Abstract
Craniofacial and jaw bones have unique physiological specificities when compared to axial and appendicular bones. However, the molecular profile of the jaw osteoblast (OB) remains incomplete. The present study aimed to decipher the bone site-specific profiles of transcription factors (TFs) expressed in OBs in vivo. Using RNA sequencing analysis, we mapped the transcriptome of confirmed OBs from 2 different skeletal sites: mandible (Md) and tibia (Tb). The OB transcriptome contains 709 TF genes: 608 are similarly expressed in Md-OB and Tb-OB, referred to as “OB-core”; 54 TF genes are upregulated in Md-OB, referred to as “Md-set”; and 18 TF genes are upregulated in Tb-OB, referred to as “Tb-set.” Notably, the expression of 29 additional TF genes depends on their RNA transcript variants. TF genes with no previously known role in OBs and bone were identified. Bioinformatics analysis combined with review of genetic disease databases and a comprehensive literature search showed a significant contribution of anatomical origin to the OB signatures. Md-set and Tb-set are enriched with site-specific TF genes associated with development and morphogenesis (neural crest vs. mesoderm), and this developmental imprint persists during growth and homeostasis. Jaw and tibia site-specific OB signatures are associated with craniofacial and appendicular skeletal disorders as well as neurocristopathies, dental disorders, and digit malformations. The present study demonstrates the feasibility of a new method to isolate pure OB populations and map their gene expression signature in the context of OB physiological environment, avoiding in vitro culture and its associated biases. Our results provide insights into the site-specific developmental pathways governing OBs and identify new major OB regulators of bone physiology. We also established the importance of the OB transcriptome as a prognostic tool for human rare bone diseases to explore the hidden pathophysiology of craniofacial malformations, among the most prevalent congenital defects in humans.
Keywords: transcriptome, RNA-seq, jawbone, tibia, transcription factor, bone disease
Introduction
Driven by a complex network of transcription factors (TFs), the osteoblast (OB) is traditionally considered a unique differentiated mesenchymal cell that synthesizes various peptides in charge of matrix deposition, biomineralization, and remodeling during bone growth, homeostasis, and healing (reviewed in Lian et al. 2006). However, craniofacial and jawbones exhibit a number of physiological specificities when compared to axial and appendicular bones (reviewed in Wang et al. 2020). Craniofacial bones and long bones primarily develop through different ossification processes: intramembranous ossification for most craniofacial bones and endochondral ossification for long bones. Long bone growth and homeostasis, which ensure skeletal mobility and load bearing, are mainly regulated by mechanical strains and hormones. Jawbone development also depends on teeth, as evidenced by its reduced volume in inherited tooth agenesis or after tooth extraction (Bertl et al. 2018; Nowwarote et al. 2019). Jawbones and long bones also respond differently to various agents. Estrogens and parathyroid hormone imbalances affect mineral density in long bones disproportionately (Liu et al. 2009; Coutel et al. 2019; Watanabe et al. 2020). Bisphosphonates and other antiresorptive drugs have few adverse effects on long bones but alter jawbone metabolism, sometimes culminating in jaw osteonecrosis (reviewed in Katsarelis et al. 2015). Finally, autografts taken from craniofacial bones offer better outcomes regardless of the acceptor site when compared with grafts from long bones (Akintoye et al. 2006; Leucht et al. 2008).
Unsurprisingly then, jawbones and long bones show major differences at the cellular level. Craniofacial bone progenitors show differential proliferation and osteogenic potential when compared to long bone progenitors (Matsubara et al. 2005; Leucht et al. 2008; Aghaloo et al. 2010; Kelder et al. 2020). Their cell lineages also differ: while most craniofacial OBs derive from cranial neural crest, long bone OBs are of mesodermal origin (Le Douarin 1980; Gans and Northcutt 1983; Chai et al. 2000; Yoshida et al. 2008). Furthermore, in vivo and in vitro analyses suggest the existence of cell site-specific signatures between jawbones and long bones (Kasperk et al. 1995), notably for a number of TFs (Rawlinson et al. 2009; Kingsmill et al. 2013; Reichert et al. 2013; Lee et al. 2015). However, to date, the transcriptome of the jaw OB in its physiological environment has remained elusive, as the studies focusing on jaws were performed using either cell culture or whole-tissue extracts. Moreover, jawbone cells, including OBs but also osteocytes and bone marrow cells, have been omitted from large-scale omics studies on the tissue-specific landscape (Zhou et al. 2017; Tabula Muris Consortium et al. 2018; Youlten et al. 2021). Here, we prove the feasibility of a new isolation method to obtain pure populations of functional OBs within their physiological environment. This strategy allowed us to define the complete repertoires of TF genes expressed in jaw- and tibia-OBs in vivo. These data provide insights into the developmental and genetic pathways governing site-specific OB differentiation and activity, as well as identify major OB regulators involved in jawbone physiology and disorders.
Materials and Methods
All experiments were approved by the Charles Darwin ethics committee (CEEACD/05) and conducted in accordance with the European legislation for the care and use of laboratory animals. This study adheres to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for reporting animal. For ARRIVE guidelines checklist and detailed Materials and Methods, see the Appendix. Protocols of RNA sequencing (RNA-seq) and RNA-seq raw and processed data files are publicly available in the Gene Expression Omnibus (GEO) (accession GSE186535).
Results
OB Expression Profiles Include TFs Conserved among Bone Sites and Site-Specific TFs
The in vivo TF signatures expressed in functional OBs were defined by using a global gene profiling approach based on RNA-seq of flow-sorted green fluorescent protein (GFP)–positive cells from mandibular (Md) and tibia (Tb) bones of 9-d-old (P9) Col1a1*2,3-GFP mice (Fig. 1A). Our RNA-seq results were cross-referenced with a list of 1,388 TFs (GEO accession GSE186535). Md-OB or/and Tb-OB expressed 709 TF genes (977 transcripts), that is, more than 50% of the known TF genes (Appendix Table 2). The largest group, termed “OB-core,” comprises 608 TF genes (86%) similarly expressed in Md-OBs and Tb-OBs. The remaining TF genes were differentially expressed between the 2 skeletal sites: “Md-set” consists of 54 TF genes (8%) upregulated in Md-OBs, and “Tb-set” consists of 18 TF genes (2%) upregulated in Tb-OBs (Fig. 1B). Notably, for 29 TF genes (4%), the bone site specificity depended on their RNA transcript variants (TVs) (Appendix Fig. 1). To decipher how the anatomical origin contributed to the OB signatures, the 3 OB groups were further investigated with an enrichment analysis based on the biological processes (BPs) of the functional Gene Ontology (GO) annotation using DAVID and a literature review (Fig. 1C–E, Appendix Figs. 2 and 3).
Figure 1.
Osteoblasts display a bone site-specific transcriptional profile and mesenchymal identity in their physiological environment. (A) Workflow of the RNA sequencing transcriptional study of Md-OBs and Tb-OBs. (B) Total number of TFs commonly expressed (OB-core) or overexpressed by Md-OBs (Md-set) or by Tb-OBs (Tb-set). (C–E) Genes are classified according to their tissue affinities. (C) GO and enrichment analysis of TFs associated with mesenchymal tissues/cells in OB-core, Md-set, and Tb-set (data are presented as –log10 [P value]) pos., positive; neg. negative. (D) RPKM expression heat map of TF genes in Md-set associated with mesenchymal tissues/cells (only RPKM values >1 are shown). RPKM, reads per kilobase of transcript per million mapped reads. *Site-specific genes (all the expressed transcripts of a given gene are increased in Md-OB). (E) Genes known to be involved in different mesenchymal tissues but reported here for the first time as regulators of OBs and bone for the Md-set. (F) Quantitative reverse transcriptase–polymerase chain reaction analysis of in vivo gene expression of Sp6 in tissues isolated from C57BL/6JR mice at E10.5, E14.5, E16.5, E18.5, P1, and P9. Messenger RNA levels are expressed as percentage of TF expression in P9 Md-bone. ND, Non detected, ***p ≤ 0.001. (G–I) Fluorescence imaging of functional OBs (GFP+) and Sp6-expressing cells (pink+) in the BA1 at E10.5 (G) and in the JB at E15.5 (H) and P9 (I) in Col1a1*2,3-GFP mice (scale bars: 200 μm for the main images, 50 μm for the inserts). BA1, first branchial arch; BP, biological process; GO, Gene Ontology; HL, hindlimb; Inc, incisor; JB, jawbone; M, Meckel’s cartilage; Md, mandible; Mo, molar; NT, neural tube; OB, osteoblast; OV, otic vesicle; Tb, tibia; TF, transcription factor; vXX, transcript variant identified by the last 2 numbers of the messenger RNA sequence NM number.
OB and bone BPs
Analysis of the OB transcriptome showed that OB-core and Md-set were enriched with TF genes associated with OB and bone-related BPs (Fig. 1C, Table). In OB-core, 21% of the TF genes (135/637) were previously established as regulators of OB (e.g., Atf3, Klf6, Runx2, Sp7, Tsc22d) (Appendix Fig. 2). The highest expressed TF genes of OB-core (with mean [RPKM] >100) were all previously known for their involvement in OB (Atf3/4, Tsc22d1/3, Egr1, FosB, Hnrnpk, Klf6, and Nr4a). However, the percentage of TF genes with known roles in OB decreased along with the TF expression level, and between 75% and 80% of the TFs with mean (RPKM) <25 have no previously known role in OB and bone (Appendix Fig. 2). To validate these results, quantitative polymerase chain reaction (qPCR) and immunofluorescence (IF)analyses were performed. In line with our RNA-seq results, no significant difference in messenger RNA (mRNA) levels between Md- and Tb-bones isolated from P9 mice was detected for the 8 investigated TF genes (Appendix Figs. 2 and 4). Runx2 and Sp7 IF analyses showed a similar protein expression pattern, with almost all the functional OBs expressing both Sp7 and Runx2 independently of their localization (Appendix Fig. 4). In Md-set, 62% of the TF genes (46/74) were previously known to regulate OB and bone (Fig. 1D). Among them, 22 TF genes were expressed specifically in Md-OBs (including Alx genes [Alx1/3/4], Dlx genes [Dlx1/2], Pax genes [Pax3/9], and Hand2, Msx2, Six2, Sox5), and 24 TF genes showed lower expression in Tb-OBs (including Dlx genes [Dlx3/4/5], Sox genes [Sox9/11], and Msx1, Osr2, Pbx1, Sp6) (Fig. 1D).
Table.
Functional Enrichment Analysis of GO Terms in the OB-Core and Md-Set of TF Genes Associated with Biological Processes Categorized into “Osteoblast Development and Differentiation” and “Ossification and Mineralization.”
| Bone Site | BP GO Term | Category | No. of Genes | Gene Name | Fold Enrichment | P Value |
|---|---|---|---|---|---|---|
| OB-core | Osteoblast differentiation | GO:0001649 | 24 | Cbfb, Cebpb, Cebpd, Creb3l1, Dlx5, Gli1, Gli2, Hey1, Junb, Jund, Lef1, Mef2c, Mef2d, Nfatc1, Phb, Runx2, Satb2, Smad1, Smad3, Smad5, Snai1, Snai2, Sp7, Twist1 | 3.2 | 1.6E-06 |
| OB development and differentiation | Osteoblast development | GO:0002076 | 6 | Gli2, Hey1, Jund, Runx2, Satb2, Smad3 | 10.0 | 2.4E-04 |
| Regulation of osteoblast differentiation | GO:0045667 | 14 | Cebpb, Cebpd, Dlx5, Gli1, Hey1, Jund, Mef2c, Nfatc1, Runx2, Smad1, Smad3, Smad5, Snai2, Twist1 | 3.1 | 5.2E-04 | |
| Positive regulation of osteoblast differentiation | GO:0045669 | 9 | Cebpb, Cebpd, Dlx5, Hey1, Jund, Mef2c, Runx2, Smad1, Smad5 | 4.0 | 1.9E-03 | |
| Osteoblast fate commitment | GO:0002051 | 3 | Runx2, Smad1, Smad5 | 17.9 | 1.0E-02 | |
| Ossification and mineralization | Ossification | GO:0001503 | 39 | Cbfb, Cebpb, Cebpd, Creb3l1, Dlx5, Foxc1, Gata1, Gli1, Gli2, Hey1, Hif1a, Junb, Jund, Klf10, Lef1, Mef2c, Mef2d, Nfatc1, Nfe2, Osr1, Phb, Rbpj, Runx1, Runx2, Runx3, Satb2, Scx, Smad1, Smad3, Smad5, Smad6, Snai1, Snai2, Sp1, Sp3, Sp7, Tcf7l2, Thra, Twist1 | 2.9 | 7.0E-09 |
| Regulation of ossification | GO:0030278 | 23 | Cebpb, Cebpd, Creb3l1, Dlx5, Egr2, Gata1, Gli1, Hey1, Hif1a, Jund, Mef2c, Nfatc1, Nfe2, Osr1, Pbx1, Rbpj, Runx2, Smad1, Smad3, Smad5, Smad6, Snai2, Twist1 | 3.2 | 3.9E-06 | |
| Positive regulation of ossification | GO:0045778 | 11 | Cebpb, Cebpd, Creb3l1, Dlx5, Egr2, Gata1, Gli1, Hey1, Hif1a, Jund, Mef2c, Nfatc1, Nfe2, Osr1, Pbx1, Rbpj, Runx2, Smad1, Smad3, Smad5, Smad6, Snai2, Twist1 | 3.3 | 1.9E-03 | |
| Negative regulation of ossification | GO:0030279 | 9 | Gata1, Hif1a, Mef2c, Nfatc1, Nfe2, Rbpj, Smad3, Smad6, Twist1 | 3.1 | 8.6E-03 | |
| Replacement ossification | GO:0036075 | 5 | Dlx5, Mef2c, Mef2d, Runx2, Scx | 5.0 | 1.7E-02 | |
| Endochondral ossification | GO:0001958 | 5 | Dlx5, Mef2c, Mef2d, Runx2, Scx | 5.0 | 1.7E-02 | |
| Bone mineralization | GO:0030282 | 8 | Gata1, Hif1a, Klf10, Mef2c, Nfe2, Osr1, Smad3, Tcf7l2 | 2.6 | 3.4E-02 | |
| Regulation of bone mineralization | GO:0030500 | 7 | Gata1, Hif1a, Mef2c, Nfe2, Osr1, Smad3, Twist1 | 2.8 | 3.9E-02 | |
| Md-set | Regulation of osteoblast differentiation | GO:0045667 | 7 | Dlx5, Gli3, Hand2, Msx2, Smad5, Sox11, Twist2 | 13.4 | 1.3E-05 |
| OB development and differentiation | Positive regulation of osteoblast differentiation | GO:0045669 | 5 | Dlx5, Gli3, Msx2, Smad5, Sox11 | 18.7 | 1.4E-04 |
| Osteoblast differentiation | GO:0001649 | 7 | Dlx5, Gli3, Hand2, Msx2, Smad5, Sox11, Twist2 | 8.0 | 2.2E-04 | |
| Regulation of ossification | GO:0030278 | 11 | Dlx5, Gli3, Hand2, Msx2, Osr2, Pbx1, Six2, Smad5, Sox11, Sox9, Twist2 | 12.9 | 1.1E-08 | |
| Ossification and mineralization | Ossification | GO:0001503 | 10 | Dlx5, Gli3, Hand2, Msx2, Osr2, Smad5, Sox11, Sox9, Tcf7l2, Twist2 | 6.4 | 2.2E-05 |
| Positive regulation of ossification | GO:0045778 | 6 | Dlx5, Gli3, Msx2, Osr2, Smad5, Sox11 | 15.3 | 4.3E-05 | |
| Negative regulation of ossification | GO:0030279 | 3 | Hand2, Sox9, Twist2 | 8.8 | 4.5E-02 | |
| Bone mineralization | GO:0030282 | 3 | Osr2, Sox9, Tcf7l2 | 8.3 | 4.9E-02 |
BP, biological process; GO, Gene Ontology; Md, mandible; No. number; OB, osteoblast; Tb, tibia; TF, transcription factor.
Other connective/mesenchymal tissue BPs
OB-core was significantly enriched with TF genes associated with cartilage, skeletal muscle, and fat BPs, whereas enrichment with TF genes associated with dental BPs was low (Fig. 1C, Appendix Fig. 2). Although Md-set displayed an enrichment profile similar to that of OB-core for bone- and cartilage-related BPs, dental BPs were overrepresented in Md-set, whereas skeletal- and fat-related BPs were low and mostly associated with negative regulation (Fig. 1C, Appendix Fig. 3). Gene-by-gene analysis of Md-set confirmed the high contribution of dental-associated genes, as 26% of the TF genes (19/74) have known roles in odontogenesis and odontoblast differentiation (e.g., Dlx4, Alx3, Lhx8, Dlx1) (Fig. 1D).
In Tb-set, a gene-by-gene literature analysis revealed bone-related TF genes known for their regulatory roles in cartilage (e.g., Tbx18, Hoxa5/d8, Nfia), skeletal muscle (e.g., Heyl, Hoxa7, Crem, Nfya), and fat (e.g., Creb3l3, Hoxa9/c5, Tbx15). Shox2 was the only TF known to have a role in odontogenesis but also in bone, cartilage, muscle, and fat (Appendix Fig. 3). Notably, TFs known for their involvement in the regulation of mesenchymal cells and tissues other than OBs and bone and never described in differentiated OBs were identified in the RNA-seq panel. These novel OB TF genes include Arid5a/5b, Ybx3, Meox2, and Creb5 in OB-core; Zfp90/110, Sp6, Rxrb, and Bhlhb9 in Md-set; and Sox7 and Irf7 in Tb-set (Fig. 1E, Appendix Figs. 2 and 3). mRNA expression of some of these TF genes was validated in raw bones from P9 mice (Appendix Figs. 2 and 3). To investigate one of the novel OB TFs in Md-set, the expression of Sp6, a known odontogenesis regulator, was analyzed by qPCR and IF from E10.5 to P9. In line with our RNA-seq results, Sp6 was detected at mRNA and protein levels in craniofacial tissues, including in Md-bone and Md-OBs from E14.5 to P9, while no expression was detected in appendicular skeleton (Fig. 1F–I, Appendix Fig. 5).
Site-Specific OB-Omics: Blueprints of Embryonic Signatures
Development and morphogenesis BPs
To address the contribution of embryonic BPs to OB signatures at postnatal stage P9, we first performed enrichment analysis of the 3 OB sets (Fig. 2A). The 3 sets were enriched in TF genes associated with the following BPs: skeletal system, limb and appendage development, and morphogenesis. Only OB-core and Md-set displayed enrichment in TF genes associated with head and craniofacial developmental BPs, while “limb bud formation” and “embryonic digit morphogenesis” were enriched exclusively in Md-set. OB-core was enriched with TF genes associated with mesoderm development BPs and Md-set with neural crest (NC) BPs, whereas Tb-set displayed no enrichment in any of these BPs. RNA-seq data were also analyzed in light of 278 TFs associated with NC, based on information generated from the literature (Fig. 2B–C, Appendix Table 3). Thirty-nine percent of Md-set TF genes (29/74) were associated with NC, and all these genes were directly associated with cranial NC (CNC) (e.g., Alx1/3/4, Dlx1/2/3/5, Msx1/2, Sox5/9/11) or with organogenesis/patterning of craniofacial skeletal elements (Pax9) or NC differentiation (Nfatc4). Six Tb-set TF genes were associated with caudal NC and participated in positional identity in the anterior–posterior axis (Hoxa5/a7/c10/c5, Tbx18) and the epithelial–mesenchymal transition (Akna). The expression of 2 TFs associated with CNC, Dlx3 and Sox9 was further investigated from E10.5 to P9. Dlx3 and Sox9 showed high mRNA expression levels as previously described (Appendix Table 4) and, in line with our RNA-seq data, were significantly higher in head and craniofacial tissues, including Md-bone, when compared to appendicular skeleton (Fig. 2D–E, Appendix Fig. 5). In situ IF imaging of Sox9 confirmed its known localization in cartilaginous tissues and demonstrated its protein expression in Md-bone and Md-OBs (Fig. 2F–H).
Figure 2.
The osteoblast transcriptome is characterized by site-specific developmental imprints. (A) GO and enrichment analysis of TF genes associated with developmental processes and morphogenesis in OB-core, Md-set, and Tb-set. (B) Venn diagram of TF genes associated with neural crest (NC) cells in OB-core, Md-set, and Tb-set. (C) RPKM expression heatmap of TF genes in Md-set and Tb-set associated with NC cells (only RPKM values >1 are shown). RPKM, reads per kilobase of transcript per million mapped reads; vXX, transcript variant identified by the last 2 numbers of the mRNA sequence NM number. (D, E) Quantitative reverse transcriptase–polymerase chain reaction analysis of in vivo gene expression of Dlx3 (D) and Sox9 (E) in tissues isolated from C57BL/6JR mice at E10.5, E14.5, E16.5, E18.5, P1, and P9. Messenger RNA levels are expressed as percentage of TF expression in P9 Md-bone. **p ≤ 0.01, ***p ≤ 0.001. (F–H) Fluorescence imaging of functional OBs (GFP+) and Sox9-expressing cells (pink+) in BA1 at E10.5 (F) and JB at E15.5 (G) and P9 (H) in Col1a1*2,3-GFP mice (scale bars: 200 μm for the main images, 50 μm for the inserts). BA1, first branchial arch; BP, biological process; CNC, cranial neural crest; EMT, epithelial to mesenchymal transition; GO, Gene Ontology; HL, hindlimb; Inc, incisor; JB, jawbone; M, Meckel’s cartilage; Md, mandible; Mo, molar; NC, neural crest; NT, neural tube; OB, osteoblast; OV, otic vesicle; Tb, Tibia; TF, transcription factor.
Long-term maintenance of in vivo and ex vivo OB embryonic positional identity
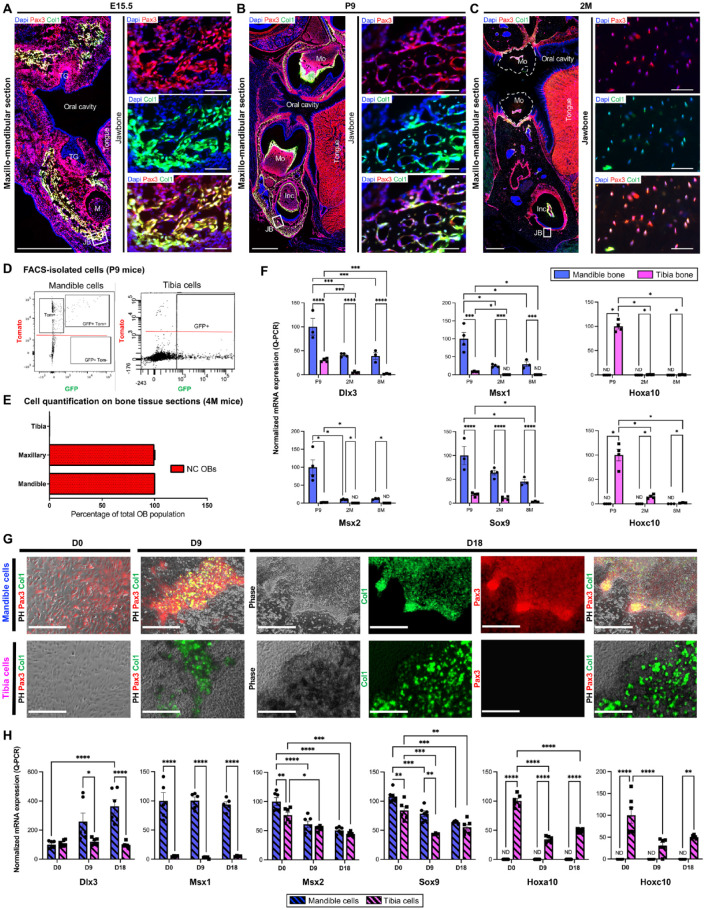
To determine whether Md-OB origin remains neuroectodermal with age, we examined the distribution of NC-derived cells in the growing oral bones at embryonic (E15.5), postnatal (P9), and homeostasis stages (2 mo [2M], 4 mo [4M], and 8 mo [8M]), taking advantage of the individual cell visibility of fluorescent markers in Pax3-cre::Col1a1*2,3-GFP::Rosatomato mice (Fig. 3 and Appendix Fig. 5). Microscopic observations (Fig. 3A–C) and cell quantification using colocalization of tomato, GFP, and DAPI signals on fluorescence-activated cell sorting–isolated cells (Fig. 3D) and tissue sections (Fig. 3E) revealed that OBs from both jaws (upper and lower) were of NC origin from development to adulthood. Dlx3, Msx1, Msx2, and Sox9 mRNA levels were significantly higher in mandible bone when compared to tibia bone at P9, 2M, and 8M, whereas Hoxa10 and Hoxc10 mRNA were only detected in tibia bone (Fig. 3F). To investigate this embryonic signature ex vivo, we cultured primary osteoprogenitors from mandible (Md-cells) and tibia (Tb-cells) of P9 Pax3-cre::Col1a1*2,3-GFP::Rosatomato mice (Fig. 3G–H). While the Md-cells that differentiated in vitro were double positive (GFP+, Tom+), Tb-cells displayed only GFP signal (Fig. 3G). Differential mRNA expression of Dlx3, Msx1, Msx2, and Sox9 in Md-cells and of Hoxa10 and Hoxc10 in Tb-cells persisted in vitro (Fig. 3H).
Figure 3.
Osteoblast embryonic positional identity is maintained in vivo and ex vivo. (A–D) Fluorescence imaging of functional OBs (GFP+) and NC-derived cells (Tomato+) in jawbone at embryonic (E15.5) (A), postnatal (P9) (B), and 2 mo (2M) (C) stages in Pax3-cre::Col1a1*2,3-GFP::Rosatomato mice (scale bars: 500 μm for the main images, 50 μm for the inserts). (D) Double labeling flow cytometry for GFP and Tomato of cells isolated from mandible and tibia of P9 Pax3-cre::Col1a1*2,3-GFP::Rosatomato mice. (E) Quantification (cell counting) of NC-derived cells (tomato+) in mandible, maxillary, and tibia from Pax3-cre::Col1a1*2,3-GFP::Rosatomato mice at 4M. (F) Quantitative reverse transcriptase–polymerase chain reaction (RT-qPCR) analysis of in vivo gene expression of Dlx3, Msx1, Msx2, Sox9, Hoxa10, and Hoxc10 in mandible and tibia bone tissues isolated from C57BL/6JR mice at P9, 2M, and 8 mo (8M). Messenger RNA (mRNA) levels are expressed as percentage of TF expression in P9 Md-bone (for Dlx3, Msx1, Msx2, Sox9) or in P9 Tb-Bone (for Hoxa10 and Hoxc10). (G) Primary cell culture of osteoprogenitors isolated from mandible and tibia of P9 Pax3-cre::Col1a1*2,3-GFP::Rosatomato mice. At confluency, cells were grown in osteogenic medium and observed at day 0 (D0), day 9 (D9), and day 18 (D18) (scale bar: 400 μm). (H) RT-qPCR analysis of ex vivo gene expression of Dlx3, Msx1, Msx2, Sox9, Hoxa10, and Hoxc10 in mandible and tibia osteoprogenitors. mRNA levels are expressed in percentage of TF expression in D0 mandible cells (for Dlx3, Msx1, Msx2, Sox9) or in D0 tibia cells (for Hoxa10 and Hoxc10). ND, Non detected, *p < 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001. Inc, incisor; JB, jawbone; M, Meckel’s cartilage; Md, mandible; Mo, molar; NC, neural crest; OB, osteoblast; PH, phase; Tb, tibia; TF, transcription factor; TG, tooth germ.
Site-Specific OB-Omics: A Prognostic Tool for Rare Diseases
Using the OMIM database and a comprehensive literature review, we identified 29 TFs associated with human genetic disorders (out of 101 differentially expressed genes) in our omics data (Fig. 4A, Appendix Table 5). Twenty-six of these TF genes are directly associated with mineralized tissue pathologies, whereas 3 are linked to deficiencies in hematopoiesis and/or immune function (KLF1, IRF1, and IRF7) (Fig. 4B, C).
Figure 4.
Human mutations associated with TF genes of jaw and tibia site-specific osteoblast signatures and their consequences in pathophysiology. (A) RPKM expression heatmap of TF genes in Md-set and Tb-set associated with human genetic disorders (only RPKM values >1 are shown). Rpkm: Reads Per Kilobase of transcript per Million mapped reads, *site-specific genes (all the expressed transcripts of a given gene are commonly expressed in Md-OBs and Tb-OBs), vXX: transcript variant identified by the last 2 numbers of the mRNA sequence NM number. (B, C) OMIM- and Orphanet-based gene-by-gene analysis of genetic disorders and conditions caused by human mutations of Md-set and Tb-set according to tissue type and anatomical region. Pathological growth modification patterns are indicated with yellow arrows. (D) Quantitative reverse transcriptase–polymerase chain reaction analysis of in vivo gene expression of Alx3, Pax3, and Pax9 in mandible and tibia bone tissues isolated from C57BL/6JR mice at postnatal (P9), 2 mo (2M), and 8 mo (8M) stages. Messenger RNA (mRNA) levels are expressed as percentage of TF expression in P9 Md-bone. Md, mandible; OB, osteoblast; Tb, tibia; TF, transcription factor; ND, Non detected, p > 0.5 (ns), *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001. Abbreviations of human genetic disorders: AI, amelogenesis imperfecta; AML, acute myelogenous leukemia; AMS, ablepharon-macrostomia syndrome; BRMUTD, brain malformations with or without urinary tract defects/1p31p32 microdeletion syndrome; CAKUTHED, congenital anomalies of kidney and urinary tract syndrome with or without hearing loss, abnormal ears, or developmental delay; CDAN, congenital dyserythropoietic anemia (type IV); CDHS, craniofacial-deafness-hand syndrome; CRS, craniosynostosis (types 3 and 5); DRRS, Duane-radial ray syndrome (Okihiro syndrome); FFDD3, focal facial dermal dysplasia 3; FND, frontonasal dysplasia (types 1, 2, and 3); GCPS, Greig cephalopolysyndactyly syndrome; ICF2, immunodeficiency-centromeric instability-facial anomalies syndrome 2; INLU, blood group–Lutheran inhibitor; IVIC, Instituto Venezolano de Investigaciones Cientificas; MDS, myelodysplastic syndrome; OFC, orofacial cleft (type 5); PAP, polydactyly, postaxial (types A1 and B); PFM, parietal foramina 1; PFMCCD, parietal foramina with cleidocranial dysplasia; PHS, Pallister–Hall syndrome; PPD, preaxial postdactyly (type 4); PFM, parietal foramina; RMS, rhabdomyosarcoma 2, alveolar; SAMS, short stature, auditory canal atresia, mandibular hypoplasia, skeletal abnormalities; SHFM, split hand/foot malformation; STHA, selective tooth agenesis (type 3); TDO, trichodontoosseous syndrome; WS, Waardenburg syndrome, type 1.
Twenty neurocristopathies are known to be caused by 9 TFs in Md-set (ALX1/3/4, DLX3, GLI3, GSC, MSX2, PAX3, SOX9). Except for IRF1, all the human disorders associated with Md-set genes display orofacial features. Thirteen TF genes are associated with jawbone phenotypes (11 in mandible, 5 in maxilla) as well as cranial bone pathologies (13/13), orofacial clefts (9/13), and dental abnormalities (6/13) (Fig. 4B). In humans, mutations of some Md-set TF genes are not associated with clinical jawbone features but instead with defects in cranial bones, palate, and/or teeth (ALX3/4, DLX4, MSX1/2, PAX9, PBX1, SALL4, SOX11, SP6, TBX3) (Fig. 4C). Mutations of the Md-set genes DLX3, DLX5, GLI3, GSC, MSX2, SALL4, SOX9, and TBX3 cause not only craniofacial defects in humans but also disorders in long bones and/or shoulder girdle/gluteal regions (Fig. 4C, Appendix Table 5).
The dysmorphological patterns of these human rare diseases are mirrored by the differential mRNA levels of TFs measured in mouse jaw and tibia OBs and bones by RNA-seq and qPCR (Figs. 1–4) and the site-specific in situ localization of TFs identified by IF (Figs. 1 and 2) and previously reported (Appendix Table 4). For example, mutations of TF genes highly expressed in Tb-OBs (Dlx3/5, Sox9) (Fig. 2D–E, Appendix Fig. 5) are associated with long-bone disorders in humans. Mutations of genes weakly expressed in both Md-OBs and Tb-OBs or with relatively low fold changes between the 2 OB populations affect both craniofacial bones and long bones (e.g., GSC, SALL4, TBX3) and/or are included in clinical synopses of short stature (GLI3, GSC, PBX1, SOX11, MYCN) (Appendix Table 5). This is also the case for NFIA and TBX15, which are more significantly expressed in Tb-set but at low levels and whose mutations in humans affect both long bones and craniofacial bones. Among the genes we found by RNA-seq and that cause both oral and appendicular bone disorders in humans are Dlx5, Pbx1, and Nfia; these genes are characterized by both bone site-specific TVs as well as TVs commonly expressed in OB-core (Appendix Fig. 1). Finally, in line with our bioinformatics analyses showing enrichment in Md-set with TF genes associated with limb bud formation and digit morphogenesis (Fig. 2A), mutations of 14 Md-set TF genes have been implicated in human digit malformations. Together, these data suggest that the OB transcriptome may be used as a diagnostic tool to predict the affected bone sites and other disorders such as dental anomalies, neurocristopathies, or digit malformations associated with TF mutations.
Discussion
This study defined the complete repertoires of TF genes expressed in jaw and tibia OBs in their native physiological environments. To do so, we established a new and easily reproducible approach by isolating functional OBs directly from tissues and thus avoiding cell culture, which may introduce biases in gene expression (Ryu et al. 2017). Notably, our results evidenced differentially expressed transcript variants from a given gene between bone sites, including coding and noncoding RNA sequences, of key OB regulators such as Dlx5 and Runx2. These results are in line with osteocyte transcriptome mapping and strongly suggest additional levels of regulation of proteins synthesized by bone cells (Youlten et al. 2021).
In these OB populations free of contaminating cell types and stages, we identified a large TF set conserved between mandible and tibia OBs. This supports a model in which a central transcriptional network, OB-core, operates in all OBs of the skeleton independently of their localization. Other TF genes were site specific, highlighting the importance of the experimental models chosen when studying bone disorders, notably those of the jaws. In line with previous studies (Aïoub et al. 2007; Duverger et al. 2013; Reichert et al. 2013; Nassif et al. 2014; Lee et al. 2015), the preferential expression of Msx/Dlx genes in mandible and Hox genes in tibia is supported here, and these genes are identified as being specifically related to functional OBs in vivo. Msx/Dlx/Hox homeoproteins operate under different contexts: 1) in early stages, they drive rostro-caudal patterning and morphogenesis, and 2) thereafter, they trigger key OB functions, including the secretion of a number of noncollagenous peptides (Molla et al. 2010; Duverger et al. 2012; Isaac et al. 2014). Overall, the maintained spatial expression of several TFs supports the existence of developmental imprinting in differentiated OBs at postnatal and adult stages. Furthermore, in vivo NC cell tracking demonstrated the exclusivity of CNC and the absence of mesoderm participation in jaw OBs. In tibia, CNC participation was excluded. Thus, OBs maintain both a spatial and an embryologic identity from growth to advanced postnatal stages. This implies that OBs differentiate from resident cell niches during growth and homeostasis, as fibroblasts do (Rinkevich et al. 2015; Mah et al. 2017).
In OB-core, although the cardinal bone TFs were found (e.g,. Runx2, Sp7, Twist1), most TF genes (almost 80%) had not been previously described as being expressed in differentiated OBs or associated with OB or bone. The same observation holds true for the Md and Tb sets. The present exhaustive intersite comparative approach therefore completes the previously incomplete OB transcriptional profile and provides a better understanding of OBs within their physiological environments. The newly identified genes in the OB-core and site-specific TF sets will allow reevaluation of the bone symptoms of the associated genetic diseases.
We confirmed the relevance of the jaw and tibia OB signatures by comparing the detected TFs to genes implicated in monogenic skeletal diseases. Genes and rare diseases affecting other organs were also analyzed. Such was the case for the teeth, which share an embryological origin with orofacial bones. Jaw OBs display a unique mesenchymal profile highly enriched with dental-related TF genes (e.g., Pax9, Msx2, Dlx2, Sp6), consistent with human tooth and bone genes/phenotypes (de La Dure-Molla et al. 2019). In addition, our bioinformatics analyses showed enrichment of biological processes involved with limb bud formation and digit morphogenesis in Md-set. Analysis of human mutations confirmed that 14 of these genes are associated with digit malformations. Thus, in addition to a number of genes consistently associated with neurocristopathies and craniofacial development, the present study also suggests molecular similarities between craniofacial bones and digits.
Notably, mutations of some TF genes of Md-set have not yet been associated with jawbone disorders in humans. These synopses appear to contradict not only the RNA-seq, qPCR, and IF data for OB and raw jaw/tibia bones reported here but also the described mouse jawbone phenotypes for various mutations, such as Alx3 and Alx4 (Beverdam et al. 2001), Dlx4 (Wu et al. 2015), Msx1 (Nassif et al. 2014), and Msx2 (Aïoub et al. 2007). These observations support that jawbone abnormalities might also be present but not diagnosed in human disorders and highlight the need for systematic exploration of the jawbones while investigating skeletal and dental diseases.
Apart from rare diseases, this molecular signature may underlie known site-specific bone behavior in pathophysiology. For instance, the exclusive CNC origin and Hox-negative status of jaw OBs are shared with other oral mesenchymal cells such as gingival fibroblasts (Isaac et al. 2018). This singular profile has been shown to provide these cells with increased stemness and regenerative potential (Wang et al. 2009; Jiang et al. 2018). These qualities are crucial for tissue regeneration and stem cell therapies, as consistently reflected in the superior efficiency of jaw- versus long-bone grafts (Akintoye et al. 2006; Leucht et al. 2008).
In conclusion, the OB TF transcriptome provides insights into the pathways governing site-specific OB development and homeostasis, and it identifies novel OB regulators involved in bone physiology and human rare diseases. Integrative analysis of the OB reveals that its TF signatures appear to be developmental imprints of distinct embryonic origin (NC/mesoderm) that may directly influence the behavior of bones and dictate their anatomical site specificities.
Author Contributions
A. Nassif, G. Lignon, contributed to data acquisition and interpretation, drafted and critically revised the manuscript; A. Asselin, contributed to conception, design, data acquisition and interpretation, and critically revised the manuscript; C.C. Zadikian, S. Petit, C. Klein, contributed to data acquisition, critically revised the manuscript; H.W. Sun, F.C. Ferré, M.I. Morasso, contributed to data analysis, critically revised the manuscript; A. Berdal, B.P.J. Fournier, contributed to data analysis and interpretation, critically revised the manuscript; J. Isaac, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_00220345221074356 for Transcriptional Regulation of Jaw Osteoblasts: Development to Pathology by A. Nassif, G. Lignon, A. Asselin, C.C. Zadikian, S. Petit, H.W. Sun, C. Klein, F.C. Ferré, M.I. Morasso, A. Berdal, B.P.J. Fournier and J. Isaac in Journal of Dental Research
Acknowledgments
We thank Dr. Benoit Robert and Dr. Yvan Lallemand of the Institut Pasteur; the INSERM UMRS 1138 Histology, Cell Imaging and Flow Cytometry Platform (CICH); and the animal core facilities of INSERM UMRS 1138 for their expert technical support.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Université de Paris and the French National Research Agency (ANR) (IdEx Université de Paris [grant ANR-18-IDEX-0001] and the Osteodiversity-ANR consortium [ANR-12-BSV1-0018]) and grants from “Fondation des Gueules Cassées” and the Federation Hospitalo-Universitaire FHU DDS-ParisNet, Assistance Publique-Hôpitaux de Paris (AP-HP), Inserm, and Université de Paris.
ORCID iDs: G. Lignon  https://orcid.org/0000-0002-6670-5690
https://orcid.org/0000-0002-6670-5690
References
- Aghaloo TL, Chaichanasakul T, Bezouglaia O, Kang B, Franco R, Dry SM, Atti E, Tetradis S. 2010. Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J Dent Res. 89(11):1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aïoub M, Lézot F, Molla M, Castaneda B, Robert B, Goubin G, Néfussi JR, Berdal A. 2007. Msx2–/– transgenic mice develop compound amelogenesis imperfecta, dentinogenesis imperfecta and periodental osteopetrosis. Bone. 41(5):851–859. [DOI] [PubMed] [Google Scholar]
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. 2006. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 38(6):758–768. [DOI] [PubMed] [Google Scholar]
- Bertl K, Bertl MH, Heimel P, Burt M, Gahleitner A, Stavropoulos A, Ulm C. 2018. Alveolar bone resorption after primary tooth loss has a negative impact on straightforward implant installation in patients with agenesis of the lower second premolar. Clin Oral Implants Res. 29(2):155–163. [DOI] [PubMed] [Google Scholar]
- Beverdam A, Brouwer A, Reijnen M, Korving J, Meijlink F. 2001. Severe nasal clefting and abnormal embryonic apoptosis in Alx3/Alx4 double mutant mice. Development. 128(20):3975–3986. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. 2000. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 127(8):1671–1679. [DOI] [PubMed] [Google Scholar]
- Coutel X, Delattre J, Marchandise P, Falgayrac G, Béhal H, Kerckhofs G, Penel G, Olejnik C. 2019. Mandibular bone is protected against microarchitectural alterations and bone marrow adipose conversion in ovariectomized rats. Bone. 127:343–352. [DOI] [PubMed] [Google Scholar]
- de La Dure-Molla M, Fournier BP, Manzanares MC, Acevedo AC, Hennekam RC, Friedlander L, Boy-Lefèvre M-L, Kerner S, Toupenay S, Garrec P, et al. 2019. Elements of morphology: standard terminology for the teeth and classifying genetic dental disorders. Am J Med Genet A. 179(10):1913–1981. [DOI] [PubMed] [Google Scholar]
- Duverger O, Isaac J, Zah A, Hwang J, Berdal A, Lian JB, Morasso MI. 2013. In vivo impact of Dlx3 conditional inactivation in neural crest-derived craniofacial bones. J Cell Physiol. 228(3):654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverger O, Zah A, Isaac J, Sun HW, Bartels AK, Lian JB, Berdal A, Hwang J, Morasso MI. 2012. Neural crest deletion of Dlx3 leads to major dentin defects through down-regulation of Dspp. J Biol Chem. 287(15):12230–12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gans C, Northcutt RG. 1983. Neural crest and the origin of vertebrates: a new head. Science. 220(4594):268–273. [DOI] [PubMed] [Google Scholar]
- Isaac J, Erthal J, Gordon J, Duverger O, Sun HW, Lichtler AC, Stein GS, Lian JB, Morasso MI. 2014. DLX3 regulates bone mass by targeting genes supporting osteoblast differentiation and mineral homeostasis in vivo. Cell Death Differ. 21(9):1365–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J, Nassif A, Asselin A, Taïhi I, Fohrer-Ting H, Klein C, Gogly B, Berdal A, Robert B, Fournier BP. 2018. Involvement of neural crest and paraxial mesoderm in oral mucosal development and healing. Biomaterials. 172:41–53. [DOI] [PubMed] [Google Scholar]
- Jiang D, Correa-Gallegos D, Christ S, Stefanska A, Liu J, Ramesh P, Rajendran V, De Santis MM, Wagner DE, Rinkevich Y. 2018. Two succeeding fibroblastic lineages drive dermal development and the transition from regeneration to scarring. Nat Cell Biol. 20(4):422–431. [DOI] [PubMed] [Google Scholar]
- Kasperk C, Wergedal J, Strong D, Farley J, Wangerin K, Gropp H, Ziegler R, Baylink DJ. 1995. Human bone cell phenotypes differ depending on their skeletal site of origin. J Clin Endocrinol Metab. 80(8):2511–2517. [DOI] [PubMed] [Google Scholar]
- Katsarelis H, Shah NP, Dhariwal DK, Pazianas M. 2015. Infection and medication-related osteonecrosis of the jaw. J Dent Res. 94(4):534–539. [DOI] [PubMed] [Google Scholar]
- Kelder C, Kleverlaan CJ, Gilijamse M, Bakker AD, de Vries TJ. 2020. Cells derived from human long bone appear more differentiated and more actively stimulate osteoclastogenesis compared to alveolar bone-derived cells. Int J Mol Sci. 21(14):5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsmill VJ, McKay IJ, Ryan P, Ogden MR, Rawlinson SC. 2013. Gene expression profiles of mandible reveal features of both calvarial and ulnar bones in the adult rat. J Dent. 41(3):258–264. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. 1980. The ontogeny of the neural crest in avian embryo chimaeras. Nature. 286(5774):663–669. [DOI] [PubMed] [Google Scholar]
- Lee J-T, Choi S-Y, Kim H-L, Kim J-Y, Lee H-J, Kwon T-G. 2015. Comparison of gene expression between mandibular and iliac bone-derived cells. Clin Oral Investig. 19(6):1223–1233. [DOI] [PubMed] [Google Scholar]
- Leucht P, Kim JB, Amasha R, James AW, Girod S, Helms JA. 2008. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 135(17):2845–2854. [DOI] [PubMed] [Google Scholar]
- Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. 2006. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 7(1–2):1–16. [DOI] [PubMed] [Google Scholar]
- Liu H, Guo J, Wang L, Chen N, Karaplis A, Goltzman D, Miao D. 2009. Distinctive anabolic roles of 1,25-dihydroxyvitamin D(3) and parathyroid hormone in teeth and mandible versus long bones. J Endocrinol. 203(2):203–213. [DOI] [PubMed] [Google Scholar]
- Mah W, Jiang G, Olver D, Gallant-Behm C, Wiebe C, Hart DA, Koivisto L, Larjava H, Häkkinen L. 2017. Elevated CD26 expression by skin fibroblasts distinguishes a profibrotic phenotype involved in scar formation compared to gingival fibroblasts. Am J Pathol. 187(8):1717–1735. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, Nishimura M, Saito M, Nakagawa K, Yamanaka K, et al. 2005. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 20(3):399–409. [DOI] [PubMed] [Google Scholar]
- Molla M, Descroix V, Aïoub M, Simon S, Castañeda B, Hotton D, Bolaños A, Simon Y, Lezot F, Goubin G, et al. 2010. Enamel protein regulation and dental and periodontal physiopathology in MSX2 mutant mice. Am J Pathol. 177(5):2516–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif A, Senussi I, Meary F, Loiodice S, Hotton D, Robert B, Bensidhoum M, Berdal A, Babajko S. 2014. Msx1 role in craniofacial bone morphogenesis. Bone. 66:96–104. [DOI] [PubMed] [Google Scholar]
- Nowwarote N, Osathanon T, Kanjana K, Theerapanon T, Porntaveetus T, Shotelersuk V. 2019. Decreased osteogenic activity and mineralization of alveolar bone cells from a patient with amelogenesis imperfecta and FAM83H 1261G>T mutation. Genes Dis. 6(4):391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson SC, McKay IJ, Ghuman M, Wellmann C, Ryan P, Prajaneh S, Zaman G, Hughes FJ, Kingsmill VJ. 2009. Adult rat bones maintain distinct regionalized expression of markers associated with their development. PLoS One. 4(12):e8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert JC, Gohlke J, Friis TE, Quent VM, Hutmacher DW. 2013. Mesodermal and neural crest derived ovine tibial and mandibular osteoblasts display distinct molecular differences. Gene. 525(1):99–106. [DOI] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, et al. 2015. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 348(6232):aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu AH, Eckalbar WL, Kreimer A, Yosef N, Ahituv N. 2017. Use antibiotics in cell culture with caution: genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci Rep. 7(1):7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabula Muris Consortium. 2018. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature. 562(7727):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Gilbert JR, Zhang X, Zhao B, Ker DFE, Cooper GM. 2020. Calvarial versus long bone: implications for tailoring skeletal tissue engineering. Tissue Eng Part B Rev. 26(1):46–63. [DOI] [PubMed] [Google Scholar]
- Wang KC, Helms JA, Chang HY. 2009. Regeneration, repair and remembering identity: the three Rs of Hox gene expression. Trends Cell Biol. 19(6):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Lewis S, Guo X, Ni A, Lee BS, Deguchi T, Kim D-G. 2020. Regional variations of jaw bone characteristics in an ovariectomized rat model. J Mech Behav Biomed Mater. 110:103952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Mandal S, Choi A, Anderson A, Prochazkova M, Perry H, Gil-Da-Silva-Lopes VL, Lao R, Wan E, Tang PL-F, et al. 2015. DLX4 is associated with orofacial clefting and abnormal jaw development. Hum Mol Genet. 24(15):4340–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. 2008. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 125(9–10):797–808. [DOI] [PubMed] [Google Scholar]
- Youlten SE, Kemp JP, Logan JG, Ghirardello EJ, Sergio CM, Dack MRG, Guilfoyle SE, Leitch VD, Butterfield NC, Komla-Ebri D, et al. 2021. Osteocyte transcriptome mapping identifies a molecular landscape controlling skeletal homeostasis and susceptibility to skeletal disease. Nat Commun. 12(1):2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Liu M, Xia X, Gong T, Feng J, Liu W, Liu Y, Zhen B, Wang Y, Ding C, et al. 2017. A mouse tissue transcription factor atlas. Nat Commun. 8:15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_00220345221074356 for Transcriptional Regulation of Jaw Osteoblasts: Development to Pathology by A. Nassif, G. Lignon, A. Asselin, C.C. Zadikian, S. Petit, H.W. Sun, C. Klein, F.C. Ferré, M.I. Morasso, A. Berdal, B.P.J. Fournier and J. Isaac in Journal of Dental Research