Abstract
Despite our best intentions, physically salient but entirely task-irrelevant stimuli can sometimes capture our attention. With learning, it is possible to more efficiently ignore such stimuli, although specifically how the visual system accomplishes this remains to be clarified. Using a sample of young-adult participants, we examined the time course of eye movements to targets and distractors. We replicate a reduced frequency of eye movements to the distractor when appearing in a location at which distractors are frequently encountered. This reduction was observed even for the earliest saccades, when selection tends to be most stimulus-driven. When the distractor appeared at the high-probability location, saccadic reaction time was slowed specifically for distractor-going saccades, suggesting a slowing of priority accumulation at this location. In the event that the distractor was fixated, disengagement from the distractor was also faster when it appeared in the high-probability location. Both proactive and reactive mechanisms of distractor suppression work together to minimize attentional capture by frequently-encountered distractors.
Keywords: selective attention, attentional capture, selection history, signal suppression, eye movements
Although we are capable of focusing our attention on stimuli that are relevant to our current goals and needs (e.g., Kiss et al., 2009; Wolfe & Horowitz, 2017), we can at times be distracted by stimuli that we know to be task-irrelevant even when we try our best to ignore them. Certain stimuli are more likely than others to break our focus and capture our attention. Such stimuli include previously reward-associated (e.g., Anderson et al., 2011) and aversively-conditioned stimuli (e.g., Schmidt et al., 2015; Anderson & Britton, 2020). Physically salient stimuli have long been observed to function as potent distractors (see Theeuwes, 2010, for a review), with a well-characterized time course in which the attentional priority of such stimuli is initially high and then dissipates over time as the priority of the task-relevant target increases, reflecting the more sluggish influence of task goals on biased competition (e.g., Donk & van Zoest, 2008; Godjin & Theeuwes, 2002; van Zoest et al., 2004; van Zoest & Donk, 2005). Distraction by task-irrelevant stimuli can have significant health, occupational, and educational consequences (e.g., Namian et al., 2018; Strayer & Drews, 2004; Taneja et al., 2015), and so it is important to understand the mechanisms by which distraction can be mitigated.
Under certain conditions, the processing of physically salient stimuli can be suppressed (e.g., Gaspelin et al., 2015, 2017; Geng & Diquattro, 2010), a process believed to play a key role in the control of attention (Luck et al., 2021). Recent research demonstrates a powerful role for learning in the ability to resist distraction via suppressive mechanisms. When a physically salient but task-irrelevant distractor is more likely to appear at a particular location in space, attentional capture by that distractor is substantially reduced when appearing in this high-probability location (e.g., Wang & Theeuwes, 2018a, 2018b, 2018c). This spatially-specific reduction in capture persists once the biased spatial probabilities are removed (Britton & Anderson, 2020; Kim & Anderson, 2021), implicating learning-dependent processes. A similar reduction in the magnitude of attentional capture by physically salient stimuli has been observed as a function of the frequency with which distractors appear in a particular color (Stilwell et al., 2019; Vatterott & Vecera, 2012; see also Failing et al., 2019).
Specifically how the visual system implements such statistical learning in the mitigation of distraction remains to be clarified, including whether a single or multiple mechanisms of distractor suppression are implicated and the manner in which they are leveraged to modulate information processing. Both proactive and reactive mechanisms of attentional control contribute to the mitigation of distraction, reconfiguring how visual information will be processed in advance of seeing a display and quickly adjusting based on what is detected in the environment, respectively (see Geng, 2014). One study related statistically-learned distractor suppression to pre-trial neural oscillations in the alpha band as measured using electroencephalography (EEG), suggesting a proactive mechanism of suppression (Wang et al., 2019b). The time course of this putatively proactive influence of suppression on stimulus selection, and whether it can mitigate capture even at the earliest stages of competition within the attention system, is not known. Mechanisms of reactive attentional control could also be subject to learning from statistical regularities and assist with the mitigation of attentional capture by frequent distractors, although such mechanisms have received little consideration in the emerging literature linking distractor suppression to statistical learning.
To gain additional insights into these issues, we examined the time course of statistically-learned distractor suppression as a function of saccadic reaction time (sRT) in an oculomotor attentional capture task. Statistical learning of a high-probability distractor location has been shown to modulate the frequency of distractor and target fixations, with selection more strongly favoring the target when the distractor appears in the high-probability location (Wang et al., 2019a). Leveraging the well-characterized time course of eye movements to physically salient distractors and targets as a function of sRT (Donk & van Zoest, 2008; Godjin & Theeuwes, 2002; van Zoest et al., 2004; van Zoest & Donk, 2005), we looked for evidence for proactive and reactive mechanisms of mitigating distraction tied to statistical learning. Proactive attentional control would be evident in a reduced frequency of distractor fixations for distractors appearing at the high-probability location even for the most rapidly triggered eye movements, reflecting an influence of statistical learning on the most stimulus-driven saccades. Relatedly, a slowing of sRT for distractor-going saccades would be consistent with a slowing of priority accumulation at this location. Distinctly reactive attentional control would be evident in speeded disengagement from a distractor when appearing at the high-probability location (Wang et al., 2019a).
Methods
Participants
Thirty-six participants (26 female), between the ages of 18 and 33 inclusive (M = 20.5, SD = 3.0), were recruited from the local University community. All participants were English-speaking and reported normal or corrected-to-normal visual acuity and normal color vision. All procedures were approved by the University Institutional Review Board and were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained for each participant. Our sample size was based off a power analysis (G*Power 3.1, α = 0.05, 1−β > 0.8). From Wang et al. (2019a), the effect size dz (t/sqrt(n)) for the difference in oculomotor capture for distractors appearing in the high- vs. low-probability distractor locations was dz = 1.493; for (manual) response time (RT) it was dz = 1.765 and for oculomotor dwell time it was dz = 1.305. Since the effect size for oculomotor capture in smaller bins of trials (based on sRT) may be somewhat reduced, we powered our study to detect an effect with power (1−β) > 0.8 as small as dz = 0.481, which is less than one-third the size of the effect over all trials reported in Wang et al. (2019a). Our sample provided power (1−β) > 0.98 to replicate even the smallest pairwise comparison reported as significant in Wang et al. (2019a).
Apparatus
A Dell OptiPlex 7040 (Dell, Round Rock, TX, USA) equipped with Matlab software (Mathworks, Natick, MA, USA) and Psychophysics Toolbox extensions (Brainard, 1997) was used to present the stimuli on a Dell P2717H monitor. The participants viewed the monitor from a distance of approximately 70 cm in a dimly lit room. Eye-tracking was conducted using the EyeLink 1000 Plus system (SR Research Ltd., Ottawa, Ontario, Canada), and head position was maintained using a manufacturer-provided chin rest (SR Research Ltd.).
Stimuli and Task
Each trial consisted of a gaze-contingent fixation display, a visual search array, and an inter-trial-interval (ITI; see Figure 1). The fixation display consisted of a fixation cross (0.7° × 0.7° visual angle) at the center of the screen. The fixation display remained on screen until eye position was registered within 1.1° of the center of the fixation cross for a continuous period of 500 ms (as in Anderson & Kim, 2019a, 2019b). The visual search array was then presented for 1500 ms or until a fixation on the target was registered. If a target was not fixated within the timeout limit, the words “Too Slow” would appear in the center of the screen for 1500 ms. Lastly, the ITI consisted of a blank screen for 1000 ms. During the visual search task, participants had to search for the unique shape (one circle among diamonds, or vice versa). Each circle in the search array was 4.5° visual angle in diameter and diamonds were 4.1° × 3.7° visual angle. Each shape was placed at equal intervals along an imaginary circle with a radius of 10.2°. The color of the shapes was either red or green. In contrast to a prior study examining the influence of statistical learning of distractor location on oculomotor capture and sRT (Wang et al., 2019a; see also Gaspelin et al., 2017), our task used gaze-contingent displays in which the only action participants needed to perform was to fixate the target, with the task directly probing eye movements. In the context of the influence of reward on attention, such an approach produces a measure of oculomotor capture with high test-retest reliability (Anderson & Kim, 2019b) and the direct linking between eye movements and the requirements of the task might facilitate a more sensitive test of the relationship between oculomotor capture and sRT as a function of learning history. This approach is also more similar to studies that have historically examined oculomotor capture as a function of sRT (e.g., Donk & van Zoest, 2008; Godjin & Theeuwes, 2002; van Zoest et al., 2004; van Zoest & Donk, 2005).
Figure 1.
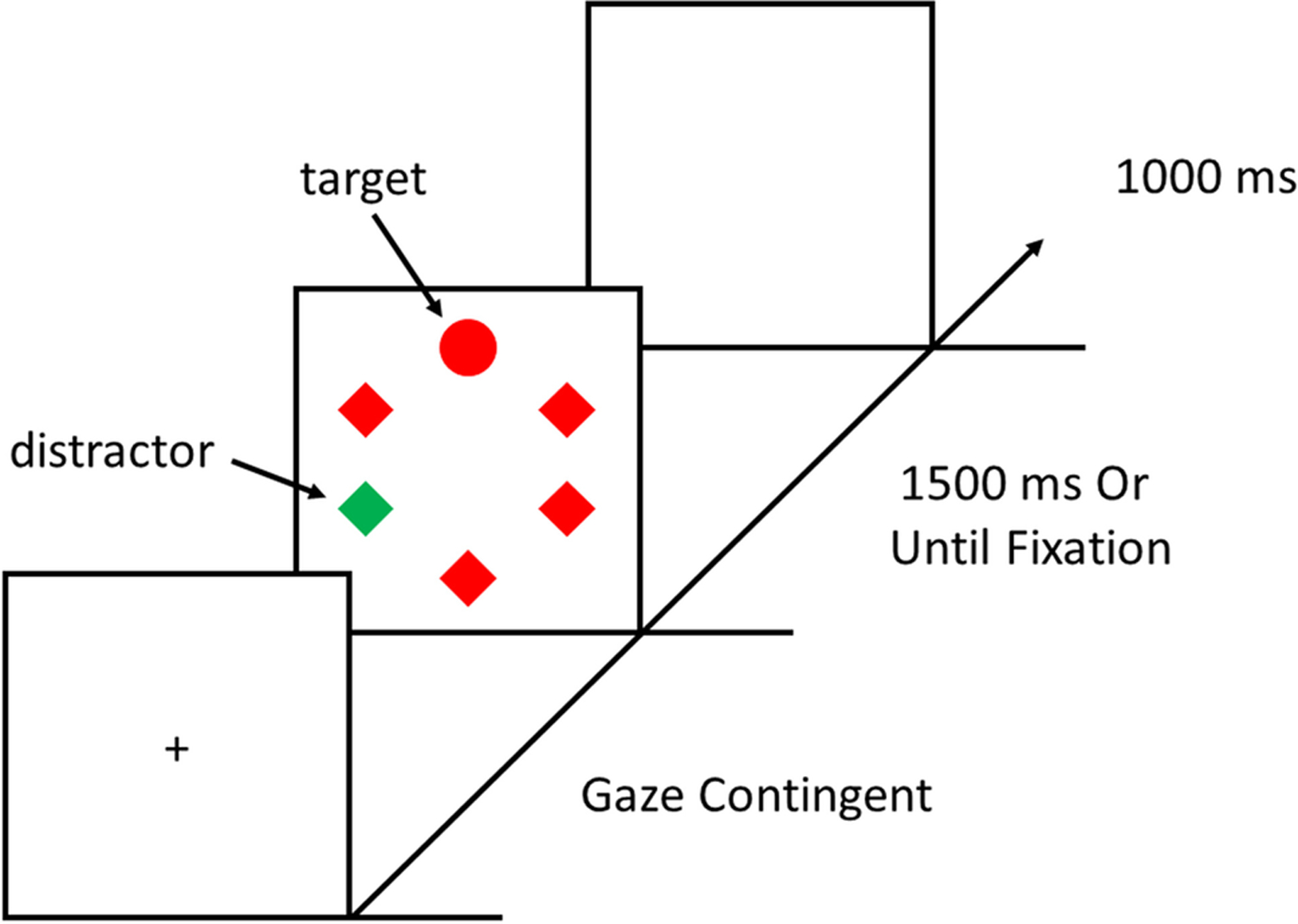
Sequence of trial events. The search array shows an example of a distractor-present trial.
Design
Each participant completed 6 runs of 111 trials each (total 666 trials). The target was present in each trial and a uniquely colored distractor singleton was presented in 67.6% of trials (same shape as other non-targets but a different color). On distractor-absent trials, the target position, shape, and color were fully counter-balanced. On distractor-present trials, the distractor was in one position 2/3 of the time (high-probability location) and equally often in all other locations (thus, the distractor appeared in the high-probability location on 45% of all trials). The high-probability location was counter-balanced across participants. The target position was fully crossed in respect to the distractor position, and each target shape/color combination was used equally-often over distractor-present trials. Trials were presented in a random order.
Data Analysis
Eye position was calibrated prior to each run using 9-point calibration and was manually drift corrected by the experimenter as necessary during the initial fixation display (as in Anderson & Kim, 2019a, 2019b). During the presentation of the search array, the X and Y position of the eyes was continuously monitored in real time with respect to the six stimulus positions, such that fixations were coded online (as in Anderson & Kim, 2019a, 2019b). The EyeLink 1000 Plus also exported an EDF file at the end of the experiment that contained detailed measurements concerning the saccades and fixations made on each trial in addition to markers for the beginning of each trial, presentation of visual search array, and the end of the trial for offline analysis. Fixation of a stimulus was registered if eye position remained within a region extending 0.7° around the stimulus for a continuous period of at least 50 ms (100 ms on the target trigger the termination of the stimulus array; see, e.g., Anderson & Kim, 2019a, 2019b). RT was measured from the onset of the stimulus array until a valid target fixation was registered. RTs in fixating the target that exceeded three standard deviations of the mean for a given condition for a given participant were trimmed (Anderson & Kim, 2019b; Kim & Anderson, 2020).
We measured which of the six shape stimuli was initially fixated on each trial (i.e., the first stimulus fixated) in addition to the time to fixate the target (i.e., response time, RT) from the eye data coded online. From this data, we calculated the proportion of initial fixations on the target and distractor when the distractor is in either a high-probability or low-probability location. Oculomotor dwell time on first fixations to the distractor (in high- and low-probability location) in addition to non-salient non-targets was computed as the average duration that the eyes remained within the fixation window surrounding the stimulus, also from the eye data coded online. Lastly, saccadic RTs (sRTs) were computed as the time that the first saccade that landed outside of the fixation zone began relative to stimulus onset. This was computed offline using the EDF file. Saccades were defined as occurring when velocity exceeded 35°/s and acceleration exceeded 9,500°/s2 (Anderson & Kim, 2018; Wang et al., 2019a). For each participant, we computed mean sRT for each distractor condition and additionally Vincintized distractor-present trials by sRT into 10 equally-sized bins (regardless of distractor condition), from which the proportion of oculomotor capture was computed for each of the two distractor conditions separately for each bin; this data was submitted to an analysis of variance (ANOVA) with distractor condition (distractor at high-probability location, distractor at low-probability location) and bin (1–10) as factors.
Results
Time to Fixate the Target
Time to fixate the target varied as a function of distractor condition (distractor at high-probability location, distractor at low-probability location, distractor absent), F(2,70) = 259.20, p < 0.001, η2p = 0.881 (Figure 2A). A priori contrasts revealed that time to fixate the target was slower for both of the distractor-present conditions relative to distractor-absent trials, reflective of stimulus-driven attentional capture, ts > 8.65, ps < 0.001, dzs > 1.44, but was faster for high-probability distractor trials compared to low-probability distractor trials, t(35) = 15.17, p < 0.001, dz = 2.53, reflective of statistically-learned distractor suppression. On distractor-absent trials, time to fixate the target was slower when the target appeared in the high-probability distractor location compared to one of the other, low-probability distractor locations, t(35) = 3.80, p = 0.001, dz = 0.63 (Figure 2B), also indicative of suppression.
Figure 2.
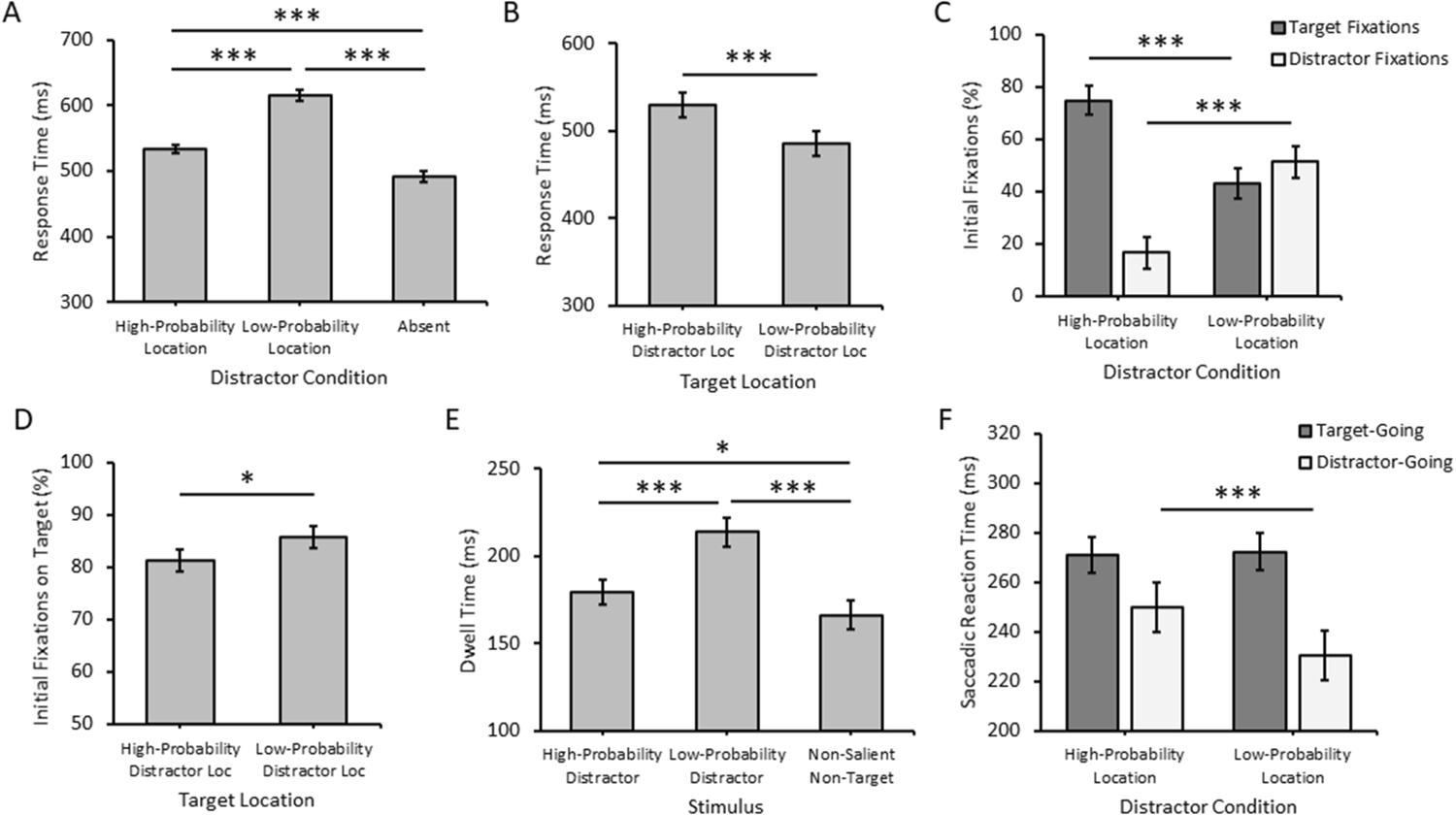
Behavioral results. Time to fixate the target (response time) on (A) distractor-present trials and (B) distractor-absent trials. (C) Percent initial fixations on targets and distractors as a function of the location of the distractor. (D) Initial fixations on the target on distractor-absent trials. (E) Dwell time as a function of the type of non-target. (F) Mean saccadic reaction time on distractor-present trials. Error bars depict within-subject confidence intervals calculated using the Cousineau method with a Morey correction. *p<0.05. ***p<0.001.
Oculomotor Capture and Target Selection
Oculomotor capture was significantly less frequent when the distractor appeared in the high-probability compared to one of the other, low-probability distractor locations, t(35) = 16.89, p < 0.001, dz = 2.81 (Figure 2C). This reduction in oculomotor capture came at the benefit of initial fixations being on the target, which were correspondingly more frequent in the high-probability distractor condition, t(35) = 16.82, p < 0.001, dz = 2.78 (Figure 2C). On distractor-absent trials, the target was less likely to be the first stimulus fixated when appearing in the high-probability distractor location, t(35) = 2.66, p = 0.012, dz = 0.44 (Figure 2D), also indicative of location-specific inhibition.
Distractor Dwell Time
Dwell time on a non-target varied as a function of stimulus type (distractor at high-probability location, distractor at low-probability location, non-salient non-target), F(2,70) = 39.84, p < 0.001, η2p = 0.532 (Figure 2E). Most critically, dwell time was shorter on high-probability compared to low-probability distractors, t(35) = 6.59, p< 0.001, dz = 1.10, indicative of accelerated distractor rejection. Both types of distractors were fixated longer than non-salient non-targets, ts > 2.51, ps < 0.018, dzs > 0.42.
Relating Oculomotor Suppression to Saccadic Reaction Time (sRT)
Distractor-present trials were separated into 10 equally-sized bins (Vincintized) based on sRT regardless of distractor condition, such that the probability of oculomotor capture could be compared between the high- and low-probability distractor condition with sRT equated in each bin. There was a main effect of distractor condition that reiterates the effect collapsed over all trials described above, F(1,35) = 306.44, p < 0.001, η2p = 0.897 (Figure 3). There was a main effect of bin in which capture tended to decrease with increasing sRT, F(9,315) = 32.79, p < 0.001, η2p = 0.484, reflecting the well-established relationship between oculomotor capture by physically salient stimuli and sRT (e.g., Donk & van Zoest, 2008; Godjin & Theeuwes, 2002; van Zoest et al., 2004; van Zoest & Donk, 2005). There was also a significant interaction between distractor condition and bin, F(9,315) = 9.85, p < 0.001, η2p = 0.220, which was well-accounted for by a linear trend with respect to the interaction term, F(1,35) = 26.78, p < 0.001, η2p = 0.433, reflecting a strong dependence between sRT and capture for distractors in the low-probability location while capture was generally infrequent across sRT for distractors appearing in the high-probability location. Most importantly, a significant difference between distractor conditions was evident for each of the ten bins, ts > 3.81, ps < 0.002, dzs > 0.63, including even in the fastest bin in which eye movements tend to be most stimulus-driven, t(35) = 12.13, p < 0.001, dz = 2.02.
Figure 3.
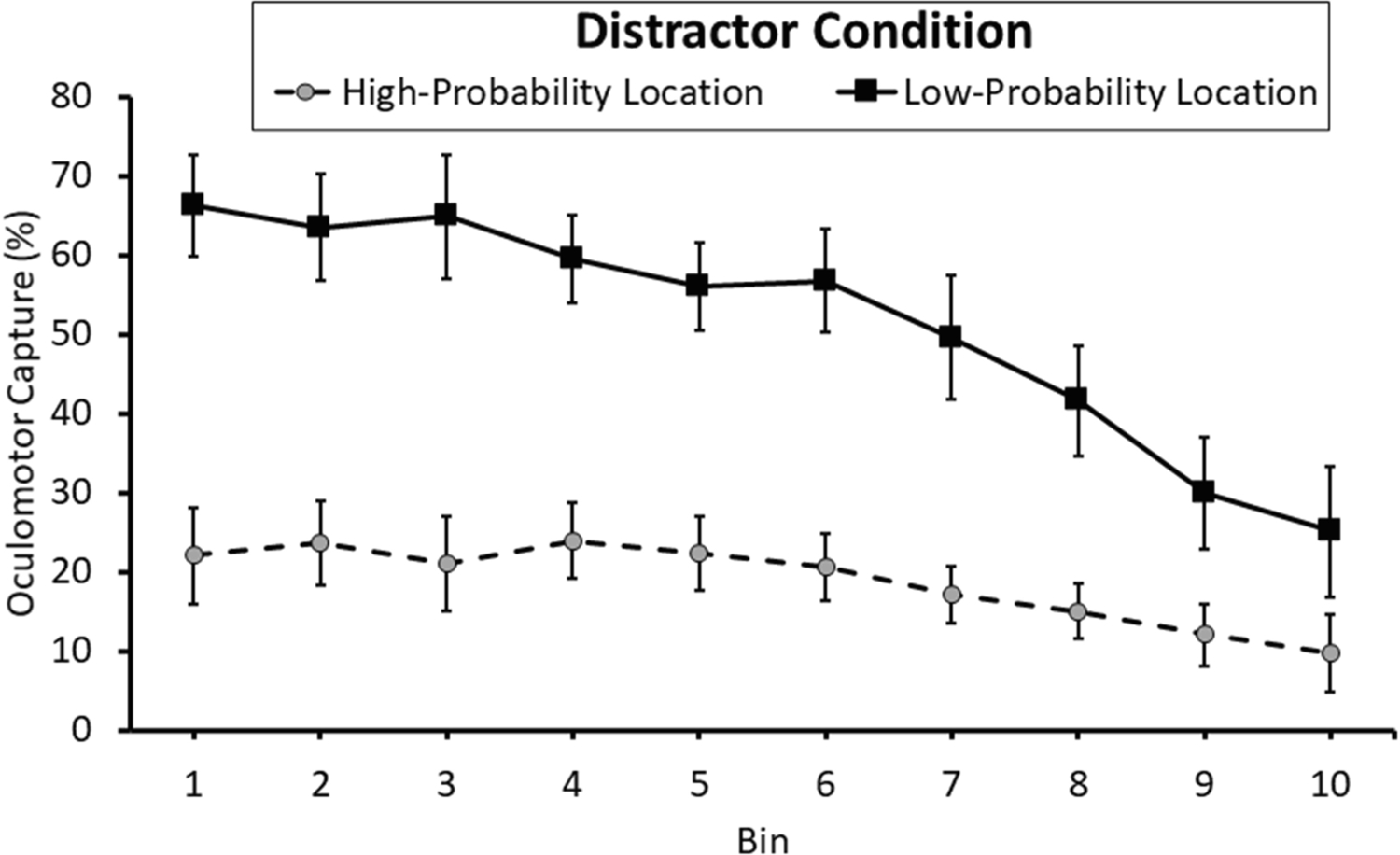
Oculomotor capture as a function of saccadic reaction time (sRT; Vincintized into 10 bins with bin 1 containing the fastest 10% of sRTs for each participant, bin 2 the next 10%, etc.). Error bars depict within-subject confidence intervals calculated using the Cousineau method with a Morey correction.
We further examined mean sRT as a function of distractor condition to test whether the time to initiate a saccade differed as a function of distractor condition. We first examined sRT over all trials for each distractor condition, regardless of stimulus fixated (as in Wang et al., 2019a), which revealed a slowing of sRT in the high-probability compared to the low-probability distractor condition, t(35) = 4.38, p < 0.001, dz = 0.73. That is, even though participants were overall significantly faster to fixate the target in the high-probability distractor condition, likely due to substantially less frequent oculomotor capture by the distractor, the opposite was true of how quickly they initiated an eye movement in this condition. Further analysis broke sRT down additionally by whether the saccade was a target-going or distractor-going saccade. This analysis revealed that the slowing of sRT on high-probability distractor trials was entirely accounted for by a slowing of sRT for distractor-going saccades, t(35) = 4.72, p < 0.001, dz = 0.79; sRT did not differ for target-going saccades, t(35) = −0.26, p = 0.799 (Figure 2F). This suggests that attentional priority for the distractor accumulated more slowly when appearing at the high-probability location, such that if the distractor did reach the threshold for triggering a saccade (which it did significantly less often as revealed by analysis of mean oculomotor capture), it took significantly longer to reach that threshold.
Examining the Role of Inter-trial Priming of Distractor Location
All of the above analyses remain significant and essentially unchanged if all trials on which the position of the distractor immediately repeats from the prior trial are removed from analysis, consistent with prior research (Wang et al., 2019a), suggesting that our findings are not reducible to more frequent repetitions of distractor position in the high-probability distractor condition.
Examining the Speed with Which Effects of Statistical Learning on Distractor Suppression Emerge
To probe the speed with which the observed effects of statistical learning emerged, we performed the same analyses specifically on the first block of trials (with the exception of the analysis binning sRT since that analysis requires a large number of trials to represent each bin). All of the above-reported effects were significant when restricted to the first block of trials, with the exception of RT to fixate the target when appearing in the high-probability vs. a low-probability distractor location, t(35) = 1.54, p = 0.133, and the proportion of first saccades on the target when comparing these same two conditions, t(35) = 0.66, p = 0.512; it is worth noting that in each of these two cases, the analysis relies on trials on which the target appears in the high-probability distractor location which occur infrequently, and so these particular analyses may be underpowered at the level of a single block of trials. Overall, the findings here suggest that the observed effects of statistically learned distractor suppression can emerge quickly, consistent with a prior report (Wang et al., 2019a).
Discussion
By examining oculomotor capture as a function of both sRT and the probability of a distractor appearing at a particular location, the present study provides novel insights into the mechanisms underlying statistically-learned distractor suppression. In combination with other oculomotor measures, we provide evidence for multiple mechanisms of distractor suppression working in tandem to support a reduction in the frequency of attentional capture as a function of statistical learning. Most strikingly, even the saccades that participants were the fastest to initiate were significantly less likely to be directed to the salient distractor, as if the salience of the distractor was blunted as a result of statistical learning. Our findings here provide straightforward behavior evidence of proactive distractor suppression, demonstrating a reduced consequence of salience at the earliest stage of stimulus selection, confirming interpretations of pre-trial EEG data using a similar paradigm (Wang et al., 2019b).
Replicating the well-established relationship between sRT and oculomotor capture (e.g., Donk & van Zoest, 2008; Godjin & Theeuwes, 2002; van Zoest et al., 2004; van Zoest & Donk, 2005), saccades that were initiated more slowly were generally less susceptible to capture. It was also the case that distractor-going saccades were slower to initiate when the distractor appeared at the high-probability compared to a low-probability location. This suggests that attentional priority accumulated more slowly for the distractor when appearing at the high-probability location which, given with overall relationship between sRT and oculomotor capture, may have served to further reduce the likelihood that a distractor at the high-probability location would reach the threshold for triggering a saccade.
Replicating previous results (Wang et al., 2019a), we also found that participants were faster to disengage attention from a distractor when appearing in a high-probability location. This is a clear case of reactive attentional control, reflecting a shift in the speed with which a stimulus can be rejected as a non-target. Although such an effect does not itself influence the likelihood of attentional capture by the distractor, it mitigates the consequences of attentional capture as a result of statistical learning.
A significant effect of distractor probability on sRT was not observed in Wang et al. (2019a), in contrast to the present study. However, in that study, a 10 ms difference collapsed across distractor- and target-going saccades was observed in the same direction, which is numerically quite similar to the significant 12 ms effect observed in the present study. With a sample size more than twice as large in the present study, this apparent discrepancy may simply reflect a consequence of reduced statistical power obscuring a comparatively smaller effect, as indeed the study of Wang et al. (2019a) was powered to detect the much larger consequence of distractor location probability on RT in target report (from Wang et al., 2018a). The explicitly gaze-contingent aspect of our experimental design may have also resulted in greater sensitivity to detect a link between sRT and oculomotor capture (see Methods). Otherwise, we fully replicate the findings of Wang et al. (2019a), demonstrating highly robust overall effects of statistical learning on oculomotor capture.
The present study lends important insights into why statistical learning can have such substantial effects on the mitigation of distraction, and how distraction can be mitigated through learning more generally. By providing straightforward evidence linking both proactive and reactive mechanisms of distractor suppression to statistical learning in the same experiment, our results demonstrate that multiple mechanisms are recruited to manage distraction that are subject to learning from prior experience or selection history (see Anderson et al., 2021; Awh et al., 2012). Our findings suggest that the reduction in distraction observed in prior studies in which the probability of different distractor events is manipulated (e.g., Britton & Anderson, 2020; Kim & Anderson, 2021; Wang & Theeuwes, 2018a, 2018b, 2018c; Wang et al., 2019a, 2019b) reflects the sum total of multiple underlying mechanisms, with at least three different time courses of information processing impacted. Attentional priority accumulates more slowly for distractors appearing at the high-probability location, such that it takes longer for a saccade to be initiated to the distractor in the event that it wins the competition for oculomotor selection. In addition, for any given response speed, the attentional priority of the distractor and its corresponding ability to compete for selection is overall reduced. These two mechanisms result in a substantial reduction in the frequency with which distractors at high-probability locations are fixated, producing facilitated visual search performance as reflected in overall less time to localize the target. In the event that attention is still captured by the distractor in spite of these learning-dependent changes, the speed of distractor rejection is further subject to selection history, facilitating the disengagement of attention from more frequently encountered distractor events and thereby further facilitating visual search performance.
Maximally efficient ignoring of task-irrelevant stimuli will optimize all three of these aspects of attentional processing, which should be the focus of any learning procedure or intervention designed to mitigate distraction. It does not appear to be the case that learning-dependent ignoring can be reduced to a single consequence of learning on the attention system, or likewise that only select components of the attention system are subject to learning from selection history. Instead, our findings suggest that selection history reflects a systemic shift in how information is processed on multiple levels. Future research should further explore the scope and limits of such learning, in addition to how these different mechanisms of learned suppression are related to one another (e.g., Born et al., 2011). Our findings suggest that learning to ignore is a multifaceted process, which should be more substantively taken into account in the study of selection history.
Public Relevance Statement.
Distraction can have serious health, occupational, and educational consequences. When a specific distracting event is experienced frequently, individuals can learn to better ignore it. Specifically what it is that changes about how a person directs their attention in order to facilitate this ignoring is not well understood. In the present study, we demonstrate a widespread influence of learning on the ability to resist distraction across multiple aspects of attentional control. Our findings reveal that learning to ignore is a systemic process, which efforts to facilitate learned ignoring should take into account.
Open Practices Statement:
The experiment reported in this article was not formally preregistered.
Neither the data nor the materials have been made available on a permanent third-party archive; requests for the data or materials can be sent via email to the lead author at andyk@usc.edu.
Funding:
This study was supported by NIH grant R01-DA046410 to BAA.
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Conflicts of Interest Statement: The authors declare no conflict of interest.
References
- Anderson BA, & Britton MK (2020). On the automaticity of attentional orienting to threatening stimuli. Emotion, 20, 1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (2019a). On the relationship between value-driven and stimulus-driven attentional capture. Attention, Perception, and Psychophysics, 81, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (2019b). Test-retest reliability of value-driven attentional capture. Behavior Research Methods, 51, 720–726. [DOI] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (2018b). On the representational nature of value-driven spatial attentional biases. Journal of Neurophysiology, 120, 2654–2658. [DOI] [PubMed] [Google Scholar]
- Anderson BA, Kim H, Kim AJ, Liao M-R, Mrkonja L, Clement A, & Grégoire L (2021). The past, present, and future of selection history. Neuroscience & Biobehavioral Reviews, 130, 326–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2011). Value-driven attentional capture. Proceedings of the National Academy of Sciences, USA, 108, 10367–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E Belopolsky AV, & Theeuwes J (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences, 16, 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born S, Kerzel D, & Theeuwes J (2011). Evidence for a dissociation between the control of oculomotor capture and disengagement. Experimental Brain Research, 208, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox, Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Britton MK, & Anderson BA (2020). Specificity and persistence of statistical learning in distractor suppression. Journal of Experimental Psychology: Human Perception and Performance, 46, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donk M, & van Zoest W (2008). Effects of salience are short-lived. Psychological Science, 19, 733–739. [DOI] [PubMed] [Google Scholar]
- Failing M, Feldmann-Wustefeld T, Wang B, Olivers C, & Theeuwes J (2019). Statistical regularities induce spatial as well as feature-specific suppression. Journal of Experimental Psychology: Human Perception and Performance, 45, 1291–1303. [DOI] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, & Luck SJ (2017). Suppression of overt attentional capture by salient-but-irrelevant color singletons. Attention, Perception, and Psychophysics, 79, 45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, & Luck SJ (2015). Direct evidence for active suppression of salient-but-irrelevant sensory inputs. Psychological Science, 22, 1740–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ (2014). Attentional mechanisms of distractor suppression. Current Directions in Psychological Science, 23, 147–153. [Google Scholar]
- Geng JJ, & DiQuattro NE (2010). Attentional capture by a perceptually salient non-target facilitates target processing through inhibition and rapid rejection. Journal of Vision, 10(6):5, 1–12. [DOI] [PubMed] [Google Scholar]
- Godijn R, & Theeuwes J (2002). Programming of endogenous and exogenous saccades: Evidence for a competitive integration model. Journal of Experimental Psychology: Human Perception and Performance, 28, 1039–1054. [DOI] [PubMed] [Google Scholar]
- Kim AJ, & Anderson BA (2020). Threat reduces value-driven but not salience-driven attentional capture. Emotion, 20, 874–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, & Anderson BA (2021). Combined influence of valence and statistical learning on the control of attention: Evidence for independent sources of bias. Cognition, 208, 104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M, Driver J, & Eimer M (2009). Reward priority of visual target singletons modulates event-related potential signatures of attentional selection. Psychological Science, 20, 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Gaspelin N, Folk CL, Remington RW, & Theeuwes J (2021). Progress toward resolving the attentional capture debate. Visual Cognition, 29, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namian M, Albert A, & Feng J (2018). Effect of distraction on hazard recognition and safety risk perception. Journal of Construction Engineering and Management, 144(4), 04018008. [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015). Attentional capture by signals of threat. Cognition and Emotion, 29, 687–694. [DOI] [PubMed] [Google Scholar]
- Stilwell BT, Bahle B, & Vecera SP (2019). Feature-based statistical regularities of distractors modulate attentional capture. Journal of Experimental Psychology: Human Perception and Performance, 45, 419–433. [DOI] [PubMed] [Google Scholar]
- Strayer DL, & Drews FA (2004). Profiles in driver distraction: Effects of cell phone conversations on younger and older drivers. Human Factors, 46, 640–649. [DOI] [PubMed] [Google Scholar]
- Taneja A, Fiore V, & Fischer B (2015). Cyber-slacking in the classroom: Potential for digital distraction in the new age. Computers & Education, 82, 141–151. [Google Scholar]
- Theeuwes J (2010). Top-down and bottom-up control of visual selection. Acta Psychologica, 135, 77–99. [DOI] [PubMed] [Google Scholar]
- van Zoest W, & Donk M (2005). The effects of salience on saccadic target selection. Visual Cognition, 12, 353–375. [Google Scholar]
- van Zoest W, Donk M, & Theeuwes J (2004). The role of stimulus-driven and top-down control in saccadic visual selection. Journal of Experimental Psychology: Human Perception and Performance, 30, 746–759. [DOI] [PubMed] [Google Scholar]
- Vatterott DB, & Vecera SP (2012). Experience-dependent attentional tuning of distractor rejection. Psychonomic Bulletin and Review, 19, 871–878. [DOI] [PubMed] [Google Scholar]
- Wang B, Samara I, & Theeuwes J (2019a). Statistical regularities bias overt attention. Attention, Perception, and Psychophysics, 81, 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, & Theeuwes J (2018a). Statistical regularities modulate attentional capture. Journal of Experimental Psychology: Human Perception and Performance, 44, 13–17. [DOI] [PubMed] [Google Scholar]
- Wang B, & Theeuwes J (2018b). How to inhibit a distractor location? Statistical learning versus active, top-down suppression. Attention, Perception, and Psychophysics, 80, 860–870. [DOI] [PubMed] [Google Scholar]
- Wang B, & Theeuwes J (2018c). Statistical regularities modulate attentional capture independent of search strategy. Attention, Perception, and Psychophysics, 80, 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, van Driel J, Ort E, & Theeuwes J (2019b). Anticipatory distractor suppression elicited by statistical regularities in visual search. Journal of Cognitive Neuroscience, 31, 1535–1548. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, & Horowitz TS (2017). Five factors that guide attention in visual search. Nature Human Behaviour, 1, 0058. [DOI] [PMC free article] [PubMed] [Google Scholar]


