Abstract
Introduction
Neonates appear to be less affected by COVID-19 than adults, yet COVID-19 has been a challenge for all medical specialties, including neonatal intensive care unit (NICU) specialists. Unfortunately, current knowledge about the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is limited. This case report explains how COVID-19 neonatal sepsis was treated with immunomodulatory agents.
Case presentation
In this case, we present a premature male newborn who was ill. He was born to a mother with a negative nasopharyngeal swab test for SARS-CoV-2. On the fifth day of life, the baby developed respiratory distress, and a nasopharyngeal swab test for SARS-CoV-2 tested positive. The baby was Intubated, and intratracheal surfactant was administered. The infant was treated with intravenous immunoglobulin (IVIg) and corticosteroids for 14 days.
Patient's demographics
Age: under 1 month, Sex: Male, Ethnicity: Iranian.
Conclusion
The basics of treatment for neonatal COVID-19 is supportive care. Some studies have treated infants with various drugs such as Hydroxychloroquine, Favipiravir, and Remedsivir; however, in our case, a 5-day-old baby boy was treated with corticosteroids and IVIg. We achieved good outcomes after 2 weeks of treatment with dexamethasone 0.3 mg/kg per day and IVIg 2 g/kg/day (for 3 days). It appears that these treatments, along with adjuvant ventilation and the administration of endotracheal surfactant, can improve a patient's general condition.
Keywords: Neonate, Sepsis, Pneumonia, COVID-19, SARS-CoV-2, Case report
1. Introduction
Available scientific reports, up to now, suggest that neonates appear to be less affected by Coronavirus Disease (COVID-19) than adults; yet, COVID-19 has been a challenge for all medical specialties, including the neonatal intensive care unit (NICU) specialists (Wu and McGoogan, 2020). Unfortunately, current knowledge about severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is limited. Based on studies and case studies, most newborns with this infection were asymptomatic or demonstrated mild illnesses, but a small percentage of patients require admission to the neonatal intensive care unit (NICU). Due to the lack of global treatment guidelines at the beginning of the pandemic, many infected infants were admitted to the NICU. As the experience of health workers gradually increased, several local guidelines were introduced.
In some cases, supportive care and antibiotics did not work effectively; therefore, additional therapies were implemented to improve the outcomes of the babies. The next section introduces an infant boy in Iran with COVID-19. The baby's parents signed a written informed consent form regarding this case report.
2. Case presentation
A preterm 34 weeks + 3 days infant boy was born via a cesarean section on October 13, 2020, to Iranian parents in Tehran, Iran. He was born with a birth weight of 1610 g with Apgar scores of 9 and 10 in the 1st and 5th minute after birth, respectively. His 45-year-old mother had three previous abortions: the first was aborted spontaneously at 8 weeks of gestation, the second was aborted medically at 18 weeks of gestation due to Down's syndrome, and the third was aborted at 16 weeks of gestation due to PPROM. This pregnancy (gravid 4) resulted from IVF with a donated egg. The mother had no signs or symptoms of the COVID-19 infection fourteen (Moolasart et al., 2020) days prior to delivery nor 14 days post-delivery and tested negative via a nasopharyngeal swab for SARS-CoV-2 (by RT-PCR assay).
At first, the baby did well but gradually became ill during the first day of life. He developed mild respiratory distress and needed supplemental oxygen via hood at times. On chest auscultation, rales were noted; therefore, the patient was transferred to the NICU of Bahrami hospital. Laboratory tests and imaging (Fig. 1 A) were performed, and antibiotic therapy was started (Ampicillin + Cefotaxime). The baby's respiratory distress gradually improved on the second day of life, and he did not need supplemental oxygen. Low-volume feeding was then started.
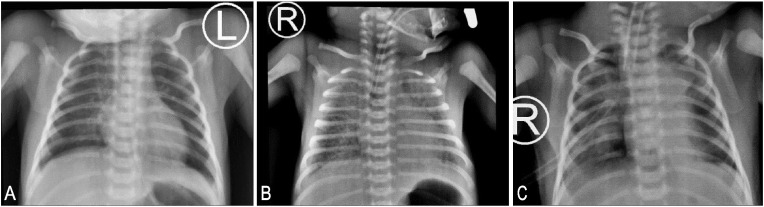
Fig. 1.
A: A normal chest x-ray without opacities after NICU admission:
B: Chest x-ray after the onset of respiratory distress with intubation showing diffuse opacities in both lungs.
C: Chest x-ray after 10 days showing better aeration with some diminished opacities.
On the fifth day of his life, while increasing daily feeding volumes, the baby developed respiratory distress, crackles via chest auscultation, mottling, and a decreased pulse pressure. He was subsequently intubated due to worsening respiratory distress and received supportive care. Suddenly, the infant began having status seizures (tonic -clonic movement + upward gaze), which could not be controlled with one loading dose of Levetiracetam and two loading doses of phenobarbital. One Phenytoin loading dose and infusion finally suppressed the seizure activity. Lab tests were again obtained, and more potent antibiotics (Vancomycin + Meropenem) were administered. The infant boy had hypokalemia, hypocalcemia, respiratory acidosis, lymphopenia, elevated LDH, thrombocytopenia, and elevated INR levels (Table 1 ). FFP and platelets were transfused, and the infant was treated with potassium and calcium supplements. A chest X-ray showed diffuse opacities in both lungs (Fig. 1B), and surfactant (4 mL Crossruff ®) was administered twice via endotracheal tube. The general status of the baby improved thanks to this early intervention and ventilatory support.
Table 1.
Laboratory test on the first and fifth day of life.
| Lab tests | 1st day | 5th day |
|---|---|---|
| WBC count/mm3 | 9.9 × 103 | 5.8 × 103 |
| Lymphocyte count/mm3 | 2.17 × 103 | 1.04 × 103 |
| Hemoglobin gr/dL | 16 | 13.2 |
| platelet count/mm3 | 157 × 103 | 40 × 103 |
| CRP, mg/dl | 4 | 3 |
| Potassium, mEq/L | 3.6 | 2.6 |
| Calcium, mg/dl | 8.1 | 6.3 |
| LDH, IU/L | 888 | 727 |
| AST(U/L) | – | 49 |
| ALT (U/L) | – | 16 |
| PT, sec | – | 16.9 |
| INR | – | 1.5 |
| PTT, sec | – | 45 |
| Arterial Blood gas | ||
| PH | 7.27 | 7.16 |
| PCO2, mmHg | 39 | 56 |
| HCO3, mEq/L | 17.7 | 19.7 |
| BE, mEq/L | −3 | −7 |
Since the baby boy tested positive for SARS-CoV-2, the patient was isolated in a separate room with strict protective protocols. The following day, the infant suddenly developed severe respiratory distress with a pneumothorax requiring a chest tube insertion. Chest ultrasonography was performed, showing a mild pleural effusion.
The patient was treated with daily oral Sildenafil due to increased pulmonary artery hypertension (diagnosed via echocardiography). A lumbar puncture showed normal values with a negative CSF culture; therefore, bacterial meningitis was ruled out. Both blood and urine culture tests were negative. During the next few weeks, after obtaining a normal brain ultrasound, the consulting neurologist discontinued phenytoin and phenobarbital, and maintenance treatment with Levetiracetam was continued.
Infectious disease specialists suggested treatment with corticosteroid and IVIG for Covid-19 disease, so the baby was treated with Dexamethasone 0.3mg/kg/day intravenously (twice a day) for the next 14 days and IVIg 1 g/kg/day for 3 days (for a total of 6 g). After 4 days of Sildenafil treatment (at 10 days old), echocardiography showed normal pulmonary artery pressure. A repeat Chest X-ray showed improved aeration, and some opacities were diminished (Fig. 1C).
During the following days, ventilator settings were reduced, and the baby was weaned from the ventilator. Next, NIPPV therapy was started, and the chest tube was removed after 3 days (at 19 days old). A nasopharyngeal swab test for SARS-COV-2 was repeated, which was negative. A serologic lab test result was negative (IgM and IgG against SARS-COV-2). Gradually, Dexamethasone was tapered over 5 days, and the patient began to feed with breast milk and was finally discharged from the hospital at the age of 30 days. At the age of 28 days, an eye exam showed that the baby boy had ROP stage I in Zone III; however, the ROP examination was normal at the follow-up visit, as was the ABR test. At 6 months of age, his neurologic state was normal, as well as EEG and brain ultrasound, and the antiepileptic therapy with Levetiracetam was tapered.
3. Conclusions and discussion
There is still no consensus on the vertical transmission of COVID-19. Some studies have found that mother-to-child transmission of COVID-19 is impossible, whereas others claim that vertical transmission is possible (Zhu et al., 2020). In our case, the mother had no signs and symptoms of infection, and her nasopharyngeal swab test was negative. Although, some reports suggest the possible vertical transmission of COVID-19 from mothers with a history of COVID-19 in the 6 weeks before delivery (Hascoët et al., 2020). Also, considering the COVID-19 epidemic condition, disease transmission from the asymptomatic carrier mother to the fetus may not be excluded.
Several studies reported diagnosis and management of COVID-19 sepsis in neonates. Zeng et al. reported that COVID-19 presented clinically in 9% of neonates as early-onset sepsis in China (Zeng et al., 2020). Zhu et al. suggested that SARS-COV-2 in neonates can cause respiratory distress, thrombocytopenia, liver function test abnormalities, or even neonatal death (Zhu et al., 2020). In our study, newborns had respiratory distress, lymphopenia, elevated LDH, hypocalcemia, hypokalemia, thrombocytopenia, and elevated INR on the fifth day of life. CRP level did not rise. Saeedi et al. showed that elevated inflammatory markers are less common in infants, unlike adults. They also reported that CRP does not increase in neonates, and leukopenia and lymphopenia are less common (Saeedi et al., 2021). Serology tests (IgM and IgG against SARS-COV-2) were negative. It has been reported that a significant proportion of neonates with positive RT-PCR results had negative antibody tests, possibly due to host factors that affect the immune response to SARS-CoV-2 (Guo et al., 2020).
In our case, radiographic findings were nonspecific. Moreover, we could not get a chest CT scan (computed tomography) due to the patient's instability. Some studies showed that radiographic findings in neonates could be normal and may show lung consolidation, mild pulmonary infection, ground glass opacity, and patchy shadow under pleura. The chest CT scan may show subpleural lesions with localized inflammatory infiltration (Zeng et al., 2020; Saeedi et al., 2021).
A few reports of neonatal infection of SARS-COV-2 were published during the COVID-19 pandemic. Premature neonates may be at risk of more severe signs and symptoms. Up to now, no valid guideline has been published for the treatment of COVID-19 in neonates. Therefore, the management of COVID-19 in neonates usually varies among hospitals (De Luca, 2020). In each country, medical groups have developed guidelines for neonatal COVID-19, including Italy (Management of the newborn with), the United Kingdom (https://www.rcog.org.uk/globalassets/documents/guidelines/2021-02-19-coronavirus-covid-19-infection-in-pregnancy-v13.pdf, 2021), the United States (https://irimc.org/Portals/0/Images/News/%20%20%20%20%20%20%20-%20%20%20%20%2099.pdf, 2099), and the Islamic Republic of Iran (Sagheb et al., 2020).
These are urgent steps against the pandemic, but health systems have difficulty determining the best guidelines due to constant updates and controversial data. In addition, our knowledge about infection with SARS-CoV-2 is undoubtedly incomplete in many ways.
This paper reports our experience using corticosteroids and IVIg in COVID-19 pneumonia in a neonate. No specific medication is approved for COVID-19; thereby, its management is principally supportive (including oxygen supplementation, electrolyte maintenance, acid-base balance, and nutritional support). Most studies for newborns with severe acute respiratory syndrome used surfactant, nitric oxide, and mechanical ventilation. According to some studies, high-frequency oscillating ventilation is recommended in neonates with COVID-19 (Saeedi et al., 2021). In our study, we first used an intratracheal surfactant and Assisted Control (A/C) mode ventilation, then Synchronized intermittent mandatory ventilation (SIMV) mode ventilation until extubation.
Some studies reported different experiences. Sagheb reported a good outcome of using Hydroxychloroquine in treating COVID-19 pneumonia in two cases (Kamali Aghdam et al., 2020). Kamali administered Oseltamivir to a 15-day-old neonate, and the baby was discharged in good condition (Coronado Munoz et al., 2020). However, there is insufficient data to suggest the superiority of any of these medications (Moolasart et al., 2020). Coronado et al. used Hydroxychloroquine and azithromycin for a 3-week-old patient (Moolasart et al., 2020). Moolasart treated a 47-day-old male newborn with Favipiravir, Hydroxychloroquine, and Lopinavir/Ritonavir. He claimed that a Favipiravir-based regimen might be the drug of choice for COVID-19 pneumonia in newborns (Hopwood et al., et al.). Hopwood used Remdesivir, corticosteroid, and plasma exchange in a 4-day-old neonate, which showed a proper outcome (Hopwood et al., 2020). In our case, we used corticosteroids and IVIg to treat a 5-day-old neonate. IVIg has been used in some children with COVID-19 in special conditions (Yu and Chen, 2020). In our case, the proper outcome was achieved after 2 weeks of treatment with Dexamethasone 0.3 mg/kg/day and 2 gm/kg/day IVIg (for three days). This treatment, along with adjuvant ventilation and intratracheal surfactants, appeared to improve the patient's lungs and pleural involvement.
Authors’ contributions
KM is the chief manager of Bahrami hospital NICU, and he managed this newborn medically. SSM prepared the primary draft of the manuscript and the laboratory tests and prepared pictures and tables. Again KM reviewed and edited the text. Both authors read and approved the submitted manuscript and have critical roles in caring for and treating the patients.
Funding
There was no funding resource.
Availability of data and materials
Data Security: All data, including patients’ medical records, images, and laboratory data, are stored in our hospital for a minimum of 10 years based on the local regulations.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient's legal guardian to publish this case report and any accompanying images. A copy of the written informed consent is available for review by the Editor-in-Chief of this journal.
Declaration of competing interest
All the authors declare no competing interest in this manuscript.
Acknowledgments
We would like to announce our sincere thanks to all nursing staff who have a critical role in taking care of these patients.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ABR
auditory brainstem response
- BE
Base excess
- COVID-19
Coronavirus disease 2019
- CPK
Creatine phosphokinase
- CRP
C-reactive protein
- CSF
Cerebrospinal fluid
- EEG
Electroencephalogram
- HCO3
Bicarbonate
- IVF
In vitro fertilization
- IVIg
Intravenous immunoglobulin
- IgM
Immunoglobulin M
- IgG
Immunoglobulin G
- LDH
Lactate dehydrogenase
- NICU
Neonatal intensive care unit
- NIPPV
Non Invasive positive pressure ventilation
- PCO2
Partial pressure of carbon dioxide;
- PT
Prothrombin time;
- PTT
Partial thromboplastin time;
- PPROM
Preterm premature rupture of the membranes
- ROP
Retinopathy of Prematurity
- RTPCR
Reverse transcription polymerase chain reaction
- SARS-COV-2
Severe acute respiratory syndrome Coronavirus-2
- WBC
White blood cells
References
- Coronado Munoz A., Nawaratne U., McMann D., Ellsworth M., Meliones J., Boukas K. Late-onset neonatal sepsis in a patient with Covid-19. N. Engl. J. Med. 2020;382(19):e49. doi: 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca D. Managing neonates with respiratory failure due to SARS-CoV-2. Lancet Child & Adolesc. Health. 2020;4(4):e8. doi: 10.1016/S2352-4642(20)30073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Ren L., Yang S., Xiao M., Chang D., Yang F., et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin. Infect. Dis. 2020;71(15):778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascoët J.-M., Jellimann J.-M., Hartard C., Wittwer A., Jeulin H., Franck P., et al. Case series of COVID- 19 asymptomatic newborns with possible intrapartum transmission of SARS-CoV-2. Front. Pediatr. 2020;8 doi: 10.3389/fped.2020.568979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood AJ, Jordan-Villegas A, Gutierrez LD, Cowart MC, Vega-Montalvo W, Cheung WL, et al. [DOI] [PMC free article] [PubMed]
- https://irimc.org/Portals/0/Images/News/%20%20%20%20%20%20%20-%20%20%20%20%2099.pdf (MoHotIRoIGfdatoC-inacteAf.)
- https://www.rcog.org.uk/globalassets/documents/guidelines/2021-02-19-coronavirus-covid-19-infection-in-pregnancy-v13.pdf RCoOaGCC-iipIfhpVAf.10.2021 AAoPCuoC-Afhsaoep-n-c-c--iAM.
- Hopwood Andrew J, Jordan-Villegas Alejandro, Gutierrez Liliana D, Cowart Mallory C, Vega-Montalvo Wilfredo, Cheung Wang Leung, McMahan Michael J, Gomez Michael R, Laham Federico R. Severe acute respiratory syndrome coronavirus-2 pneumonia in a newborn treated with Remdesivir and coronavirus disease 2019 convalescent plasma. J. Pediatr. Infect. Dis. 2020;electronic publish doi: 10.1093/jpids/piaa165. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7798965/?report=classic XX-XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali Aghdam M., Jafari N., Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect. Dis. 2020;52(6):427–429. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Management of the Newborn with Suspected or Confirmed SARS-CoV-2 Infection. Italy: Magazine of the Italian Society of Neonatology SAfhwnn.
- Moolasart V., Wongsawat J., Phokhom P., Thienthong V. vol. 8. SAGE open medical case reports; 2020. (Favipiravir-based Regimen for Coronavirus Disease 2019 Pneumonia for a 47-Day-Old Male Newborn). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi M., Sangsari R., Mirnia K. COVID‐19 in neonates: a review. Iran. J. Pediatr. 2021;31(1) [Google Scholar]
- Sagheb S., Lamsehchi A., Jafary M., Atef-Yekta R., Sadeghi K. Two seriously ill neonates born to mothers with COVID-19 pneumonia-a case report. Ital. J. Pediatr. 2020;46(1):1–6. doi: 10.1186/s13052-020-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yu Y., Chen P. Coronavirus disease 2019 (COVID-19) in neonates and children from China: a review. Front. Pediatr. 2020;8:287. doi: 10.3389/fped.2020.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., et al. Neonatal early-onset infection with SARS-CoV- 2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020;9(1):51. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data Security: All data, including patients’ medical records, images, and laboratory data, are stored in our hospital for a minimum of 10 years based on the local regulations.