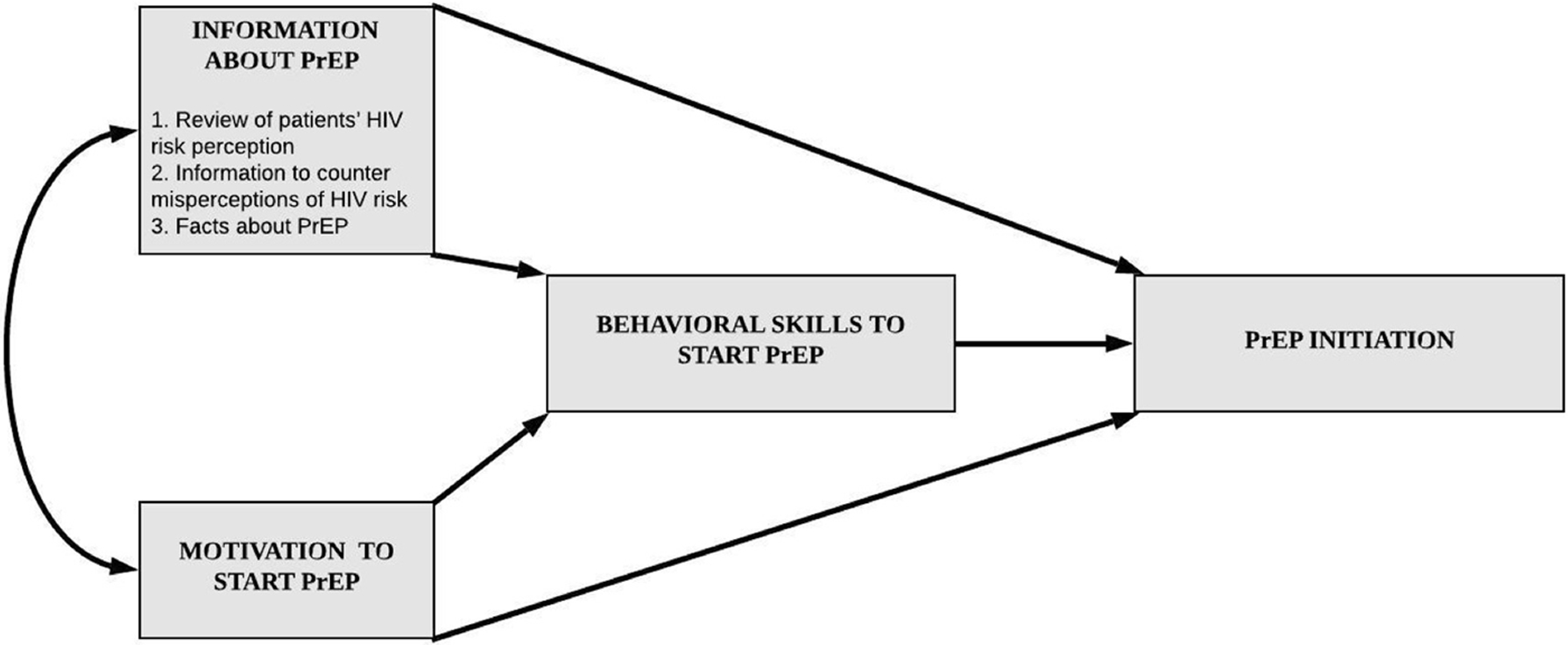
Information-Motivation-Behavioral (IMB) skills theoretical model by Fisher et al. adapted for PrEP initiation.
PrEP = Pre-Exposure Prophylaxis
We iteratively developed, refined, and finalized the codebook as a team (Assoumou et al, 2021b; Vaismoradi et al, 2013) and then applied final codes to interview transcripts using NVivo. For this analysis, we summarized data coded for HIV risk perceptions, testing, and prevention including PrEP. Anonymized quotes illustrate key findings.
3.1. RESULTS
Sample Characteristics and Findings Overview
Among 24 participants, mean age was 37 years and 54% identified as male (Table 1). Two-thirds (67%) identified as White (13% as Black, 8% Latinx, 4% Native Hawaiian/Pacific Islander, and 8% Other), and 71% reported injecting drugs in the past 6 months (Table 1). None of participants had taken PrEP before and heroin was most commonly used in the past 3 months. Additionally, 62% reported condomless sex over the previous 6 months. Ten key informants included case managers, physicians, registered nurses, nurse practitioners, and harm reduction educators. From analysis of coded interview data, we identified three themes: (1) mixed HIV risk perceptions, (2) limited PrEP interest, and (3) opportunities for delivering PrEP information and services in detoxification centers.
Table 1:
Characteristics of patients interviewed at a drug detoxification center, Boston, Massachusetts (n=24)
| Characteristics | n (%) |
|---|---|
|
| |
| Race/ethnicity | |
| White | 16 (66.7) |
| Black or African American | 3 (12.5) |
| Latinx | 2 (8.3) |
| Native Hawaiian/Pacific Islander | 1 (4.2) |
| Other | 2 (8.3) |
|
| |
| Sexual orientation | |
| Heterosexual | 20 (83.3) |
| Bisexual | 2 (8.3) |
| Homosexual or gay | 2 (8.3) |
|
| |
| Educational attainment | |
| Some high school | 5 (20.8) |
| Completed high school or graduate equivalent degree | 11 (45.8) |
| Some college | 8 (33.3) |
|
| |
| Housing status, past 6 months | |
| House or apartment | 7 (29.2) |
| On the street | 7 (29.2) |
| Overnight shelter | 4 (16.6) |
| Residential treatment facility | 3 (12.5) |
| Other (friend or relative’s home) | 3 (12.5) |
|
| |
| Employment | |
| Employed full-time (30+ hours per week) | 4 (16.7) |
| Employed part-time (<30 hours per week) | 2 (8.3) |
| Unemployed | 14 (58.3) |
| Disabled | 4 (16.7) |
|
| |
| Drug use, past 3 months (categories are not mutually exclusive) | |
| Crack (“snow”) | 18 (75) |
| Marijuana (“pot, 420”) | 17 (70.8) |
| Alcohol | 16 (66.6) |
| Heroin | 16 (66.6) |
| Cocaine | 16 (66.6) |
| Downers or sedatives (Valium, Xanax) | 12 (50) |
| Crystal methamphetamine (“speed, ice, tina”) | 7 (29.2) |
| Prescribed painkillers | 7 (29.2) |
| Street methadone | 3 (12.5) |
| Viagra, Cialis, or Levitra | 1 (4.2) |
| Other drugs NOT prescribed to participant d | 5 (20.8) |
|
| |
| Any distributive or receptive syringe sharing, past month | 7 (29.2) |
benzodiazepines n=2; methamphetamine n=1; buprenorphine n=2; alprazolam n=2
3.2. Mixed HIV risk perceptions:
Participants described mixed levels of HIV risk perception. Judah (White, age 31) noted, “[HIV] risk is a lot higher now. [There have been] a lot more new infections recently. So [people need to] be extra cautious with sharing cookers and cottons and [using] new needles.” While some individuals reported using “all brand-new needles,” others described higher risk injection practices including sharing injection equipment and rinse water even when clean water was available (e.g., “I’m really not taking precautions,” Josie, Black and Italian, age 31). In addition to a range of risk practices, participants described unreliable strategies for assessing risk. For example, John (White, age 28), explained that he could discern whether his sex partners “were probably clean,” later explaining, “My girlfriend is HIV negative as well [so] the risk of me getting [HIV] is a very low percentile.” Several suggested that they were already using standard HIV prevention practices. As Janine (Lebanese, age 42) said, “You know, I felt that I had been, like I said, 95% safe.” One key informant, a physician from a nearby clinic, helped contextualize participants’ low HIV risk perceptions, stating, “People feel like HIV is a hoax, that it’s a government-made disease.”
3.3. Limited PrEP interest:
Participants’ PrEP interest varied and appeared linked to their mixed HIV risk perceptions. For example, Jack (Black, age 32) stated, “I don’t think I would be a good candidate [for PrEP] because I don’t engage in any of that behavior.” Others perceived PrEP as an indicator of HIV risk, as Jim (White, age 34) explained, “People I was using with [were] taking PrEP every day, and that threw me off [and] made me think [they] had [HIV].” For others, PrEP was not a priority because they had more important health concerns. As Jessica (White, age 26) stated, “I need to worry about different things with my body right now.” More generally, Joseph (White, age 60) stated, “I’m really not afraid of dying.” Additionally, some participants were not interested in PrEP because they were entering recovery and felt they would no longer experience HIV risk. Others were distrustful of PrEP’s safety during a time when they were focused on improving their health. As Jerry (White, age 29) articulated, “I just don’t want to be a guinea pig. I put all kinds of shit in my body, but when I’m clean, I’m not willing to do something like that.”
3.4. Opportunities for delivering PrEP information and services in drug detoxification centers:
Some key informants believed PrEP should be integrated into clinical programming at detoxification centers. A clinician informant explained, “Usually we’re doing multiple cycles of PEP [post exposure prophylaxis] rather than just [starting] PrEP. It’s been difficult to interest them [in PrEP], even if they’re interested in the Hep C treatments.” Key informants described detoxification centers as ideal venues for increasing PrEP knowledge and interest because patients often reflect on their health during their stay at the center. Some participants’ narratives demonstrated this sentiment as they expressed interest in PrEP. For example, Julie (White, age 36) described a renewed investment in her health and protecting it for her family members: “When you’re doing drugs, you don’t want to do nothin’ else but the drugs…but now I am getting clean, and I don’t want to kill myself. I want to be healthy for my daughter.” For Julie, PrEP represented another method of caring for her health. Similarly, Joan (White, age 49) acknowledged that her HIV risk could change even during recovery and expressed interest in PrEP, stating, “Screwing up from time to time, I’d rather have that protection.”
4.1. DISCUSSION
Drug detoxification centers offer potential for PrEP information and service delivery. Just as medications for opioid use disorder (MOUD) such as buprenorphine have been shown to decrease drug-related HIV risk behavior, PrEP represents an additional HIV prevention tool for this population and setting (Sullivan et al, 2008). To leverage the readiness of some participants to seek a healthier lifestyle as they entered recovery, services along the PrEP delivery cascade including HIV testing, PrEP education, PrEP uptake, and adherence supports could be framed as key components of comprehensive care for patients with OUD.
To our knowledge, this is the first qualitative study to evaluate the potential of drug detoxification centers as settings for PrEP implementation. While prior research using quantitative surveys has suggested limited awareness of PrEP among patients in this setting (Assoumou et al., 2021a), our study provides more in-depth information on how mixed HIV risk perceptions may suppress PrEP interest despite the potential ability of drug detoxification centers to deliver PrEP information and services. Additionally, this research builds on prior studies that have explored PrEP knowledge and interest among PWID in community-based and other non-medical settings (Bazzi et al, 2018). Limitations include the small sample and single site from which we recruited participants, which could limit generalizability. However, we believe this work highlights opportunities for future research investigating the feasibility and acceptability of PrEP delivery to patients with OUD within drug detoxification centers.
5.1. CONCLUSION
In the context of intertwining opioid overdose and HIV epidemics among PWID, we found that drug detoxification center patients had mixed HIV risk perceptions and PrEP interest but some narratives highlighted the promise of this setting for PrEP delivery. Additional research should explore the feasibility and acceptability of various PrEP education and delivery strategies for patients and staff at drug detoxification centers, as these settings present important opportunities to improve the health of patients with OUD.
ACKNOWLEDGEMENTS
The authors would like to thank study participants and our research team for their contributions to this work. Special thanks to Imaan Umar for assistance with reviewing transcripts. This work was supported by the National Institute of Drug Abuse [K23DA044085 and K23 DA044085-03S1 to S.A.A, and K01DA043412 to A.R.B] and a Boston University School of Medicine Department of Medicine Career Investment and Evans Junior Faculty Research Merit Awards to SAA. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institutes of Health.
Footnotes
CONFLICT OF INTEREST: The authors have no conflicts of interest to declare.
REFERENCES
- Allen ST, O’Rourke A, White RH, Smith KC, Weir B, Lucas GM, Sherman SG, & Grieb SM (2020). Barriers and Facilitators to PrEP Use Among People Who Inject Drugs in Rural Appalachia: A Qualitative Study. AIDS Behav. 2020; 24(6):1942–1950. doi: 10.1007/s10461-019-02767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpren C, Dawson EL, John B, Cranston K, Nivedha Panneer H, Fukuda D, Roosevelt RK, Klevens M, Bryant J, Peters PJ, Lyss SB, Switzer WM, Burrage A, Murray A, Agnew-Brune C, Stiles T, McClung P, Campbell EM, Breen C, Randall LM, …, & Buchacz K (2020). Opioid Use Fueling HIV Transmission in an Urban Setting: An Outbreak of HIV Infection Among People Who Inject Drugs—Massachusetts, 2015–2018. American Journal of Public Health. 2019; 110:37–44. doi: 10.2105/AJPH.2019.305366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoumou SA, Paniagua SM, Gonzalez P, Wang J, Beckwith CG, White LF, Taylor JL, Coogan K, Samet JH, & Linas BP. (2021a). HIV Pre-Exposure Prophylaxis and Buprenorphine at a Drug Detoxification Center during the Opioid Epidemic: Opportunities and Challenges. AIDS Behav. 2021 Aug; 25(8):2591–2598. doi: 10.1007/s10461-021-03220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoumou SA, Paniagua SM, Linas BP, Wang J, Samet JH, Hall J, White LF, & Beckwith CG. (2020). Rapid Versus Laboratory-Based Testing for HIV and Hepatitis C at a Drug Detoxification Treatment Center: A Randomized Trial. J Infect Dis. 2020 Sep; 222(Suppl 5):S376–S383. doi: 10.1093/infdis/jiaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoumou SA, Sian CR, Gebel CM, Linas BP, Samet JH, & Bernstein JA. (2021b). Patients at a drug detoxification center share perspectives on how to increase hepatitis C treatment uptake: A qualitative study. Drug and alcohol dependence. 2021 Mar 1; 220:108526. doi: 10.1016/j.drugalcdep.2021.108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi AR, Biancarelli DL, Childs E, Drainoni ML, Edeza A, Salhaney P, Mimiaga MJ, & Biello KB (2018). Limited Knowledge and Mixed Interest in Pre-Exposure Prophylaxis for HIV Prevention Among People Who Inject Drugs. AIDS Patient Care STDS. 2018; 32(12):529–537. doi: 10.1089/apc.2018.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello KB, Bazzi AR, Mimiaga MJ, Biancarelli DL, Edeza A, Salhaney P, Childs E, & Drainoni ML (2018). Perspectives on HIV pre-exposure prophylaxis (PrEP) utilization and related intervention needs among people who inject drugs. Harm Reduct J. 2018; 15, 55. doi: 10.1186/s12954-018-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2020). Recent HIV Clusters and Outbreaks Across the United States Among People Who Inject Drugs and Considerations During the COVID-19 Pandemic. Accessed on December 21, 2021. Available at. https://emergency.cdc.gov/han/2020/han00436.asp. [Google Scholar]
- Center for Substance Abuse Treatment. Detoxification and Substance Abuse Treatment. (2006). Treatment Improvement Protocol (TIP) Series 45. DHHS Publication No. (SMA) 06–4131. Rockville, MD: Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- Earlywine JJ, Bazzi AR, Biello KB, & Klevens RM (2020). High Prevalence of Indications for Pre-exposure Prophylaxis Among People Who Inject Drugs in Boston, Massachusetts. American Journal of Preventive Medicine. 2021 Mar; 60(3):369–378. doi: 10.1016/j.amepre.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Chaipung B, Worrajittanon D, Leethochawalit M, Chiamwongpaet S, Kittimunkong S, Gvetadaze RJ, McNicholl JM, Paxton LA, Curlin ME, Holtz TH, Samandari T, & Choopanya K (2016). Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, Chawarski MC, Pantalon MV, Barry D, O’Connor PG, Schottenfeld RS, & Fiellin DA. (2008). Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. Journal of substance abuse treatment. 2008; 35(1):87–92. doi: 10.1016/j.jsat.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Preventive Services Task Force (USPSTF). (2019). Preexposure Prophylaxis for the Prevention of HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019; 321(22):2203–2213. doi: 10.1001/jama.2019.6390. [DOI] [PubMed] [Google Scholar]
- Vaismoradi M, Turunen H, & Bondas T. (2013). Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs Health Sci 2013; 15(3):398–405. doi: 10.1111/nhs.12048. [DOI] [PubMed] [Google Scholar]
- Walters SM, Kral AH, Simpson KA, Wenger L, & Bluthenthal RN. (2020). HIV Pre-Exposure Prophylaxis Prevention Awareness, Willingness, and Perceived Barriers among People Who Inject Drugs in Los Angeles and San Francisco, CA, 2016–2018. Subst Use Misuse. 2020; 55(14):2409–2419. doi: 10.1080/10826084.2020.1823419. [DOI] [PMC free article] [PubMed] [Google Scholar]


