Abstract
Background
Aging is a phenomenon universally involving all organisms, genetically determined, and epigenetically influenced by the environment. Numerous observational studies have shown the positive impact of non-pharmacological approaches started in younger age on chronic conditions affecting the elderly health and survival. This meta-analysis aimed to investigate the effect of beta-carotene on the total and cause-specific mortality as reported by randomized controlled trials (RCTs).
Methods
We searched Medline, Scopus, Web of Science, and CENTRAL Cochrane from inception to September 2021. Studies were eligible if enrolled adults with any health condition, compared beta-carotene supplements at any dose with placebo or no intervention, provided information on deaths from any cause, and were RCTs, in English. The risk of bias was assessed by the Cochrane risk of bias tool and the GRADE. Risk ratios and their 95% confidence intervals were used and a P-value less than 0.05 was considered statistically significant.
Results
Among 3,942 articles searched, 44 articles on 31 RCTs, which included 216,734 total subjects, 108,622 in beta-carotene supplement groups, and 108,112 in the placebo or no-intervention groups, were involved in the final analyses. In a random-effects meta-analysis of all 31 trials, beta-carotene supplements were found to have no preventive effect on mortality (risk ratio 1.02, 95% confidence interval 0.98–1.05, I2 = 42%). Further, the analysis showed no preventive effect on cancer, cardiovascular, cerebrovascular, and other mortality causes. Instead, beta-carotene supplementation significantly increased the risk of lung cancer mortality (RR 1.14, 95% CI 1.02, 1.27, I2 = 3%) but decreased the risk of human immunodeficiency virus-related mortality (RR 0.55, 95% CI 0.33, 0.92, I2 = 0).
Conclusion
More studies should be performed to better define the role of beta-carotene on survival, to confirm or deny our results. Therefore, the possible beneficial or harmful effects of the beta-carotene supplementation on mortality must not be overstated.
Systematic Review Registration
[https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=259354], identifier [CRD42021259354].
Keywords: mortality, meta-analysis, randomized controlled trials, aging, beta-carotene
Introduction
Aging is a phenomenon universally involving all organisms, genetically determined, epigenetically influenced by the environment, and characterized by a progressive decline of physiological function, mainly the cardiovascular and metabolic profile, leading to death. Numerous observational studies have shown the positive impact of non-pharmacological approaches started in younger age on chronic conditions affecting the elderly health and survival (1–4).
Nutrition is a modifiable lifestyle factor that has been consistently associated with various aspects, including greater adherence to healthy dietary patterns, the intake of specific nutrients, or the consumption of specific foods (5).
Beta-carotene is a fat-soluble phytochemical found naturally in yellow/orange and green leafy plants, and also produced by some microorganisms (6). It is a single homolog of nearly 600 known carotenoids, several of which can be converted into vitamin A and occur as cis-trans forms at a varying ratio (7, 8). As the main carotenoids, beta-carotene can be metabolized into bioactive retinol and other beta-carotene compounds essential for maintaining homeostasis and human physiology (9). Several studies reveal that the beta-carotene is a potent antioxidant, able to function against oxidative stress, maintaining health, and preventing diseases such as cancer and cardiovascular disease (CVD) (10–15). Observational evidence also suggests that a high dietary intake of beta-carotene is associated with a reduced risk of cancer and CVD (16). Moreover, serum beta-carotene has also been inversely correlated with systemic inflammation and insulin resistance (17, 18). However, there is also evidence that beta-carotene may possess a pro-oxidant property and act as a cocarcinogen (19).
Several studies, including meta-analyses, assessing the health effects of beta-carotene showed inconsistent results in humans. Although there have been mixed results for the risk of mortality from cancer (20–22), several observational studies indicated that individuals with a high dietary intake or high circulatory levels of beta-carotene have a lower risk of all-cause (21) and CVD mortality (20, 23, 24). According to a meta-analysis of prospective studies, dietary or circulating beta-carotene has an inverse association with total mortality (25). In addition, in another recent dose-response meta-analysis of observational studies, higher circulating concentrations of beta-carotene were significantly associated with a lower risk of CVD mortality, whereas higher dietary intake of beta-carotene did not appear to have protective effects (26). As a supplement, the findings were inconsistent. Large controlled trials reported either no benefits or unpredicted adverse effects of beta-carotene supplementation, including increased lung cancer incidence and mortality among subjects exposed to asbestos and tobacco (27–30). In these treatment trials, beta-carotene also led to a small but significant increase in CVD and augmented total mortality. In 2012, a meta-analysis of RCTs was conducted by the Cochrane group. In trials with a low risk of bias, the results demonstrated that beta-carotene used singly or in combination with other antioxidants significantly increases overall mortality (31). Furthermore, the same review group performed a meta-regression analysis and reported significant effects of the dose of beta-carotene on mortality (32).
There has been substantial attention to the health effects of beta-carotene, and a systematic review and meta-analysis of the association between beta-carotene supplementation and all-cause mortality in RCTs have already been reported (31). However, the last analyses referred to data available until 2012, and a better and more updated understanding of the beta-carotene-mortality association to examine cause-specific mortality is needed. Therefore, this meta-analysis investigates the association between beta-carotene supplementation and the risk of cause-specific mortality among population subgroups in RCTs, including the most recent results in the literature.
Materials and Methods
This study was performed following Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 (33). The protocol for this review was registered on PROSPERO (CRD42021259354).
Inclusion and Exclusion Criteria
Studies were eligible if they enrolled adults (age ≥ 18) with any health condition; if they compared beta-carotene supplements at any dose with placebo or no intervention, provided information on deaths from any cause; and if they were randomized controlled trials (RCTs). On the contrary, we excluded studies if all the participants received beta-carotene; if they included pregnant women or critically ill patients; and if they used beta-carotene analogs.
Search Strategy
We searched four databases: Medline, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) of the Cochrane library, from inception to September 2021. We also checked the bibliography of identified studies and systematic reviews to increase the search for relevant articles. We applied English language restriction. No restriction on the type of publication was used. We selected the following keywords for the literature search: “carotenoid*,” or “beta-carotene,” or “b-carotene” and “mortality,” or “death.” At the same time, similar queries were, respectively, used for controlled vocabulary search: “beta-carotene” [Mesh] AND “mortality” [Mesh], INDEX TERMS “beta-carotene” AND “mortality.”
Study Selection and Data Extraction
After removing duplicates with reference management software EndNote X9 (Clarivate Analytics, Philadelphia, PA, United States), Two raters screened the title/abstract of articles independently. Potentially eligible articles were then accessed in full. Divergences between raters on article eligibility were resolved by a third rater, who screened the studies independently (100% consensus on article eligibility was reached). A data extraction spreadsheet was then developed, and the information from the included studies was extracted and tabulated. When RCTs had more than two arms, data from the separate treatment arms were pooled. The following data were extracted: study name (along with the year of publication), country, study characteristics (participant number, age, gender, health status, and study design), treatment duration/follow-up period, intervention and dosage, mortality causes, and the amount of death/number of participants in each intervention group.
Study Quality Assessment
The quality of all included trials was assessed using the Cochrane Collaboration risk of bias tool (34). The Cochrane risk of bias tool is made up of 7 components: (1) sequence generation, (2) allocation sequence concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective outcome reporting, and (7) other bias. Moreover, we also performed the GRADEpro GDT (GRADEpro Guideline Development Tool Software (35) assessment for the quality of evidence.
Statistical Analyses
We performed statistical analyses using RevMan (version 5.3.3; The Cochrane Collaboration) and the meta package in R Software, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), and the interface R-Studio version 1.4.1717 (R studio, PBC, Boston, MA, United States). We used risk ratios and their associated 95% confidence intervals to assess outcomes and considered a P-value less than 0.05 to be statistically significant. We assessed heterogeneity using the I2-test (34). We used random-effects models for our analysis and the possibility of small study effects was assessed qualitatively by a visual estimate of the funnel plot and quantitatively by calculation of the Egger and Begg’s tests (36).
We evaluated the effects of beta-carotene supplements according to mortality cause (cancer mortality, CVD mortality, cerebrovascular disease mortality, and mortality from other causes). Besides, we performed several additional subgroup analyses to test interactions according to: the number of participants (≥1,000 and <1,000, by using the median value for stratification), the number of events (≥100 and <100 by using the median value for stratification), the gender (men, women, and both), the mean age (≥65 and < 65 years to evaluate the aging effect), the beta-carotene dose (>20 and <20 mg/day by using the median value for stratification), the length of follow-up (at least four years and less than four years, by using the median value for stratification), the intervention (beta-carotene singly and beta-carotene combined with vitamins, minerals, or other interventions), the participant health status (healthy and unhealthy), and the control group (placebo and no intervention) in all the included trials. Moreover, a subgroup analysis was also performed by country (Supplementary Figure 7).
Results
Study Selection
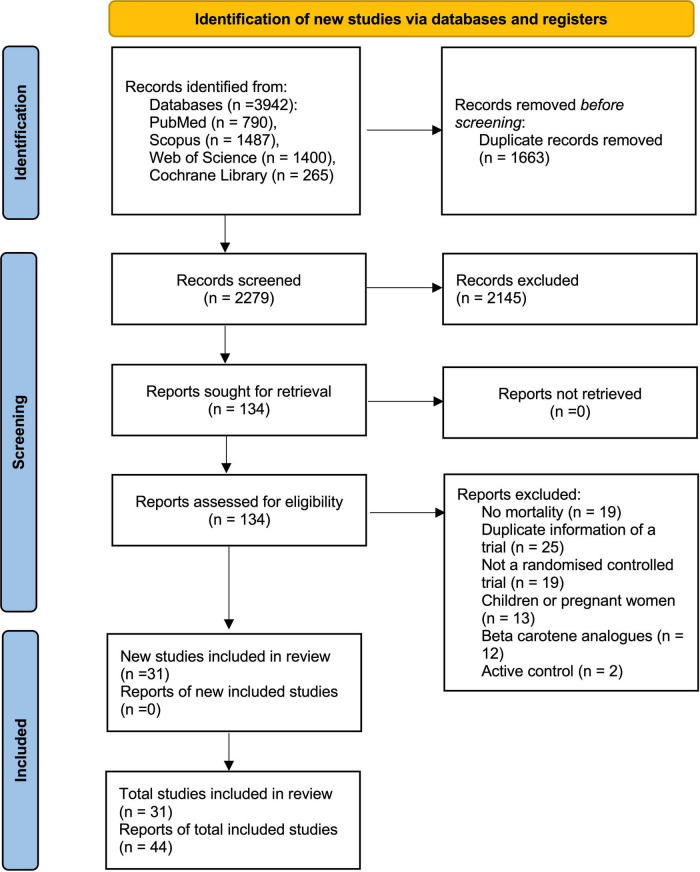
We initially identified 3,942 records after searching databases and relevant bibliographies. After excluding 1,663 duplicated articles and 2,145 articles that did not satisfy the selection criteria, we reviewed the full texts of 134 articles and included 44 articles (27, 29, 30, 37–77) on 31 RCTs in the final analysis (Figure 1).
FIGURE 1.
Search strategy and final included and excluded studies by the PRISMA flowchart.
Study Characteristics
Table 1 summarizes the characteristics of included trials, and Table 2 gives details of those trials. The final analysis comprised 216,734 participants, 108,622 in the beta-carotene supplement group and 108,112 in the placebo or no intervention groups, from 31 RCTs reported in 44 articles. In the studies in which age and gender were reported, the median age was 60.2 years (age range 32–85 years), and 49% of the subjects were women. The median treatment and follow-up periods were 3 and 4.6 years, respectively. There were 45,907 deaths, of which 4,609 deaths were from cancer, 3,796 deaths were from CVD, and 956 deaths were from cerebrovascular disease.
TABLE 1.
Summary characteristics of included studies.
| Characteristics | No. of trials (No. of participants) |
| Eligible studies | |
| Total No. of trials (No. of participants) Median (IQR) follow-up (years) Follow-up at least 4 years Median (IQR) No. of participants Total No. of deaths Median (IQR)% women Median (IQR) age (years) |
31 (216,734) 4.6 (1.7–8.8) 16 (171,578) 382 (85, 5,883) 45,907 49 (15.45–58.44) 60.2 (54.2–67.7) |
| Country | |
| American European Asian-pacific |
17 (119,297) 9 (63,937) 5 (33,500) |
TABLE 2.
Data summary of randomized controlled trials assessing the effects of beta-carotene supplementation on mortality (n = 44).
| References | Country | Study characteristics | Treatment duration/follow-up period (median) |
Intervention (dose) | Mortality cause | Intervention (death/total) |
Control (death/total) |
| Albanes et al. (37) | Finland |
N = 29,133 (mean age 57.2 y) Women: 0% Health status: smokers (5 + cigarettes/day) Design: 2.2 factorial |
6.1/6.1 y | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Colorectal cancer | 23/14,560 | 23/14,573 |
| Austin et al. (38) | Canada |
N = 331 (median age 39.5 y) Women: 10.5% Condition: acquired immunodeficiency syndrome Design: Parallel |
13/13 m | Beta-carotene (72 mg/d) + multivitamins and trace elements vs. beta-carotene placebo |
HIV-related mortality | 13/165 |
23/166 |
| Bairati et al. (39) | Canada |
N = 156 (mean age 62.5 y) Women: 21% Condition: stage I or II head and neck cancer Design: Parallel |
3.1/6.5 y | Beta-carotene (30 mg/d) + alpha-tocopherol (400 UI/d) vs. placebo |
All-cause | 37/79 |
30/77 |
| Blot et al. (40) | China |
N = 29,450 (age range 40–69 y) Women: 55% Health status: at risk of esophageal/gastric cardia cancer Design: 2.2.2.2 factorial |
5.25/5.25 y |
Beta-carotene (15 mg/d) + vitamin E and selenium + micronutrients vs. beta-carotene placebo |
Cancer Cerebrovascular disease |
369/14,729 249/14,729 |
423/14,721 274/14,721 |
| Brown et al. (41) | United States |
N = 80 (mean age 53 y) Women: 13% Health status: coronary disease Design: 2.2 factorial |
3/3 y | Antioxidant vitamins (beta-carotene 25 mg/d) vs. placebo |
All-cause Cardiovascular cause |
11/42 3/42 |
12/38 7/38 |
| Chew et al. (42) | United States |
N = 4,757 (median age 69 y) Women: 56% Health status: age-related eye disease Designs: 2.2 factorial |
6.3/10 y | Beta-carotene (15 mg/d) + vitamin C (500 mg/d) + vitamin E (400 IU/d) ± zinc (80 mg/d) vs. placebo |
All-cause | 439/2,370 | 427/2,387 |
| Chylack et al. (43) | United States |
N = 297 (mean age 68 y) Women: 59% Condition: age-related cataract Design: Parallel |
3/3 y | Antioxidant micronutrients (beta-carotene 18 mg/d) vs. placebo |
All-cause | 9/149 |
3/148 |
| Garbagnati et al. (44) | Italy | N = 34 (mean age 66.75 y) Women: 44.5% Condition: stroke Designs: 2.2 factorial |
1/1 y | Antioxidants (beta-carotene 19 mg/d) vs. placebo |
Cardiovascular disease | 1/16 | 3/18 |
| Gaziano et al. (45) | United States |
N = 14,641 (mean age 64.3 y) Women: 0% Health status: General population Design: 2.2.2.2 factorial |
11.2/11.2 y | Beta-carotene (50 mg/alternate days) + multivitamins vs. beta-carotene placebo |
All-cause Cancer |
1,345/7,317 403/7,317 |
1,412/7,324 456/7,324 |
| Girodon et al. (46) | France |
N = 362 (mean age 83.9 y) Women: 74.58% Condition: Institutionalized elderly Design: 2.2 factorial |
2/2 y | Vitamins (beta-carotene 6 mg/d) vs. placebo |
All-cause | 45/180 | 51/182 |
| Goodman et al. (47) | United States |
N = 18,314 (median age 58 y) Women: 34% Health status: Smoker or asbestos exposed Designs: Parallel |
4/10 y | Beta-carotene (30 mg/d) + retinyl palmitate (25,000 IU/d) vs. placebo |
All-cause Lung cancer Cardiovascular disease |
1,855/9,420 294/9,420 354/9,420 |
1,509/8,894 227/8,894 319/8,894 |
| Graat et al. (48) | Netherlands |
N = 316 (mean age 73.2 y) Women: 48.5% Condition: non-institutionalized elderly Design: 2.2 factorial |
15/15 m | Multivitamin-mineral capsule (beta-carotene 2.4 mg/d) vs. placebo |
All-cause | 0/163 | 5/153 |
| Greenberg et al. (49) | United States |
N = 1,805 (mean age 63.2 y) Women: 30% Health condition: Basal cell or squamous cell carcinoma Designs: Parallel |
4.3/8.2 y | Beta-carotene (50 mg/d) vs. placebo |
All-cause Cardiovascular disease Cancer |
146/913 68/913 38/913 |
139/892 59/892 44/892 |
| Grieger et al. (50) | Australia |
N = 115 Women: 52% Health condition: aged care residents Designs: Parallel |
6/6 m | Multivitamin (beta-carotene 3 mg/d) vs. placebo |
All-cause | 3/58 | 4/57 |
| Heart Protection Study Collaborative Group (51) | United Kingdom |
N = 20,536 (age range 40–80 y) Women: 24.74% Health status: coronary disease, occlusive arterial disease, or diabetes Design: 2.2 factorial |
5/5 y | Antioxidant vitamins (20 mg/d beta-carotene) vs. placebo |
All-cause Coronary heart disease Stroke |
1,446/10,269 664/10,269 108/10,269 |
1,389/10,267 630/10,267 107/10,267 |
| Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group (27) | Finland |
N = 29,133 (mean age 57.2 y) Women: 0% Health status: Smokers (5 + cigarettes/day) Design: 2.2 factorial |
6.1/6.1 y | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Cancer Lung cancer |
582/14,560 302/14,560 |
534/14,573 262/14,573 |
| Heinonen et al. (52) | Finland |
N = 29,133 (mean age 57.2 y) Women: 0% Health status: Smokers (5 + cigarettes/day) Design: 2.2 factorial |
6.1/6.1 y | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Prostate cancer | 33/14,560 | 29/14,573 |
| Hennekens et al. (29) | United States |
N = 22,071 (mean age 53 y) Women: 0% Health status: General population Design: 2.2 factorial |
12/12 y | Beta-carotene (50 mg/alternate days) + aspirin vs. beta-carotene placebo |
All-cause Cardiovascular disease Malignant neoplasm |
979/11,036 338/11,036 386/11,036 |
968/11,035 313/11,035 380/11,035 |
| Hercberg (53) | France |
N = 13,017 (mean age 49 y) Women: 60.5% Health status: General population Designs: Parallel |
7.5/12.5 y | Antioxidant vitamins and minerals (beta-carotene 6 mg/d) vs. placebo |
All-cause | 156/6,481 | 178/6,536 |
| Jiamton et al. (54) | Thailand |
N = 481 (mean age 32 y) Women: 61% Health status: HIV-infected Designs: Parallel |
48/48 w | Immunace Micronutrient supplement (beta-carotene 6 mg/d) vs. placebo |
HIV-related mortality | 8/242 | 15/239 |
| Kataja-Tuomola et al. (55) | Finland |
N = 1,700 (mean age 57.2 y) Women: 0% Health status: Smokers (5 + cigarettes/day) with diabetes Design: 2.2 factorial |
6.1/6.1 y | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Diabetes-related mortality | 168/877 | 150/823 |
| Lai et al. (56) | Finland |
N = 29,133 (mean age 57.2 y) Women: 0% Health status: Smokers (5 + cigarettes/day) Design: 2.2 factorial |
6.1/24 y | beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Chronic liver disease | 121/14,560 | 116/14,573 |
| Lamas et al. (57) | United States |
N = 1,708 (median age 65 y) Women: 18% Health status: Post myocardial infarction Design: 2.2 factorial |
31/55 m | Multivitamin and multimineral mixture (beta-carotene 25,000 IU/d) + IV chelation infusions vs. placebo |
All-cause Cardiovascular disease |
87/853 45/853 |
93/855 56/855 |
| Lee et al. (30) | United States |
N = 39,876 (mean age 54.6 y) Women: 100% Health status: Healthy Design: 2.2.2 factorial |
2.1/4.1 y | Beta-carotene (55 mg on alternate days) + aspirin and vitamin E vs. beta-carotene placebo |
All-cause Cardiovascular disease Cancer |
59/19,939 14/19,939 31/19,939 |
55/19,937 12/19,937 28/19,937 |
| Leppälä et al. (58) | Finland |
N = 28,519 (mean age 57.2 y) Women: 0% Health status: Stroke-free smokers (5 + cigarettes/day) Design: 2.2 factorial |
6.1/6.1 y | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Stroke | 82/14,246 | 78/14,273 |
| Li et al. (59) | China |
N = 3,318 (mean age 54 y) Women: 56% Health status: Esophageal dysplasia Design: Parallel |
6/6 y | Vitamins and minerals (15 mg/d beta-carotene) vs. placebo |
All-cause Cancer Cerebrovascular disease |
157/1,657 87/1,657 22/1,657 |
167/1,661 89/1,661 35/1,661 |
| Lin et al. (60) | United States |
N = 8,171 (mean age 60.4 y) Women: 100% Health status: High risk of cardiovascular disease Design: 2.2.2.2 factorial |
9.4/9.4 y | Beta-carotene (50 mg every other day) + antioxidants vs. beta-carotene placebo |
Cancer | 80/4,084 | 96/4,087 |
| Liu et al. (61) | Canada |
N = 763 (mean age 85 y) Women: 70% Health status: Institutionalized elderly Design: Parallel |
19/19 m | Multivitamin and multimineral (beta-carotene 16 mg/d) vs. placebo |
All-cause | 96/379 | 97/384 |
| Margalit et al. (62) | United States |
N = 383 (median age 73 y) Women: 0% Health status: Prostate cancer Design: 2.2 factorial |
12/22.5 y | Beta-carotene (50 mg/alternate days) ± aspirin vs. placebo |
Prostate cancer |
20/192 |
25/191 |
| Mayne et al. (63) | United Kingdom |
N = 264 (mean age 68 y) Women: 19% Health status: Head and neck cancer Design: Parallel |
4.25/4.25 y | Beta-carotene (50 mg/d) vs. placebo |
All-cause | 21/135 | 26/129 |
| Papadimitrakopoulou et al. (64) | United States |
N = 84 (mean age 56 y) Women: 48.9% Health status: Oral premalignancy Design: Parallel |
3/5 y | Beta-carotene (50 mg/d) + retinyle palmitate vs. beta-carotene placebo |
All-cause | 1/47 | 0/37 |
| Age-Related Eye Disease Study 2 Research Group (65) | United States |
N = 4,203 (median age 74 y) Women: 56.75% Health status: AMD Design: 2.2 factorial |
5/5 y | Macular xanthophylls (10 mg/d lutein + 2 mg/d zeaxanthin) + omega-3 fatty acids (350 mg/d DHA + 650 mg/d EPA) vs. macular xanthophylls placebo |
All-cause | 746/2,123 PC | 727/2,080 PC |
| Pathak et al. (66) | India |
N = 136 (median age 56 y) Women: 14.6% Health status: Advanced non-small cell lung cancer Design: Parallel |
2/2 y | Antioxidants (60 mg/d beta-carotene) + chemotherapy vs. chemotherapy |
All-cause | 54/64 | 64/72 |
| Plummer et al. (67) | Venezuela |
N = 1,980 (mean age 35–69 y) Women: 52.7% Condition: Precancerous gastric lesions Design: Parallel |
3/3 y | Antioxidant vitamins (beta-carotene 18 mg/d) vs. placebo |
All-cause | 16/990 | 11/990 |
| Prince et al. (68) | United Kingdom |
N = 61 (mean age 58 y) Women: 92% Health condition: primary biliary cirrhosis Design: Cross-over |
12/12 w | Antioxidant supplementation (beta-carotene 3 mg/d) vs. placebo |
Ischemic heart disease | 1/29 | 0/32 |
| Qu et al. (69) | China |
N = 29,450 (age range 40–69 y) Women: 55% Health status: At risk of esophageal or stomach cancer Design: 24 partial factorials |
5.25/15.2 y | Beta-carotene (15 mg/d) + vitamin E and selenium vs. placebo |
Liver cancer | 68/14,729 | 83/14,721 |
| Rautalahti et al. (70) | Finland |
N = 29,133 (mean age 75.7 y) Women: 0% Health status: Smokers (5 + cigarettes/day) Design: 2.2 factorial |
6.1/6.1 y | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Pancreatic carcinoma | 35/14,560 | 48/14,573 |
| Richer et al. (71) | United States |
N = 60 (mean age 75.3 y) Women: 5% Condition: Atrophic age-related macular degeneration Design: Parallel |
12/12 m | Lutein (10 mg/d) vs. placebo |
All-cause | 1/29 | 2/31 |
| Toma et al. (72) | Italy |
N = 214 (median age 60.5 y) Women: 9.8% Health condition: Stage I-II head and neck cancer Design: Parallel |
3/4.9 y | Beta-carotene (75 mg/d) vs. no treatment |
All-cause Head and neck tumor |
9/104 5/104 |
15/110 6/110 |
| Törnwall et al. (73) | Finland |
N = 29,133 (mean age 57.7 y) Women: 0% Health status: Smokers at risk of major coronary event Design: 2.2 factorial |
6.1/6.1 | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Coronary heart disease | 456/14,560 | 449/14,573 |
| Virtamo et al. (74) | Finland |
N = 29,133 (mean age 57.7 y) Women: 0% Health status: Smokers (5 + cigarettes/day) Design: 2.2 factorial |
6.1/6.1 y | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Urothelial cancer Renal cell cancer |
13/14,560 16/14,560 |
11/14,573 25/14,573 |
| Virtamo et al. (75) | Finland |
N = 29,133 (mean age 57.7 y) Women: 0% Health status: Smokers (5 + cigarettes/day) Design: 2.2 factorial |
6.1/14.1 y | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
All-cause | 5,555/14,560 | 5,276/14,573 |
| Wang et al. (76) | China |
N = 29,450 (median age 52 y) Women: 55% Health status: At risk of esophageal/gastric cardia cancer Design: 2.2.2.2 factorial |
5.25/30 y | Beta-carotene (15 mg/d) + vitamin E and selenium + micronutrients vs. beta-carotene placebo |
All-cause | 9,910/14,729 | 9,824/14,721 |
| Wright et al. (77) | Finland |
N = 29,133 (mean age 57.7 y) Women: 0% Health condition: Smokers (5 + cigarettes/day) Design: 2.2 factorial |
6.1/6.1 y | Beta-carotene (20 mg/d) + alpha-tocopherol (50 mg/d) vs. beta-carotene placebo |
Oral/pharyngeal cancer Esophageal cancer laryngeal cancer |
10/14,560 6/14,560 5/14,560 |
7/14,573 9/14,573 5/14,573 |
ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; NIT1, Nutrition Intervention Trial (NIT); The General Population Trial; HATS, The HDL-Atherosclerosis Treatment Study; AREDS, Age Related Eye Disease Study; REACT, The Roche European American Cataract Trial; PHSII, Physicians Health Study; CARET, The Beta-Carotene and Retinol Efficacy Trial; SCPS, Skin Cancer Prevention Study; HPS, Heart Protection Study; PHS, Physicians Health Study; SUVIMAX, The Supplementation en Vitamines et Mineraux Antioxydants; WHS, Women’s Health Study; AREDS2, Age-Related Eye Disease Study 2; PC, personal contact; LAST, Lutein Antioxidant Supplementation Trial.
The selected articles were published from 1993 through 2018, spanning 25 years. The countries in which the studies were conducted were as follows: United States (n = 13), Canada (n = 3), United Kingdom (n = 3), China (n = 2), France (n = 2), Italy (n = 2), Finland (n = 1), Netherlands (n = 1), Venezuela (n = 1), India (n = 1), Thailand (n = 1), and Australia (n = 1). The studies included healthy subjects (general population, physicians, and nurses); patients with oral premalignancy, skin, lung, and head and neck cancer; adults with underlying CVD or cerebrovascular diseases, and acquired immunodeficiency syndrome (AIDS), primary biliary cirrhosis, and age-related eye diseases; persons at risk of esophageal/gastric cardia cancer; smokers or asbestos-industry workers; and institutionalized elderlies.
Among the 31 trials, 30 had a placebo group, and 1 had a no-intervention group as the control (77). Further, 16 trials used the parallel design, 14 used the factorial design, and one study used a cross-over design (67). The following 3 trials were reported in 16 articles: the Alpha-Tocopherol Beta-Carotene Prevention Study (n = 11), Nutrition Intervention Trial; The General Population Trial (n = 3), and the Physicians’ Health Study (n = 2).
Quality of the Included Trials
Supplementary Figures 1, 2 show the quality of the included trials. Twenty-four trials were classified as having a low risk of bias. The remaining 4 trials had one or more inadequate components (64, 66, 72, 76), and 1 trial had an unclear risk of bias (63). Supplementary Figures 3, 4 show the GRADE assessment of the quality. The overall results showed a high quality of the studies.
Meta-Analysis of the Effect of Beta-Carotene Supplements on Mortality Risk
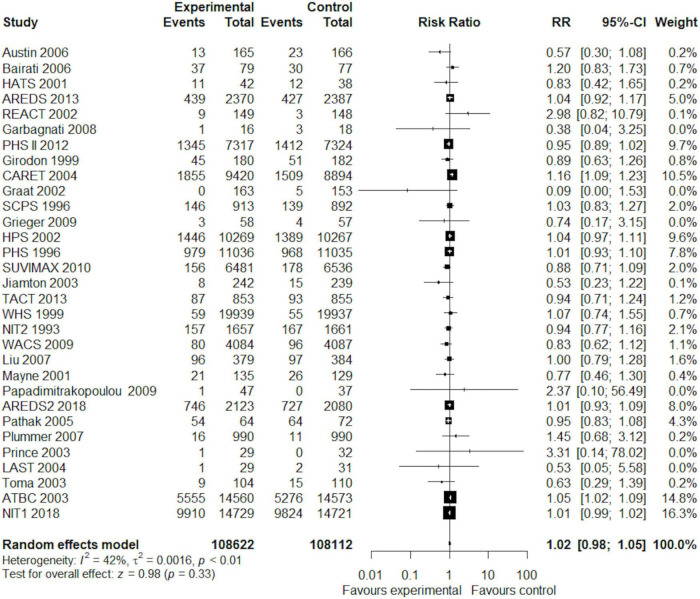
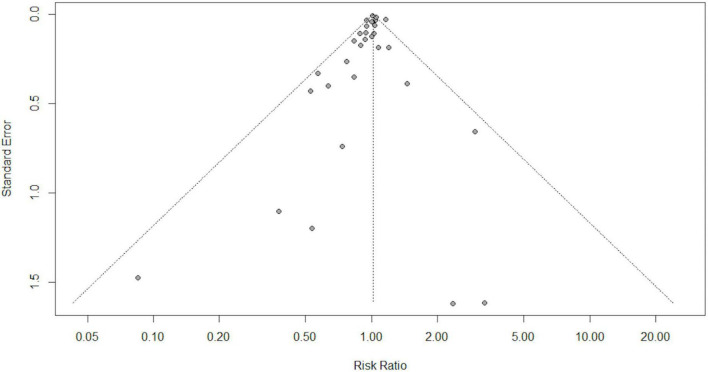
Overall, in a random-effects model meta-analysis of all the 31 trials (27, 29, 30, 37–77), there was no statistically significant difference in total mortality between the beta-carotene supplementation group and the control group (RR 1.02, 95% CI 0.98–1.05, I2 = 42%; Figure 2). Funnel plot analysis showed no asymmetry (Figure 3); additionally, the Egger test (P = 0.25) and Begg’s test (P = 0.85) detected no significant small-study effects.
FIGURE 2.
Forest plot showing the effect of beta-carotene supplementation on total mortality in 31 randomized controlled trials.
FIGURE 3.
Funnel plot for publication bias in 31 randomized controlled trials.
Subgroup analyses according to the number of participants, the number of events, gender, age groups, beta-carotene dose, follow-up duration, type of intervention (singly or combined beta-carotene supplements), participant health status, and the control group did not show any difference in total mortality among the participants (Table 3). Table 4 shows the results of the subgroup analyses on cause-specific mortality. Beta-carotene supplementation was not associated with cancer mortality (RR 0.98, 95% CI 0.90–1.07, I2 = 37%). However, the use of beta-carotene supplements significantly increased mortality among lung cancer patients (RR 1.14, 95% CI 1.02, 1.27, I2 = 3%). As for CVD mortality, we found no statistically significant difference between the groups (RR 1.04, 95% CI 0.98, 1.11, I2 = 0%). Similarly, beta-carotene supplementation did not reduce the risk of death from cerebrovascular disease (RR 0.94, 95% CI 0.82, 1.06, I2 = 0%). However, a significant beneficial effect of beta-carotene on mortality risk was observed in participants with human immunodeficiency virus (HIV) infection (RR 0.55, 95% CI 0.33, 0.92, I2 = 0%).
TABLE 3.
Subgroup analyses of the effect of beta-carotene on total mortality.
| Subgroup title | No. of trials | No. of participants | I2 (%) | Risk ratio (95% CI) | P-value |
| Overall | 31 | 216,734 | 42.0 | 1.02 (0.98, 1.05) | 0.3 |
| No of participants | |||||
| ≥1,000 <1,000 |
15 16 |
212,980 3,754 |
58.0 4.0 |
1.02 (0.98, 1.05) 0.93 (0.83, 1.04) |
0.1 0.2 |
| No of events | |||||
| ≥100 <100 |
16 15 |
211,899 4,835 |
55.0 15.0 |
1.02 (0.99, 1.05) 0.88 (0.71, 1.09) |
0.2 0.3 |
| Age (years) | |||||
| ≥65 <65 |
11 20 |
12,879 203,855 |
0.0 56.0 |
1.00 (0.94, 1.07) 1.02 (0.98, 1.06) |
0.9 0.3 |
| Gender | |||||
| Women Men Women and men |
2 3 26 |
48,047 65,845 102,842 |
9.0 73.0 39.0 |
0.92 (0.72, 1.17) 1.01 (0.95, 1.08) 1.02 (0.97, 1.06) |
0.5 0.8 0.5 |
| Daily dose equivalent (mg) | |||||
| ≥ 20 < 20 |
15 16 |
155,812 60,922 |
55.0 0.0 |
1.02 (0.97, 1.08) 1.01 (0.99, 1.02) |
0.5 0.4 |
| Follow up | |||||
| At least 4 years Less than 4 years |
16 15 |
171,578 45,156 |
57.0 2.0 |
1.02 (0.98, 1.06) 0.94 (0.85, 1.04) |
0.3 0.2 |
| Intervention | |||||
| Beta carotene alone Combined |
4 27 |
2,343 214,391 |
0.0 47.0 |
0.96 (0.79, 1.16) 1.02 (0.98, 1.05) |
0.6 0.3 |
| Participant health status | |||||
| Healthy Unhealthy |
5 26 |
89,921 126,813 |
19.0 42.0 |
0.97 (0.91, 1.04) 1.03 (0.99, 1.07) |
0.4 0.1 |
| Control group | |||||
| Placebo No intervention |
30 1 |
216,520 214 |
43 - |
1.02 (0.99, 1.05) 0.63 (0.29, 1.39) |
0.3 0.25 |
TABLE 4.
Effects of beta-carotene supplements vs. placebo or no intervention on cause-specific mortality.
| Mortality cause | No. of trials | Risk ratio (95% CI) | I2 (%) | Model used |
| Cancer | 13 | 0.98 (0.90, 1.07) | 37.0 | Random effects |
| Colorectal cancer | 2 | 0.97 (0.68, 1.38) | 0.0 | Random effects |
| Esophagus and stomach cancer | 2 | 0.93 (0.82, 1.06) | 0.0 | Random effects |
| Prostate cancer | 3 | 0.93 (0.73, 1.18) | 0.0 | Random effects |
| Lung cancer | 5 | 1.14 (1.02, 1.27)* | 3.0 | Random effects |
| Lung cancer in smokers | 2 | 1.14 (1.03, 1.27)* | 0.0 | Random effects |
| Lung cancer in mixed smokers and non-smokers | 3 | 0.94 (0.74, 1.20) | 0.0 | Random effects |
| Urinary tract cancer | 2 | 0.82 (0.55, 1.21) | 0.0 | Random effects |
| Pancreatic cancer | 2 | 0.85 (0.62, 1.16) | 0.0 | Random effects |
| Other cancer | 6 | 0.86 (0.70, 1.06) | 0.0 | Random effects |
| Cardiovascular disease | 12 | 1.04 (0.98, 1.11) | 0.0 | Random effects |
| Cerebrovascular disease | 5 | 0.94 (0.82, 1.06) | 0.0 | Random effects |
| HIV-related causes | 2 | 0.55 (0.33, 0.92)* | 0.0 | Random effects |
| Non-cancer, non-vascular cause | 5 | 1.04 (0.95, 1.14) | 0.0 | Random effects |
*Statistically significant.
Discussion
The current meta-analysis found that the administration of beta-carotene supplements had no preventive effect on total mortality, mortality from cancer, and vascular and non-vascular diseases. Furthermore, no association was found within subgroup meta-analyses based on the number of participants, the number of events, sex, age groups, beta-carotene dose, follow-up duration, type of intervention (singly or combined beta-carotene supplements), participant health status, and control group. However, beta-carotene supplementation was significantly related to an increased risk of lung cancer mortality (RR 1.14, 95% CI 1.02, 1.27, I2 = 3%, n = 5). The effects of beta-carotene supplementation on increased lung cancer incidence and mortality among smokers have already been described, and several possible biological mechanisms have been proposed. In general, beta-carotene supplementation has not been shown to positively impact cancer prevention. In a systematic review and meta-analysis, no effect of beta-carotene supplementation was observed on the incidence of the total, pancreatic, colorectal, prostate, breast, melanoma, and non-melanoma skin cancers. However, a significant harmful effect of beta-carotene supplementation on the incidence of lung and stomach cancers was observed in people supplemented with beta-carotene at 20–30 mg/day, in smokers and asbestos workers compared to placebo (78). Beta-carotene may act as a pro-oxidant in the presence of chronic oxidative stress such as smoking (79) and it may enhance the oxidative stress initiated by cigarette smoking and stimulate toxic effects in tissues (80). Our study also found significant inverse associations of beta-carotene supplementation with the risk of HIV-related mortality; however, this was reported in only two studies. This is in line with previous evidence illustrating that persons in all stages of HIV infection generally have low circulating levels of micronutrients, including carotenoids, and low micronutrient concentrations are correlated with HIV disease progression and mortality (38).
Overall, the findings of the present meta-analysis of RCTs are inconsistent with previous meta-analyses of observational studies suggesting beneficial effects from high dietary or circulatory beta-carotene-rich fruits and vegetables on all-cause and CVD mortality (25, 26). Intervention studies are commonly considered to provide conclusive answers, whereas observational studies represent a better picture of the real-world population. There are evident differences between the findings of published trials, which could be explained by population characteristics (general, ill, or at high-risk subjects), the different doses of supplementation (dietary levels or higher), which can be associated with harmful health effects (81), and the type of supplement (alone or in association). In this last condition, when subgroup analysis was performed, only 4 out of 31 studies reported the use of beta-carotene alone. Although no significant difference was found in all-cause mortality (p = 0.64), a very low heterogeneity was discovered among these studies (I2 = 3.60%) with a trend in reduced mortality with beta-carotene supplementation (RR = 0.95, 95% CI 0.74. 1.16, Supplementary Figure 5). Indeed, it appears that optimal effects may be obtained with a combination of nutrients at similar levels to a healthy diet. A single antioxidant, such as beta-carotene, given at high doses in subjects with a high risk of diseases, such as smokers and asbestos-exposed workers, might not have considerable benefits and can even have adverse outcomes (82). Another possible reason for the harmful effect in clinical trials involving beta-carotene may be attributed to the purified synthetic form (83, 84). The effective uptake of synthetic all-trans beta-carotene seems to make the synthetic form more suitable for efficient absorption. However, the fact that synthetic beta-carotene can change normal serum trans/cis ratios favoring the trans-isomer may lead to an unfavorable effect. The effects of using all-trans synthetic beta-carotene are still not well-understood (84). It is assumed that synthetic beta-carotene rather than natural mixed carotenoids may stimulate cancer formation (85). Ultimately, higher antioxidant intakes, including beta-carotene, are associated with a better diet quality, which indicates higher intakes of nutrients such as fibers, minerals, and flavonoids, and lower intakes of unhealthy nutrients.
The present study has several possible limitations. Firstly, in the majority of the studies, synthetic beta-carotene was used. Clinical consequences of using natural beta-carotene are not well-understood because RCTs have yet to be conducted. Additional trials are required to understand the differential results of synthetic beta-carotene as an alternative to natural beta-carotene. Secondly, the results were accompanied by some evidence of heterogeneity. However, the subgroup analyses were performed to overcome this problem, implying that some of the study and participant characteristics were possible sources of the heterogeneity in the data. Thirdly, the database sources did not include EMBASE. However, CENTRAL and Scopus include several articles from EMBASE as the original source.
Our study has several strengths, as well. We updated the association of beta-carotene with total mortality, assessed its effects on cause-specific mortality, and showed a significant inverse association between beta-carotene intake and HIV-related mortality. Second, because of no evidence of publication bias, the results have not been altered by this type of bias.
In conclusion, we found no evidence of an overall preventive effect of beta-carotene supplements on total, cancer, CVD, and cerebrovascular mortality risk in our meta-analysis of RCTs published over the past 25 years. Instead, beta-carotene supplementation increased the risk of lung cancer mortality but decreased the risk of HIV-related mortality. Surely more studies should be performed to better define this issue, by confirming or denying our results. Therefore, beta-carotene supplementation’s possible beneficial or harmful effects on mortality must not be overstated.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
GC and SD conceived of the presented manuscript. SA, SM, MI, and GS analyzed each article and performed the data extraction independently. VC, MI, and SM drafted the method and result section with the input of GC and SD. GS and VC drafted the introduction and discussion section with the input of SA, GC, and SD. All authors discussed the results and contributed to the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This work was supported by the Italian Ministry of Health funds (FFABR_Corbi 2017).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.872310/full#supplementary-material
Quality of the included trials by using the Cochrane Collaboration risk of bias tool.
Quality of the included trials by using the Cochrane Collaboration risk of bias tool in summary with the studies.
The GRADE assessment of the study’s quality for the all-cause mortality outcome.
The GRADE assessment of the study’s quality for the all-cause mortality outcome with beta carotene alone.
Subgroup analysis by the type of supplement (alone or in association).
PRISMA checklist.
Subgroup analysis by country.
Publication bias evaluation of each study by using the Cochrane Collaboration risk of bias tool.
References
- 1.Davinelli S, Trichopoulou A, Corbi G, De Vivo I, Scapagnini G. The potential nutrigeroprotective role of Mediterranean diet and its functional components on telomere length dynamics. Ageing Res Rev. (2019) 49:1–10. 10.1016/j.arr.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 2.Davinelli S, Corbi G, Zarrelli A, Arisi M, Calzavara-Pinton P, Grassi D, et al. Short-term supplementation with flavanol-rich cocoa improves lipid profile, antioxidant status and positively influences the AA/EPA ratio in healthy subjects. J Nutr Biochem. (2018) 61:33–9. 10.1016/j.jnutbio.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 3.Conti V, Corbi G, Manzo V, Pelaia G, Filippelli A, Vatrella A. Sirtuin 1 and aging theory for chronic obstructive pulmonary disease. Anal Cell Pathol (Amst). (2015) 2015:897327. 10.1155/2015/897327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russomanno G, Corbi G, Manzo V, Ferrara N, Rengo G, Puca AA, et al. The anti-ageing molecule sirt1 mediates beneficial effects of cardiac rehabilitation. Immun Ageing. (2017) 14:7. 10.1186/s12979-017-0088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davinelli S, Ali S, Solfrizzi V, Scapagnini G, Corbi G. Carotenoids and cognitive outcomes: a meta-analysis of randomized intervention trials. Antioxidants (Basel). (2021) 10:223. 10.3390/antiox10020223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. (2005) 1740:101–7. 10.1016/j.bbadis.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 7.Schieber A, Carle R. Occurrence of carotenoid cis-isomers in food: technological, analytical, and nutritional implications. Trends Food Sci Technol. (2005) 16:416–22. [Google Scholar]
- 8.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. (2005) 26:459–516. 10.1016/j.mam.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 9.Bendich A. From 1989 to 2001: what have we learned about the “biological actions of beta-carotene”? J Nutr. (2004) 134:225S–30S. 10.1093/jn/134.1.225S [DOI] [PubMed] [Google Scholar]
- 10.Paiva SA, Russell RM. Beta-carotene and other carotenoids as antioxidants. J Am Coll Nutr. (1999) 18:426–33. 10.1080/07315724.1999.10718880 [DOI] [PubMed] [Google Scholar]
- 11.Dugas TR, Morel DW, Harrison EH. Dietary supplementation with beta-carotene, but not with lycopene, inhibits endothelial cell-mediated oxidation of low-density lipoprotein. Free Radic Biol Med. (1999) 26:1238–44. 10.1016/s0891-5849(98)00321-9 [DOI] [PubMed] [Google Scholar]
- 12.Corbi G, Conti V, Komici K, Manzo V, Filippelli A, Palazzo M, et al. Phenolic plant extracts induce sirt1 activity and increase antioxidant levels in the rabbit’s heart and liver. Oxid Med Cell Longev. (2018) 2018:2731289. 10.1155/2018/2731289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy Y, Kaplan M, Ben-Amotz A, Aviram M. Effect of dietary supplementation of beta-carotene on human monocyte-macrophage-mediated oxidation of low density lipoprotein. Isr J Med Sci. (1996) 32:473–8. [PubMed] [Google Scholar]
- 14.Levy Y, Zaltsberg H, Ben-Amotz A, Kanter Y, Aviram M. Dietary supplementation of a natural isomer mixture of beta-carotene inhibits oxidation of LDL derived from patients with diabetes mellitus. Ann Nutr Metab. (2000) 44:54–60. 10.1159/000012821 [DOI] [PubMed] [Google Scholar]
- 15.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. (2004) 44:275–95. 10.1080/10408690490468489 [DOI] [PubMed] [Google Scholar]
- 16.van Poppel G. Epidemiological evidence for beta-carotene in prevention of cancer and cardiovascular disease. Eur J Clin Nutr. (1996) 50 Suppl 3:S57–61. [PubMed] [Google Scholar]
- 17.Erlinger TP, Guallar E, Miller ER, III, Stolzenberg-Solomon R, Appel LJ. Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch Intern Med. (2001) 161:1903–8. 10.1001/archinte.161.15.1903 [DOI] [PubMed] [Google Scholar]
- 18.Higuchi K, Saito I, Maruyama K, Eguchi E, Mori H, Tanno S, et al. Associations of serum β-carotene and retinol concentrations with insulin resistance: the toon health study. Nutrition. (2015) 31:975–80. 10.1016/j.nut.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 19.Paolini M, Abdel-Rahman SZ, Sapone A, Pedulli GF, Perocco P, Cantelli-Forti G, et al. Beta-carotene: a cancer chemopreventive agent or a co-carcinogen? Mutat Res. (2003) 543:195–200. 10.1016/s1383-5742(03)00002-4 [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, et al. A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants. Asian Pac J Cancer Prev. (2006) 7:533–46. [PubMed] [Google Scholar]
- 21.Hashim D, Gaughan D, Boffetta P, Lucchini RG. Baseline serum β-carotene concentration and mortality among long-term asbestos-exposed insulators. Cancer Epidemiol Biomarkers Prev. (2015) 24:555–60. 10.1158/1055-9965.EPI-14-0952 [DOI] [PubMed] [Google Scholar]
- 22.Goyal A, Terry MB, Siegel AB. Serum antioxidant nutrients, vitamin A, and mortality in U.S. Adults. Cancer Epidemiol Biomarkers Prev. (2013) 22:2202–11. 10.1158/1055-9965.EPI-13-0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karppi J, Laukkanen JA, Mäkikallio TH, Ronkainen K, Kurl S. Low β-carotene concentrations increase the risk of cardiovascular disease mortality among Finnish men with risk factors. Nutr Metab Cardiovasc Dis. (2012) 22:921–8. 10.1016/j.numecd.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 24.Buijsse B, Feskens EJ, Kwape L, Kok FJ, Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr. (2008) 138:344–50. 10.1093/jn/138.2.344 [DOI] [PubMed] [Google Scholar]
- 25.Zhao LG, Zhang QL, Zheng JL, Li HL, Zhang W, Tang WG, et al. Dietary, circulating beta-carotene and risk of all-cause mortality: a meta-analysis from prospective studies. Sci Rep. (2016) 6:26983. 10.1038/srep26983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayedi A, Rashidy-Pour A, Parohan M, Zargar MS, Shab-Bidar S. Dietary and circulating vitamin C, vitamin E, β-carotene and risk of total cardiovascular mortality: a systematic review and dose-response meta-analysis of prospective observational studies. Public Health Nutr. (2019) 22:1872–87. 10.1017/S1368980018003725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. (1994) 330:1029–35. 10.1056/NEJM199404143301501 [DOI] [PubMed] [Google Scholar]
- 28.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin a on lung cancer and cardiovascular disease. N Engl J Med. (1996) 334:1150–5. 10.1056/NEJM199605023341802 [DOI] [PubMed] [Google Scholar]
- 29.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. (1996) 334:1145–9. 10.1056/NEJM199605023341801 [DOI] [PubMed] [Google Scholar]
- 30.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. J Natl Cancer Inst. (1999) 91:2102–6. 10.1093/jnci/91.24.2102 [DOI] [PubMed] [Google Scholar]
- 31.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. (2008) 12:CD007176. 10.1002/14651858.CD007176 [DOI] [PubMed] [Google Scholar]
- 32.Bjelakovic G, Nikolova D, Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin a, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One. (2013) 8:e74558. 10.1371/journal.pone.0074558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane bias methods group; cochrane statistical methods group. the cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime; (2022). Available online at: gradepro.org [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albanes D, Malila N, Taylor PR, Huttunen JK, Virtamo J, Edwards BK, et al. Effects of supplemental alpha-tocopherol and beta-carotene on colorectal cancer: results from a controlled trial (Finland). Cancer Causes Control. (2000) 11:197–205. 10.1023/a:1008936214087 [DOI] [PubMed] [Google Scholar]
- 38.Austin J, Singhal N, Voigt R, Smaill F, Gill MJ, Walmsley S, et al. CTN 091/CRIT Cartenoids Study Group. a community randomized controlled clinical trial of mixed carotenoids and micronutrient supplementation of patients with acquired immunodeficiency syndrome. Eur J Clin Nutr. (2006) 60:1266–76. 10.1038/sj.ejcn.1602447 [DOI] [PubMed] [Google Scholar]
- 39.Bairati I, Meyer F, Jobin E, Gélinas M, Fortin A, Nabid A, et al. Antioxidant vitamins supplementation and mortality: a randomized trial in head and neck cancer patients. Int J Cancer. (2006) 119:2221–4. 10.1002/ijc.22042 [DOI] [PubMed] [Google Scholar]
- 40.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. (1993) 85:1483–92. 10.1093/jnci/85.18.1483 [DOI] [PubMed] [Google Scholar]
- 41.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. (2001) 345:1583–92. 10.1056/NEJMoa011090 [DOI] [PubMed] [Google Scholar]
- 42.Chew EY, Clemons TE, Agrón E, Sperduto RD, Sangiovanni JP, Kurinij N, et al. Age-Related eye disease study research group. long-term effects of vitamins c and e, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology. (2013) 120:1604–11. 10.1016/j.ophtha.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chylack LT, Jr., Brown NP, Bron A, Hurst M, Köpcke W, Thien U, et al. The Roche european american cataract trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol. (2002) 9:49–80. 10.1076/opep.9.1.49.1717 [DOI] [PubMed] [Google Scholar]
- 44.Garbagnati F, Cairella G, De Martino A, Multari M, Scognamiglio U, Venturiero V, et al. Is antioxidant and n-3 supplementation able to improve functional status in poststroke patients? Results from the Nutristroke Trial. Cerebrovasc Dis. (2009) 27:375–83. 10.1159/000207441 [DOI] [PubMed] [Google Scholar]
- 45.Gaziano JM, Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, et al. Multivitamins in the prevention of cancer in men: the physicians’. health study ii randomized controlled trial. JAMA. (2012) 308:1871–80. 10.1001/jama.2012.14641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girodon F, Galan P, Monget AL, Boutron-Ruault MC, Brunet-Lecomte P, Preziosi P, et al. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN. VIT. AOX. geriatric network. Arch Intern Med. (1999) 159:748–54. 10.1001/archinte.159.7.748 [DOI] [PubMed] [Google Scholar]
- 47.Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL, Jr., Omenn GS, et al. The Beta-Carotene and retinol efficacy trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. (2004) 96:1743–50. 10.1093/jnci/djh320 [DOI] [PubMed] [Google Scholar]
- 48.Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons: a randomized controlled trial. JAMA. (2002) 288:715–21. 10.1001/jama.288.6.715 [DOI] [PubMed] [Google Scholar]
- 49.Greenberg ER, Baron JA, Karagas MR, Stukel TA, Nierenberg DW, Stevens MM, et al. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA. (1996) 275:699–703. 10.1001/jama.1996.03530330043027 [DOI] [PubMed] [Google Scholar]
- 50.Grieger JA, Nowson CA, Jarman HF, Malon R, Ackland LM. Multivitamin supplementation improves nutritional status and bone quality in aged care residents. Eur J Clin Nutr. (2009) 63:558–65. 10.1038/sj.ejcn.1602963 [DOI] [PubMed] [Google Scholar]
- 51.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. (2002) 360:23–33. 10.1016/S0140-6736(02)09328-5 [DOI] [PubMed] [Google Scholar]
- 52.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. (1998) 90:440–6. 10.1093/jnci/90.6.440 [DOI] [PubMed] [Google Scholar]
- 53.Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, Touvier M, Favier A, Latino-Martel P, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. (2010) 127:1875–81. 10.1002/ijc.25201 [DOI] [PubMed] [Google Scholar]
- 54.Jiamton S, Pepin J, Suttent R, Filteau S, Mahakkanukrauh B, Hanshaoworakul W, et al. A randomized trial of the impact of multiple micronutrient supplementation on mortality among HIV-infected individuals living in Bangkok. AIDS. (2003) 17:2461–9. 10.1097/00002030-200311210-00008 [DOI] [PubMed] [Google Scholar]
- 55.Kataja-Tuomola MK, Kontto JP, Männistö S, Albanes D, Virtamo JR. Effect of alpha-tocopherol and beta-carotene supplementation on macrovascular complications and total mortality from diabetes: results of the ATBC Study. Ann Med. (2010) 42:178–86. 10.3109/07853890903508887 [DOI] [PubMed] [Google Scholar]
- 56.Lai GY, Weinstein SJ, Taylor PR, McGlynn KA, Virtamo J, Gail MH, et al. Effects of α-tocopherol and β-carotene supplementation on liver cancer incidence and chronic liver disease mortality in the ATBC study. Br J Cancer. (2014) 111:2220–3. 10.1038/bjc.2014.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lamas GA, Boineau R, Goertz C, Mark DB, Rosenberg Y, Stylianou M, et al. TACT (Trial to Assess Chelation Therapy) Investigators. Oral high-dose multivitamins and minerals after myocardial infarction: a randomized trial. Ann Intern Med. (2013) 159:797–805. 10.7326/0003-4819-159-12-201312170-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leppälä JM, Virtamo J, Fogelholm R, Huttunen JK, Albanes D, Taylor PR, et al. Controlled trial of alpha-tocopherol and beta-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler Thromb Vasc Biol. (2000) 20:230–5. 10.1161/01.atv.20.1.230 [DOI] [PubMed] [Google Scholar]
- 59.Li JY, Taylor PR, Li B, Dawsey S, Wang GQ, Ershow AG, et al. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst. (1993) 85:1492–8. 10.1093/jnci/85.18.1492 [DOI] [PubMed] [Google Scholar]
- 60.Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. (2009) 101:14–23. 10.1093/jnci/djn438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu BA, McGeer A, McArthur MA, Simor AE, Aghdassi E, Davis L, et al. Effect of multivitamin and mineral supplementation on episodes of infection in nursing home residents: a randomized, placebo-controlled study. J Am Geriatr Soc. (2007) 55:35–42. 10.1111/j.1532-5415.2006.01033.x [DOI] [PubMed] [Google Scholar]
- 62.Margalit DN, Kasperzyk JL, Martin NE, Sesso HD, Gaziano JM, Ma J, et al. Beta-carotene antioxidant use during radiation therapy and prostate cancer outcome in the Physicians’ Health Study. Int J Radiat Oncol Biol Phys. (2012) 83:28–32. 10.1016/j.ijrobp.2011.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayne ST, Cartmel B, Baum M, Shor-Posner G, Fallon BG, Briskin K, et al. Randomized trial of supplemental beta-carotene to prevent second head and neck cancer. Cancer Res. (2001) 61:1457–63. [PubMed] [Google Scholar]
- 64.Papadimitrakopoulou VA, Lee JJ, William WN, Jr., Martin JW, Thomas M, Kim ES, et al. Randomized trial of 13-cis retinoic acid compared with retinyl palmitate with or without beta-carotene in oral premalignancy. J Clin Oncol. (2009) 27:599–604. 10.1200/JCO.2008.17.1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Age-Related Eye Disease Study 2 Research Group, Papudesu C, Clemons TE, Agrón E, Chew EY. Association of mortality with ocular diseases and visual impairment in the age-related eye disease study 2: age-related eye disease study 2 report number 13. Ophthalmology. (2018) 125:512–21. 10.1016/j.ophtha.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pathak AK, Bhutani M, Guleria R, Bal S, Mohan A, Mohanti BK, et al. Chemotherapy alone vs. chemotherapy plus high dose multiple antioxidants in patients with advanced non small cell lung cancer. J Am Coll Nutr. (2005) 24:16–21. 10.1080/07315724.2005.10719438 [DOI] [PubMed] [Google Scholar]
- 67.Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst. (2007) 99:137–46. 10.1093/jnci/djk017 [DOI] [PubMed] [Google Scholar]
- 68.Prince MI, Mitchison HC, Ashley D, Burke DA, Edwards N, Bramble MG, et al. Oral antioxidant supplementation for fatigue associated with primary biliary cirrhosis: results of a multicentre, randomized, placebo-controlled, cross-over trial. Aliment Pharmacol Ther. (2003) 17:137–43. 10.1046/j.1365-2036.2003.01398.x [DOI] [PubMed] [Google Scholar]
- 69.Qu CX, Kamangar F, Fan JH, Yu B, Sun XD, Taylor PR, et al. Chemoprevention of primary liver cancer: a randomized, double-blind trial in Linxian, China. J Natl Cancer Inst. (2007) 99:1240–7. 10.1093/jnci/djm084 [DOI] [PubMed] [Google Scholar]
- 70.Rautalahti MT, Virtamo JR, Taylor PR, Heinonen OP, Albanes D, Haukka JK, et al. The effects of supplementation with alpha-tocopherol and beta-carotene on the incidence and mortality of carcinoma of the pancreas in a randomized, controlled trial. Cancer. (1999) 86:37–42. [PubMed] [Google Scholar]
- 71.Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, et al. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry. (2004) 75:216–30. 10.1016/s1529-1839(04)70049-4 [DOI] [PubMed] [Google Scholar]
- 72.Toma S, Bonelli L, Sartoris A, Mira E, Antonelli A, Beatrice F, et al. beta-carotene supplementation in patients radically treated for stage I-II head and neck cancer: results of a randomized trial. Oncol Rep. (2003) 10:1895–901. [PubMed] [Google Scholar]
- 73.Törnwall ME, Virtamo J, Korhonen PA, Virtanen MJ, Taylor PR, Albanes D, et al. Effect of alpha-tocopherol and beta-carotene supplementation on coronary heart disease during the 6-year post-trial follow-up in the ATBC study. Eur Heart J. (2004) 25:1171–8. 10.1016/j.ehj.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 74.Virtamo J, Edwards BK, Virtanen M, Taylor PR, Malila N, Albanes D, et al. Effects of supplemental alpha-tocopherol and beta-carotene on urinary tract cancer: incidence and mortality in a controlled trial (Finland). Cancer Causes Control. (2000) 11:933–9. 10.1023/a:1026546803917 [DOI] [PubMed] [Google Scholar]
- 75.Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, Virtanen MJ, et al. ATBC Study Group. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. (2003) 290:476–85. 10.1001/jama.290.4.476 [DOI] [PubMed] [Google Scholar]
- 76.Wang SM, Taylor PR, Fan JH, Pfeiffer RM, Gail MH, Liang H, et al. Effects of nutrition intervention on total and cancer mortality: 25-year post-trial follow-up of the 5.25-year linxian nutrition intervention trial. J Natl Cancer Inst. (2018) 110:1229–38. 10.1093/jnci/djy043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wright ME, Virtamo J, Hartman AM, Pietinen P, Edwards BK, Taylor PR, et al. Effects of alpha-tocopherol and beta-carotene supplementation on upper aerodigestive tract cancers in a large, randomized controlled trial. Cancer. (2007) 109:891–8. 10.1002/cncr.22482 [DOI] [PubMed] [Google Scholar]
- 78.Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, et al. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. (2010) 127:172–84. [DOI] [PubMed] [Google Scholar]
- 79.Palozza P, Luberto C, Calviello G, Ricci P, Bartoli GM. Antioxidant and prooxidant role of beta-carotene in murine normal and tumor thymocytes: effects of oxygen partial pressure. Free Radic Biol Med. (1997) 22:1065–73. 10.1016/s0891-5849(96)00498-4 [DOI] [PubMed] [Google Scholar]
- 80.Palozza P, Serini S, Trombino S, Lauriola L, Ranelletti FO, Calviello G. Dual role of beta-carotene in combination with cigarette smoke aqueous extract on the formation of mutagenic lipid peroxidation products in lung membranes: dependence on pO2. Carcinogenesis. (2006) 27:2383–91. 10.1093/carcin/bgl074 [DOI] [PubMed] [Google Scholar]
- 81.Verkaik-Kloosterman J, McCann MT, Hoekstra J, Verhagen H. Vitamins and minerals: issues associated with too low and too high population intakes. Food Nutr Res. (2012) 56: *p, 10.3402/fnr.v56i0.5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hercberg S, Galan P, Preziosi P, Alfarez MJ, Vazquez C. The potential role of antioxidant vitamins in preventing cardiovascular diseases and cancers. Nutrition. (1998) 14:513–20. 10.1016/s0899-9007(98)00040-9 [DOI] [PubMed] [Google Scholar]
- 83.Ben-Amotz A, Levy Y. Bioavailability of a natural isomer mixture compared with synthetic all-trans beta-carotene in human serum. Am J Clin Nutr. (1996) 63:729–34. 10.1093/ajcn/63.5.729 [DOI] [PubMed] [Google Scholar]
- 84.Patrick L. Beta-carotene: the controversy continues. Altern Med Rev. (2000) 5:530–45. [PubMed] [Google Scholar]
- 85.Drisko JA, Chapman J, Hunter VJ. The use of antioxidant therapies during chemotherapy. Gynecol Oncol. (2003) 88:434–9. 10.1016/s0090-8258(02)00067-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality of the included trials by using the Cochrane Collaboration risk of bias tool.
Quality of the included trials by using the Cochrane Collaboration risk of bias tool in summary with the studies.
The GRADE assessment of the study’s quality for the all-cause mortality outcome.
The GRADE assessment of the study’s quality for the all-cause mortality outcome with beta carotene alone.
Subgroup analysis by the type of supplement (alone or in association).
PRISMA checklist.
Subgroup analysis by country.
Publication bias evaluation of each study by using the Cochrane Collaboration risk of bias tool.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.