Methamphetamine (METH) is a potent psychostimulant and one of the most commonly used substances worldwide. METH produces strong rewarding and psychostimulant effects by increasing dopamine (DA) release in the brain reward system. Specifically, METH is first taken into the cytoplasm via the membrane DA transporter (DAT) and then binds to the vesicular monoamine transporter (called “VMAT2”), which blocks DA loading into vesicles and causes cytoplasm DA accumulation. This rapid increase in cytoplasm DA reverses the functional direction of the DAT and releases DA into the extracellular space [1]. As such, DAT has been regarded as a major target in medication development for the treatment of psychostimulant use disorder [2]. Developing new pharmacotherapies for METH addiction is urgent as there are currently none approved by the United States Food and Drug Administration or other authorities. The research highlighted herein presents an exciting new target for METH addiction medication development, Clk1 (clock-1, also called Coq7), a mitochondrial hydroxylase involved in energy metabolism and DAT internalization [3].
In this paper, Yan et al. [3] demonstrated that METH exposure increased Clk1 expression, while genetic deletion of the Clk1 gene produced a significant reduction in METH reward as assessed by an animal model of addiction called conditioned place preference, alongside an increase in membrane DAT expression in the striatum and hippocampus. This is an important finding that demonstrates, for the first time, that mitochondrial Clk1 is involved in METH reward. If these mutant mice behave similarly in other animal models of drug addiction such as METH self-administration and reinstatement of drug seeking behavior, it will open a new avenue of research for the treatment of psychostimulant abuse. Along these lines, selective Clk1 inhibitors should produce a similar reduction in METH reward as Clk1 deletion or deficiency (Fig. 1) and may be a first test of this medication development hypothesis.
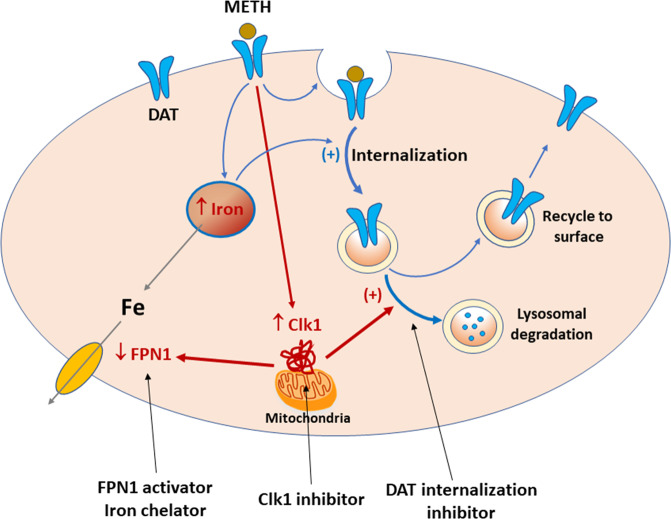
Fig. 1. Schematic diagram showing how METH exposure alters cytoplasm iron content, membrane DAT expression, and METH reward.
Briefly, METH exposure increases Clk1 expression, which subsequently decreases the iron exporter (FPN1) expression, causing an increase in cytoplasm iron content, and also increases lysosomal DAT degradation, causing a reduction in membrane DAT expression. Increased iron content may also facilitate DAT internalization. Accordingly, genetic deletion or pharmacological inhibition of mitochondria Clk1 causes a reduction in cytoplasm iron content and an increase in membrane DAT expression. Increased membrane DAT facilitates DA reuptake from extracellular space and decreases DA response to METH, producing a reduction in METH reward. Similarly, a pharmacological agent that removes excess cytoplasm iron (such as an iron chelator or FPN1 activator) or inhibits DAT internalization would be also effective in attenuation of METH reward and dependence.
It was further demonstrated that Clk1 mutation-induced reduction in METH reward is accompanied by a decrease in cytoplasm iron content [3]. A series of mechanistic experiments revealed that a Clk1 deficit upregulated expression of an iron exporter called ferroportin (FPN1) and decreased expression of hepcidin, a negative FPN1 regulator. As such, the decrease in iron content via Clk1 mutation is likely mediated by augmented iron export activity. These data parallel recent reports showing enhanced iron deposition in the basal ganglia, a critical brain region involved in drug reward and addiction [2], following psychostimulant use [4, 5]. Yan et al. [3] provide the first clear evidence linking mitochondrial Clk1 to altered iron metabolism and intracellular iron levels, which strongly suggests that a Clk1-dependent mechanism could also be involved in METH-induced iron deposition in the basal ganglia. Accordingly, an iron chelator or other pharmacological agents that can remove excess iron accumulation in METH users could also be an effective treatment of METH dependence and addiction (Fig. 1). Prior work has demonstrated that application of the iron chelator deferoxamine can attenuate METH-induced hyperthermia and hyperactivity in rats [6], supporting this line of inquiry. However, further research is required to test the value of iron-depleting agents as pharmacotherapeutics for METH addiction.
As a final consideration, the current DAT-based medication development strategy has been exclusively focused on developing selective DAT inhibitors that block METH action on DAT [7]. This approach has met with limited success, as these compounds were not approved for the treatment of psychostimulant use disorders. In this report, the reduction in METH reward in Clk1 mutant mice was mediated by decreased DAT internalization and subsequent increases in membrane DAT expression [3]. Thus, pharmacological agents that interrupt the process of DAT internalization may represent another target for reducing METH use and abuse (Fig. 1), via increased reuptake of DA, and weakened METH-enhanced striatal DA release. Given that METH exposure decreases surface DAT availability by inducing DAT internalization from the plasma membrane [8], compounds targeting this process may be particularly efficacious in treating METH addiction.
In summary, this research paper is an exciting addition to the literature. The major finding is that mitochondrial Clk1 modulates METH reward by regulating intracellular iron content and DAT expression. This work not only increases our understanding of how Clk1 alters the rewarding properties of METH, but also introduces a number of new targets and opportunities for medication development in the treatment of METH and other psychostimulant use disorders. Several key issues remain to be determined. For example, does Clk1 deficiency alter METH self-administration and relapse to drug seeking? How does Clk1 modulate iron exporter expression and subsequent intracellular iron content? Why does Clk1 selectively regulate iron homeostasis and DAT expression in the striatum and hippocampus, but not other brain regions? Additional research is needed to further address these questions and determine whether the Clk1-iron-DAT regulation pathway plays an essential role in METH use disorder.
Acknowledgements
This work is supported by the National Institute on Drug Abuse (NIDA), Intramural Research Progema (IRP) (Z1A DA000633-01), National Institutes of Health (NIH).
Competing interests
The authors declare no competing interests.
References
- 1.Freyberg Z, Sonders MS, Aguilar JI, Hiranita T, Karam CS, Flores J, et al. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat Commun. 2016;7:10652. doi: 10.1038/ncomms10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman AH, Ku T, Jordan CJ, Bonifazi A, Xi ZX. New drugs, old targets: tweaking the dopamine system to treat psychostimulant use disorders. Annu Rev Pharmacol Toxicol. 2021;61:609–28. doi: 10.1146/annurev-pharmtox-030220-124205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan PJ, Ren ZX, Shi ZF, Wan CL, Han CJ, Zhu LS, et al. Dysregulation of iron homeostasis and methamphetamine reward behaviors in Clk1-deficient mice. Acta Pharmacol Sin. 2021. 10.1038/s41401-021-00806-1. [DOI] [PMC free article] [PubMed]
- 4.Melega WP, Lacan G, Harvey DC, Way BM. Methamphetamine increases basal ganglia iron to levels observed in aging. Neuroreport. 2007;18:1741–5. doi: 10.1097/WNR.0b013e3282f0d4f4. [DOI] [PubMed] [Google Scholar]
- 5.Adisetiyo V, McGill CE, DeVries WH, Jensen JH, Hanlon CA, Helpern JA. Elevated brain iron in cocaine use disorder as indexed by magnetic field correlation imaging. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:579–88.. doi: 10.1016/j.bpsc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Park MJ, Lee SK, Lim MA, Chung HS, Cho SI, Jang CG, et al. Effect of alpha-tocopherol and deferoxamine on methamphetamine-induced neurotoxicity. Brain Res. 2006;1109:176–82. doi: 10.1016/j.brainres.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Tanda G, Hersey M, Hempel B, Xi ZX, Newman AH. Modafinil and its structural analogs as atypical dopamine uptake inhibitors and potential medications for psychostimulant use disorder. Curr Opin Pharmacol. 2021;56:13–21. doi: 10.1016/j.coph.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.German CL, Hanson GR, Fleckenstein AE. Amphetamine and methamphetamine reduce striatal dopamine transporter function without concurrent dopamine transporter relocalization. J Neurochem. 2012;123:288–97. doi: 10.1111/j.1471-4159.2012.07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]