Abstract
Although cases of secondary membranous nephropathy associated with autoimmune thyroid disease (AITD) have been reported, most of them, if not all, present with symptomatic thyroid disease. Here we report an asymptomatic case of AITD complicated with secondary membranous nephropathy. A 16-year-old girl was referred to our institute because of proteinuria found by an annual medical checkup. Urinalysis showed a urinary protein creatinine ratio (UPCR) of 3.0 g/gCre. Blood examination revealed that she had Graves’ disease, although she did not have any symptoms of hyperthyroidism such as weight loss, anxiety, tremor, tachycardia, or eye symptoms. In a kidney biopsy, periodic acid silver-methenamine staining showed spike formation in the basement membrane. Electron microscopy showed electron-dense deposits on the epithelial side of the glomerular basement membrane. Immunofluorescent staining showed co-localization of thyroid peroxidase and IgG deposition along the glomerular capillary walls. A diagnosis of membranous nephropathy secondary to asymptomatic Graves’ disease was made on the basis of results of the examinations. Treatment with thiamazole added to enalapril improved proteinuria (reduction of UPCR to 0.83 g/gCr) and hypoalbuminemia. Consideration should be given to the possibility of AITD in differential diagnosis of etiologies of membranous nephropathy even when typical symptoms of AITD are lacking.
Keywords: Membranous nephropathy, Graves’ disease, Thiamazole
Introduction
Membranous nephropathy is characterized by the deposition of immune complexes along the subepithelial region of the glomerular basement membrane. Approximately 10–20% of cases of membranous nephropathy have an extra-renal etiology such as malignant tumor, drug, and autoimmune disease and such cases are referred to as secondary membranous nephropathy. Autoimmune thyroid diseases (AITDs) including Hashimoto disease [1, 2] and Graves’ disease [3, 4] have been reported to be associated with membranous nephropathy, in which an immunocomplex of thyroglobulin and/or thyroid peroxidase (TPO) and their antibodies are reportedly involved. In most cases of Graves’ disease, patients show hyperthyroidism-related symptoms. However, there are cases with no or few such symptoms. Here, we report a case of membranous nephropathy secondary to asymptomatic Graves’ disease. This case was diagnosed by electron-dense deposits in the glomerular basement membrane and co-localization of both TPO and IgG along glomerular capillary-loop walls and the case was successfully treated with an anti-thyroid drug.
Case report
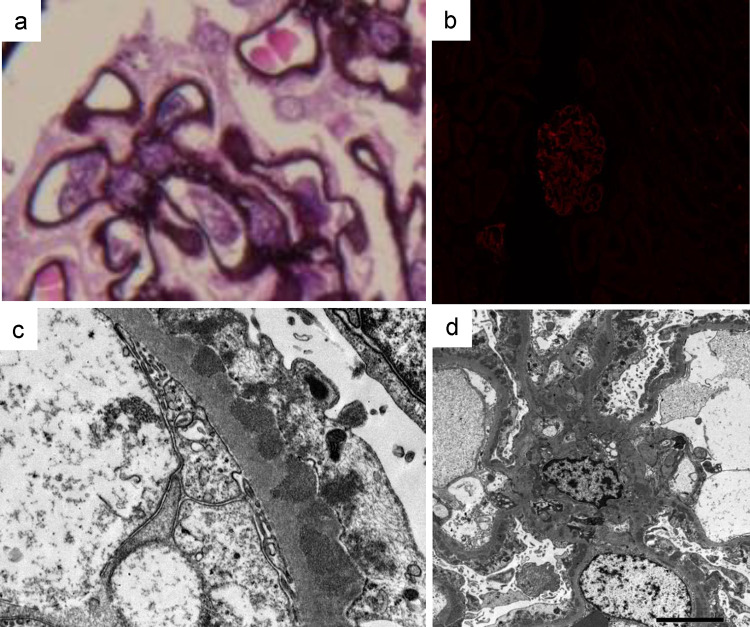
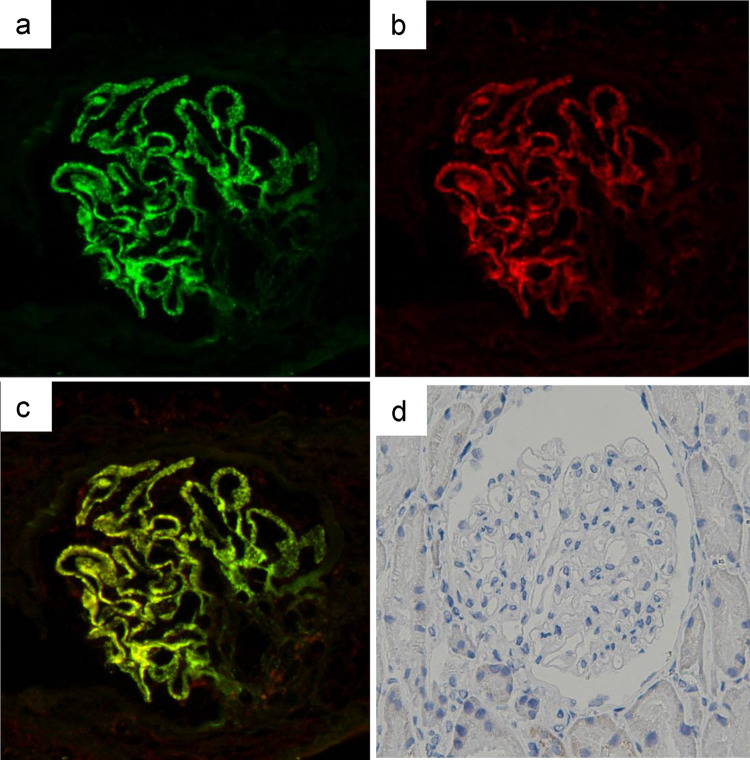
A previously healthy 16-year-old girl was referred to Sapporo Medical University Hospital because of positivity for proteinuria in an annual medical checkup in high school. She did not have any symptoms of hyperthyroidism such as weight loss, anxiety, tremor, tachycardia, increased bowel movements, or any eye symptoms including light sensitivity, double vision, or vision loss. She played hockey as a club activity almost every day. Her blood pressure was 128/78 mm Hg, pulse was 62/min, and body temperature was 36.4 degrees Celsius. Her height was 161.7 cm, weight was 53.0 kg, and body mass index was 20.3 kg/m2. She did not have goiter or bulging eyes. Blood examinations showed normal blood cell counts, normal levels of blood urea nitrogen (10.0 mg/dl), serum creatinine (0.41 mg/dl), and total cholesterol (169 mg/dl), low levels of total protein (6.2 g/dl) and serum albumin (2.7 g/dl), increased levels of serum free thyroxine (T3) (4.47 pg/ml, reference range 1.71–3.71 pg/ml) and free triiodothyronine (T4) (1.55 ng/dl, reference range 0.70–1.48 ng/dl), and suppression of thyroid-stimulating hormone (TSH) production (< 0.01 μU/ml, reference range 0.35–4.94 lU/ml). In urine examination, urine protein/creatinine ratio (UPCR) was 3.0 g/gCre and there was microhematuria (10–19 red blood cells/high-power field). A renal biopsy was performed for analyses with light and electron microscopy. Periodic acid silver-methenamine (PAM) staining showed spike formation in the basement membrane (Fig. 1a). Immunofluorescence staining showed granular deposits of IgG (2 ), IgA (1+), C3 (1+), kappa light chain (1+) and lambda light chain (1+) along glomerular capillary-loop walls, though increased signals for IgM (−), fibrinogen (±), and phospholipase A2 receptor (PLA2R) ( ±) were not detected (Fig. 1b). Subclass analysis of IgG showed 2+ signal for IgG1 and IgG4, 1 for IgG2 and no signal for IgG3. Electron microscopy showed electron-dense deposits on the epithelial side of the glomerular basement membrane (Fig. 1c) as well as in the mesangial region (Fig. 1d). The Ehrenleich-Churg stage was III. Examination for differential diagnosis of hyperthyroidism revealed increased serum levels of anti-TPO antibody (601.7 IU/ml; normal level: < 5.2 IU/ml), anti-thyroglobulin antibody (1655.2 IU/ml; normal level: < 40.6 IU/ml), thyroid-stimulating hormone receptor antibody (TRAb) (10.9 IU/l; normal level: < 0.9 IU/l), and thyroid-stimulating antibody (TSAb) (271%, reference range 0–120%). 99mTc-pertechnetate scintigraphy showing increased bilateral and diffuse uptake of the radioactive tracer (5.09%, reference range 0.4–3.0%, Fig. 2). Based on these results, she was diagnosed as having asymptomatic Graves’ disease. Because we speculated that antigen–antibody complexes associated with Graves’ disease were involved in the development of membranous nephropathy, the biopsy samples were further examined by immunofluorescence staining for TPO and by immunohistochemistry for thyroglobulin. Frozen sections were incubated with anti-human TPO antibody (TPO47; BioCytex, Marseilles, France) and then stained with fluorescein isothiocyanate-labeled goat anti-mouse IgG. Formaldehyde-fixed paraffin sections were stained using a primary antibody against human thyroglobulin (code: 422,691, NICHIREI BIOSCIENCES Inc., Tokyo, Japan) after deparaffinization and heat-induced antigen retrieval with ULTRA Cell Conditioning Solution (ULTRA CC1, Ventana Medical Systems, Inc., Arizona, USA) for 64 min at 95 °C using a VENTANA BenchMark Ultra automated staining instrument (Ventana Medical Systems, Inc., Arizona, USA). Sections from a human thyroid gland were used as positive controls. The primary antibodies were omitted in the negative controls. All histological analyses other than TPO staining were performed at Hokkaido Renal Pathology Center. Immunofluorescence staining for TPO was performed at Wakayama Medical University. Positive granular deposition of TPO along the capillary-loop wall was observed, and double immunofluorescence staining for TPO and IgG revealed co-localization of TPO and IgG along the loop wall (Fig. 3a–c). On the other hand, immunohistochemistry for thyroglobulin was negative (Fig. 3d).
Fig. 1.
Periodic acid silver-methenamine (PAM) staining of a biopsy specimen showed spike formation of the basement membrane (a). Immunofluorescence staining for phospholipase A2 receptor (PLA2R) was negative (b). Electron microscopy showed electron-dense deposits on the epithelial side of the glomerular basement membrane (c) as well as in the mesangial region (d). Original magnification is 800× for a, 400× for b, 5000× for c, and 1500× for d
Fig. 2.
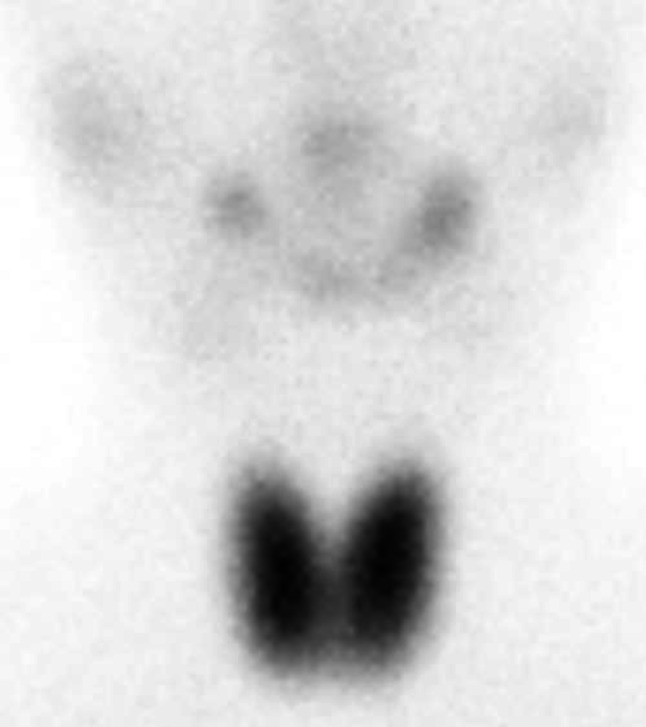
99mTc-pertechnetate scintigraphy showing elevated bilateral and diffuse uptake of the radioactive tracer (5.09%, reference range 0.4–3.0%)
Fig. 3.
Immunofluorescence staining for IgG (a), TPO (b) and merged image (c). Immunohistochemistry for thyroglobulin (d). Original magnification is 400×
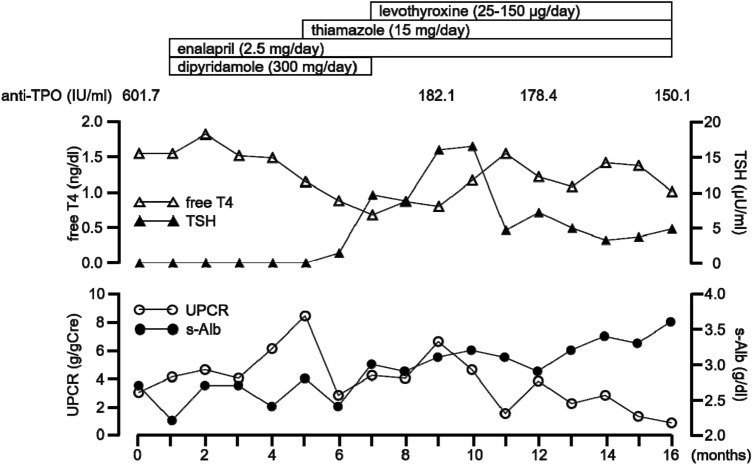
Figure 4 shows her clinical course. She was first treated with enalapril (2.5 mg/day) and dipyridamole (300 mg/day) for 4 months, but proteinuria was not reduced. Thus, based on the results of TPO staining, thiamazole (15 mg/day) was administered. After commencement of treatment with thiamazole, UPCR started to fall and serum albumin gradually recovered. Levothyroxine (25–150 µg/day) was needed to maintain a euthyroid state. One year after commencement of thiamazole administration, proteinuria and hypoalbuminemia were considerably improved (UPCR of 0.83 g/gCre and serum albumin level of 3.6 g/dl) with reduction in levels of serum anti-TPO antibody (150.1 IU/ml), anti-thyroglobulin antibody (807.0 IU/ml), TRAb (0.9 IU/l) and TSAb (121%).
Fig. 4.
Clinical course of the present case. TPO thyroid peroxidase, T4 thyroxine, TSH thyroid-stimulating hormone, UPCR urinary protein albumin ratio, s-Alb serum albumin
Discussion
Membranous nephropathy is a common cause of proteinuria and nephrotic syndrome. Idiopathic membranous nephropathy is diagnosed when causes of secondary membranous nephropathy are excluded, and the treatment strategies are different between the two types of membranous nephropathy. The differential diagnosis in cases with membranous nephropathy requires thorough medical examination, but it is sometime difficult in atypical cases.
It has been reported that AITDs including Hashimoto disease and Graves’ disease are sometimes associated with glomerulopathy. Membranous nephropathy is the most common type (20–48%) of AITD associated with nephropathy [5, 6]. Both Hashimoto disease [1, 2] and Graves’ disease [3, 4] have been reported to be associated with membranous nephropathy. In previous studies, some cases showed positive staining for both thyroglobulin and TPO [7, 8] and some cases showed positive staining for either thyroglobulin [9, 10] or TPO [3, 4] in AITDs associated with membranous nephropathy, although both thyroglobulin and TPO were elevated in serum. These findings indicate that not all elevated thyroid antigens deposit on the glomerulus basement membrane. In our case, at least one of the culprit antigens of membranous nephropathy was not thyroglobulin but TPO. The findings of previous studies and our study also indicate the possibility that the deposition of an immunocomplex of thyroglobulin and/or TPO and their antibodies contributes to the development of membranous nephropathy regardless of the presence of Hashimoto disease or Graves’ disease.
Deposition of IgA, kappa and lambda light chains in addition to IgG and negative staining for PLA2R seem to be signs of “secondary” membranous nephropathy, not “idiopathic” membranous nephropathy [11]. The fact that some electron-dense deposits were seen in the mesangial region as well as on the epithelial side of the glomerular basement membrane by electron microscopy also indicates “secondary” membranous nephropathy [11]. However, a limitation of this case study is that we did not evaluate the deposition of other antigens of membranous nephropathy, such as thrombospondin type 1 domain-containing 7A (THSD7A), NELL1, Semaphorin 3B, or exostosins 1/2 [12].
Although the prevalence of asymptomatic Graves’ disease is not known, it is likely that the entity is under-diagnosed [13]. A possible explanation for the lack of symptoms in asymptomatic hyperthyroidism is resistance of the peripheral tissues to thyroid hormone action. Elderly patients with hyperthyroidism tend to show fewer symptoms than do younger patients [13], and young adults or children with asymptomatic hyperthyroidism are very rare. To the best of our knowledge, a case of asymptomatic Graves’ disease complicated with membranous nephropathy, like the present case, has not been previously reported.
Treatment of membranous nephropathy associated with Graves’ disease has not been established. Theoretically, hemodynamic effects of hyperthyroidism and glomerular deposition of immune complexes possibly underlie the phenotype of membrane nephropathy induced by Graves’ disease. Shima et al. reported that treatment with thiamazole and lisinopril without a corticosteroid for 6 months resulted in the complete remission of nephrotic syndrome with reductions in anti-TPO antibody, anti-thyroglobulin antibody and TRAb from 1600 to 400 T, from 400 to 100 T, and from 84 to 7.5%, respectively [3]. In the present case, anti-TPO antibody, anti-thyroglobulin antibody and TRAb were reduced from 601.7 to 150.1 IU/ml, from 1655.2 to 807.0 IU/ml and from 10.9 to 0.9 IU/l, respectively, after treatment with thiamazole and enalapril. Sasaki et al. [4] reported that 131I treatment resulted in successful control of hyperthyroidism and proteinuria. On the other hand, Graves’ disease cases in which membranous nephropathy developed after 131I treatment presumably via the antigen released from the damaged thyroid have been reported [7, 8]. Efficacy of corticosteroid treatment has been shown for cases of Graves' disease-associated membranous nephropathy in which an immune complex formed by the thyroid antigen was involved [7, 8, 11], and corticosteroid treatment appears to be a possible option for cases refractory to anti-thyroid treatment and/or treatment with an ACE inhibitor.
Conclusion
We described a case of membranous nephropathy secondary to asymptomatic Graves’ disease with co-localization of TPO and IgG deposition in the glomerular capillaries. Administration of thiamazole and enalapril without a corticosteroid improved proteinuria and hypoalbuminemia. Awareness of asymptomatic AITD is needed in differential diagnosis of etiologies of membranous nephropathy.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal participation
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient described.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Horikoshi T, Tamura J, Kaneko Y, Maezawa A, Kanai H, Kaji T, Matsushima T, Sawamura M, Murakami H, Yano S, Kubota K, Naruse T. Membranous nephropathy associated with chronic thyroiditis. Nephron. 1993;63:246. doi: 10.1159/000187202. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi Y, Yorioka N, Katsutani M, Nagano R, Yokoyama R, Okubo M, Yamakido M. Hemophagocytic syndrome in a patient with Hashimoto’s thyroiditis and membranous nephritis. Nephron. 1999;81:246–247. doi: 10.1159/000045287. [DOI] [PubMed] [Google Scholar]
- 3.Shima Y, Nakanishi K, Togawa H, Obana M, Sako M, Miyawaki M, Nozu K, Iijima K, Yoshikawa N. Membranous nephropathy associated with thyroid-peroxidase antigen. Pediatr Nephrol. 2009;24:605–608. doi: 10.1007/s00467-008-0973-0. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki K, Yasuda K, Nakanishi K, Rakugi H, Isaka Y, Yamato M. Membranous nephropathy secondary to Graves’ disease with deposits of thyroid peroxidase in an adult. CEN Case Rep. 2014;3:90–93. doi: 10.1007/s13730-013-0093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoro D, Vadalà C, Siligato R, Buemi M, Benvenga S. Autoimmune thyroiditis and glomerulopathies. Front Endocrinol (Lausanne) 2017;8:119. doi: 10.3389/fendo.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain D, Aggarwal HK, Pavan Kumar YM, Jain P. Evaluation of thyroid dysfunction in patients with nephrotic syndrome. Med Pharm Rep. 2019;92:139–144. doi: 10.15386/mpr-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan SC, Buckingham B, Sakai R, Olson D. Studies of immune-complex glomerulonephritis mediated by human thyroglobulin. N Engl J Med. 1981;304:1212–1215. doi: 10.1056/NEJM198105143042006. [DOI] [PubMed] [Google Scholar]
- 8.Iwaoka T, Umeda T, Nakayama M, Shimada T, Fujii Y, Miura F, Sato T. A case of membranous nephropathy associated with thyroid antigens. Jpn J Med. 1982;21:29–34. doi: 10.2169/internalmedicine1962.21.29. [DOI] [PubMed] [Google Scholar]
- 9.Ploth DW, Fitz A, Schnetzler D, Seidenfeld J, Wilson CB. Thyroglobulin-anti-thyroglobulin immune complex glomerulonephritis complicating radioiodine therapy. Clin Immunol Immunopathol. 1978;9:327–334. doi: 10.1016/0090-1229(78)90104-6. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Sasaki M, Kan R, Osaku A, Koyama S, Shibayama S, Sato M, Narumiya K, Takagi T, Kojima M. Thyroid antigen-mediated glomerulonephritis in Graves’ disease. Clin Nephrol. 1989;31:49–52. [PubMed] [Google Scholar]
- 11.Moroni G, Ponticelli C. Secondary membranous nephropathy. A narrative review. Front Med (Lausanne). 2020;7:611317. doi: 10.3389/fmed.2020.611317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronco P, Plaisier E, Debiec H. Advances in membranous nephropathy. J Clin Med. 2021;10:607. doi: 10.3390/jcm10040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mooradian AD. Asymptomatic hyperthyroidism in older adults: is it a distinct clinical and laboratory entity? Drugs Aging. 2008;25:371–380. doi: 10.2165/00002512-200825050-00002. [DOI] [PubMed] [Google Scholar]