Abstract
Objective
This study aimed to determine the efficacy, effectiveness, and cost-effectiveness of inactivated COVID-19 vaccines (CoronaVac and BBIBP-CorV) in China using existing international clinical trials and real-world evidence.
Methods
Through a search of PubMed, Embase, Web of Science, and CNKI, studies investigating the effectiveness of inactivated COVID-19 vaccines were identified, and a meta-analysis was undertaken to synthesize the vaccine efficacy and effectiveness data. Moreover, a decision-analytic model was developed to estimate the cost-effectiveness of inactivated vaccines for combating the COVID-19 pandemic in the Chinese context from a societal perspective. Results of the meta-analysis, along with cost data from official websites and works of literature were used to populate the model. Sensitivity analysis was performed to test the robustness of the model results.
Results
A total of 24 studies were included in the meta-analysis. In comparison to no immunization, the effectiveness of inactivated vaccine against COVID-19 infection, hospitalization, ICU admission and death were 65.18% (95% CI 62.62, 67.75), 79.10% (95% CI 71.69, 86.51), 90.46% (95% CI 89.42, 91.50), and 86.69% (95% CI 85.68, 87.70); and the efficacy against COVID-19 infection and hospitalization were 70.56% (95% CI 57.87, 83.24) and 100% (95% CI 61.72, 100). Inactivated vaccine vaccination prevented more infections, hospitalizations, ICU admissions, and deaths with lower total costs, thus was cost-saving from a societal perspective in China. Base-case analysis results were robust in the one-way sensitivity analysis, and the percentage of ICU admission or death and direct medical cost ranked the top influential factors in our models. In the probabilistic sensitivity analysis, vaccination had a 100% probability of being cost-effective.
Conclusion
Inactivated vaccine is effective in preventing COVID-19 infection, hospitalization, ICU admission and avoiding COVID-19 related death, and COVID-19 vaccination program is cost-saving from societal perspective in China.
Keywords: effectiveness, cost-effectiveness, inactivated COVID-19 vaccine, RCT, real-world evidence, efficacy
Box 1. Summary Box.
What is already known?
Coronavirus disease 2019 (COVID-19) has had a huge impact on the global economy and has resulted in a significant disease burden.
The inactivated vaccines (Sinovac CoronaVac and BBIBP-CorV) have been used worldwide, and both have been validated for emergency use by the WHO. The CoronaVac is the most extensively used COVID-19 vaccine worldwide.
Vaccination strategies have been proven to be not only cost-effective but also cost-saving in countries such as the United States, Denmark, and Turkey.
What are the new findings?
This study is more comprehensive and contains more inactivated vaccine effectiveness data than other meta-analyses or systematic reviews, and can partially address the weakness that vaccine efficacy varies significantly across countries.
It is the first evaluation using synthesized data pooled from randomized control trial data and real-world evidence to estimate the cost-effectiveness of inactivated vaccines compared to no vaccination in the Chinese setting.
What do the new findings imply?
Two-dose inactivated vaccination strategy is effective and cost-saving in China.
This study offers compelling evidence to support the free COVID-19 vaccination program in China.
Background
Coronavirus disease 2019 (COVID-19) is a novel infectious disease caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It is a serious crisis and a severe test for the whole world, and studies in the United States (1), the United Kingdom (2), India (3), and China (4) have shown that COVID-19 has had a huge impact on the global economy, especially the loss of productivity due to business suspension, school suspension, and business closure. As of 3 June 2022, the WHO reported about 528.82 million cumulative cases and 6.29 million cumulative deaths worldwide (5), with China reporting 2.83 million confirmed cases and 17,271 deaths (6). COVID-19 pandemic has resulted in a significant disease burden, and the virus exhibits characteristics of high infectivity, concealment, and community aggregation; most notably, outbreaks of the Omicron strain in Tianjin, Shenzhen, Shanghai, and other locations throughout China since December 2021.
To safeguard the Chinese population and economy throughout the epidemic prevention and control stage, China altered its prevention and control strategy away from medical treatment and lockdown and toward vaccination and immune barrier establishment. Since January 2021, China has offered free vaccinations, namely, inactivated vaccines (Sinovac CoronaVac and BBIBP-CorV), adenovirus-vectored vaccine (CanSino Ad5-nCoV), and protein subunit vaccine (ZF2001). BBIBP-CorV was widely used in Asia, Africa, South America, and Europe and was approved for emergency use by the WHO on 7 May 2021. Meanwhile, CoronaVac, as the most extensively used COVID-19 vaccine worldwide, was validated for emergency use by the WHO on 1 June 2021 and is currently being utilized in nations throughout Asia, America, and Eastern Europe.
Both inactivated vaccines have been proven to be effective in both Phase III clinical trials and real-world studies in many countries, such as Brazil, Chile, Turkey, Malaysia, Indonesia, and Argentina. However, the efficacy and effectiveness rates varied significantly across countries. Numerous studies investigating the efficacy of inactivated COVID-19 vaccines are now underway, although synthesized evidence of inactivated vaccine effectiveness is rare. Additionally, only a limited number of studies have been done to evaluate the cost-effectiveness of vaccination strategies, particularly the inactivated vaccine, and no studies have been identified utilizing synthesized effectiveness data. To address this gap, this study aims to summarize the effectiveness of the Chinese inactivated vaccine by combining randomized control trial (RCT) data and real-world evidence (RWE), as well as estimate the cost-effectiveness of two-dose inactivated vaccine compared to no vaccination in China from a societal perspective, in order to provide evidence for prevention and control strategy decision-making in China and globally.
Methods
Meta Analysis
As inactivated vaccines were the most commonly used vaccines in China, taking up to more than 90% of the market share, and Sinovac CoronaVac and BBIBP-CorV were commonly vaccinated, this study only focused on inactivated vaccines. We conducted a meta-analysis to summarize the vaccine efficacy and effectiveness (VE) of the two Chinese inactivated vaccines (CoronaVac and BBIBP-CorV) in preventing COVID-19 infection, hospitalization, ICU admission, and avoiding COVID-19-related death compared with non-vaccination groups from a societal perspective.
Search Strategy
This study searched English and Chinese databases, including PubMed, Embase, Web of Science, and CNKI, for studies published by 1 June 2022, using the following search terms: (effectiveness OR efficacy) AND (COVID-19 OR SARS-CoV-2) AND (vaccine OR vaccination) AND (CoronaVac OR BBIBP OR inactivated vaccine). Reference lists from relevant primary studies and review articles were also searched manually. In addition, data shared by the pharmaceutical companies was also included.
Study Eligibility and Selection
The target population was people susceptible to COVID-19. We focused on studies that discussed the outcomes after two doses of CoronaVac or BBIBP-CorV compared with no vaccination. The outcome measures were vaccine efficacy of RCT and the effectiveness rate of RWE against COVID-19 infection, hospitalization, ICU admission, and death. Observational studies and clinical trial studies were both included. The exclusion criteria were as follows: (a) no separate evidence for CoronaVac or BBIBP-CorV reported; (b) only geometric mean concentration (GMC) or seroconversion of neutralizing antibody data available; (c) guideline, conference, and oral report; and (d) full-text unavailable.
After removing duplicates, all initial records were screened for titles and abstracts by two independent reviewers (YF and JZ). Following this, the full texts of the shortlisted abstracts were retrieved to assess eligibility for inclusion. Any disagreement was resolved by a third reviewer (PH). VE data were extracted for relevant outcomes in pre-defined tables.
Statistical Analysis
Random-effects or fixed-effects models were used to pool the VE data, based on the heterogeneity between estimates (I2). The meta-analysis was conducted using the Review Manager software (version 5.4).
Cost-Effectiveness Analysis
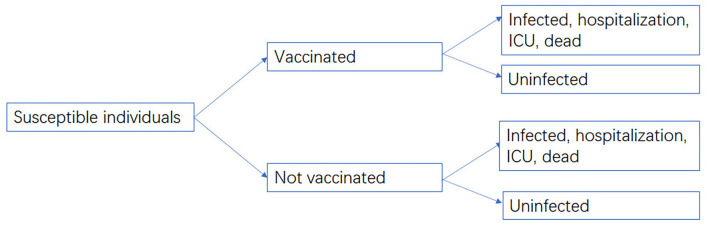
The cost-effectiveness between vaccination groups and non-vaccination groups were compared from a societal perspective, as the efficacy and effectiveness data of vaccines were compared with non-vaccination groups. Non-pharmaceutical interventions (NPIs) were not included in this study. A decision-analytic model was developed using TreeAge Pro 2021 (Figure 1). The cost data included vaccination cost, medical cost, and indirect cost, while the effectiveness of the vaccine against infection, hospitalization, ICU admission, and death was derived from our meta-analysis. The target population of our model was people aged over 3 years who had completed two-dose inactivated vaccination in China. The vaccinated population was 1,256.86 million, accounting for 89.15% of the total population (7). Cost data were not discounted due to the short time period.
Figure 1.
Decision-analytic model.
Natural Infection Rate and Percentage of Each Status
There was a paucity of data on the natural infection rate of unvaccinated people. Only an RCT of BBIBP-CorV in UAE and Bahrain (8) and a real-world study of CoronaVac in Chile (9) reported the natural infection rate. The natural infection rate from the RWE research in Chile was adopted since it encompassed approximately 80% of its population and was more representative. Furthermore, the declining exponential approximation of life expectancy (DEALE) method (10) was applied to convert the incidence in a cohort (per person-year) to the probability of infection in 1 year. The calculation of 1-year infection probability is p = 1−e−r. Here, p is the 1-year infection probability and r (person-year) is the incidence rate.
As for other transition probabilities, the transition pattern between infection, hospitalization, and ICU or death was obtained from the patient proportion in China (11).
Costs
Vaccination cost, medical cost, and indirect cost were included in this study. Due to the lack of a systematic review of the economic burden of COVID-19 in China, medical and indirect cost data mostly came from real-world sources, such as the literature of the Wuhan pandemic. Specifically, vaccination costs comprised vaccine procurement, cold-chain transportation, refrigeration, and administration, whereas medical costs covered diagnosis, treatment, hospitalization, and care expenditures associated with sickness. Although the expenses of COVID-19 vaccination and treatment are fully covered by basic medical insurance and the Ministry of Finance, this analysis included all the vaccination and medical costs incurred through the health system. Additionally, the productivity loss due to illness or premature death was included as the indirect cost from a societal perspective.
The cost of each dose of inactivated vaccine came from the lowest global purchase price published by the WHO (12). The transportation cost of vaccine was assumed to be 6% of the purchase cost (13), the refrigeration cost was calculated based on WHO recommendation, and the vaccine administration fee (injection service fee) was US$1.55 (14) (US$1 = ¥6.449).
Medical costs increased as disease severity grew from mild to severe to critical, according to data from an economic impact study of COVID-19 during the early stages of the Wuhan outbreak, and were US$868.51, US$8,210.73, and US$23,467.82 for infection, hospitalization, and ICU or death, respectively (4). Productivity losses were calculated based on the proportion of the labor force and the average daily salary (15).
Base Case Analysis
The outcome of the cost-effectiveness analysis was the number of infection cases, hospitalization, ICU admissions, and deaths avoided. The incremental cost-effectiveness ratio (ICER) was calculated to determine whether vaccination was cost-effective or not, and the threshold was set at the gross domestic product (GDP) per capita of China in 2021 (US$ 12,556.37) (15).
Sensitivity Analysis
One-way sensitivity analysis and probabilistic sensitivity analysis (PSA) were performed for the base case results from a societal perspective. The range of each parameter was derived from the published literature or our meta-analysis. The results were shown as tornado diagrams. As for the PSA, Monte Carlo simulation (N = 1,000 iterations) was used to assess the effects of changing multiple parameters simultaneously. Gamma and Dirichlet distributions were assigned to costs, with Beta distributions to vaccine efficacy and proportions of different infection severities. The PSA results were presented as cost-effectiveness acceptability curves.
Patient and Public Involvement
Not applicable.
Results
Meta Analysis of Vaccine Effectiveness
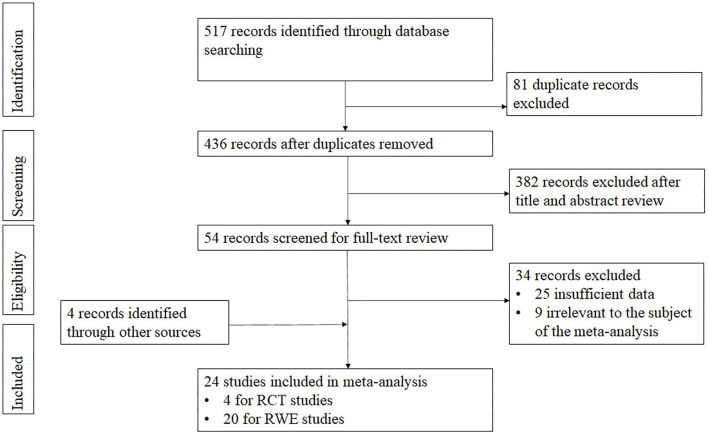
A total of 517 articles from four databases were extracted. Of them, 81 articles of duplication were excluded. Following a review of the title and abstract, we excluded 382 records not related to inactivated vaccine or only mentioning GMC or seroconversion of neutralizing antibody. Among the 54 studies under full-text review, 34 studies were excluded (Figure 2). Ultimately, with four records identified through other sources included, the meta-analysis comprised 24 eligible studies finally, including four phase 3 studies (8, 16–18) and 20 real-world studies (9, 19–35) (including 2 RWE shared by company). The studies mentioned virus strains were as follows: four of Alpha (22, 23, 31, 32), one of Beta (31), three of Gamma (19, 22, 27), five of Delta (20, 21, 25, 28, 29), one of Omicron (30), while the others did not mention the virus type. The majority of the included studies focused on adults, one focused on children aged 3–5 years (30), and three focused on the elderly aged over 60 years (22, 23, 33, 35).
Figure 2.
PRISMA flowchart for review of the study selection.
In the meta-analysis fixed-effects model was used when I2 <50%, and the random-effects model was used when the I2 > 50% to pool the VE data with 95% confidence intervals (CIs). Among the included articles, RCT evidence from Turkey (16), Brazil (18, 36), Indonesia (17), and the United Arab Emirates and Bahrain (8); RWE data from Chile (9, 30, 34), Brazil (19, 24, 27, 35), Argentina (22), Thailand (21), Serbia (23), United Arab Emirates (25, 31), Iran (26), China (20, 28, 29), Colombia (33), Hungary, and Turkey (32) were included. As the heterogeneity among RCT and RWE was huge, and I2 was consistently higher than 90%, this study was not able to pool efficacy and effectiveness data together; therefore, we have done subgroup analysis for RCT and RWE studies of adult, and for the elderly and children. The effectiveness of inactivated vaccine in real-world settings of adults against COVID-19 infection, hospitalization, ICU admission, and death were 65.18% (95% CI: 62.62, 67.75), 79.10% (95% CI: 71.69, 86.51), 90.46% (95% CI: 89.42, 91.50), and 86.69% (95% CI: 85.68, 87.70), while the efficacy in clinical trials against COVID-19 infection and hospitalization were 70.56% (95% CI: 57.87, 83.24) and 100% (95% CI: 61.72, 100; Table 1). The forest plots are shown in Figure 3.
Table 1.
Model parameters.
| Item | Parameter | Value | Lower* | Upper* | Distribution | Data source |
|---|---|---|---|---|---|---|
| Vaccination cost per dose (USD) | Acquisition | 4.00 | 4 | 5.5 | Gamma | WHO (12) |
| Cold-chain freight | 0.24 | / | / | Chen et al. (13) | ||
| Refrigerator storage | 0.18 | / | / | Jiang et al. (10) | ||
| Administration | 1.55 | / | / | National medical insurance bureau (14) | ||
| Direct medical cost (USD) | Infection/Non-severe | 868.51 | 613.58 | 1,788.03 | Dirichlet | Jin et al. (4); Zhao et al. (11) |
| Hospitalization/Severe | 8,210.73 | 5,800.74 | 16,905.10 | Dirichlet | ||
| ICU/Critical/death | 23,467.82 | 16,710.96 | 48,700.88 | Dirichlet | ||
| Percentage of each status | Infection | 81.50% | 60% | 90% | Beta | Jin et al. (4); Zhao et al. (11) |
| Hospitalization | 13.80% | 10% | 20% | Beta | ||
| ICU /death | 4.70% | 1% | 10% | Beta | ||
| Work time loss/day | Infection | 37 | / | / | Jin et al. (4); Zhao et al. (11) |
|
| Hospitalization | 41 | / | / | |||
| ICU | 42 | / | / | |||
| Death | 44 | / | / | |||
| Average salary per day (USD) | 42.18 | 31.79 | 75.36 | Gamma | National bureau of statistics (15) | |
| Labor force participation | Aged 18–60 | 60.90% | / | / | The seventh national population census (37) | |
| Nature rate | Infection rate | 0.10430 | / | / | Calculated | |
| Mortality rate | 0.00164 | / | / | Calculated | ||
| Vaccine effectiveness of adult (%) | Against infection | 65.18% | 62.62 | 67.75 | Beta | Meta-analysis |
| Against hospitalization | 79.10 | 71.69 | 86.51 | Beta | Meta-analysis | |
| Against ICU admission | 90.46% | 89.42 | 91.50 | Beta | Meta-analysis | |
| Against death | 86.69% | 85.68 | 87.70 | Beta | Meta-analysis | |
| Vaccine effectiveness of elderly (%) | Against infection | 66.52 | 28.30 | 100.00 | Beta | Meta-analysis |
| Against hospitalization | 50.58 | 42.70 | 58.45 | Beta | Meta-analysis | |
| Against death | 82.42 | 81.53 | 83.31 | Beta | Meta-analysis | |
| Vaccine efficacy (%) | Against infection | 70.56 | 57.87 | 83.24 | Beta | Meta-analysis |
| Against hospitalization | 100.00 | 61.72 | 100.00 | Beta | Meta-analysis | |
| No. of population | Vaccinated with two doses (million) | 1,256.86 | / | / | State council (7) | |
| Threshold (USD) | GDP per capital | 12556.37 | / | / | National bureau of statistics (15) |
Upper and lower bound are obtained from published literature and meta-analysis.
Figure 3.
Forest plots for the vaccine effectiveness (VE) of inactivated vaccine. (A) Forest plots against COVID-19 infection, (B) Forest plots against COVID-19 hospitalization, (C) Forest plots against COVID-19 ICU admission, (D) Forest plots against COVID-19 related death. *Internal data shared by company.
Cost-Effectiveness Analysis
Base Case Analysis
The cost-effectiveness analysis showed that although the RCT and RWE data cannot be pooled together, two-dose inactivated vaccination were consistently cost-saving in preventing COVID-19 infection, hospitalization, ICU admission, and avoiding COVID-19-related death. The ICER were calculated using RWE data of adults and were US$-3,739 per preinfected case avoided, US$-12,364 per severe case avoided, US$-21,853 per ICU case avoided, and US$-16,197 pre death avoided (Table 2). The same trend was seen while using RCT data. To summarize, vaccination strategy is consistently cost-saving and cost-effective in the Chinese setting from a societal perspective, and it is necessary to continue adhering to vaccination strategy to protect population health.
Table 2.
Cost-effectiveness of inactivated vaccine against COVID-19 infection, hospitalization, ICU admission, and death.
| Vaccine effectiveness | Group | Cost (US$) | Effectiveness (No. of events) |
Incremental cost (US$) |
Incremental effectiveness (No. of events) |
ICER (US$/event) |
|---|---|---|---|---|---|---|
| Against infection (RCT) | Two doses | 168,553,644,312 | 39,166,629 | −352,229,625,481 | 93,872,193 | −3,752 |
| Non-vaccination | 520,783,269,793 | 133,038,822 | / | / | / | |
| Against infection (RWE) | Two doses | 196,571,784,227 | 46,324,118 | −324,211,485,566 | 86,714,704 | −3,739 |
| Non-vaccination | 520,783,269,793 | 133,038,822 | / | / | / | |
| Against hospitalization (RCT) | Two doses | 15,235,049,685 | 0 | −308,329,723,032 | 24,612,182 | −12,528 |
| Non-vaccination | 323,564,772,717 | 24,612,182 | / | / | / | |
| Against hospitalization (RWE) | Two doses | 82,860,087,183 | 5,143,946 | −240,704,685,534 | 19,468,236 | −12,364 |
| Non-vaccination | 323,564,772,717 | 24,612,182 | / | / | / | |
| Against ICU admission (RWE) | Two doses | 29,877,607,019 | 596,519 | −123,608,318,918 | 5,656,305 | −21,853 |
| Non-vaccination | 153,485,925,937 | 6,252,825 | / | / | / | |
| Against death (RWE) | Two doses | 22,083,877,846 | 278,430 | −29,372,381,818 | 1,813,456 | −16,197 |
| Non-vaccination | 51,456,259,664 | 2,091,886 | / | / | / |
Sensitivity Analysis
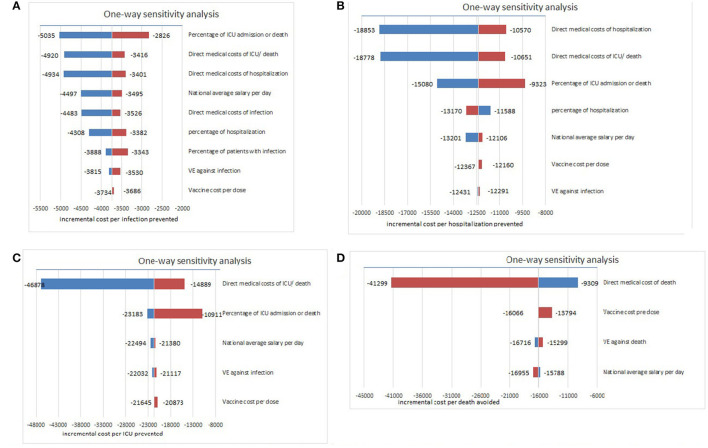
The model results were found to be robust in the one-way sensitivity analysis, indicating that two-dose inactivated vaccine strategy was always cost-saving. First, for infection prevention, percentage of ICU admission or death, direct medical costs of ICU/death, and direct medical costs of hospitalization for critical cases were the most influential parameters (Figure 4A). Second, direct medical costs of hospitalization, ICU/death, and percentage of ICU admission or death influenced the model most when considering hospitalization prevention (Figure 4B). Third, for ICU prevention, direct medical costs of ICU/death, and percentage of ICU admission, and national average salary per day were the most influential factors (Figure 4C). Finally, for death prevention, direct medical costs of death affected the model most (Figure 4D), indicating that with the decrease in medical costs and the declining severity of disease, vaccination strategies could be more cost-saving from a societal perspective, and more effective treatment methods should be adopted.
Figure 4.
One-way sensitivity analyses for the model on ICER (US$/event). (A) Sensitivity analysis for model against COVID-19 infection, (B) Sensitivity analysis for model against COVID-19 hospitalization, (C) Sensitivity analysis for model against COVID-19 ICU admission, (D) Sensitivity analysis for model against COVID-19 related death.
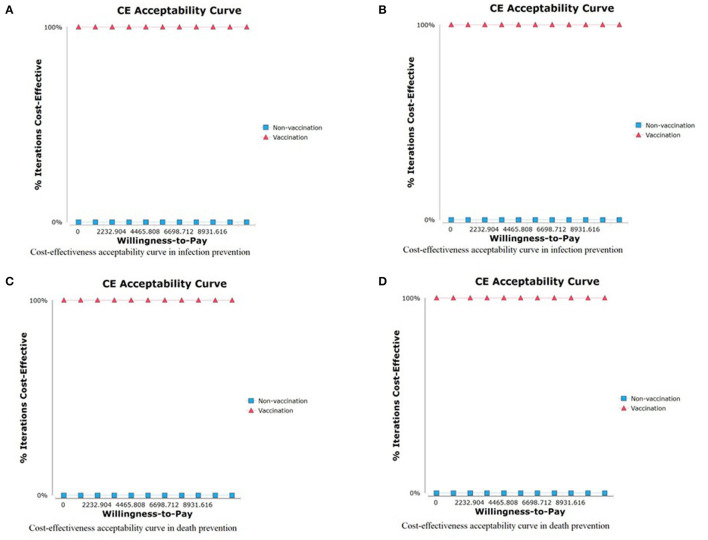
In the PSA, the probability of the vaccination strategy being cost-effective was 100% in preventing infection, hospitalization, ICU admission, and avoiding death, regardless of the willingness to pay (Figure 5).
Figure 5.
Cost-effectiveness acceptability curve. (A) CEA curve of COVID-19 infection prevention, (B) CEA curve of COVID-19 hospitalization prevention, (C) CEA curve of COVID-19 ICU admission prevention, and (D) CEA curve of COVID-19 related death COVID-19.
Discussion
This study summarized the inactivated COVID-19 vaccine efficacy and effectiveness evidence from 24 RCT and real-world studies and is, to our knowledge, more extensive and contained other meta-analyses or systematic reviews on inactivated vaccine (38–42). As the pandemic control strategy, study population, and sample size vary among countries, we synthesized both RCT and RWE data to generate more representative evidence. Our findings corroborate prior analyses of the literature, and the pooled VE indicated that inactivated vaccine is highly effective in preventing COVID-19-related infections, hospitalization, ICU admission, and death. The cost-effectiveness analysis showed that the vaccination strategy was cost-saving compared with non-vaccination in China.
Our results were consistent with previous cost-effectiveness studies and can improve their findings as these studies were done at the early stage of the COVID-19 pandemic with insufficient data on the efficacy and vaccine cost. Specifically, evidence from high-income countries showed that in general vaccination was cost-saving (43–45) and cost-effective (46, 47), and the whole society can benefit from vaccination even when the coverage rate was at 60% (44). As for low- and middle-income countries, vaccination was cost-saving from a societal perspective (10, 48). A Ukraine study illustrated that the priority strategy for older adults was more cost-effective when vaccine supply was insufficient (49). However, the majority of these studies used a vaccine with hypothetical efficacy, coverage rate, and cost data, and none of the studies mentioned the type of vaccine. There is a limited number of studies focusing on a specific type of vaccine, such as a Taiwan study evaluating mRNA vaccines (BNT162b2 and mRNA-1273) and adenovirus vaccines (AZD1222) (50), and a cross-country/region study examining inactivated vaccines (10), and the results are consistent with previous findings. In conclusion, there is limited evidence on the economic evaluation of inactivated vaccines, especially in low- and middle-income settings.
China has offered free vaccination to cover the whole population, and the first step was to cover people aged 18–59 years, which gradually extended to those aged over 60 years in April 2021, to adolescents aged 12–17 years in July 2021, and to children aged 3–11 years in November 2021. Previous study focusing on the cost-effectiveness of inactivated vaccine suggested that mass vaccination program should be encouraged (10), and our findings indicated that the high vaccination coverage rate can also benefit Chinese society. Moreover, due to the fact that both of the inactivated vaccines have been validated for emergency use by the WHO at a relatively low price, the results of this study can be used for countries or regions where vaccination coverage is high.
By strictly adhering to the containment strategy to safeguard its population, as detailed in the pamphlet Fighting COVID-19 China in Action (51), the pandemic has been effectively controlled in China mainland. The cost-effectiveness results in our study can provide compelling evidence to support the current vaccination strategy as well as evidence for subsequent decision-making, particularly in health sectors.
When the long-term effects of virus strains in human, the shock to the medical system (52) or health insurance system (53), and the uncertainty impacts on social stability are considered, vaccination is likely to be more cost-effective than the non-vaccination strategies, and the ICER could be much lower than our estimates. In addition, as the cost of treatment affected the model most in the one-way sensitivity analysis, encouraging asymptomatic patients and mild patients to stay at home or be treated at cabin hospitals instead of crowding into hospitals could save costs from a societal perspective and save medical resources. Therefore, it is necessary to continue adhering to the vaccination strategy to protect population health and maintain social stability and economic development.
Except for the free COVID-19 vaccination program, such as mask wearing, social distancing, quarantine, contact tracing, business closure, and lockdown, are adopted by the Chinese government. Additionally, the government also takes measures to release the financial burden of its population by capping nucleic acid testing costs at US$6.20 per individual test and US$1.55 per mixed test (54), increasing labor productivity by encouraging telecommuting, and encouraging small and medium-sized enterprises by reducing the taxation and increasing subsidy (55).
It is also worth noting that further studies on the exploration of the most cost-effective combination of vaccines and other NPIs strategies in real-world scenarios, the reduction of unnecessary lockdown and containment policies, and maximizing the smooth operation of society and economic development are needed.
This study has some limitations. First, statistics on medical costs were gathered during the early stage of the COVID-19 outbreak in Wuhan. Medical cost data may alter as the pandemic progresses, diagnosis and treatment guidelines improve, and the infectivity and pathogenicity of the virus change. However, this study contains the most comprehensive and robust data on medical costs. Second, the VE are from overseas studies and may not be representative for the Chinese population; however, due to the limited number of trials conducted and the small number of COVID-19 cases in China, the results of this study remain the most reliable estimate. Third, the efficacy and effectiveness data were collected throughout the pandemic, so the protection rate cannot be simply extrapolated to Delta or Omicron strain virus; however, under the Delta and Omicron virus pandemic, attention should be paid to the effectiveness of booster vaccination after the initial immunization procedure. Finally, neither the influence of NPIs nor the effects of pandemic control, economic sustainability, and social stability were considered in this study. In light of the long-term effects, this study may underestimate the importance of immunization.
Conclusion
The inactivated vaccine is effective in preventing COVID-19 infection, hospitalization, ICU admission, and avoiding COVID-19-related death, and free COVID-19 vaccination program is consistently cost-saving from both health system and societal perspective in China. Therefore, the entire Chinese population should receive two doses of inactivated vaccine. Moreover, further studies on booster vaccination are necessary to determine the most cost-effective and long-lasting approach of COVID-19 prevention and control.
Data Availability Statement
The modeling presented in this study is parameterized by data from public sources.
Author Contributions
LY conceived this analysis article. YF and JZ collected the evidence and drafted the manuscript. LY, XW, and PH reviewed and edited the manuscript. LY, TR, SZ, and LL coordinated the overall process. All authors approved the final manuscript.
Funding
This study was supported by a grant from the National Natural Science Foundation of China [72174010], the Beijing Natural Science Foundation [M22033], and the Capital Health Research and Development of Special Fund [2021-1G-4091].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to China National Biotec Group for sharing effectiveness rate data in real-world settings.
Glossary
Abbreviations
- COVID-19
Coronavirus disease 2019
- CIs
Confidence intervals
- DEALE
Declining exponential approximation of life expectancy
- GMC
Geometric mean concentration
- ICERs
Incremental cost-effectiveness ratios
- NPIs
Non-pharmaceutical interventions
- RCT
Randomized control trial
- RWE
Real-world evidence
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- VE
Vaccine effectiveness.
References
- 1.Miller IF, Becker AD, Grenfell BT, Metcalf CJE. Disease and healthcare burden of COVID-19 in the United States. Nat Med. (2020) 26:1212–17. 10.1038/s41591-020-0952-y [DOI] [PubMed] [Google Scholar]
- 2.Keogh-Brown MR, Jensen HT, Edmunds WJ, Smith RD. The impact of Covid-19, associated behaviours and policies on the UK economy: a computable general equilibrium model. SSM Population Health. (2020) 12:100651. 10.1016/j.ssmph.2020.100651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John D, Narassima MS, Menon J, Rajesh JG, Banarjee A. Estimation of the economic burden of COVID-19 using disability-adjusted life years (DALYs) and productivity losses in Kerala, India: a model-based analysis. BMJ Open. (2021) 11:e049619. 10.1136/bmjopen-2021-049619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin H, Wang H, Li X, Zheng W, Ye S, Zhang S, et al. Economic burden of COVID-19, China, January-March, 2020: a cost-of-illness study. Bull World Health Organ. (2021) 99:112–24. 10.2471/BLT.20.267112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . Coronavirus (COVID-19) Dashboard. Available online at: https://covid19.who.int/ (accessed April 5, 2022).
- 6.Real-Time Big Data Reporting on the COVID-19 Pandemic In China. Available online at: https://voice.baidu.com/act/newpneumonia/newpneumonia/?from=osari_aladin_banner (accessed April 5, 2022).
- 7.Council S. Vaccination Status. Available online at: http://www.gov.cn/xinwen/gwylflkjz190/index.htm (accessed April 5, 2022).
- 8.Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. (2021) 326:35–45. 10.1001/jama.2021.8565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. (2021) 385:875–84. 10.1056/NEJMoa2107715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y, Cai D, Shi S. Economic evaluations of inactivated COVID-19 vaccines in six Western Pacific and South East Asian countries and regions: a modeling study. Infect Dis Model. (2022) 7:109–21. 10.1016/j.idm.2021.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Jin H, Li X, Jia J, Zhang C, Zhao H, et al. Disease burden attributable to the first wave of COVID-19 in China and the effect of timing on the cost-effectiveness of movement restriction policies. Value Health. (2021) 24:615–24. 10.1016/j.jval.2020.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organization WH . Vaccine Purchase Data. Available online at: https://www.who.int/teams/immunization-vaccines-and-biologicals/vaccine-access/mi4a/mi4a-vaccine-purchase-data (accessed February 20, 2022).
- 13.Chen C, Cervero Liceras F, Flasche S, Sidharta S, Yoong J, Sundaram N, et al. Effect and cost-effectiveness of pneumococcal conjugate vaccination: a global modelling analysis. Lancet Glob Health. (2019) 7:e58–67. 10.1016/S2214-109X(18)30422-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bureau NMI. Notice on the Effective Implementation of Phased Liquidation of Coronavirus Vaccines and Vaccination Costs. Available online at: http://www.nhsa.gov.cn/art/2021/8/23/art_53_5856.html (accessed February 20, 2022).
- 15.Statistics NBo. China Statistical Data. Available online at: https://data.stats.gov.cn/easyquery.htm?cn (accessed April 10, 2022).
- 16.Tanriover MD, Doganay HL, Akova M, Guner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. (2021) 398:213–22. 10.1016/S0140-6736(21)01429-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baraniuk C. What do we know about China's COVID-19 vaccines? BMJ. (2021) 373:n912. 10.1136/bmj.n912 [DOI] [PubMed] [Google Scholar]
- 18.Characterisation WHOWGotC Management Management of C-i . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. (2020) 20:e192–7. 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hitchings MDT, Ranzani OT, Torres MSS, de Oliveira SB, Almiron M, Said R, et al. Effectiveness of coronavac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: a test-negative case-control study. Lancet Reg Health Am. (2021) 1:100025. 10.1016/j.lana.2021.100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X-N, Huang Y, Wang W, Jing Q-L, Zhang C-H, Qin P-Z, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg Microbes Infect. (2021) 10:1751–59. 10.1080/22221751.2021.1969291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A, et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: a test-negative case-control study. Emerg Microbes Infect. (2022) 11:585–92. 10.1080/22221751.2022.2037398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rearte A, Castelli JM, Rearte R, Fuentes N, Pennini V, Pesce M. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and death due to COVID-19 in people older than 60 years in Argentina: a test-negative, case-control, and retrospective longitudinal study. Lancet. (2022) 399:1254–64. 10.1016/S0140-6736(22)00011-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrović V, Vuković V, Marković M, Ristic M. Early effectiveness of four SARS-CoV-2 vaccines in preventing COVID-19 among adults aged ≥60 years in Vojvodina, Serbia. Vaccines. (2022) 10:389. 10.3390/vaccines10030389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paixao ES, Wong KLM, Alves FJO, Oliveira VdA, Cerqueira-Silva T, Junior JB, et al. CoronaVac vaccine is effective in preventing symptomatic and severe COVID-19 in pregnant women in Brazil: a test-negative case-control study. BMC Med. (2022) 20:146. 10.1186/s12916-022-02353-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mousa M, Albreiki M, Alshehhi F, AlShamsi S, Marzouqi NA, Alawadi T, et al. Similar effectiveness of the inactivated vaccine BBIBP-CorV (Sinopharm) and the mRNA vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19 related hospitalizations during the delta outbreak in the United Arab Emirates. J Travel Med. (2022) 29:taac036. 10.1093/jtm/taac036. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirahmadizadeh A, Heiran A, Bagheri Lankarani K, Serati M, Habibi M, Eilami O, et al. Effectiveness of coronavirus disease 2019 vaccines in preventing infection, hospital admission, and death: a historical cohort study using Iranian registration data during vaccination program. Open Forum Infect Dis. (2022) 9:ofac177. 10.1093/ofid/ofac177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marra AR, Miraglia JL, Malheiros DT, Guozhang Y, Teich VD, Victor EdS, et al. Effectiveness of two COVID-19 vaccines (viral vector and inactivated viral vaccine) against SARS-CoV-2 infection in a cohort of healthcare workers. Infect Control Hosp Epidemiol. (2022) 43:1–20. 10.1017/ice.2022.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma C, Sun W, Tang T, Jia M, Liu Y, Wan Y, et al. Effectiveness of adenovirus type 5 vectored and inactivated COVID-19 vaccines against symptomatic COVID-19, COVID-19 pneumonia, and severe COVID-19 caused by the B.1.617.2 (delta) variant: evidence from an outbreak in Yunnan, China, 2021. Vaccine. (2022) 40:2869–74. 10.1016/j.vaccine.2022.03.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang M, Yi Y, Li Y, Sun L, Deng A, Hu T, et al. Effectiveness of inactivated COVID-19 vaccines against illness caused by the B.1.617.2 (delta) variant during an outbreak in Guangdong, China: a cohort study. Ann Intern Med. (2022) 175:533–40. 10.7326/M21-3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jara A, Undurraga EA, Zubizarreta JR, Gonzalez C, Acevedo J, Pizarro A, et al. Effectiveness of coronavac in children 3 to 5 years during the SARS-CoV-2 omicron outbreak in Chile. Nat Med. (2022) 28:1–4. 10.1038/s41591-022-01874-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ismail AlHosani F, Eduardo Stanciole A, Aden B, Timoshkin A, Najim O, Zaher WA, et al. Impact of the sinopharm's BBIBP-CorV vaccine in preventing hospital admissions and death in infected vaccinees: results from a retrospective study in the emirate of Abu Dhabi, United Arab Emirates (UAE). Vaccine. (2022) 40:2003–10. 10.1016/j.vaccine.2022.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Can G, Acar HC, Aydin SN, Balkan II, Karaali R, Budak B, et al. Waning effectiveness of coronavac in real life: a retrospective cohort study in health care workers. Vaccine. (2022) 40:2574–79. 10.1016/j.vaccine.2022.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arregocés-Castillo L, Fernández-Niño J, Rojas-Botero M, Palacios-Clavijo A, Galvis-Pedraza M, Rincon-Medrano L. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. (2022) 3:e242–52. 10.1016/S2666-7568(22)00035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, et al. Immunogenicity and safety of a third dose of coronavac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. (2021) 22:483–95. 10.1016/S1473-3099(21)00681-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranzani OT, Hitchings MDT, Dorion M, D'Agostini TL, Cardoso de Paula R, Pereira de Paula OF, et al. Effectiveness of the coronavac vaccine in older adults during a gamma variant associated epidemic of COVID-19 in Brazil: test negative case-control study. BMJ. (2021) 374:n2015. 10.1136/bmj.n2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO . Evidence Assessment: Sinovac/CoronaVac COVID-19 Vaccine. Available online at: https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf?fbclid=IwAR3dm2dCYAba–supV91KiIZ-6xPa1Mam55fep67v_GQZm2f8TNVxdfdMcY2021 (accessed February 20, 2022).
- 37.Bulletin of the seventh National Census. Available online at: http://www.stats.gov.cn/tjsj/tjgb/rkpcgb/qgrkpcgb/202106/t20210628_1818827.html (accessed February 21, 2022).
- 38.Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. (2022) 114:252–60. 10.1016/j.ijid.2021.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling Y, Zhong J, Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. J Med Virol. (2021) 93:6486–95. 10.1002/jmv.27203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv M, Luo X, Shen Q, Lei R, Liu X, Liu E, et al. Safety, immunogenicity, and efficacy of COVID-19 vaccines in children and adolescents: a systematic review. Vaccines. (2021) 9:1102. 10.1101/2021.09.11.21262855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khandker SS, Godman B, Jawad MI, Megha BA, Tisha TA, Khondoker MU, et al. A systematic review on COVID-19 vaccine strategies, their effectiveness, and issues. Vaccines. (2021) 9:1387. 10.3390/vaccines9121387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q, Qin C, Liu M, Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. (2021) 10:132. 10.1186/s40249-021-00915-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaker M, Abrams EM, Greenhawt M. A cost-effectiveness evaluation of hospitalizations, fatalities, and economic outcomes associated with universal vs. anaphylaxis risk-stratified COVID-19 vaccination strategies. J Allergy Clin Immunol Pract. (2021) 9:2658–68.e3. 10.1016/j.jaip.2021.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padula WV, Malaviya S, Reid NM, Cohen BG, Chingcuanco F, Ballreich J, et al. Economic value of vaccines to address the COVID-19 pandemic: a US cost-effectiveness and budget impact analysis. J Med Econ. (2021) 24:1060–69. 10.1080/13696998.2021.1965732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohli M, Maschio M, Becker D, Weinstein MC. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine. (2021) 39:1157–64. 10.1016/j.vaccine.2020.12.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marco-Franco JE, Pita-Barros P, González-de-Julián S, Sabat I, Vivas-Consuelo D. Simplified mathematical modeling of uncertainty: cost-effectiveness of COVID-19 vaccines in Spain. Mathematics. (2021) 9:566. 10.3390/math9050566 [DOI] [Google Scholar]
- 47.Debrabant K, Gronbaek L, Kronborg C. The cost-effectiveness of a COVID-19 vaccine in a Danish context. Clin Drug Investig. (2021) 41:975–88. 10.1007/s40261-021-01085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagens A, Inkaya AC, Yildirak K, Sancar M, van der Schans J, Sancar AA, et al. COVID-19 vaccination scenarios: a cost-effectiveness analysis for Turkey. Vaccines. (2021) 9:19. 10.3390/vaccines9040399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volodymyrovych T, Ivanovich S, Tetiana K, Yaroslavovych TB. Pharmaco economics analysis of COVID-19 vaccines in Ukraine. J Pharm Res Int. (2021) 33:140–7. 10.9734/jpri/2021/v33i32A3172735024509 [DOI] [Google Scholar]
- 50.Wang W-C, Fann JC-Y, Chang R-E, Jeng Y-C, Hsu C-Y, Chen H-H, et al. Economic evaluation for mass vaccination against COVID-19. J Formos Med Assoc. (2021) 120:S95–105. 10.1016/j.jfma.2021.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Council S. Fighting COVID-19 China in Action. Available online at: http://www.scio.gov.cn/ztk/dtzt/42313/43142/index.htm (accessed February 20, 2022).
- 52.Chang AY, Cullen MR, Harrington RA, Barry M. The impact of novel coronavirus COVID-19 on noncommunicable disease patients and health systems: a review. J Intern Med. (2021) 289:450–62. 10.1111/joim.13184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zuo F, Zhai S. The influence of China's COVID-19 treatment policy on the sustainability of its social health insurance system. Risk Manag Healthc Policy. (2021) 14:4243–52. 10.2147/RMHP.S322040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Working Conference on Health Care. Available online at: http://www.nhsa.gov.cn/art/2022/1/14/art_14_7780.html (accessed February 25, 2022).
- 55.Notice on Tax Policy in Support of Prevention and Control of Pneumonia in Coronavirus Infections. Available online at: http://www.chinatax.gov.cn/chinatax/n810341/n810755/c5143465/content.html (accessed March 9, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The modeling presented in this study is parameterized by data from public sources.