Abstract
Objectives
Vulval Intraepithelial Neoplasia 3 (VIN) is a chronic, premalignant condition affecting the vulval skin. The age standardised incidence is approximately one per 100,000 women, with a peak at 30–49 years of age, and has risen over recent decades. This study would analyse the pattern of presentation, diagnosis, treatment and follow up of patients diagnosed with VIN 3 over a period of ten years at a tertiary care centre in India.
Materials and Methods
This was a retrospective study conducted on all patients diagnosed to have VIN 3 between 1 January 2010 to 30 November 2019 in the Department of Gynaecologic Oncology, Christian Medical College, Vellore were included in this study. The outpatient records of the patients were obtained from an electronic registry.
Results
A total of 18 patients were diagnosed of VIN 3 during this time period. Sixteen patients were older than 50 years. Abnormal PAP was noted in 10 patients (HSIL-7, LSIL-2, ASC-H-1). Four patients had coexisting VAIN 3. About 16 patients underwent primary simple vulvectomy or wide local excision. Two patients were managed conservatively. Nine patients had recurrence with mean disease free interval of 12.5 months (4–36 months). Cryotherapy was used in 2 patients. Imiquimod was used in 3 patients. Surgical margins was achieved in 7 patients out of which 5 patients had recurrence. About 50% of patients with involved margins on biopsy had recurrence. Mean duration of follow up was 17 months (4–105 months). About 8 patients developed squamous cell carcinoma of genital tract on follow up.
Conclusion
VIN 3 has a high rate of progression to invasive SCC. Regression of VIN is rare. Proper follow up and treatment of VIN 3 goes a long way in preventing the morbidity associated with vulval cancer.
Keywords: Management, Recurrence, VIN3, Vulva
Introduction
Vulval intra-epithelial neoplasia (VIN 3) is a forerunner to carcinoma vulva [1]. While its incidence is showing a rising trend, it is estimated at 1/1,00,000 women with peak in late reproductive age group (30–45 years) [1]. While the treatment modalities have expanded it is still a taboo in present day. Many women present late in the course of illness and already have advanced disease.
VIN classification has changed over time with the initial International Society for the Study of Vulvar Disease (ISSVD) classification in 1976 which was updated in 2004.Recent nomenclature by Lower Anogenital Squamous Terminology (LAST) 2012/World Health Organisation (WHO) 2014/ISSVD 2015 classify VIN into 2 broad categories namely Low Grade Squamous Intraepithelial Lesion (LSIL) and High Grade Squamous Intraepithelial Lesion (HSIL).VIN 3 is a component of HSIL which also includes VIN 2, moderate to severe dysplasia, Bowens disease, Bowenoid dysplasia and Carcinoma In situ (CIS) [2, 3].
We did this study to look into the presentation, diagnosis, management and follow up in women diagnosed to have VIN 3 over a period of ten years at a tertiary care centre in India. The study was undertaken specifically in regard to follow up patients as it was associated with a social stigma wherein patients were likely to defer treatment and subsequent follow up.
Materials and Methods
The study was carried at department of Gynaecologic Oncology at Christian Medical College and Hospital, Vellore a tertiary care centre in southern India. The study period was from 1 January 2010 to 30 November 2019.All data concerning these patients which were collected from electronic registry were reviewed retrospectively. Women were included if biopsy proven VIN 3 was reported in our hospital reports.
Ethical Clearance was obtained from the Institutional Review Board and Ethics Committee (IRB No. 13122). The consent was waived off as it was a retrospective study. Data were analysed using SPSS software version 21(IBM, Armonk, New York, USA). Recurrence free survival was plotted using the Kaplan Meier curve.
Results
A total of 18 cases of VIN 3 were identified in last 10 years. Table 1 illustrates the demographic profile of the patients. Sixteen patients were greater than 50 years, 17 patients were parous. One patient had lichen sclerosus, two patients were PLWHA (people living with HIV and AIDS) and two patients had concomitant carcinoma cervix.
Table 1.
Demographic profile of the patients
| Patient Characteristics | N = 18 |
|---|---|
| 1. Age distribution | |
| a. ≤ 50 years | 2 (11.1%) |
| b. > 50 years | 16 (88.9%) |
| 2. Parity | |
| a.Nulliparous | 1 (5.5%) |
| b.Parous | 17 (94.5%) |
| 3. Co-morbidities | |
| Diabetes mellitus | 2 (11.1%) |
| Hypertension | 7 (28%) |
| Hypothyroid | 2 (11.1%) |
| PLWHAa | 2 (11.1%) |
| Hepatitis B | 1 (5.5%) |
| Tuberculosis | 3 (16.6%) |
| Lichen Sclerosus | 1 (5.5%) |
| Carcinoma cervix | 2 (11.1%) |
| 4. Menopausal status | |
| Premenopause | 2 (11.1%) |
| Menopausal | 16 (88.9%) |
apeople living with HIV and AIDS
Majority (77.8%) presented with complaints of pruritus and growth in the genitalia area. Two cases were diagnosed incidentally to have lesions on vulva on examination. The mean duration of symptoms was 7 months with the majority of patients (61%) having multifocal lesions. Pap smear was tested in all patients, HSIL was seen in 38% of patients. On further biopsy Cervical Intra-epithelial neoplasia (CIN 3) was found in 7 patients, Vaginal Intra-epithelial neoplasia (VAIN 3) in four patients, cancer cervix in 2 patients. High risk HPV was present in 9 patients. Table 2 illustrates the initial symptomatology and examination findings.
Table 2.
Presenting complaints and primary investigations
| Characteristics | N = 18 |
|---|---|
| 1. Presenting complaints | |
| a. Pruritus | 2 (11.1%) |
| b. Incidental diagnosis | 2 (11.1%) |
| c. Pruritus and growth | 14 (77.8%) |
| 2. Laterality of lesion | |
| a. Unifocal | 7 (38.4%) |
| b. Multifocal | 11 (61.1%) |
| 3. Duration of complaints-mean (range) | 7 months (14 days-5 years) |
| 4. Pap Smear | |
| a. Negative | 6 (45%) |
| b. LSIL | 2 (11.1%) |
| c. ASC-H | 1 (5.5%) |
| d. HSIL | 7 (38.4%) |
| 5. High Risk HPV | |
| a. Present | 9 (50%) |
| b. Not done | 9 (50%) |
| 6. Associated multi-centric lesions | |
| a. VIN 3 | 13 |
| b. Carcinoma vulva | 3 |
| c. Normal cervix | 4 |
| d. CIN 1 | 3 |
| e. CIN 3 | 7 |
| f. Carcinoma cervix | 2 |
| g. Normal vagina | 12 |
| h. VAIN 3 | 4 |
| i. Vaginal cancer | 0 |
Out of 18 patients who were initially diagnosed to have VIN 3, 16 patients underwent surgical resection and two patients had ablative therapy with cryotherapy due to smaller lesions. Wide local excision (WLE) was done in 10 patients and simple vulvectomy in 6 patients due to larger size lesions as shown in Table 3. Three patients were excluded from further analysis as post surgery biopsy showed incidental micro-invasive carcinoma vulva.
Table 3.
Initial management of lesions
| Total cases | 18 |
|---|---|
| 1. Surgery type | |
| a. Wide local excision | 10 (55.5%) |
| b. Simple Vulvectomy | 6 (33.3%) |
| 2. Cryotherapy | 2 (11.2%) |
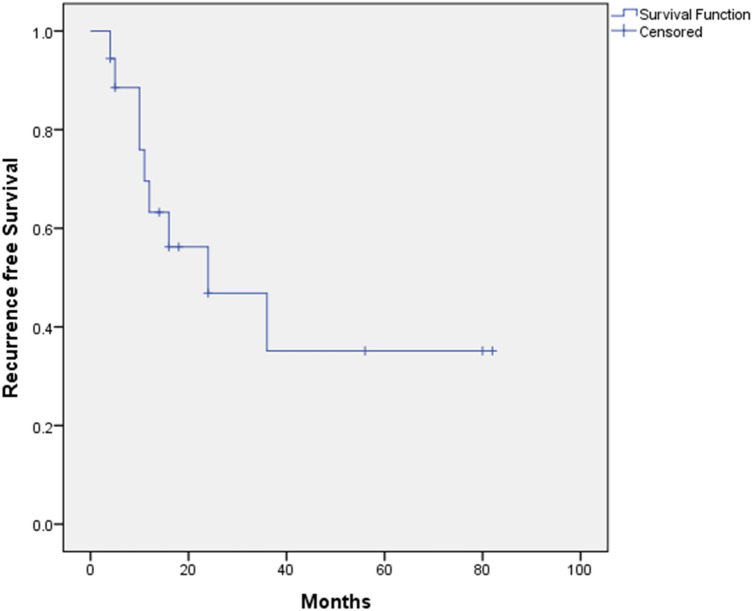
Of the rest 13 patients, after excluding the three carcinomas, we achieved free surgical margins (5 mm margins) in 7 patients and involved surgical margins in 6 patients. Nine of these patients had recurrence of which 5 patients had free margin on histopathology, 3 patients in involved margins group had involved margins on histopathology and 1 person in cryotherapy group. Nine patients further underwent adjuvant therapy, of that 6 patients had imiquimod therapy and 3 patients had 5FU. Patient who recurred had multifocal nature of lesions and positive margins (8 out of 9 patients).This was plotted in the Kaplan Meier curve is shown in Fig. 1. Of the 9 patients who had recurrence, 3 were treated with imiquimod and 6 patients had a repeat surgical excision (Wide local excision).Of the 6 patients 5 patients again recurred and clear margins were achieved in four patients. Overall mean disease free interval was 12.5 months (4–36 months) and mean duration of follow up was 17 months (4–105 months). On follow up 3 patients developed vulval cancer, 3 patients had carcinoma cervix and 2 patients developed vaginal cancer. The follow up details are mentioned in Table 4.
Fig. 1.
Kaplan Meier curve to show Recurrence free survival curve
Table 4.
Follow up of lesions
| Number | Surgical margins free | Involved surgical margins | Cryotherapy |
|---|---|---|---|
| Follow up | |||
| No of patients | 7/13(53.8%) | 6/13(46.2%) | 2 |
| Recurrence | 5(71.4%) | 3(50%) | 1(50%) |
| 1. Treatment of recurrences | N = 9 | ||
| a. Imiquimod | 3(33%) | ||
| b. Repeat Surgery-WLE | 6(67%) | ||
| c. Repeat recurrence | 5 | ||
| 2. Mean(range)Disease free interval | 12.5 months (4–36 months) | ||
| 3. Mean (range)of follow up | 17 months (4–105 months) | ||
| 4. Subsequent cancer development- | |||
| a. Vulval cancer | 3 | ||
| b. Carcinoma cervix | 3 | ||
| c. Vaginal cancer | 2 | ||
| 5.Mortality | 3 |
Three patients had expired during the follow up period. They developed multiple recurrences, one was treated with Imiquimod and expired 15 months later, one had recurrence treated with surgical excision and was treated with RT for concomitant cervix cancer and had expired 49 months later. The third patient was treated with 5 FU/Brachytherapy for concomitant vaginal cancer and had expired 54 months later.
Discussion
This study was undertaken to provide a valuable insight into patients presenting with VIN 3 with regard to their management and outcomes. VIN 3 has a varied, individual clinical profile and histological appearance. In a study done by Peter Sykes et al. the median age of presentation was 38 years with a wide age range [4], whereas study by Mario Preti et al. showed a bimodal peak at 40–44 years and over 55 years [5]. Our group of patients were mostly post menopausal. According to a study done by Jones et al. up to 50% of cases of VIN 3 had concomitant lower genital tract neoplasia [5, 6], this was also evident in a number of patients having multiple lesions in cervix, vagina and vulva. This is well explained by the “mullerian field” effect in which the entire genital tract develops from a common embryological precursor with squamous epithelium extending from cervix to anus. This was also supported in numerous other studies [7–11]. Our study too conferred with the above concept with the observing of multiple genital tract pre malignant and malignant conditions in the same setting.
Peter Sykes et al. showed majority of patients (79%) were symptomatic for a median period of 9.5 months [4]. In our study also most of patients had a long duration of symptoms, this was expected in view of the nature of presenting complaints i.e. pruritus and genitalia lesions which are usually not reported early in our strata of patients; unlike cancer cervix where bleeding prompts early health care visits. Sixty percent of our patients had multifocal lesions which are usually the presentation where HPV acts a predisposing factor, this is also supported by 9 patients who had tested positive for HPV also. Failure of the immune system to effectively clear HPV is evident in PLWHA thus leading to an increased risk of conversion of VIN to vulval cancer [4]. We too found various immune-deficient conditions such as PLWHA and tuberculosis in 5 patients. Lichen sclerosus as a causal factor of undifferentiated VIN was seen in one patient. Differentiated VIN (dVIN) is an uncommon diagnosis due to common histological features shared with lichen sclerosus, mild dysplasia or inflammatory dermatosis [12–14]. Positive p53 staining can help in differentiation dVIN in such cases [15]
Surgical excision is an accepted standard of treatment in VIN, with focus shifted towards more cosmetic and conservative procedures preventing distortion of anatomy and function [5]. Wide local excision is the preferred surgery which achieves the goal of gross margins of 0.5–1 cm and depth of 4 mm which may be reduced to avoid damage to important structures like clitoris, urethra and anus maintaining psycho-sexual function. Up to 22% of women had occult carcinoma in the final biopsy when operated for VIN 3 [9], we had 3 cases of occult carcinoma in final biopsy. A study done by Modesitt et al. showed mere presence of negative margins on the biopsy were still prone to develop recurrence although comparatively lesser than those cases where margins were positive [9]. Another study by van Seters et al. also inferred that free margins did not alter the progression to neoplasia [16]. In our study, 71% of patients with free margins recurred, whereas 50% of cases with involved margins recurred. Recurrence was associated with multi-focal lesions which were also seen in our study. Cryotherapy was used as the primary modality in two patients, one patient had recurrence 6 months later treated with wide local excision, and other patient was lost to follow up.
As long as all macroscopic disease have been removed, re excision is not justified for positive margins. Recurrence rates up to 32% have been documented in a study by Kesterson and Lele [17]. Other studies show a recurrence rate up to 50%which was associated with positive margins and multiple lesions [6, 16, 18–20]. The recurrence rates with positive margins and multifocal lesions were 50% in our study. Three ring vulvoscopy plays an important role in follow up visits [21]. It is recommended to follow up at 6 months and 12 months after initial treatment and yearly thereafter [22]. Studies have documented recurrence as early as 9 months [17]. This also should be highlighted to patients at the time of discharge stressing the need for an annual inspection after documentation of complete response.
Few studies have achieved a negative margin rate up to 87.5% [23, 24], compared to our negative margins rate of 54%. About 5 patients recurred and underwent repeat excision. The repeated high rate of recurrences establishes the need for programmed follow up visits.
Topical imiquimod 5% is a well-known immunomodulator which targets the immune mediated clearance of HPV which is effective for treatment of vulval HSIL (usual type) [24]. Recommended duration of usage was 12–20 weeks, in our study the mean duration was 2 weeks, the limiting factors being burning sensation experienced by patients and need for application by a trained health care worker. 5-FU creams has been used been used with varying success in RCTs [23, 24].We had three patients treated with 5 FU, this was in view of these patients having concomitant multi-focal lesions on cervix and vagina. These patient recurred within 24–54 months post treatment. Side effects such as burning sensation were common. RECIST criteria should be calculated at 6, 12, 18, 24 weeks and each post treatment visit to assess response to therapy [25].
It is reported up to 36.7% of vulval cancers are associated with VIN 3 [6, 22, 23]. Data on rates of progression to malignancy are scanty as most cases are operated but may be up to 5% per year or 1–2% with surgery [16]. We had 3 cases of concomitant carcinoma vulva and 3 patients developed vulval cancer on long term follow up. One patient underwent repeated wide local excision and developed cancer nearly 9 years from initial diagnosis, another patient had concomitant carcinoma cervix and was treated with radiotherapy and developed cancer vulva 3 years later and the last patient developed cancer vulva one year later. We had 3 deaths in our study which were attributed to advanced cancer cervix, advanced vaginal cancer and last patient due to natural death.
The quadrivalent vaccine is 100% effective in HPV 16/18 related high-grade lesions in young women and up to 49% effective against all high-grade VIN irrespective of HPV status; this also stresses the need for a proper vaccination programme keeping in mind the benefits.
Our limitation of the study has been the small number of patients and being a retrospective study we could not assess certain aspects such as impact of disease on patient. However, due to the small size we were able to effectively follow up of our patients.
Declarations
Conflict of Interest
The authors had declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Judson PL, Habermann EB, Baxter NN, et al. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018–1022. doi: 10.1097/01.AOG.0000210268.57527.a1. [DOI] [PubMed] [Google Scholar]
- 2.Sideri M, Jones RW, Wilkinson EJ, et al. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. J Reprod Med. 2005;50(11):807–810. [PubMed] [Google Scholar]
- 3.Wilkinson EJ, Kneale B, Lynch PJ. Report of the ISSVD terminology committee. J Reprod Med Obstet Gynecol. 1986;31(10):973–974. [Google Scholar]
- 4.Sykes P, Smith N, McCormick P, Frizelle FA. High-grade vulval intraepithelial neoplasia (VIN 3): a retrospective analysis of patient characteristics, management, outcome and relationship to squamous cell carcinoma of the vulva 1989–1999. Aust New Zealand J Obstet Gynaecol. 2002;42(1):75–80. doi: 10.1111/j.0004-8666.2002.00075.x. [DOI] [PubMed] [Google Scholar]
- 5.Preti M, Igidbashian S, Costa S, et al. VIN usual type—from the past to the future. ecancermedicalscience. 2015 doi: 10.3332/ecancer.2015.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones RW. Vulval intraepithelial neoplasia: current perspectives. Eur J Gynaecol Oncol. 2001;22(6):393–402. [PubMed] [Google Scholar]
- 7.Hørding U, Daugaard S, Iversen AK, et al. Human papillomavirus type 16 in vulvar carcinoma, vulvar intraepithelial neoplasia, and associated cervical neoplasia. Gynecol Oncol. 1991;42(1):22–26. doi: 10.1016/0090-8258(91)90224-S. [DOI] [PubMed] [Google Scholar]
- 8.Hørding U, Junge J, Poulsen H, et al. Vulvar intraepithelial neoplasia III: a viral disease of undetermined progressive potential. Gynecol Oncol. 1995;56(2):276–279. doi: 10.1006/gyno.1995.1046. [DOI] [PubMed] [Google Scholar]
- 9.Modesitt SC, Waters AB, Walton L, et al. Vulvar intraepithelial neoplasia III: occult cancer and the impact of margin status on recurrence. Obstet Gynecol. 1998;92(6):962–966. doi: 10.1016/s0029-7844(98)00350-0. [DOI] [PubMed] [Google Scholar]
- 10.Andreasson B, Bock JE. Intraepithelial neoplasia in the vulvar region. Gynecol Oncol. 1985;21(3):300–305. doi: 10.1016/0090-8258(85)90267-7. [DOI] [PubMed] [Google Scholar]
- 11.Van Beurden M, van Der Vange N, Ten Kate FJ, et al. Restricted surgical management of vulvar intraepithelial neoplasia 3: focus on exclusion of invasion and on relief of symptoms. Int J Gynecol Cancer: Off J Int Gynecol Cancer Soc. 1998;8(1):73–77. doi: 10.1046/j.1525-1438.1998.09733.x. [DOI] [PubMed] [Google Scholar]
- 12.Skapa P, Zamecnik J, Hamsikova E, et al. Human papillomavirus (HPV) profiles of vulvar lesions: possible implications for the classification of vulvar squamous cell carcinoma precursors and for the efficacy of prophylactic HPV vaccination. Am J Surg Pathol. 2007;31(12):1834–1843. doi: 10.1097/PAS.0b013e3180686d10. [DOI] [PubMed] [Google Scholar]
- 13.Van De Nieuwenhof HP, Bulten J, Hollema H, et al. Differentiated vulvar intraepithelial neoplasia is often found in lesions, previously diagnosed as lichen sclerosus, which have progressed to vulvar squamous cell carcinoma. Mod Pathol. 2011;24(2):297–305. doi: 10.1038/modpathol.2010.192. [DOI] [PubMed] [Google Scholar]
- 14.Bigby SM, Eva LJ, Fong KL, et al. The natural history of vulvar intraepithelial neoplasia, differentiated type: evidence for progression and diagnostic challenges. Int J Gynecol Pathol. 2016;35(6):574–584. doi: 10.1097/PGP.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 15.Reuschenbach M, Roos J, Panayotopoulos D, et al. Characterization of squamous cell cancers of the vulvar anterior fourchette by human papillomavirus, p16INK4a, and p53. J Low Genit Tract Dis. 2013;17(3):289–297. doi: 10.1097/LGT.0b013e31826f2b2b. [DOI] [PubMed] [Google Scholar]
- 16.van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005;97(2):645–651. doi: 10.1016/j.ygyno.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Kesterson JP, Lele S. Vulvar intraepithelial neoplasia 3 in women less than 35 years. J Low Genit Tract Dis. 2009;13(4):196. doi: 10.1097/LGT.0b013e318196bd23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 19.Hillemanns P, Wang X, Staehle S, et al. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO2 laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100(2):271–275. doi: 10.1016/j.ygyno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 20.van Esch EM, Dam MC, Osse ME, et al. Clinical characteristics associated with development of recurrence and progression in usual-type vulvar intraepithelial neoplasia. Int J Gynecol Cancer. 2013 doi: 10.1097/IGC.0b013e3182a57fd6. [DOI] [PubMed] [Google Scholar]
- 21.Harni V, Babic D, Barisic D. Three rings vulvoscopy–a new approach to the vulva. Cryosurg Colposc. 2016.
- 22.American College of Obstetricians and Gynecologists. Management of vulvar intraepithelial neoplasia. Committee Opinion No. 675. Obstet Gynecol. 2016;128:e178-182. [DOI] [PubMed]
- 23.Jones RW, Rowan DM. Vulvar intraepithelial neoplasia III: a clinical study of the outcome in 113 cases with relation to the later development of invasive vulvar carcinoma. Obstet Gynecol. 1994;84(5):741–745. [PubMed] [Google Scholar]
- 24.Herod JJ, Shafi MI, Rollason TP, et al. Vulvar intraepithelial neoplasia: long term follow up of treated and untreated women. BJOG: Int J Obstet & Gynaecol. 1996;103(5):446–52. doi: 10.1111/j.1471-0528.1996.tb09771.x. [DOI] [PubMed] [Google Scholar]
- 25.Tristram A, Hurt CN, Madden T, et al. Activity, safety, and feasibility of cidofovir and imiquimod for treatment of vulval intraepithelial neoplasia (RT3VIN): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2014;15(12):1361–1368. doi: 10.1016/S1470-2045(14)70456-5. [DOI] [PubMed] [Google Scholar]