Abstract
Background
The prognosis of critically ill patients with cirrhosis who require mechanical ventilation is guarded. Data are lacking for the optimal therapeutic approach to hepatic encephalopathy (HE) in the ventilated patient.
Methods
Retrospective cohort analysis of 314 encounters (298 patients) with cirrhosis who underwent mechanical ventilation in a medical ICU and were ordered at least 1 dose of lactulose. Hazard of extubation alive was determined using a competing risk model. Primary exposures were HE therapy (lactulose and rifaximin) which were adjusted for the indication for ventilation (HE, procedures, respiratory failure), age, MELD-Na, and compensation status.
Results
Indications for ventilation were 22.3% for grade 4 HE, 29.9% for procedures, and 47.8% for respiratory or cardiovascular failure. Median length of intubation was 2.63 days; death rate on ventilator was 31.2%. Relative to intubation for procedure, hazard of extubation for intubation for HE was 0.34 (95% confidence interval (CI): 0.22–0.52) and 0.33 (CI: 0.23–0.47) for respiratory failure. Hazard of extubation for rifaximin administration within 24-h after intubation was significant at 1.74 (1.21–2.50). Lactulose dosing was not significant for hazard of extubation.
Discussion
Mortality is high for all patients with cirrhosis requiring mechanical ventilation, including those intubated for grade 4 HE. Efforts to optimize the odds of successful extubation are urgently needed. Our findings suggest improved incidence of extubation associated with rifaximin administration in the first 24-h after intubation. Prospective, multi-center data to confirm these findings in this vulnerable population are warranted.
Keywords: Liver disease, Hepatic encephalopathy, Mechanical ventilation, Rifaximin, Therapy
Introduction
The prognosis of critically ill patients with decompensated cirrhosis who require mechanical ventilation is guarded [1–4]. One-year survival following a critical care admission with mechanical ventilation is as low as 11% [5]. Hepatic encephalopathy (HE) is a common reason for initiating mechanical ventilation for the purpose of airway protection. Whereas HE is often a complicating factor in the management of gastrointestinal hemorrhage or sepsis, mechanical ventilation is often utilized for ‘airway protection,’ specifically for the management of West Haven grade 4 HE, or hepatic coma [6]. Unfortunately, data are limited regarding the outcomes, optimal management, and indication for mechanical ventilation for HE.
Although prior single center and national studies have suggested that outcomes following mechanical ventilation are dismal [7–9], little is known about outcomes for airway protection in patients with HE for whom neurological status, not respiratory function, should determine the capacity for extubation. Given that HE is a reversible condition, the duration of intubation could be modifiable with therapy. Furthermore, the longer a patient remains intubated, there is both a higher cumulative dose exposure to sedative medications and a higher risk of nosocomial complications [10, 11]. While there is general guidance for the therapeutic approach to patients with overt HE [6], specific conventions for the intubated patient are lacking.
Herein, we describe the outcomes of intubation for critical illness in patients with cirrhosis, comparing those for patients with HE to those undergoing procedures and those who had respiratory indications for mechanical ventilation. We also sought to evaluate relationships between lactulose and rifaximin dosing and survival to extubation.
Methods
Patient Selection
This is a retrospective cohort study of adult (≥ 18 year old) patients with cirrhosis admitted to the University of Michigan Health System between January 2014 and December 2018. Patients were identified using DataDirect, a search tool that develops patient cohorts in the electronic medical record using international classification of diseases 10th revision (ICD-10) and international classification of diseases, 9th edition (ICD-9) billing codes. Patients were preliminarily included if they met all of the following screening criteria: (1) had a diagnosis of cirrhosis; (2) were intubated during the admission; and (3) at least one dose of lactulose was ordered during their admission. Patients were not required to have received the lactulose, only to have at least one dose ordered. To identify an expansive cohort of patients with cirrhosis, an extensive list of ICD-10 and ICD-9 billing codes was utilized to avoid inadvertent exclusion of viable patients (Supplemental Information). The initial cohort was further narrowed by only including patients who underwent intubation procedures as defined by ICD-10 and ICD-9 codes listed in the Supplemental Information.
Of the 683 unique patients, exclusion criteria included the presence of any of the following: intubated prior to transfer or in transit to our facility; chronically ventilator dependent; no evidence of cirrhosis or hepatic dysfunction on chart review; intubation was for seizure deemed not secondary to West Haven grade 4 hepatic encephalopathy (HE) on individual chart review; received a transplant prior to extubation; surgical intervention on any segment of the gastrointestinal lumen; experienced an intraparenchymal brain bleed; acute liver failure; or discharged/transferred while still intubated. This resulted in a cohort of 298 patients with a total of 314 admissions (Fig. 1).
Fig. 1.
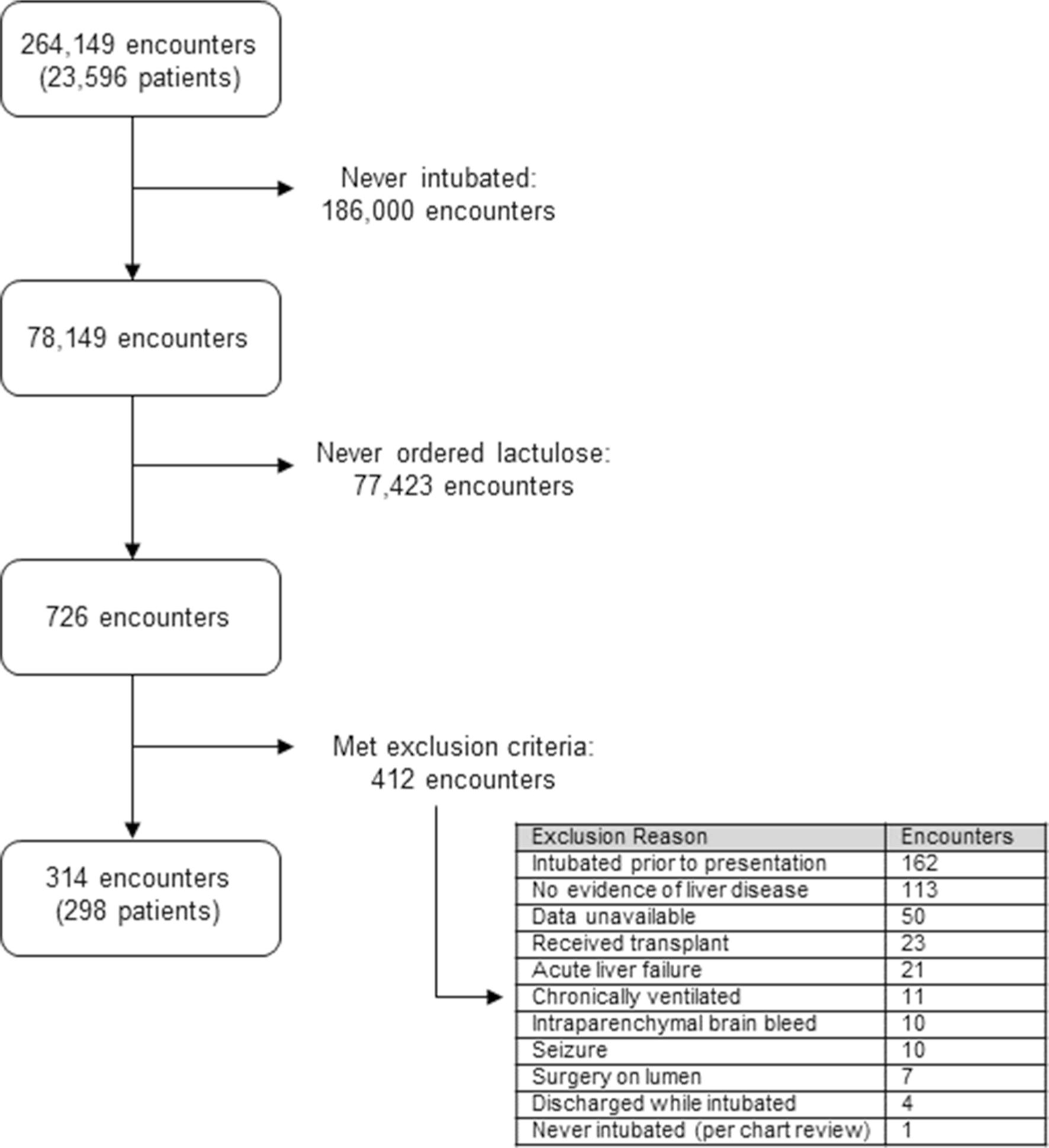
Diagram depicting the process to identify the patient population of interest
Outcomes
Our primary outcome was successful (non-terminal) extubation. Secondary outcomes measured included length of intubation, reintubation, death during admission, and length of ICU stay.
Exposures
Our primary exposures were HE therapies (lactulose and rifaximin). Lactulose was recorded in 10 g increments for oral lactulose (lactulose PO administered by orogastric or nasogastric tube), while lactulose enemas were dichotomized as administered or not owing to the lack of standardized (and measured) dwell times. Both lactulose and rifaximin administration were evaluated in the 24-h prior to intubation and the first 24-h post-intubation.
Baseline Covariates
General demographic data were collected including age, sex, and ethnicity. To characterize and compare each patient’s baseline liver disease, their MELD-Na score was calculated using the first laboratories collected during the admission. Additionally, history of hepatic decompensation events (HE, variceal hemorrhage, or ascites), lactulose prescription prior to admission, cirrhosis etiology, and Charlson Comorbidity Index were recorded. Charlson Comorbidity Index was calculated by DataDirect using ICD-9/10 codes and recorded for all patients [12]. To compare the complexity of hospital courses, the following concurrent admission diagnoses were recorded through manual chart review: pneumonia, sepsis, Clostridioides difficile infection, bacterial peritonitis, new renal failure (defined as starting hemodialysis during admission or in renal failure, but dialysis not initiated as defined by nephrology assessment), gastrointestinal bleed, and transjugular intrahepatic portosystemic shunt (TIPS) placement during admission. Sedative and analgesic exposure data at intubation, defined as within the first 15 min pre-and post-intubation, were recorded through manual review of medication administration. Sedative and analgesic data for the duration of intubation were also recorded.
The indication for intubation and mechanical ventilation was obtained through manual review of provider notes. Patients were grouped into three categories for indication for intubation: altered mental status (AMS)/HE, respiratory failure/cardiovascular instability, and procedures. Patients intubated for West Haven Grade 4 Hepatic encephalopathy or AMS with an inability to protect their airway were placed in the AMS/HE group. If patient’s AMS was secondary to shock or inadequate cerebral blood flow (as determined by provider assessment on chart review), patients were placed in the respiratory failure/cardiovascular instability group. As stated, if the etiology of AMS was seizure not deemed to be secondary to HE, patients were excluded. Patients intubated for respiratory failure, cardiovascular instability, or advanced cardiovascular life support were placed in the respiratory failure/cardiovascular instability group. Patients who were intubated for airway protection during a procedure such as esophagogastroduodenoscopy (EGD), interventional radiology arterial embolization, TIPS, or a surgical procedure (e.g., cholecystectomy) were placed in the procedure group.
Statistical Analysis
Descriptive data are presented as number (percentage) for categorical data and mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous data. The primary outcome was the hazard of successful (non-terminal) extubation. A multi-state model was used to account for the competing event of death while intubated and was conducted using Fine and Gray competing risk regression, presented as subdistribution hazard ratios (SHR) [13]. Priori, we adjusted for the covariates of age, MELD-Na, history of decompensation, indication for intubation, rifaximin use before and after intubation, and lactulose use before and after intubation. Access to data was approved by University of Michigan Institutional Review Board under study identification HUM00162860. Statistical analysis was performed using RStudio [14].
Results
Demographics and Patient Characteristics
Baseline characteristics for the 298 patients (314 admissions) are presented in Table 1. The cohort had a median age of 59 years (IQR 50–64), and was predominantly white (89.8%) and male (56.1%). The etiology of cirrhosis was primarily alcohol-related (40.1%), 84.7% had a history of a decompensation event, and the median MELD-Na was 25 (IQR 17–31). The majority of patients had a prescription for outpatient lactulose (55.4%). Common concurrent diagnoses during admission included pneumonia (43.0%), sepsis (44.6%) and gastrointestinal bleed (41.7%). The cohort was divided into three groups based on the indication for intubation: altered mental status/HE (22.3%), respiratory failure/cardiovascular instability (47.8%), and procedure (29.9%).
Table 1.
Patient demographics and characteristics
| Altered Mental Status/HE | Respiratory Failure/Cardiovascular-Instability | Procedure | All Patients | |
|---|---|---|---|---|
|
| ||||
| n | 70 | 150 | 94 | 314 |
| Female (%) | 32 (45.7) | 80 (53.3) | 26 (27.6) | 138 (43.9) |
| Median Age [IQR] | 60 [51, 65] | 58 [50, 63.8] | 58.5 [49.3, 64] | 59 [50, 64] |
| Etiology of Liver Disease | ||||
| Alcohol-Related (%) | 28 (40.0) | 55 (36.7) | 43 (45.7) | 126 (40.1) |
| Cryptogenic/NASH (%) | 21 (30.0) | 39 (26.0) | 21 (22.3) | 81 (25.8) |
| Autoimmune/PSC/PBC/Transplant Rejection (%) | 3 (4.3) | 18 (12.0) | 8 (8.5) | 29 (9.2) |
| HCV/HBV (%) | 14 (20.0) | 23 (15.3) | 19 (20.2) | 56 (17.8) |
| Hemochromatosis/A1AT/Wilson’s/Sarcoid/Cardiac (%) | 4 (5.7) | 15 (10.0) | 3 (3.2) | 22 (7.0) |
| History (Prior to Admission) | ||||
| Lactulose Home Prescription (%) | 42 (60.0) | 81 (54.0) | 51 (54.3) | 174 (55.4) |
| Hepatic Encephalopathy (%) | 46 (65.7) | 88 (58.7) | 60 (63.8) | 194 (61.8) |
| Ascites (%) | 53 (75.7) | 120 (80) | 78 (83.0) | 251 (79.9) |
| Variceal Bleed (%) | 16 (22.9) | 18 (12.0) | 43 (45.7) | 77 (24.5) |
| Bacterial Peritonitis (%) | 13 (18.6) | 19 (12.7) | 12 (12.8) | 44 (14.0) |
| History of Decompensated Cirrhosis (HE, Ascites, OR Variceal Bleed) (%) | 59 (84.3) | 124 (82.7) | 83 (88.3) | 266 (84.7) |
| COPD (%) | 10 (14.3) | 15 (10.0) | 10 (10.6) | 35 (11.1) |
| Dialysis (%) | 2 (2.9) | 10 (6.7) | 6 (6.4) | 18 (5.7) |
| Median MELD-Na [IQR] | 25 [20.3, 30] | 26 [20, 32] | 21 [15.3, 30] | 25 [17, 31] |
| Median Charlson Comorbidity Complex [IQR] | 6.5 [5, 9] | 7 [5, 10] | 7 [5, 10] | 7 [5, 9.8] |
| Ethnicity | ||||
| White, non-Hispanic (%) | 64 (91.4) | 133 (88.7) | 85 (90.4) | 282 (89.8) |
| African American (%) | 3 (4.3) | 11 (7.3) | 3 (3.2) | 17 (5.4) |
| Hispanic (%) | 3 (4.3) | 4 (2.7) | 4 (4.3) | 11 (3.5) |
| Other (%) | 0 (0.0) | 2 (1.3) | 2 (2.1) | 4 (1.3) |
| Concurrent Diagnoses During Admission | ||||
| Pneumonia (%) | 27 (38.6) | 80 (53.3) | 28 (29.8) | 135 (43.0) |
| Sepsis (%) | 25 (35.7) | 90 (60.0) | 25 (26.6) | 140 (44.6) |
| Clostridioides difficile infection (%) | 6 (8.6) | 12 (8.0) | 6 (6.4) | 24 (7.6) |
| Bacterial Peritonitis (%) | 6 (8.6) | 35 (23.3) | 11 (11.7) | 52 (16.6) |
| New Renal Failure (%) | 21 (30.0) | 70 (46.7) | 17 (18.1) | 108 (34.4) |
| Gastrointestinal Bleed (%) | 21 (30.0) | 30 (20.0) | 80 (85.1) | 131 (41.7) |
| TIPS during Admission (%) | 1 (1.4) | 6 (4.0) | 23 (24.5) | 30 (9.6) |
Admission Details
Regarding medication management for the 24 h prior to intubation, rifaximin administration varied between the three groups with those intubated for respiratory failure receiving it more often than the AMS/HE or procedure groups (Table 2). Fewer than 10% of patients received a lactulose enema in all groups, and those intubated for AMS/HE received oral lactulose at a higher rate than the other two groups.
Table 2.
Rifaximin and lactulose administration by analysis group
| Altered Mental Status/HE | Respiratory Failure/Cardiovascular-Instability | Procedure | All Patients | |
|---|---|---|---|---|
|
| ||||
| n | 70 | 150 | 94 | 314 |
| 24-h prior to intubation | ||||
| Patients given Rifaximin (%) | 16 (22.9) | 48 (32.0) | 14 (14.9) | 78 (24.8) |
| Lactulose enema received (%) | 5 (7.1) | 12 (8.0) | 5 (5.3) | 22 (7.0) |
| Patients given Lactulose PO1 (%) | 36 (51.4) | 68 (45.3) | 26 (27.7) | 130 (41.4) |
| Lactulose PO1 median number of doses2,3 [IQR] | 6 [2.75,10] | 5.5 [3, 8] | 2 [2, 4] | 4 [2, 8] |
| 24-h post-intubation | ||||
| Patients given Rifaximin (%) | 41 (58.6) | 65 (43.3) | 20 (21.3) | 126 (40.1) |
| Lactulose enema received (%) | 12 (17.1) | 11 (7.3) | 12 (12.8) | 35 (11.1) |
| Patients given Lactulose PO1 (%) | 24 (34.3) | 51 (34.0) | 11 (11.7) | 86 (27.4) |
| Lactulose PO1 median number of doses2,3 [IQR] | 10 [6.75,12.5] | 6 [4, 10] | 3 [2, 7] | 8 [4,11.5] |
Oral administration
Calculated based only on patients that received the treatment
1 dose Lactulose: 10 g
Regarding medication management for the 24 h after intubation, rifaximin was administered at a higher rate in all three groups compared to the 24 h prior to intubation with those intubated for AMS/HE receiving it at the highest rate. Lactulose enema administration rate increased in the 24 h after intubation compared to the 24 h prior for AMS/HE and procedure groups. Oral lactulose administration rate dropped in the 24 h after intubation compared to the 24 h prior to intubation for all three groups.
Of the 314 admissions, median length of intubation was 2.63 days (IQR 1.15–5.20) (Table 3). A total of 98 patients (31.2%) died while intubated and 159 patients (50.6%) died during the hospitalization. Of the sub-cohorts, median length of intubation, death during admission, and death on the ventilator were highest for respiratory failure/cardiovascular instability, followed by HE, then procedure.
Table 3.
Patient outcomes
| Altered Mental Status/HE | Respiratory Failure/ Cardiovascular Instability | Procedure | All Patients | |
|---|---|---|---|---|
|
| ||||
| n | 70 | 150 | 94 | 314 |
| Mean Length of Intubation in days (sd) | 3.82 (3.26) | 4.58 (4.36) | 2.80 (3.61) | 3.88 (3.98) |
| Median Length of Intubation in days [IQR] | 2.71 [1.67, 5.15] | 3.45 [1.44, 6.51] | 1.51 [0.70, 3.60] | 2.63 [1.15, 5.20] |
| Reintubated (%) | 7 (10.0) | 31 (20.7) | 18 (19.1) | 56 (17.8) |
| Discharged Alive and without Reintubation (%) | 32 (45.7) | 44 (29.3) | 58 (61.7) | 134 (42.7) |
| Died during admission | 37 (52.9) | 95 (63.3) | 27 (28.7) | 159 (50.6) |
| Died on the ventilator or terminally extubated (%) | 24 (34.3) | 62 (41.3) | 12 (12.8) | 98 (31.2) |
| Median Length of ICU Admission in days [IQR] | 4.13 [2.60, 6.94] | 4.93 [2.21, 9.86] | 3.68 [1.85, 6.79] | 4.19 [2.21, 8.26] |
Within the duration of intubation, the most common sedative administered was propofol (Table 4). Fentanyl was administered in approximately two-thirds of the patients in the broader cohort during intubation. Benzodiazepines (midazolam or lorazepam) were less frequently administered during intubation (17%).
Table 4.
Sedation and analgesic exposure at intubation and within the duration of intubation by analysis group
| Altered Mental Status/HE | Respiratory Failure/Cardiovascular-Instability | Procedure | All Patients | |
|---|---|---|---|---|
|
| ||||
| n | 70 | 150 | 94 | 314 |
| At intubation (15 min pre-and post-intubation) | ||||
| Propofol (%) | 54 (77.1) | 113 (75.3) | 88 (93.6) | 255 (81.2) |
| Benzodiazepine – Midazolam or Lorazepam (%) | 5 (7.1) | 17 (11.3) | 4 (4.3) | 26 (8.3) |
| Fentanyl (%) | 21 (30.0) | 47 (31.3) | 43 (45.7) | 111 (35.4) |
| Dexmedetomidine (%) | 1 (1.4) | 6 (4.0) | 2 (2.1) | 9 (2.9) |
| None (%) | 5 (7.1) | 7 (4.7) | 0 (0.0) | 12 (3.8) |
| Within duration of intubation | ||||
| Propofol (%) | 61 (87.1) | 130 (86.7) | 91 (96.8) | 282 (89.8) |
| Benzodiazepine—Midazolam or Lorazepam (%) | 9 (12.9) | 31 (20.7) | 13 (13.8) | 53 (16.9) |
| Fentanyl (%) | 40 (57.1) | 98 (65.3) | 65 (69.1) | 203 (64.7) |
| Dexmedetomidine (%) | 19 (27.1) | 38 (25.3) | 24 (25.5) | 81 (25.8) |
Primary Outcome: Factors Associated with Successful Extubation
The use of rifaximin in the 24-h prior to intubation significantly decreased the hazard of successful extubation (SHR: 0.48, CI: 0.33–0.71), while the hazard of successful extubation significantly increased with the use of rifaximin within 24-h post-intubation (SHR:1.74, CI: 1.21–2.50) (Table 5). Hazard of extubation for lactulose dosing, regardless of route, was not significant in either the 24-h prior to intubation or the first 24-h post-intubation. Compared to those intubated for a procedure, patients intubated due to respiratory failure/cardiac instability or AMS/HE had a significantly lower hazard of successful extubation (SHR 0.33 CI 0.23–0.47; SHR 0.34 CI 0.22–0.52, respectively). Additionally, for every 1-point increase in the MELD-Na score, there was a 3% decrease in the hazard of successful extubation. (Table 5, Fig. 2). There was a trend toward an increased hazard of extubation with lactulose use; however, this did not reach statistical significance.
Table 5.
Competing risk model results for extubation (alive)
| Variable | Multivariable subdistribution hazard ratio (95% confidence interval)1 |
|---|---|
|
| |
| Age (per 10 unit increase) | 0.92 (0.81–1.05) |
| MELD-Na (per unit increase) | 0.97 (0.95–0.98) |
| History of Decompensated Cirrhosis (HE, Ascites, OR Variceal Bleed) | 1.42 (0.90–2.23) |
| Indication (Referent: Procedure) | |
| AMS/HE | 0.34 (0.22–0.52) |
| Respiratory Failure/Cardiovascular Instability | 0.33 (0.23–0.47) |
| Rifaximin in the 24 h prior to intubation (Yes) | 0.48 (0.33–0.71) |
| Rifaximin in first 24 h post-intubation (Yes) | 1.74 (1.21–2.50) |
| Lactulose Enema received prior to intubation (yes) | 1.26 (0.70 –2.27) |
| Lactulose Enema received post-intubation (yes) | 1.13 (0.73–1.76) |
| Lactulose PO2 in the 24 h prior to intubation (per 10 g dose increase) | 1.03 (0.99–1.07) |
| Lactulose PO2 received in first 24 h post-intubation (per 10 g dose increase) | 1.03 (0.99–1.06) |
Statistically significant results are in bold
Oral administration
Fig. 2.
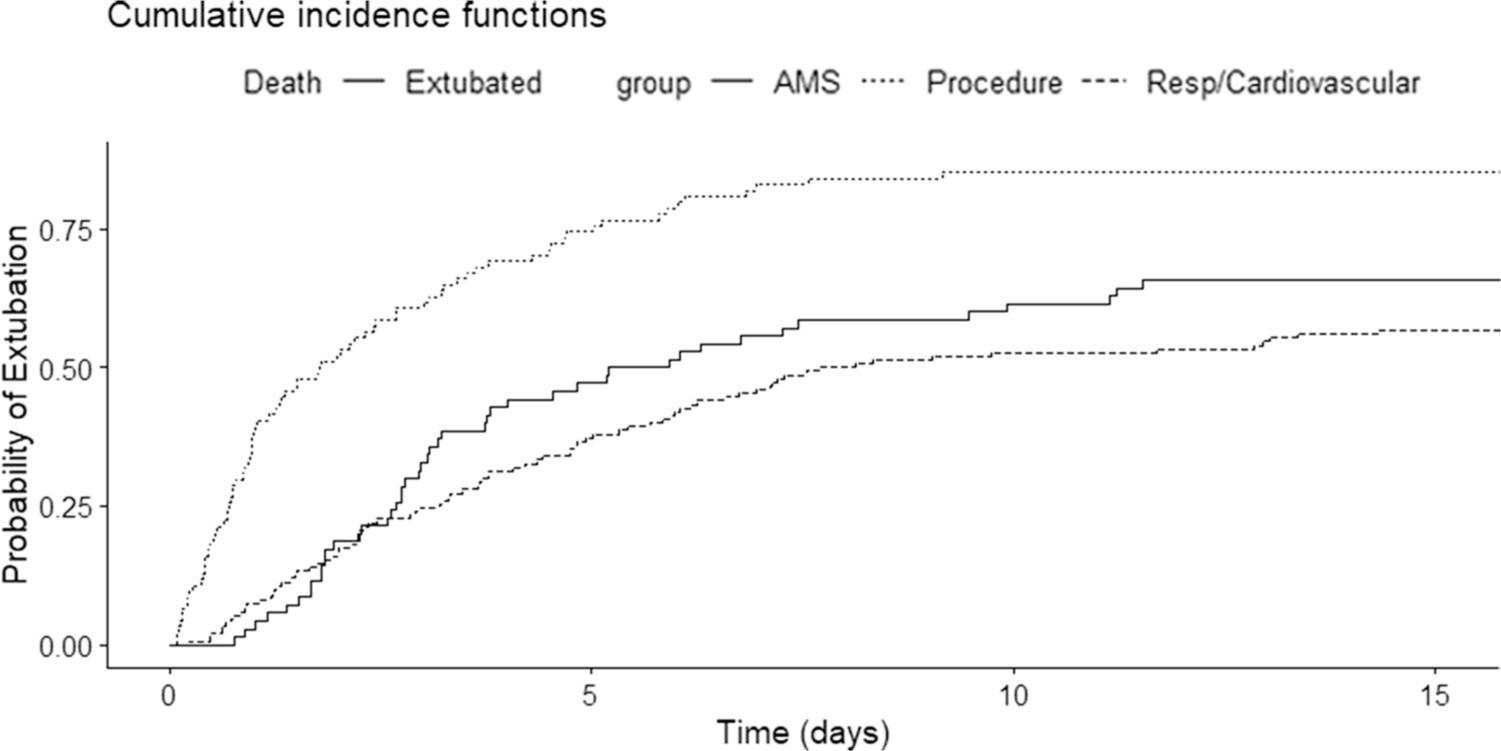
Cumulative incidence function for extubation (alive) for each sub-cohort; altered mental status/HE (AMS), procedure, and respiratory failure/cardiovascular instability (Resp/Cardiovascular)
Discussion
Although outcomes for mechanically ventilated patients with cirrhosis are poor, intubation is often necessary to reduce risk of lung injury or death. The perceived need for airway protection is common in patients with West Haven grade 4 HE and patients undergoing procedures. Unresponsive patients or those without a gag reflex may be unable to prevent aspiration of oral secretions or emesis. Intubation is believed to reduce these risks, but it may carry others relating to sedation load and procedural complications. Data are lacking for interventions that reduce intubation or result in successful extubation.
Contemporary Outcomes of Hepatic Coma
Early studies calculated the mortality rate of patients with HE admitted to the hospital to be nearly 50% but did not differentiate between ICU and non-ICU admissions [15, 16]. More recently, studies determined that the mortality rate of patients with HE requiring ICU level care was about 35% and 53% for those requiring mechanical ventilation [17, 18]. Similarly, our patient outcomes are poor with 34.3% of patients intubated for AMS/HE dying on the ventilator and 52.9% of them dying during the admission. These prior studies evaluated prognosis and predictive factors in patients with cirrhosis undergoing mechanical ventilation, with recent studies identifying extrahepatic organ failures as prognostic and older (by > 60 years) studies suggesting that antibiotic therapy with neomycin could influence outcomes [15, 17–19]. Our study extends the field by demonstrating the outcomes of intubation for HE and the contemporary management factors associated with improved rates of successful extubation.
HE Therapy and Successful Extubation
Among patients intubated for AMS/HE 34.3% died while on the ventilator or were terminally extubated; 52.9% died during the admission. These poor outcomes reinforce that optimized therapy to improve outcomes is urgently needed. The two major components of HE-directed therapy are lactulose and rifaximin. Although no significant relationship was detected between number of lactulose doses administered and incidence of extubation alive, low rates of administration and high variability in number of doses may have impacted this outcome. Further, the effect size and confidence intervals for the dose–response per 10 g lactulose were 1.03 (0.99–1.07) for the 24 h prior to intubation and 1.03 (0.99–1.06) for the 24 h after intubation, indicating that there is a high probability of benefit. Conversely, although it was utilized in 40% of cases, the incidence of successful extubation increased when rifaximin was administered during the first 24-h post-intubation. While number of bowel movements typically influences administration of lactulose, it is likely a lagging indicator of therapy. Since our study considers only the first 24-h peri-intubation, the clinical decision making is done in an intention-to-treat fashion. We cannot exclude unmeasured confounding factors; however, intubation is a common landmark eliminating the possibility of immortal time bias. The key unmeasured factors likely include concerns regarding oral administration of medication or knowledge of appropriate therapy.
Study Limitations
Our study considered the outcomes of critically ill patients with cirrhosis at a single-center academic health system over 4 years, and therefore, the results may not be representative of the broader patient population. The study cohort does not include patients with grade IV HE that were successfully managed without mechanical ventilation. Furthermore, analysis of rifaximin and lactulose administration was constrained to the 24-h prior to, and the first 24-h post-intubation to support robust statistical analysis; approximately 25% of the cohort was intubated for 24 h or less. While not included in the current analysis, rifaximin and lactulose administration for 24–48 h post-intubation and 48–72 h post-intubation was recorded (Tables 6 and 7). Potential implications of rifaximin and lactulose dosing beyond the first 24-h post-intubation remain unknown. Additionally, sedation medication may influence length of intubation and hazard of extubation. Sedation and analgesic data were recorded; however, the varied timing and combinations of medications at intubation and within the duration intubation did not allow for incorporation into the current analysis. Expansion to a multi-center study would allow for confirmation of our findings. Additional data provided by a multi-center study would also permit further exploration of sub-cohort dosing and outcomes. Further, increased data from a multi-center study may allow for statistical exploration of sedation/analgesic administration.
Table 6.
Rifaximin and lactulose administration by analysis group 24–48 h post-intubation
| Altered Mental Status/HE | Respiratory Failure/Cardiovascular Instability | Procedure | All Patients | |
|---|---|---|---|---|
|
| ||||
| n | 70 | 150 | 94 | 314 |
| 24-h prior to intubation | ||||
| Patients given Rifaximin (%) | 40 (57.1) | 63 (42.0) | 24 (25.5) | 127 (40.4) |
| Lactulose enema received (%) | 3 (4.3) | 4 (4.7) | 14 (14.9) | 24 (7.6) |
| Patients given Lactulose PO1 (%) | 49 (70.0) | 87 (58.0) | 45 (47.9) | 181 (57.6) |
| Lactulose PO1 median number of doses2,3 [IQR] | 8 [6, 10] | 6 [3, 8] | 4 [2, 6] | 6 [3, 8] |
Oral administration
Calculated based only on patients that received the treatment
1 dose Lactulose: 10 g
Table 7.
Rifaximin and lactulose administration by analysis group 48–72 h post-intubation
| Altered Mental Status/HE | Respiratory Failure/Cardiovascular Instability | Procedure | All Patients | |
|---|---|---|---|---|
|
| ||||
| n | 70 | 150 | 94 | 314 |
| 24-h prior to intubation | ||||
| Patients given Rifaximin (%) | 41 (58.6) | 62 (41.3) | 31 (33.0) | 134 (42.7) |
| Lactulose enema received (%) | 3 (4.3) | 5 (3.3) | 7 (7.4) | 15 (4.8) |
| Patients given Lactulose PO1 (%) | 46 (65.7) | 84 (56.0) | 61 (64.9) | 191 (60.8) |
| Lactulose PO1 median number of doses2,3 [IQR] | 6 [4, 10] | 6 [3, 8] | 4 [2, 6] | 6 [2.5,8] |
Oral administration
Calculated based only on patients that received the treatment
1 dose Lactulose: 10 g
Conclusion
The poor outcomes of hepatic coma remain an unmet need for patients with cirrhosis. Our data highlight the potential for benefit in optimizing our therapeutic approach. There are two clear future directions. First, given the event rate and potential effects of therapy observed in this study, it would be relatively straightforward to design a multi-center trial powered to detect improvements in outcomes. Second, reevaluation of the need for intubation in the management of hepatic coma may be warranted as the benefits of mechanical ventilation are uncertain and the risks substantial.
Supplementary Material
Funding
Elliot Tapper receives funding from the National Institutes of Health through the NIDDK (1K23DK117055-01A1). Jeremy Louissaint received support through the T32DK062708.
Abbreviations
- HE
Hepatic encephalopathy
- Lactulose PO
Oral lactulose
- TIPS
Transjugular intrahepatic portosystemic shunt
- AMS
Altered mental status
- EGD
Esophagogastroduodenoscopy
- SD
Standard deviation
- IQR
Interquartile range
- SHR
Subdistribution hazard ratios
Footnotes
Conflict of interest Elliot Tapper has served as a consultant to Norvartis, Kaleido, and Allergan, has served on advisory boards for Mallinckrodt, Rebiotix, and Bausch Health, and has received unrestricted research grants from Gilead.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10620-022-07400-3.
References
- 1.O’Leary JG, Reddy KR, Garcia-Tsao G et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology. 2018;67:2367–2374. [DOI] [PubMed] [Google Scholar]
- 2.Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care 2011;17:165–169. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal A, Ong JP, Younossi ZM et al. Predictors of mortality and resource utilization in cirrhotic patients admitted to the medical ICU. Chest 2001;119:1489–1497. [DOI] [PubMed] [Google Scholar]
- 4.Moreau R, Jalan R, Gines P et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis Gastroenterology. 2013;144:1426–1437, 1437 e1421–1429. [DOI] [PubMed] [Google Scholar]
- 5.Levesque E, Saliba F, Ichai P, Samuel D. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol. 2014;60:570–578. [DOI] [PubMed] [Google Scholar]
- 6.Vilstrup H, Amodio P, Bajaj J et al. Hepatic encephalopathy in chronic liver disease: (2014) Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 7.Lee KC, Chiang AA. The outcome of terminal liver cirrhosis patients requiring mechanical ventilation. Zhonghua Yi Xue Za Zhi (Taipei) 1997;59:88–94. [PubMed] [Google Scholar]
- 8.Juneja D, Gopal PB, Kapoor D, Raya R, Sathyanarayanan M. Profile and outcome of patients with liver cirrhosis requiring mechanical ventilation. J Intensive Care Med. 2012;27:373–378. [DOI] [PubMed] [Google Scholar]
- 9.Lai CC, Ho CH, Cheng KC, Chao CM, Chen CM, Chou W. Effect of liver cirrhosis on long-term outcomes after acute respiratory failure: A population-based study. World J Gastroenterol. 2017;23:2201–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaillette E, Girault C, Brunin G et al. Impact of tapered-cuff tracheal tube on microaspiration of gastric contents in intubated critically ill patients: a multicenter cluster-randomized cross-over controlled trial. Intensive Care Med. 2017;43:1562–1571. [DOI] [PubMed] [Google Scholar]
- 11.Wahab EA, Hamed EF, Ahmad HS, Abdel Monem SM, Fathy T. Conscious sedation using propofol versus midazolam in cirrhotic patients during upper GI endoscopy: A comparative study. JGH Open. 2019;3:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 13.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 14.RStudio Team (2020). RStudio: Integrated Development for R RStudio, PBC, Boston, MA: URL http://www.rstudio.com/. City. [Google Scholar]
- 15.Prytz H, Sloth K. Hepatic coma in cirrhosis of the liver. The course and prognosis of 100 consecutive coma episodes. Scand J Gastroenterol. 1973;8:229–233. [PubMed] [Google Scholar]
- 16.Sherlock S Pathogenesis and management of hepatic coma. Am J Med. 1958;24:805–813. [DOI] [PubMed] [Google Scholar]
- 17.Sasso R, Lauzon S, Rockey DC. Cirrhotic Patients on Mechanical Ventilation Have a Low Rate of Successful Extubation and Survival. Dig Dis Sci. 2020;65:3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fichet J, Mercier E, Genee O et al. Prognosis and 1-year mortality of intensive care unit patients with severe hepatic encephalopathy. J Crit Care. 2009;24:364–370. [DOI] [PubMed] [Google Scholar]
- 19.Stormont JM, Mackie JE, Davidson CS. Observations on antibiotics in the treatment of hepatic coma and on factors contributing to prognosis. N Engl J Med. 1958;259:1145–1150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


